Abstract
Purpose
To compare palbociclib + letrozole and palbociclib + fulvestrant with chemotherapy agents in postmenopausal women with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) advanced/metastatic breast cancer (ABC/MBC) who had no prior systemic treatment for advanced disease (first line) or whose disease progressed after prior endocrine therapy or chemotherapy (second line).
Methods
A systematic search identified randomized controlled trials (RCTs) published from January 2000 to January 2016 that compared endocrine-based therapies, chemotherapy agents, and/or chemotherapy agents + biological therapies in the first- and second-line treatment of postmenopausal women with HR+/HER2− ABC/MBC. The main outcome of interest was progression-free survival (PFS)/time to progression (TTP). Bayesian network meta-analyses (NMAs) and pairwise meta-analyses were conducted. Heterogeneity and inconsistency were assessed.
Results
Sixty RCTs met eligibility criteria and were stratified by line of therapy. In the first line, palbociclib + letrozole showed statistically significant improvements in PFS/TTP versus capecitabine [intermittent: HR 0.28 (95% CrI 0.11–0.72)] and mitoxantrone [HR 0.28 (0.13–0.61)], and trended toward improvements versus paclitaxel [HR 0.59 (0.19–1.96)], docetaxel [HR 0.51 (0.14–2.03)] and other monotherapy or combination agents (HRs ranging from 0.24 to 0.99). In the second line, palbociclib + fulvestrant showed statistically significant improvements in PFS/TTP versus capecitabine [intermittent: HR 0.28 (0.13–0.65)], mitoxantrone [HR 0.26 (0.12–0.53)], and pegylated liposomal doxorubicin [HR 0.19 (0.07–0.50)], and trended toward improvements versus paclitaxel [HR 0.48 (0.16–1.44)], docetaxel [HR 0.71 (0.24–2.13)] and other monotherapy or combination agents (HRs ranging from 0.23–0.89). NMA findings aligned with direct evidence and were robust to sensitivity analyses.
Conclusions
Palbociclib + letrozole and palbociclib + fulvestrant demonstrate trends in incremental efficacy compared with chemotherapy agents for the first- and second-line treatment of HR +/HER2− ABC/MBC.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-017-4404-4) contains supplementary material, which is available to authorized users.
Keywords: Advanced/metastatic breast cancer, Chemotherapy, Network meta-analysis, Palbociclib, Progression-free survival, Time to progression
Introduction
Postmenopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor type 2-negative (HER2−) tumors represent the majority of patients with advanced/metastatic breast cancer (ABC/MBC) [1–3]. Despite the sometimes indolent course of the disease, HR+/HER2− ABC/MBC remains incurable [1–3]. Guidelines suggest that endocrine therapy should be offered as standard first-line treatment in patients who do not have visceral crises [1–3]. After receiving first-line endocrine therapy, many patients experience disease progression due to endocrine resistance and are offered chemotherapy as second-line therapy [2]. Various monotherapy and combination chemotherapy regimens are available, providing treatment options for patients with endocrine resistance [4].
Palbociclib (IBRANCE®; Pfizer Inc, New York, NY, USA) is a new oral cyclin-dependent kinase 4/6 (CDK4/6) inhibitor approved by the United States (US) Food and Drug Administration (FDA) for HR+/HER2− ABC/MBC in combination with letrozole as initial endocrine-based therapy [5], or in combination with fulvestrant for patients whose disease had progressed following prior endocrine therapy [6]. The efficacy and safety of palbociclib combination therapies have been demonstrated in phase 3 clinical studies [5, 6]; however, a comparison of progression-free survival (PFS) has not been made between palbociclib and chemotherapy agents. Here, we report the results of a systematic literature review (SLR) and network meta-analysis (NMA) that evaluates the efficacy of palbociclib + letrozole and palbociclib + fulvestrant versus chemotherapy agents in postmenopausal women with HR+/HER2− ABC/MBC who had no prior systemic treatment for advanced disease (first line) or whose disease had progressed after prior endocrine therapy or chemotherapy (second line).
Methods
Systematic literature review
An SLR was conducted to identify randomized controlled trials (RCTs) published from January 2000 to January 2016. All references used in two previous NMAs by Generali et al. [7] and Chirila et al. [8] formed the starting point for the current systematic review. These represent the most recent NMAs conducted for chemotherapy agents and for endocrine therapies. The NMA by Generali et al. [7] compared everolimus + exemestane with various chemotherapy agents, and the literature search spanned from 2000 to May 2014. The NMA by Chirila et al. [8] compared palbociclib with other endocrine-based therapies, and the literature search was conducted in January 2015 with no date restrictions. An updated literature search was performed by searching MEDLINE, EMBASE, Cochrane CENTRAL, and PubMed from May 2014 (search date of Generali) to January 2016 to identify RCTs that were published since the aforementioned two reviews. A predefined search strategy (Online Appendix A) was used, based on the previous searches by Generali et al. [7] and Chirila et al. [8]. The search was designed to identify all RCTs of chemotherapy agents, chemotherapy agents + biological therapies, and endocrine therapies used to treat postmenopausal women with HR+/HER2− ABC/MBC who had not received any prior systemic anticancer treatment for advanced disease (first line) or whose disease had progressed after prior endocrine therapy or chemotherapy (second line). However, the current analysis focuses only on chemotherapy agents.
Predefined eligibility criteria were used to screen all identified studies (Online Appendix B; additional details are available upon request). Phase 2 and phase 3 RCTs and conference abstracts were included. Treatments of interest included chemotherapy agents, chemotherapy agents + biological therapies, and endocrine-based therapies. Endocrine-based therapies were included in all analyses but have not been reported here, given that the focus of this analysis is on chemotherapy agents. Outcomes of interest were PFS, time to progression (TTP), and overall survival (OS), reported as hazard ratios (HRs) with 95% confidence intervals (CIs). PFS and TTP were considered as equivalent outcomes since the definitions aligned well across studies and any heterogeneity was considered non-substantial. As the outcome of disease progression is a negative event for patients, HRs < 1 corresponded to beneficial treatment effects of the first treatment compared with the second treatment. The analysis of OS has been excluded here due to lack of availability of final OS data from the palbociclib clinical trials.
Two reviewers independently reviewed citation titles and abstracts identified in the updated literature search to assess study eligibility. Citations considered to describe potentially eligible articles were independently reviewed in full-text form. A PRISMA flow diagram documenting the process of study selection was prepared.
Network meta-analysis
Network meta-analysis is a widely used approach to derive estimates of effect among treatments that may not have been compared directly in clinical trials. Bayesian NMAs and pairwise meta-analyses were conducted to pool RCT results using well-established methods outlined by the National Institute for Health and Care Excellence (NICE) [9, 10]. Two separate evidence networks were generated to stratify studies by first and second lines of therapy. Based on the line of therapy definitions used in the palbociclib clinical trials [5, 6], first line of therapy was defined as having neither previous systemic endocrine therapy nor chemotherapy for ABC/MBC, and second line of therapy was defined as having previous systemic endocrine therapy or chemotherapy for ABC/MBC.
For each pairwise comparison, HRs with 95% credible intervals (CrIs) were used as a measure of the association between the treatment and its efficacy. Estimates with 95% CrIs that excluded the null value of 1 were considered to reflect statistically significant differences between interventions. Additional measures of effect were also generated, including Surface Under the Cumulative RAnking curve (SUCRA) values (expressed as percentages, which show the relative probability of an intervention being among the best options), probability best, and mean rank [11]. For interpretation, SUCRA values and probability best range between 0 and 1, with values closer to 1 being preferred [11].
Fixed-effects models were performed as primary analyses, given that the networks are largely composed of single-study connections. Random-effects models were performed as secondary analyses, using both informative and vague priors on the variance. Informative priors were based on an estimate of between-study variance using data from previous Cochrane systematic reviews [12]. For vague priors, we assumed a uniform distribution [i.e., Uniform (0, 5)] for between-study variance, as recommended by the NICE [9]. To assess whether the models had adequate fit to the data, the posterior residual deviance from each NMA was compared to the corresponding number of unconstrained data points; approximately equal values represented an adequate fit.
Network meta-analyses were performed using WinBUGS (version 1.4.3) and R (version 3.2.2) and were based on burn-in samples of at least 40,000 iterations and subsequent sampling iterations of at least 50,000 iterations (WinBUGS code is available upon request). Trace plots and Gelman–Rubin plots were reviewed to assess model convergence.
Assessment of heterogeneity and inconsistency
In accordance with the exchangeability assumption of NMAs [13], study and patient characteristics were assessed to ensure similarity and to investigate the potential impact of heterogeneity on effect estimates. Factors considered included mean/median age, HR status, HER2 status, menopausal status, prior therapies, crossover after disease progression, blinding, drug dosing, and endpoint definitions. Heterogeneity was assessed by summarizing relevant information using tables and by conducting sensitivity analyses where possible. The presence of several single-study connections between interventions in the evidence networks precluded us from performing meta-regression analyses or sub-group/sensitivity analyses related to certain characteristics of interest [14]. Sensitivity analyses were conducted to include both the palbociclib phase 2 and 3 studies [5, 15], and to adjust for heterogeneity in median PFS/TTP values.
The NMA results were qualitatively compared with pairwise estimates generated from traditional frequentist meta-analyses of direct evidence. Inconsistency in the networks was assessed by comparing deviance and deviance information criterion (DIC) statistics in fitted consistency and inconsistency models [16]. The posterior mean deviance of the individual data points in the inconsistency model was plotted against the corresponding posterior mean deviance in the consistency model to identify any loops where inconsistency was present (available upon request).
Results
Study selection
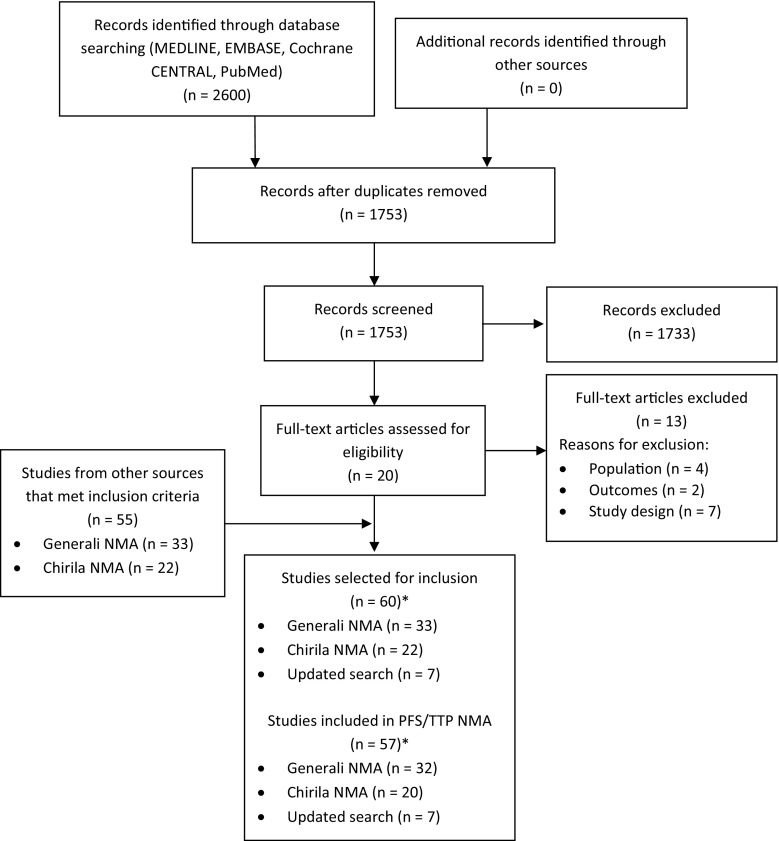
The NMA by Generali et al. [7] included 44 RCTs, which were not stratified by line of therapy. The NMA by Chirila et al. [8] included 27 RCTs, stratified by line of therapy. Of these, 53 RCTs met the eligibility criteria described above. In addition, two recently published studies that provided updated PFS results for palbociclib trials in first line [5] and second line [6] were included. Among the 2600 study records that were identified in the updated literature search, seven RCTs met the eligibility criteria and were included in the NMA.
In total, 60 RCTs (from the three SLRs) met the eligibility criteria; however, only 57 RCTs were included in the PFS/TTP NMA that is presented here (Fig. 1). In order for the evidence networks to be fully connected, some connections had to be forced based on line of therapy and patient characteristics. The three connections forced based on patient characteristics were chemotherapy trials and were due to less than 50% of patients being HR+ [17–19].
Fig. 1.
PRISMA flow diagram
NMA network meta-analysis, PRISMA preferred reporting items for systematic reviews and meta-analyses. *Two studies overlapped between the Generali et al. NMA and the Chirila et al. NMA
Study and patient characteristics
The 57 RCTs included in analyses were published between 1992 and 2016, with trials conducted on all continents. Mean age across the trials ranged from 51 to 70 years, and median follow-up ranged from 6 to 61.2 months (Online Appendix C). The percentage of HR+ patients was reported in 56 of the 57 trials and ranged from about 15 to 100%, and the proportion of patients receiving prior metastatic endocrine therapy or chemotherapy ranged from 0 to 100%. Based on this high level of heterogeneity, trials were stratified by line of therapy based on prior neoadjuvant/adjuvant and advanced/metastatic therapy received by patients (details available upon request). Assessment of other study and patient characteristics revealed that many sensitivity analyses were not feasible due to insufficient information or disconnected evidence networks. Overall, the studies included in the NMA had a low risk of bias (Online Appendix D). A summary of the median PFS/TTP values and HRs used in analyses is available upon request.
First-line therapy progression-free survival/time to progression
The evidence network for the first-line PFS/TTP NMA is shown in Fig. 2. Each intervention is represented by a node and randomized comparisons are shown as links between the nodes. Overall, 22 studies were included that enrolled a total of 8152 patients with available outcomes data. Data from head-to-head trials were available for 28 pairwise comparisons in the network, with single studies informing all of these comparisons. This analysis includes data from the PALOMA-2 trial which compares palbociclib + letrozole with letrozole [5].
Fig. 2.
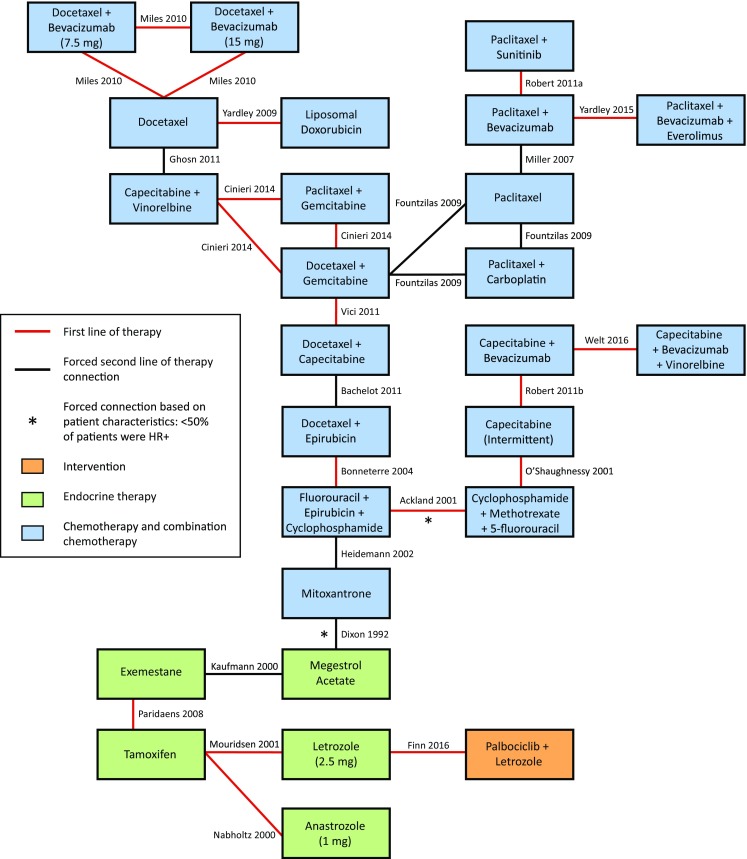
Evidence network for first-line PFS/TTP
HR+ hormone receptor positive, PFS progression-free survival, TTP time to progression
In the fixed-effects model, palbociclib + letrozole showed statistically significant improvements in PFS/TTP relative to capecitabine [intermittent: HR 0.28 (95% CrI 0.11–0.72)] and mitoxantrone [HR 0.28 (0.13–0.61)], and trended toward improvements (not statistically significant) versus paclitaxel [HR 0.59 (0.19–1.96)], docetaxel (HR 0.51 (0.14–2.03)], and other monotherapy or combination chemotherapy agents (HRs ranging from 0.24 to 0.99; Table 1). Palbociclib + letrozole ranked more favorably than all chemotherapy comparators for PFS/TTP in terms of SUCRA, probability best, and mean rank. Palbociclib + letrozole was associated with the highest SUCRA value among all treatments (96.00%), the highest probability of being the best treatment (41.70%), and a treatment ranking closest to 1. Model fit statistics from the fixed-effects model were favorable; a total residual deviance value close to the number of unconstrained data points was obtained (i.e., 25.08 vs. 25).
Table 1.
First-line therapy NMA results for PFS/TTP: palbociclib + letrozole versus comparators
| Comparisons | HR (95% CrI) Fixed-effects model |
HR (95% CrI) Random-effects model: vague priors |
HR (95% CrI) Random-effects model: informative priors |
|---|---|---|---|
| Palbociclib + letrozole | 1 | 1 | 1 |
| Single chemotherapy agents | |||
| Paclitaxel | 0.59 (0.19–1.96) | 0.59 (0.07–4.83) | 0.63 (0.07–5.48) |
| Docetaxel | 0.51 (0.14–2.03) | 0.50 (0.06–3.92) | 0.55 (0.07–4.24) |
| Capecitabine (intermittent) | 0.28 (0.11–0.72) | 0.27 (0.03–2.12) | 0.29 (0.03–2.45) |
| Mitoxantrone | 0.28 (0.13–0.61) | 0.27 (0.03–2.23) | 0.28 (0.03–2.28) |
| Combination chemotherapy agents | |||
| Paclitaxel + bevacizumab + everolimus | 0.99 (0.29–3.81) | 0.98 (0.12–8.43) | 0.93 (0.12–7.17) |
| Paclitaxel + bevacizumab | 0.98 (0.31–3.38) | 0.96 (0.13–7.29) | 0.94 (0.10–8.65) |
| Docetaxel + bevacizumab 15 mg | 0.65 (0.18–2.69) | 0.65 (0.09–4.70) | 0.72 (0.09–6.11) |
| Docetaxel + bevacizumab 7.5 mg | 0.59 (0.16–2.40) | 0.58 (0.08–4.19) | 0.64 (0.08–5.36) |
| Paclitaxel + sunitinib | 0.60 (0.18–2.14) | 0.60 (0.07–4.93) | 0.66 (0.09–5.00) |
| Docetaxel + gemcitabine | 0.59 (0.20–1.91) | 0.59 (0.08–4.32) | 0.64 (0.08–5.20) |
| Liposomal doxorubicin | 0.54 (0.14–2.29) | 0.53 (0.07–3.79) | 0.59 (0.07–4.99) |
| Paclitaxel + gemcitabine | 0.51 (0.15–1.87) | 0.49 (0.06–4.21) | 0.56 (0.08–4.06) |
| Paclitaxel + carboplatin | 0.53 (0.17–1.83) | 0.51 (0.06–4.35) | 0.57 (0.06–5.16) |
| Docetaxel + capecitabine | 0.51 (0.19–1.49) | 0.50 (0.06–4.19) | 0.54 (0.08–3.90) |
| Capecitabine + vinorelbine | 0.50 (0.16–1.72) | 0.49 (0.06–4.18) | 0.55 (0.06–4.98) |
| Capecitabine + bevacizumab + vinorelbine | 0.48 (0.19–1.27) | 0.46 (0.06–3.76) | 0.50 (0.06–4.02) |
| Docetaxel + epirubicin | 0.47 (0.20–1.19) | 0.46 (0.06–3.32) | 0.49 (0.06–4.15) |
| Capecitabine + bevacizumab | 0.40 (0.16–1.06) | 0.39 (0.05–2.82) | 0.41 (0.06–2.99) |
| Fluorouracil + epirubicin + cyclophosphamide | 0.33 (0.15–0.77) | 0.32 (0.04–2.47) | 0.34 (0.03–3.34) |
| Cyclophosphamide + methotrexate + 5-fluorouracil | 0.24 (0.11–0.57) | 0.24 (0.03–2.11) | 0.25 (0.03–1.85) |
| Model fit statistics | Residual deviance = 25.08 vs. 25 DIC = −0.02 |
Residual deviance = 25.71 vs. 25 DIC = 0.11 Heterogeneity (SD) = 0.67 (0.01–4.53) |
Residual deviance = 25.69 vs. 25 DIC = −0.05 Heterogeneity (SD) = 0.73 (0.02–3.64) |
Analyses use data from PALOMA-2 [5]. Statistically significant differences are shown in bold. Endocrine therapies have been excluded from this table, given that the focus is on chemotherapy agents. For vague priors in the random-effects model, a uniform distribution for between-study variance was assumed, as recommended by the National Institute for Health and Care Excellence [9]. Informative priors were based on an estimate of between-study variance using data from previous Cochrane systematic reviews [12]
Crl credible interval, DIC deviance information criterion, HR hazard ratio, SD standard deviation
In the random-effects models using both informative and vague priors on the variance, palbociclib + letrozole trended toward improvements (not statistically significant) versus all chemotherapy comparators. Model fit was favorable and relatively constant across both analyses (Table 1).
Second-line therapy progression-free survival/time to progression
Figure 3 presents the evidence network for the second-line PFS/TTP NMA. Overall, 44 studies were included that enrolled a total of 14,708 patients with available outcomes data. Data from head-to-head trials were available for 45 of the pairwise comparisons in the network, with single studies informing 35 of these comparisons. This analysis includes data from the PALOMA-3 trial which compares palbociclib + fulvestrant with fulvestrant 500 mg [6].
Fig. 3.
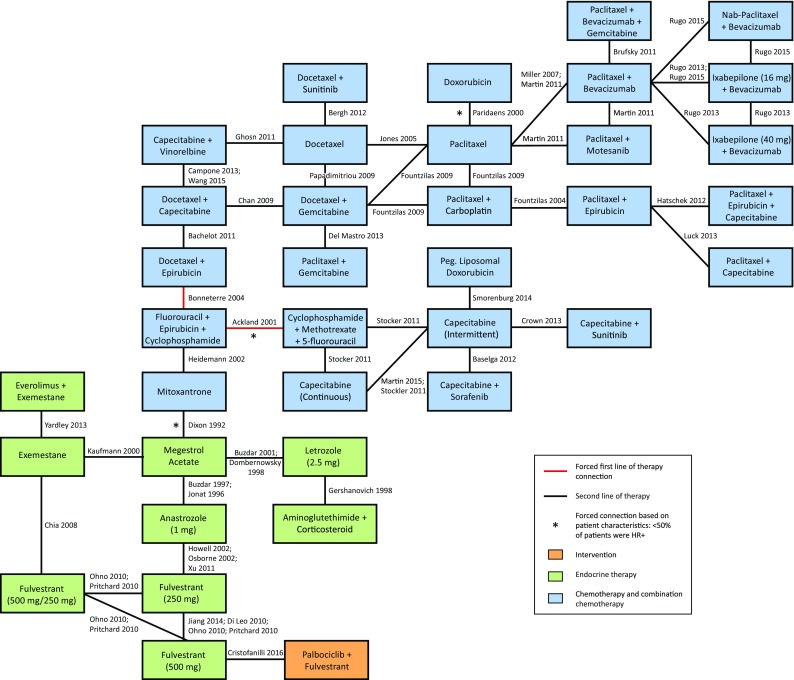
Evidence network for second-line PFS/TTP
HR+ hormone receptor positive, PFS progression-free survival, TTP time to progression
In the fixed-effects model, palbociclib + fulvestrant showed statistically significant improvements in PFS/TTP relative to capecitabine [intermittent: HR 0.28 (95% CrI 0.13–0.65); continuous: HR 0.24 (0.11–0.56)], mitoxantrone [HR 0.26 (0.12–0.53)], and pegylated liposomal doxorubicin [HR 0.19 (0.07–0.50)], and trended toward improvements (not statistically significant) versus paclitaxel [HR 0.48 (0.16–1.44)], docetaxel [HR 0.71 (0.24–2.13)], and other monotherapy or combination chemotherapy agents (HRs ranging from 0.23 to 0.89; Table 2). Palbociclib + fulvestrant ranked more favorably than all chemotherapy comparators for PFS/TTP in terms of SUCRA, probability best, and mean rank. Palbociclib + fulvestrant was associated with the highest SUCRA value among all treatments (97.20%), and an 18.90% probability of being the best treatment.
Table 2.
Second-line therapy NMA results for PFS/TTP: palbociclib + fulvestrant versus comparators
| Comparisons | HR (95% CrI) Fixed-effects model |
HR (95% CrI) Random-effects model: vague priors |
HR (95% CrI) Random-effects model: informative priors |
|---|---|---|---|
| Palbociclib + Fulvestrant | 1 | 1 | 1 |
| Single chemotherapy agents | |||
| Doxorubicin | 0.80 (0.27–2.44) | 0.81 (0.22–3.02) | 0.82 (0.19–3.40) |
| Docetaxel | 0.71 (0.24–2.13) | 0.69 (0.20–2.57) | 0.70 (0.17–2.76) |
| Paclitaxel | 0.48 (0.16–1.44) | 0.49 (0.14–1.74) | 0.49 (0.12–1.98) |
| Capecitabine (intermittent) | 0.28 (0.13–0.65) | 0.29 (0.10–0.81) | 0.29 (0.10–0.89) |
| Mitoxantrone | 0.26 (0.12–0.53) | 0.26 (0.11–0.60) | 0.26 (0.11–0.65) |
| Capecitabine (continuous) | 0.24 (0.11–0.56) | 0.25 (0.09–0.70) | 0.25 (0.09–0.78) |
| Pegylated liposomal doxorubicin | 0.19 (0.07–0.50) | 0.20 (0.06–0.64) | 0.19 (0.06–0.67) |
| Combination chemotherapy agents | |||
| Paclitaxel + bevacizumab + gemcitabine | 0.89 (0.28–2.82) | 0.89 (0.23–3.49) | 0.91 (0.20–3.95) |
| Docetaxel + sunitinib | 0.77 (0.26–2.36) | 0.75 (0.21–2.81) | 0.76 (0.18–3.12) |
| Paclitaxel + bevacizumab | 0.72 (0.24–2.20) | 0.73 (0.20–2.68) | 0.74 (0.17–3.11) |
| Paclitaxel + gemcitabine | 0.64 (0.22–1.94) | 0.67 (0.19–2.45) | 0.67 (0.16–2.77) |
| Ixabepilone 40 mg + bevacizumab | 0.61 (0.18–2.05) | 0.62 (0.15–2.53) | 0.62 (0.13–2.84) |
| Nab-paclitaxel + bevacizumab | 0.60 (0.20–1.87) | 0.60 (0.16–2.27) | 0.61 (0.13–2.61) |
| Docetaxel + gemcitabine | 0.55 (0.19–1.61) | 0.57 (0.17–1.99) | 0.58 (0.15–2.29) |
| Paclitaxel + motesanib | 0.51 (0.16–1.61) | 0.53 (0.14–2.13) | 0.53 (0.12–2.33) |
| Docetaxel + capecitabine | 0.49 (0.17–1.38) | 0.51 (0.16–1.70) | 0.51 (0.14–1.96) |
| Docetaxel + epirubicin | 0.45 (0.19–1.06) | 0.46 (0.17–1.28) | 0.47 (0.16–1.44) |
| Paclitaxel + carboplatin | 0.44 (0.14–1.38) | 0.46 (0.13–1.67) | 0.46 (0.11–1.94) |
| Ixabepilone 16 mg + bevacizumab | 0.45 (0.15–1.39) | 0.45 (0.12–1.71) | 0.46 (0.10–1.93) |
| Capecitabine + sorafenib | 0.44 (0.17–1.14) | 0.45 (0.14–1.49) | 0.44 (0.14–1.54) |
| Capecitabine + vinorelbine | 0.44 (0.15–1.28) | 0.47 (0.14–1.62) | 0.47 (0.13–1.85) |
| Paclitaxel + epirubicin | 0.35 (0.11–1.16) | 0.36 (0.09–1.44) | 0.31 (0.07–1.37) |
| Fluorouracil + epirubicin + cyclophosphamide | 0.31 (0.14–0.67) | 0.32 (0.13–0.78) | 0.31 (0.12–0.87) |
| Paclitaxel + capecitabine | 0.3 (0.09–0.98) | 0.31 (0.08–1.23) | 0.31 (0.07–1.44) |
| Paclitaxel + epirubicin + capecitabine | 0.29 (0.09–0.95) | 0.31 (0.08–1.16) | 0.37 (0.08–1.70) |
| Capecitabine + sunitinib | 0.23 (0.1–0.56) | 0.24 (0.08–0.71) | 0.23 (0.08–0.78) |
| Cyclophosphamide + methotrexate + 5-fluorouracil | 0.23 (0.1–0.51) | 0.23 (0.09–0.61) | 0.23 (0.09–0.68) |
| Model fit statistics | Residual deviance = 58.12 vs. 51 DIC = −4.11 |
Residual deviance = 52.50 vs. 51 DIC = −4.36 Heterogeneity (SD) = 0.11 (0.01–0.26) |
Residual deviance = 52.11 vs. 51 DIC = −4.63 Heterogeneity (SD) = 0.11 (0.01–0.26) |
Analyses use data from PALOMA-3 [6]. Statistically significant differences are shown in bold. Endocrine therapies have been excluded from this table, given that the focus is on chemotherapy agents. For vague priors in the random-effects model, a uniform distribution for between-study variance was assumed, as recommended by the National Institute for Health and Care Excellence [9]. Informative priors were based on an estimate of between-study variance using data from previous Cochrane systematic reviews [12]
CrI credible interval, DIC deviance information criterion, HR hazard ratio, SD standard deviation
Model fit statistics from the fixed-effects model indicated a poor fit; a total residual deviance value greater than the number of unconstrained data points was obtained (i.e., 58.12 vs. 51). This high residual deviance was largely driven by one study [20], which was removed in a sensitivity analysis and model fit improved (Online Appendix E).
In the second-line random-effects model using vague priors, palbociclib + fulvestrant showed statistically significant improvements versus capecitabine [intermittent: HR 0.29 (95% CrIR 0.10–0.81); continuous: HR 0.25 (0.09–0.70)], mitoxantrone [HR 0.26 (0.11–0.60)], and pegylated liposomal doxorubicin [HR 0.2 (0.06–0.64)] and trended toward improvements versus paclitaxel [HR 0.49 (0.14–1.74)], docetaxel [HR 0.69 (0.2–2.57)], and other monotherapy or combination chemotherapy agents (HRs ranging from 0.23 to 0.89). Similar results and statistical significance were obtained from the random-effects model using informative priors. Model fit statistics were favorable from both random-effects models (Table 2).
Sensitivity analyses
Sensitivity analyses were conducted to include both the palbociclib phase 2 and 3 studies [5, 15], and to adjust for heterogeneity in median PFS/TTP values (Online Appendix E). For each sensitivity analysis in the first line of therapy, palbociclib + letrozole was associated with improved PFS/TTP relative to all other treatments. After adjusting for heterogeneity in median PFS/TTP values in the second-line analysis, palbociclib + fulvestrant was associated with improved PFS/TTP relative to all chemotherapy comparators. Model fit was favorable across all sensitivity analyses.
Discussion
An SLR and NMAs were conducted to indirectly compare palbociclib + letrozole and palbociclib + fulvestrant with chemotherapy agents used in the first- and second-line treatment of postmenopausal women with HR +/HER2− ABC/MBC.
The first-line NMA results suggest that palbociclib + letrozole is associated with improved PFS/TTP relative to all other treatments. In the fixed-effects model, statistically significant improvements in PFS/TTP were observed in favor of palbociclib + letrozole relative to capecitabine (intermittent) and mitoxantrone, and trended toward improvements versus paclitaxel, docetaxel, and other monotherapy or combination chemotherapy agents. Findings from the random-effects models suggest that palbociclib + letrozole is associated with improved PFS/TTP relative to all other treatments, although not statistically significant.
The second-line NMA results suggest that palbociclib + fulvestrant is associated with improved PFS/TTP relative to all other chemotherapy treatments. In the fixed-effects model, statistically significant improvements in PFS/TTP were observed in favor of palbociclib + fulvestrant relative to capecitabine (intermittent and continuous), mitoxantrone, and pegylated liposomal doxorubicin, and trended toward improvements versus paclitaxel, docetaxel, and other monotherapy or combination chemotherapy agents. Results from the random-effects models aligned closely with those of the fixed-effects model.
Strengths and limitations
Palbociclib is a relatively new targeted therapy with the Palbociclib Clinical Trial Development Program still ongoing, and it is currently the only CDK inhibitor approved for use in the US. Since direct head-to-head comparisons have not been made between palbociclib and chemotherapy agents, the current NMA sought to indirectly compare these therapies. To the best of our knowledge, this is the most up-to-date systematic review and NMA to synthesize data for this population of patients with HR+/HER2− ABC/MBC. Notably, analyses were stratified by first and second line of therapy rather than considering both populations simultaneously, as was done in the NMA by Generali et al. [7]. Combining first- and second-line therapies likely violates the exchangeability assumption [13], whereas the current stratified approach adheres to best practices for the conduct of NMA [9, 21]. This study also adheres to PRISMA reporting guidelines (Online Appendix F) [22]. Thorough sensitivity analyses were conducted and yielded similar findings for both the first and second lines of therapy, providing evidence for the robustness of study results.
However, there are a few limitations associated with the analyses employed. Firstly, there is heterogeneity in patient and study characteristics, introduced primarily by the fact that the included studies span several decades. The studies included in our analyses were published between 1992 and 2016, so there is likely some heterogeneity in the diagnostic procedures that were used. Stage migration via technology may result in more patients being diagnosed with advanced stages of disease in more recent trials, which may bias survival rates. However, the structure of the evidence networks limited our ability to adjust for these factors. Despite these issues, considerable effort was taken to account for heterogeneity and inconsistency using best practices [21, 22] and approaches that are analogous to or exceed those employed by other HTA bodies [23, 24]. Various sensitivity analyses were performed, all of which yielded similar findings to the main analyses. Secondly, although analyses were stratified by line of therapy, some connections had to be forced to maintain a connected network. For example, the study by Bachelot et al. [25] was classified as a second-line study, but it was also forced into first-line networks so that chemotherapy agents of interest, such as docetaxel and paclitaxel, could be included. Three studies were also forced into networks based on patient characteristics: Ackland et al. [17], Dixon et al. [19], and Paridaens et al. [18]. Although the study by Dixon et al. [19] is the oldest study included in our analyses, it was also included by Generali et al. [7] and it appears to be the only appropriate trial available that directly compares an endocrine therapy with a chemotherapy. However, only 20–27% of patients in this study were estrogen receptor-positive (ER+), and several key study design characteristics were not reported, including randomization technique, concealment of treatment allocation, or blinding of participants and outcome assessors. Therefore, there is an elevated risk of bias associated with this trial.
Network meta-analyses were also conducted for overall survival; however, these results have been excluded from the current analysis due to immature data in the palbociclib clinical trials.
Conclusions
Palbociclib + letrozole and palbociclib + fulvestrant demonstrate trends in incremental efficacy compared with chemotherapy agents for the first- and second-line treatment of postmenopausal HR+/HER2− ABC/MBC. Both palbociclib combination therapies consistently showed statistically significant improvements in PFS/TTP versus capecitabine and mitoxantrone, and trended toward improvements versus paclitaxel, docetaxel, and other monotherapy or combination chemotherapy agents. Findings from network meta-analyses were robust to sensitivity analyses, lending credibility to the analyses and conclusions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was sponsored by Pfizer, Inc. The authors would like to acknowledge Dagmara Chojecki (contract information specialist for Cornerstone Research Group) for conducting the literature search.
Funding
This study was sponsored by Pfizer, Inc.
Abbreviations
- ABC
Advanced breast cancer
- HER2−
Human epidermal growth factor receptor type 2 negative
- HR+
Hormone receptor positive
- MBC
Metastatic breast cancer
- NMA
Network meta-analysis
- PFS
Progression-free survival
- RCT
Randomized control trial
- TTP
Time to progression
Compliance with ethical standards
Conflicts of interest
Debanjali Mitra and Shrividya Iyer are employees of Pfizer, Inc. Florence R. Wilson, Abhishek Varu, and Chris Cameron are employees of Cornerstone Research Group, Inc., who were paid contractors to Pfizer in the development of this manuscript. This research was performed under a research contract between Cornerstone Research Group and Pfizer, and was funded by Pfizer.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-017-4404-4) contains supplementary material, which is available to authorized users.
Contributor Information
Florence R. Wilson, Email: fwilson@cornerstone-research.com
Abhishek Varu, Email: avaru@cornerstone-research.com.
Debanjali Mitra, Email: debanjali.mitra@pfizer.com.
Chris Cameron, Phone: 613-852-2374, Email: ccameron@cornerstone-research.com.
Shrividya Iyer, Email: shrividya.iyer@pfizer.com.
References
- 1.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, Cardoso MJ, Cufer T, El Saghir N, Fallowfield L, Fenech D, Francis P, Gelmon K, Giordano SH, Gligorov J, Goldhirsch A, Harbeck N, Houssami N, Hudis C, Kaufman B, Krop I, Kyriakides S, Lin UN, Mayer M, Merjaver SD, Nordstrom EB, Pagani O, Partridge A, Penault-Llorca F, Piccart MJ, Rugo H, Sledge G, Thomssen C, Van’t Veer L, Vorobiof D, Vrieling C, West N, Xu B, Winer E. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25(10):1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Migliaccio I, Malorni L, Hart CD, Guarducci C, Di Leo A. Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med. 2015;13(1):1–6. doi: 10.1186/s12916-015-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joy AA, Ghosh M, Fernandes R, Clemons MJ. Systemic treatment approaches in her2-negative advanced breast cancer-guidance on the guidelines. Curr Oncol. 2015;22(Suppl 1):S29–S42. doi: 10.3747/co.22.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters R, Regierer AC, Schwentner L, Geyer V, Possinger K, Kreienberg R, Wischnewsky MB, Wockel A. A comparison of international breast cancer guidelines—do the national guidelines differ in treatment recommendations? Eur J Cancer. 2012;48(1):1–11. doi: 10.1016/j.ejca.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Martin M, Rugo H, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 7.Generali D, Venturini S, Rognoni C, Ciani O, Pusztai L, Loi S, Jerusalem G, Bottini A, Tarricone R. A network meta-analysis of everolimus plus exemestane versus chemotherapy in the first- and second-line treatment of estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2015;152(1):95–117. doi: 10.1007/s10549-015-3453-9. [DOI] [PubMed] [Google Scholar]
- 8.Chirila C, Mitra D, Colosia A, Ling C, Odom D, Iyer S, Kaye JA. Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: network meta-analysis. Curr Med Res Opin. 2017 doi: 10.1080/03007995.2017.1325730. [DOI] [PubMed] [Google Scholar]
- 9.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias S, Welton NJ, Sutton AJ, Ades AE. Evidence synthesis for decision making 1: introduction. Med Decis Making. 2013;33(5):597–606. doi: 10.1177/0272989X13487604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol. 2015;68(1):52–60. doi: 10.1016/j.jclinepi.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron C, Fireman B, Hutton B, Clifford T, Coyle D, Wells G, Dormuth CR, Platt R, Toh S. Network meta-analysis incorporating randomized controlled trials and non-randomized comparative cohort studies for assessing the safety and effectiveness of medical treatments: challenges and opportunities. Syst Rev. 2015;4:147. doi: 10.1186/s13643-015-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618–640. doi: 10.1177/0272989X13485157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 16.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33(5):641–656. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackland SP, Anton A, Breitbach GP, Colajori E, Tursi JM, Delfino C, Efremidis A, Ezzat A, Fittipaldo A, Kolaric K, Lopez M, Viaro D. Dose-intensive epirubicin-based chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and fluorouracil regimen in metastatic breast cancer: a randomized multinational study. J Clin Oncol. 2001;19(4):943–953. doi: 10.1200/JCO.2001.19.4.943. [DOI] [PubMed] [Google Scholar]
- 18.Paridaens R, Biganzoli L, Bruning P, Klijn JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A, Sylvester R, Awada A, Wildiers J, Piccart M. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol. 2000;18(4):724–733. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- 19.Dixon AR, Jackson L, Chan S, Haybittle J, Blamey RW. A randomised trial of second-line hormone vs single agent chemotherapy in tamoxifen resistant advanced breast cancer. Br J Cancer. 1992;66(2):402–404. doi: 10.1038/bjc.1992.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Xu B, Yuan P, Ma F, Li Q, Zhang P, Cai R, Fan Y, Luo Y, Li Q. Capecitabine combined with docetaxel versus vinorelbine followed by capecitabine maintenance medication for first-line treatment of patients with advanced breast cancer: phase 3 randomized trial. Cancer. 2015;121(19):3412–3421. doi: 10.1002/cncr.29492. [DOI] [PubMed] [Google Scholar]
- 21.Jansen JP, Trikalinos T, Cappelleri JC, Daw J, Andes S, Eldessouki R, Salanti G. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health. 2014;17(2):157–173. doi: 10.1016/j.jval.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, Dahl M. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5(1):e35–e48. [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron C, Coyle D, Richter T, Kelly S, Gauthier K, Steiner S, Carrier M, Coyle K, Bai A, Moulton K, Clifford T, Wells G. Systematic review and network meta-analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open. 2014;4(6):e004301. doi: 10.1136/bmjopen-2013-004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachelot T, Bajard A, Ray-Coquard I, Provencal J, Coeffic D, Agostini C, Boisseau M, Kaphan R, Dramais D, Oprea C, Ferri-Dessens RM, Guastalla JP, Perol D. Final results of ERASME-4: a randomized trial of first-line docetaxel plus either capecitabine or epirubicin for metastatic breast cancer. Oncology. 2011;80(3–4):262–268. doi: 10.1159/000329066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.