Abstract
Purpose
To examine the associations between pharmaceutically treated anxiety and depression present in the year prior to breast cancer diagnosis and the risk of incident cardiovascular disease (CVD), while controlling for traditional cardiovascular risk factors and clinical characteristics in a population-based observational study.
Methods
Adult 1-year breast cancer survivors (n = 7227), diagnosed between 01-01-1999 and 12-31-2010, with no history of CVD, were selected from the Netherlands Cancer Registry. Drug dispensing data were derived from the PHARMO Database Network and used as proxy for CVD, anxiety, and depression. By multivariable Cox regression analysis, we examined the risk associated with pharmaceutically treated anxiety and depression for developing CVD after cancer diagnosis, adjusting for age, pharmaceutically treated hypertension, hypercholesterolemia, and diabetes mellitus in the year prior to cancer diagnosis, tumor stage, and cancer treatment.
Results
During the 13-year follow-up period, 193 (3%) breast cancer survivors developed CVD. Women pharmaceutically treated for anxiety in the year prior to their cancer diagnosis had a 48% increased hazard for CVD [HR = 1.48; 95% CI 1.05–1.08] after full adjustment. This association was restricted to breast cancer survivors who were 65 years or younger. Depression was not associated with CVD risk [HR = 0.89; 95% CI 0.52–1.53]. Older age [HR = 1.06; 95% CI 1.05–1.08], hypertension [HR = 1.80; 95% CI 1.32–2.46], and hypercholesterolemia [HR = 1.63; 95% CI 1.15–2.33] were associated with an increased hazard for incident CVD, whereas hormone therapy [HR = 0.59; 95% CI 0.42–0.83] was protective.
Conclusions
Anxiety present in the year prior to breast cancer diagnosis increases the risk of incident CVD in 1-year breast cancer survivors, after adjustment for depression, traditional cardiovascular risk factors, and clinical characteristics.
Keywords: Breast cancer survivors, Cardiovascular disease, Anxiety, Depression, Cardiotoxicity
Introduction
Cardiovascular disease (CVD) is a common comorbidity in breast cancer survivors [1, 2] and estimated to be responsible for many non-cancer-related mortalities [1]. This is partly due to aging, yet also a consequence of received treatment. The cardiotoxicity of cancer treatment can lead to the development of a wide range of CVDs, such as arrhythmias, heart failure, and valvular heart disease [3, 4]. Furthermore, there are similar underlying risk factors for both breast cancer and CVD, such as obesity and physical inactivity [5–7].
Incidence rates of CVD in breast cancer survivors vary depending on the type of treatment and dosage [8]. Even in individuals who all received the exact same treatment and dosage not all develop CVD [9]. Hence, additional factors, other than cardiotoxic treatment, are involved and may include traditional cardiovascular risk factors, such as hypertension or diabetes mellitus, as they increase the risk of CVD in breast cancer survivors [7, 10, 11].
Psychological factors, such as anxiety and depression, may also increase the risk of CVD in breast cancer survivors. Several studies have shown that depression and anxiety are predictive for the development and progression of CVD in non-cancer patients [12–16]. A meta-analysis based on 20 studies examining the predictive value of anxiety for the incidence of CVD in healthy individuals concluded that anxious patients had a 26% increased risk of developing CVD and a 48% increased risk of cardiac death [12]. Furthermore, depression is a risk factor for recurrent cardiac events, heart failure [13, 14], major adverse cardiac events [14, 15], and cardiac death [13]. Hence, the European Society of Cardiology included psychosocial risk factors, including anxiety and depression, in the Guidelines on Cardiovascular Disease Prevention in Clinical Practice in 2012 [11]. Moreover, in a previous study among middle-aged healthy women (similar in age to breast cancer survivors), anxiety was found to be predictive of cardiac mortality [16].
CVD is understudied, underdiagnosed, and undertreated among women, yet we know that there are sex differences in the pathophysiology of CVD [17]. Simultaneously, levels of anxiety and depression are more prominent among female cancer survivors than in males [18]. Nonetheless, to our knowledge, the association between anxiety and depression with incident CVD among female breast cancer survivors has never been studied before. The aim of this study was therefore to examine the associations between anxiety and depression present in the year prior to a breast cancer diagnosis and the risk of incident CVD in 1-year breast cancer survivors, while controlling for age, traditional cardiovascular risk factors, tumor stage, and cancer treatment (i.e., chemo-, radio-, and hormone therapy). We chose to look at 1-year survivors as cancer treatment is generally finished within the first year. In addition, we explored whether the associations between anxiety and depression with incident CVD risk differed by age, traditional cardiovascular risk factors, or cancer treatment.
Methods
Procedure and participants
Data from the Southern Region of the Netherlands Cancer Registry (NCR) were used in this observational cohort study. The NCR registers cancer diagnosis, stage, and primary cancer treatment for all newly diagnosed cancer patients and is maintained by the Netherlands Comprehensive Cancer Organization [19]. The Southern Region of the NCR covers an area of 2.4 million inhabitants [19]. For this study, the NCR was linked to data from the PHARMO Database Network for cancer patients diagnosed from 1998 onwards, and a detailed description of this linkage is found elsewhere [20]. PHARMO is a large, population-based network of electronic healthcare databases and combines data from general practices, pharmacies, and hospitals which are linked on patient-level though validated algorithms. In this study, the out-patient pharmacy database comprising healthcare products prescribed by the general practitioner or specialist was used. Dispensing records used included information on product type and date. Drug dispensings are coded according to the international Anatomical Therapeutic Chemical (ATC) classification system [21].
Female adult breast cancer patients diagnosed between 01-01-1999 and 12-31-2010 were selected from the NCR. To obtain information on survival status and date of death, the NCR was linked to the municipal Personal Records database. As anonymous observational patient information was used, this study does not fall under the Medical Research Involving Human Subjects Act in the Netherlands; therefore, this study was exempted from medical ethics review and no informed consent was required. This study was performed in agreement with the Declaration of Helsinki.
Breast cancer survivors who had a history of CVD medication use (see the definition of CVD in the next section) in the 12 months prior to their cancer diagnosis were excluded, as our aim was to examine the risk of incident CVD following cancer diagnosis. In addition, to exclude the effect of detecting CVD due to increased clinical checkups and the direct and sometimes reversible effects of receiving cancer treatment, breast cancer survivors who developed CVD in the first year after diagnosis were also excluded. Follow-up for a diagnosis of CVD began 12 months after the cancer diagnosis (which was set as the index date), as primary cancer treatment is generally finalized within the first year. Hence, we excluded those who died or were lost to follow-up during the first year. Follow-up time was measured until onset of CVD, death, loss to follow-up, or until the end of the study period (31-12-2010), whichever occurred first.
Measurements
Cardiovascular disease
CVD was defined as having at least two drug dispenses of cardiac therapeutics (i.e., ATC code C01) at unique dates within 6 months. Survivors who dispensed two cardiac drugs with less than 15 days in between were classified as having CVD only when they had three cardiac dispenses at unique dates in a 6-month period. We used a strict definition based solely on using cardiac therapeutics (ATC = C01) to avoid false classifications of CVD. Therefore, usage of CVD-related drugs such as diuretics (C03) or beta-blockers was insufficient to be classified as having CVD, as these drugs have a broad treatment range including non-CVD indications.
Psychological factors—anxiety and depression
Drug dispense information for anxiety disorders (ATC = N05B) and depression (ATC = N06A) during the 12 months prior to the cancer diagnosis was included. Patients with one or more drug dispensings were categorized as anxious or depressed (yes/no).
Traditional cardiovascular risk factors
Drug dispense information on the traditional cardiovascular risk factors including hypertension (ATC = C02, C03A, CO3B (except C03C), C07, C08, C09 (except C09X)), hypercholesterolemia (ATC = C10), and diabetes mellitus (ATC = A10) during the 12 months prior to the cancer diagnosis was captured [22]. Having one or more drug dispensings for hypertension, hypercholesterolemia, and diabetes mellitus in the 12 months prior to the cancer diagnosis was categorized as having traditional cardiovascular risk factors (yes/no). We opted for the inclusion of these traditional cardiovascular risk factors already present prior to cancer diagnosis, as cardiotoxic treatment is known to increase the risk of developing these traditional cardiovascular risk factors, which can then be seen as a precursor of cancer treatment-induced CVD itself.
Age and clinical characteristics
Survivors’ age and clinical information on tumor stage and treatment (i.e., having received chemo-, radiation-, or hormone therapy (yes/no)) were obtained from the NCR.
Statistical analyses
Differences in patient characteristics (i.e., age, psychological factors, traditional cardiovascular risk factors, and clinical characteristics) between breast cancer survivors with and without incident CVD were analyzed using ANOVA, the χ 2 test, or Student’s t test for independent samples as appropriate.
The associations between pharmaceutically treated anxiety and depression with incident CVD risk were examined separately and simultaneously using multivariable Cox regression analyses. Analyses included covariates which were entered in separate steps. First, we adjusted for age (continuous) as a potential confounder. Second, traditional cardiovascular risk factors (i.e., hypertension, hypercholesterolemia, and diabetes mellitus) were added to the model. Finally, clinical information on tumor stage, chemo-, radio-, and hormone therapy was entered, which were considered possible explanatory variables. Assumptions underlying the multivariable Cox regression analysis were met (e.g., visual inspection of the KM curve allowed confirmation of the Cox proportional hazard assumptions).
We tested effect modifications for age, traditional cardiovascular risk factors, and cancer treatment (chemo-, radio-, and hormone therapy) by adding interaction terms (i.e., depression/anxiety*age/traditional cardiovascular risk/cancer treatment) to the fully adjusted model. We examined whether the effect of anxiety and depression on CVD risk differed by age (≤65 vs >65 years at the time of cancer diagnosis), traditional cardiovascular risk factors, chemotherapy, radiation, or hormonal treatment. Missing data were handled in previous steps and described elsewhere [20]. All statistical tests were two-sided with alpha set at 5%. We chose not to use a more stringent alpha level since this is the first study relating both pharmaceutically treated anxiety and depression to CVD risk in a sample of breast cancer survivors, and hence we wanted to avoid making a type 2 error. All analyses were performed using SPSS version 22.0.
Results
Patient characteristics
Of the 7889 eligible breast cancer survivors, 515 were excluded as they received prescribed CVD medications in the 12 months before or after their cancer diagnosis, and 147 were excluded as they were deceased or lost to follow-up in the first year after cancer diagnosis. After exclusion, 7227 1-year breast cancer survivors were included in statistical analyses.
The 1-year breast cancer survivors who developed CVD differed from those who did not (Table 1)—that is—they had a 1-year shorter follow-up period, had higher mortality rates, used more often drugs for anxiety and traditional cardiovascular risk factors (i.e., hypertension, hypercholesterolemia, diabetes mellitus) in the year prior to their cancer diagnosis, and were treated less often with chemo-, radio-, and hormone therapy (all p’s <0.05).
Table 1.
Patient characteristics of 7227 1-year breast cancer survivors stratified by CVD status
| CVD (n = 193) | No CVD (n = 7034) | |
|---|---|---|
| Follow-upb time in median years (range) | 3 (0–13) | 4 (0–13)* |
| Deceased | 53 (28) | 1077 (15)* |
| Demographics | ||
| Age in median years (range) | 70 (46–91) | 60 (23–102)* |
| Psychological factors | ||
| Anxiety | 45 (23) | 976 (14)* |
| Depression | 15 (8) | 553 (8) |
| Traditional CVD risk factorsa | 118 (61) | 2214 (32)* |
| Hypertension | 106 (55) | 1892 (27)* |
| Hypercholesterolemia | 49 (25) | 810 (12)* |
| Diabetes mellitus | 21 (11) | 398 (6)* |
| Clinical characteristics | ||
| Tumor stage | ||
| 1 | 93 (49) | 3119 (45) |
| 2 | 80 (42) | 2819 (41) |
| 3 | 14 (7) | 761 (11) |
| 4 | 3 (2) | 223 (3) |
| Cancer treatment | ||
| Surgery | 184 (95) | 6677 (95) |
| Chemotherapy | 29 (15) | 2661 (38)* |
| Radiation | 123 (64) | 5042 (72)* |
| Hormone therapy | 72 (37) | 3392 (48)* |
Information is provided in numbers (n) with percentages for categorical variables, whereas follow-up time and age are presented in median years (range). CVD = cardiovascular disease. Psychological and traditional CVD risk factors = being pharmaceutically treated in the 12 months prior to breast cancer diagnosis
aBeing pharmaceutically treated for at least one of the traditional cardiovascular risk factors (hypertension, hypercholesterolemia, diabetes mellitus) during the 12 months prior to cancer diagnosis, yes/no
bFollow-up for a diagnosis of CVD began 12 months after cancer diagnosis, as primary cancer treatment is generally finalized within the first year
*Significant difference (p < 0.05) between those with and without CVD
Associations with CVD risk
Analyzing the associations between anxiety and depression present in the year prior to cancer diagnosis with CVD risk separately (data not shown) showed that anxiety in the year prior to cancer diagnosis was associated with an increased risk for incident CVD in all models: age-adjusted [hazard ratio (HR) = 1.58; 95% confidence interval (95% CI) 1.13–2.20], partially adjusted (adjusted for age and CVD risk factors) [HR = 1.45; 95% CI 1.04–2.03], and fully adjusted model [HR = 1.46; 95% CI 1.04–2.04). Surprisingly, depression was not significantly associated with CVD risk in any of the models (data not shown).
When adding both anxiety and depression simultaneously to the model (Table 2), anxiety remained associated with an increased risk for incident CVD [HR = 1.60; 95% CI 1.13–2.25], while controlling for depression. The adjustment for traditional cardiovascular risk factors slightly attenuated the effect of anxiety but remained significant [HR = 1.47; 95% CI 1.04–2.07]. This did not change after adding information on tumor stage and treatment to the model [HR = 1.48; 95% CI 1.05–2.08]. Hence, women who were anxious in the year prior to their cancer diagnosis had a 48% increased CVD hazard after adjustment for depression, traditional cardiovascular risk factors, and clinical factors. Depression was not associated with incident CVD in any of the models (Table 2).
Table 2.
Associations between pharmaceutically treated anxiety and depression with incident CVD risk after breast cancer diagnosis
| Age-adjusted model | Partially adjusted model | Fully adjusted model | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Psychological factors | |||
| Anxiety | 1.60 (1.13–2.25)* | 1.47 (1.04–2.07)* | 1.48 (1.05–2.08)* |
| Depression | 0.91 (0.53–1.57) | 0.90 (0.53–1.55) | 0.89 (0.52–1.53) |
| Demographics | |||
| Age (continuous) | 1.07 (1.06–1.08)* | 1.06 (1.05–1.08)* | 1.06 (1.05–1.08)* |
| Traditional CVD risk factors | |||
| Hypertension | – | 1.77 (1.29–2.42)* | 1.80 (1.32–2.46)* |
| Hypercholesterolemia | – | 1.77 (1.29–2.42)* | 1.63 (1.15–2.33)* |
| Diabetes mellitus | – | 1.02 (0.63–1.64) | 1.05 (0.65–1.69) |
| Clinical characteristics | |||
| Tumor stage | |||
| Stage I (reference) | – | – | – |
| Stage II | – | – | 1.14 (0.81–1.61) |
| Stage III | – | – | 0.97 (0.52–1.80) |
| Stage IV | – | – | 0.93 (0.29–3.05) |
| Cancer treatment | |||
| Chemotherapy | – | – | 1.05 (0.65–1.69) |
| Radiation | – | – | 0.78 (0.57–1.06) |
| Hormone treatment | – | – | 0.59 (0.42–0.83)* |
Information is provided in hazard ratios (HR) with 95% confidence intervals (95% CI). Partially adjusted = adjusted for age and the traditional cardiovascular risk factors (i.e., hypertension, hypercholesterolemia, and diabetes mellitus) present in the 12 months prior to cancer diagnosis; fully adjusted = adjusted for traditional cardiovascular risk factors and clinical information (tumor stage and treatment information, that is chemotherapy, radiation, and hormone treatment). Psychological and traditional CVD risk factors = being pharmaceutically treated in the 12 months prior to breast cancer diagnosis. *p < 0.05
Older age [HR = 1.06; 95% CI 1.05–1.08], taking medication for hypertension [HR = 1.80; 95% CI 1.32–2.46], and hypercholesterolemia [HR = 1.63; 95% CI 1.15–2.33] were associated with an increased hazard for incident CVD, whereas being treated with hormone therapy [HR = 0.59; 95% CI 0.42–0.83] was protective for CVD (Table 2). Taking medication for depression or diabetes mellitus, tumor stage, chemo- and radiotherapy were not associated with CVD risk (Table 2).
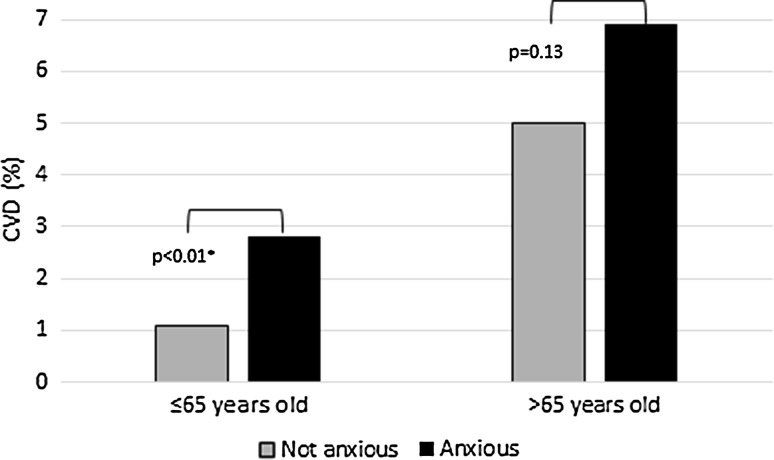
There were no cancer treatment moderation effects for anxiety on CVD risk, nor were there significant interaction effects with traditional cardiovascular risk factors. There was an age-related significant interaction effect with anxiety [HR = 0.84; 95% CI 0.74–0.96], where there remained a significant main effect of anxiety [HR = 2.04; 95% CI 1.36–3.06] on CVD risk. Stratified analyses showed that the association was restricted to women who were ≤65 years at breast cancer diagnosis [HR = 2.29; 95% CI 1.31–4.02], as there was no increased CVD risk among older survivors [HR = 1.24; 95% CI 0.80–1.93] (Fig. 1).
Fig. 1.
Association between pharmaceutically treated anxiety and incident CVD among younger (≤65 years) and older (>65) women (age at breast cancer diagnosis). Note CVD = cardiovascular disease, p = p value; *p < 0.05
Discussion
This population-based observational study showed that pharmaceutical therapy for anxiety but not depression in the year prior to breast cancer diagnosis was associated with an increased hazard of incident CVD in 1-year breast cancer survivors. This relation remained significant after adjustment for depression, traditional cardiovascular risk factors, and clinical characteristics, and was restricted to younger breast cancer patients. Associations did not differ by type of cancer treatment or the presence of traditional cardiovascular risk factors in the year prior to breast cancer diagnosis.
The association between pharmaceutical treatment for anxiety present in the year prior to cancer diagnosis and the increased risk for CVD found in the current study is consistent with studies in healthy individuals, showing that anxiety predicts the onset of CVD [11, 12]. We focused on pharmaceutically treated anxiety and depression prior to breast cancer to examine whether anxiety and depression prior to the major life event of a breast cancer diagnosis are related to an increased risk of CVD. However, we know that being diagnosed with cancer and undergoing treatment is known to have a major impact on one’s life, as many cancer survivors experience feelings of anxiety or depression [23, 24]; hence, an additional proportion of breast cancer survivors may start to use pharmaceutical treatment for anxiety. We found a higher increased risk for anxiety in our study (48%) than a meta-analysis among healthy individuals did (26%) [12]. The increased risk of anxiety in our study remained statistically significant despite adjustment for hypertension, hypercholesterolemia, diabetes mellitus, tumor stage, and received chemo-, radio-, or hormone therapy. Hence, this association seems not to be driven by the presence of traditional cardiovascular risk factors, cancer stage, or cancer treatment. It is interesting that anxiety is only associated with an increased CVD risk in younger survivors (≤65 years). This is in line with previous research where psychological factors especially seem to play a role among younger individuals, whereas among older individuals aging—and likely physiological factors related to the aging and disease process—is suggested to drive the relationship with poor health outcomes [25]. Several behavioral and pathophysiological mechanisms have been suggested to underlie the associations between anxiety and increased CVD risk in non-cancer populations. Anxiety has, for example, been related to unhealthy lifestyle behaviors such as smoking and limited exercise [26]. Furthermore, the autonomous nervous system and hypothalamic–pituitary–adrenal axis known for their role in the pathogenesis of CVD [27, 28] have been suggested to be involved, as anxious individuals have a lower heart rate variability [29] and higher cortisol levels [30]. As medication use was used as a proxy for both anxiety and CVD, we cannot rule out that the association may partly be explained by a pharmacokinetic interaction between both drug types, although little is known about this association and the usage of anxiolytics is common among CVD populations [31, 32].
The lack of an association between depression and CVD risk was unexpected, as previous studies have demonstrated that depression is a risk factor for both incident CVD and CVD progression [11, 13–15]. This dissimilarity could be because often other studies have examined either depression or anxiety, and hence they are unable to disentangle the role of each of depression and anxiety separately and together. Alternatively, the result of the low prevalence of pharmaceutically treated depressed individuals in our study (8%) could play a role in us not confirming previous results. Using drug dispense information as an indicator of depression, we could be underestimating the true prevalence of depression, as a depression and its milder form depressive symptoms are often not treated with medication. Alternatively, depression may comprise different subtypes. It has been suggested that some manifestations of depression may partly reflect cardiac disease severity [33], and hence it may have distorted the strength of the previously found associations between depression and prognosis of CVD. Nevertheless, the fact that anxiety, but not depression, was significantly associated with increased CVD risk is in line with previous findings studying poor outcomes among CVD populations [34–36].
Surprisingly, there was no effect of chemo- or radiotherapy on CVD risk, despite the known cardiotoxicity of these treatments [3, 4]. This finding may be attributed to the lack of detailed information about type and dose of systemic therapy given. As not all chemotherapeutic agents are equally cardiotoxic, cardiotoxicity of chemo- and radiotherapy is dose dependent [3]. It is thus possible that grouping all chemotherapeutic agents irrespective of type and dosage will result in an underestimation of their true effect. Moreover, we found a protective effect of hormonal cancer treatment on CVD risk. A previous study also found that hormone treatment in breast cancer survivors can lower the risk of CVD, yet only several years after diagnosis, as it increases the risk of CVD in the first years after cancer diagnosis [37]. Previous studies have suggested that estrogen hormone treatment has favorable effects on lipoproteins, coronary arteriosclerosis, endothelial function, and arterial thrombosis [37]. Exploratory post hoc analyses, indeed, showed a protective effect of hormone treatment on incident CVD 6–10 years after cancer diagnosis in our sample, whereas there was no significant relation in the first 5 years after diagnosis or after ≥11 years.
A general limitation inherent to the observational study design is the lack of information on residual confounders. Furthermore, we used drug dispense information as a proxy for anxiety, depression, and CVD. We did not have additional information on medical diagnosis or patient-reported information on anxiety or depression. Nevertheless, algorithms based on pharmacy drugs are known to be more specific, yet less sensitive than medical diagnoses [38]. Also, we used a rather tight algorithm to define CVD, as this was based on a minimum of two C01 drug dispenses within 6 months, possibly leading to an underestimation of the incidence of CVD. It is also possible that we missed CVD patients who use other drugs, such as ACE inhibitors or beta-blockers but no C01 drug, although we expect this number to be small. In addition, as we were interested in incident CVD, breast cancer survivors with CVD in the year prior to their cancer diagnosis were excluded. Hence, we are looking at a subpopulation of 1-year breast cancer survivors.
Strengths of our study include the large population-based sample of breast cancer survivors and the usage of high-quality databases of the Netherlands Cancer Registry and PHARMO enabling a 13-year follow-up period. Additionally, this is to our knowledge the first study that examined the association between pharmaceutically treated anxiety and depression with incident CVD among breast cancer survivors. Furthermore, we estimated CVD risk 1 year after breast cancer diagnosis, striking a compromise between not starting too late and missing incident CVD due to ongoing cancer treatment or increased clinical checkups.
In conclusion, 1-year breast cancer survivors with pharmaceutically treated anxiety in the year prior to their cancer diagnosis had a 48% increased risk of incident CVD, after adjustment for depression, traditional cardiovascular risk factors, tumor stage, and cancer treatment. This increased risk seems to be limited to those breast cancer survivors who were 65 years or younger at cancer diagnosis. Depression was not related to an increased risk of incident CVD. Future studies unraveling these associations are warranted in order to provide the best optimal care for women treated for breast cancer.
Acknowledgments
Funding
This study was funded by a Social Psychology Fellowship from the Dutch Cancer Society (#UVT2013-5893) granted to Dounya Schoormans.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
As anonymous observational patient information was used, this study does not fall under the Medical Research Involving Human Subjects Act in the Netherlands; therefore, this study was exempted from medical ethics review and no informed consent was required. Hence, informed consent for each participant was neither possible nor needed.
Research involving human participants and/or animals
For this type of study formal consent is not required. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Land LH, Dalton SO, Jorgensen TL, Ewertz M. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol. 2012;81(2):196–205. doi: 10.1016/j.critrevonc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Schoormans D, Czene K, Hall P, Brandberg Y. The impact of co-morbidity on health-related quality of life in breast cancer survivors and controls. Acta Oncol. 2015;54(5):727–734. doi: 10.3109/0284186X.2014.998277. [DOI] [PubMed] [Google Scholar]
- 3.Geiger S, Lange V, Suhl P, Heinemann V, Stemmler HJ. Anticancer therapy induced cardiotoxicity: review of the literature. Anticancer Drugs. 2010;21(6):578–590. doi: 10.1097/CAD.0b013e3283394624. [DOI] [PubMed] [Google Scholar]
- 4.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30(2):181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson J, Margolis KL, Muti PC, Stefanick ML, McTiernan A. Obesity, body size, and risk of postmenopausal breast cancer: the women’s health initiative (United States) Cancer Causes Control. 2002;13(8):741–751. doi: 10.1023/A:1020239211145. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137(3):869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 7.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. ArchInternMed. 2001;161(7):996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 8.Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23(30):7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 9.Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, Dumontet C, Thieblemont C, Arnaud P, Antal D, Bouafia F, Coiffier B. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. 2004;22(10):1864–1871. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 11.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F, European Association for Cardiovascular P, Rehabilitation European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Int J Behav Med. 2012;19(4):403–488. doi: 10.1007/s12529-012-9242-5. [DOI] [PubMed] [Google Scholar]
- 12.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56(1):38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22(7):613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 14.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosom Med. 2005;67(Suppl 1):S19–S25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 16.Denollet J, Maas K, Knottnerus A, Keyzer JJ, Pop VJ. Anxiety predicted premature all-cause and cardiovascular death in a 10-year follow-up of middle-aged women. J Clin Epidemiol. 2009;62(4):452–456. doi: 10.1016/j.jclinepi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK, American Heart Association Cardiovascular Disease in W, Special Populations Committee of the Council on Clinical Cardiology CoE, Prevention CoC, Stroke N, Council on Quality of C, Outcomes R Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 18.Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141(2–3):343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Janssen-Heijnen MLG, Louwman WJ, van de Poll-Franse LV, Coebergh JWW. Results of 50 years cancer registry in the South of the Netherlands: 1955-2004 (in Dutch) Eindhoven: Eindh Cancer Regist; 2005. [Google Scholar]
- 20.van Herk-Sukel MP, van de Poll-Franse LV, Lemmens VE, Vreugdenhil G, Pruijt JF, Coebergh JW, Herings RM. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer. 2010;46(2):395–404. doi: 10.1016/j.ejca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Methodology WCCfDS (2014) Guidelines for ATC classification and DDD assignment, 2015. Oslo
- 22.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F, European Association for Cardiovascular P, Rehabilitation, Guidelines ESCCfP European guidelines on cardiovascular disease prevention in clinical practice (version 2). The fifth joint task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 23.NPK-rapport werkgroep 5 (2010)
- 24.Merckaert I, Libert Y, Messin S, Milani M, Slachmuylder JL, Razavi D. Cancer patients’ desire for psychological support: prevalence and implications for screening patients’ psychological needs. Psychooncology. 2010;19(2):141–149. doi: 10.1002/pon.1568. [DOI] [PubMed] [Google Scholar]
- 25.Denollet J, Tekle FB, van der Voort PH, Alings M, van den Broek KC. Age-related differences in the effect of psychological distress on mortality: type D personality in younger versus older patients with cardiac arrhythmias. Biomed Res Int. 2013;2013:246035. doi: 10.1155/2013/246035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strine TW, Mokdad AH, Dube SR, Balluz LS, Gonzalez O, Berry JT, Manderscheid R, Kroenke K. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30(2):127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis risk in communities. Circulation. 2000;102(11):1239–1244. doi: 10.1161/01.CIR.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 28.Whitworth JA, Mangos GJ, Kelly JJ. Cushing, cortisol, and cardiovascular disease. Hypertension. 2000;36(5):912–916. doi: 10.1161/01.HYP.36.5.912. [DOI] [PubMed] [Google Scholar]
- 29.Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vreeburg SA, Zitman FG, van PJ, DeRijk RH, Verhagen JC, van DR, Hoogendijk WJ, Smit JH, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- 31.Raviele A, Giada F, Bergfeldt L, Blanc JJ, Blomstrom-Lundqvist C, Mont L, Morgan JM, Raatikainen MJ, Steinbeck G, Viskin S, Kirchhof P, Braunschweig F, Borggrefe M, Hocini M, Della Bella P, Shah DC, European Heart Rhythm A. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace. 2011;13(7):920–934. doi: 10.1093/europace/eur130. [DOI] [PubMed] [Google Scholar]
- 32.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, American College of Cardiology/American Heart Association Task Force on Practice G ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 33.Denollet J, Pedersen SS. Anger, depression, and anxiety in cardiac patients: the complexity of individual differences in psychological risk. J Am Coll Cardiol. 2009;53(11):947–949. doi: 10.1016/j.jacc.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LL, Grossman P, Krishnan R, Blumenthal JA. Anxiety reduces baroreflex cardiac control in older adults with major depression. Psychosom Med. 1999;61(3):334–340. doi: 10.1097/00006842-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Strik JJ, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. J Am Coll Cardiol. 2003;42(10):1801–1807. doi: 10.1016/j.jacc.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Janszky I, Ahnve S, Lundberg I, Hemmingsson T. Early-onset depression, anxiety, and risk of subsequent coronary heart disease: 37-year follow-up of 49,321 young Swedish men. J Am Coll Cardiol. 2010;56(1):31–37. doi: 10.1016/j.jacc.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 38.Rector TS, Wickstrom SL, Shah M, Thomas Greeenlee N, Rheault P, Rogowski J, Freedman V, Adams J, Escarce JJ. Specificity and sensitivity of claims-based algorithms for identifying members of medicare + choice health plans that have chronic medical conditions. Health Serv Res. 2004;39(6 Pt 1):1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]