Highlights
-
•
Dynamic obstruction of right ventricle outflow tract is a rare hypotension cause.
-
•
Never described before as cause of hypotension during anesthesia induction.
-
•
Echocardiography identifies this condition with or without hemodynamic monitoring.
-
•
Identified dynamic obstruction can be successfully treated with fluid therapy.
Abbreviations: RVOTO, right ventricle outflow dynamic obstruction; RVOT, right ventricle outflow tract; TEE, transesophageal echocardiogram
Keywords: Right ventricle outflow tract, Dynamic obstruction, Surgical coronary revascularization, Transesophageal echocardiogram, Arterial hypotension
Abstract
Introduction
Dynamic obstruction of right ventricle outflow tract (RVOTO) is a rare condition that may acutely cause severe heart failure. It has been reported in some hypertrophic cardiomyopathies, after lung transplantation, and in some cases of hemodynamic instability after cardiopulmonary bypass.
Presentation of case
We report the case of a 71-year-old man who developed severe hypotension during the induction of general anesthesia for surgical coronary revascularization. Hypotension did not respond to the initial treatment with vasoconstrictors and fluids. RVOTO was suspected during pulmonary artery catheterization because of the difficulty of the catheter tip to move from the right ventricle to the pulmonary artery and, successively, because of the finding of a large gradient between the systolic pressure in the right ventricle and in the pulmonary artery. The diagnosis was confirmed by transesophageal echocardiogram (TEE). Hemodynamics recovered after the infusion of cristalloids, 1 L, and the suspension of vasoconstrictors and inotropes.
Discussion
This is the first case in which RVOTO was observed during the induction of general anesthesia. Although this is a rare condition, the diagnostic suspect is of outmost importance because treatment is mainly based on fluid administration, and drugs with positive inotropic properties (like most vasoconstrictors) are contraindicated.
Conclusions
RVOTO is an unusual, but possible cause of severe arterial hypotension during general anesthesia induction. TEE is useful for the evaluation of severely hypotensive patients who do not respond to routine treatment with fluids and vasoconstrictors.
1. Introduction
In patients affected by heart diseases, severe arterial hypotension that occurs during the induction of general anesthesia is often treated with sympathomimetic amines or norepinephrine to avoid fluid loading [1], [2]. However, these drugs are contraindicated if hypotension is caused by the dynamic obstruction of ventricular outflow tract because beta adrenergic stimulation increases the obstacle due to myocardial thickening. Unfortunately, dynamic obstruction is not easily identified in the acute setting and the correct treatment based on fluid administration is carried out on the basis of a high degree of clinical suspicion. The obstruction of the left ventricle outflow tract is not unusual in patients affected by severe myocardial hypertrophy and systolic anterior motion of the mitral valve, and is usually anticipated by preoperative echocardiographic findings. Conversely, the dynamic obstruction of the right ventricular outflow (RVOTO) has only been reported during weaning from cardiopulmonary bypass and is exclusively anticipated by right ventricle hypertrophy associated with some congenital cardiopathies. We report the case of a patient who developed RVOTO during general anesthesia induction for surgical coronary revascularization. The case has been reported in line with the SCARE criteria [3].
2. Presentation of case
A Caucasian 71-year-old man was admitted to the hospital for angina attacks that occurred at rest. His medical history was positive for smoking, arterial hypertension, pulmonary emphysema, and obstructive sleep apnea syndrome. His home therapy was aspirin and a betablocker.
Coronary angiography unveiled the presence of coronary artery disease that affected the left anterior descending artery, the left circumflex artery, and the right coronary artery, as well as the distal part of the left main coronary artery. A transthoracic echocardiogram (TTE) showed a hypertrophic left ventricle with good systolic function (left ventricle ejection fraction 75%) and moderate diastolic dysfunction; the right ventricle was normal. Consequently, surgical coronary revascularization was planned.
One hour before the admission to the operating theatre, the patient received diazepam, 6 mg PO, morphine, 10 mg IM, and scopolamine, 0.25 mg IM. A few minutes after arriving, he reported a precordial pain and was given nitroglycerin oral spray. A catheter in the left radial artery and a venous peripheral 14G cannula were quickly inserted by the anesthetist in charge and general anesthesia was induced with Propofol by slow IV injection. As soon as 80 mg of the drug were injected, the patient lost consciousness and developed severe arterial hypotension (systolic blood pressure 60 mmHg), which was resistant to saline 250 mL, calcium chloride, 1 g IV, ephedrine chloride to a final dosage of 50 mg IV, and dopamine 300 mcg IV. After obtaining muscle relaxation with cisatracurium, 20 mg IV, a tracheal tube was positioned and the patient was connected to a mechanical ventilator in controlled volume modality, with 10 breaths/min, a tidal volume of 560 mL, zero end-expiratory pressure (ZEEP), and an inspired O2 fraction of 1. Meanwhile, systolic blood pressure partly recovered to about 90 mmHg. A central venous line and an introducer were positioned in the internal jugular vein and a pulmonary artery catheter was subsequently introduced into the right ventricle, but apparently did not proceed in the pulmonary artery in spite of repeated attempts. Finally, the morphology of the diastolic part of the curve (which was descending) suggested that the catheter was correctly positioned in the pulmonary artery and a gradient of 45 mmHg was registered between systolic pressure values in the ventricle (higher) and in the pulmonary artery (lower). The gradient was calculated as the difference between peak systolic right ventricular pressure and peak systolic pulmonary artery pressure directly from the monitor values.
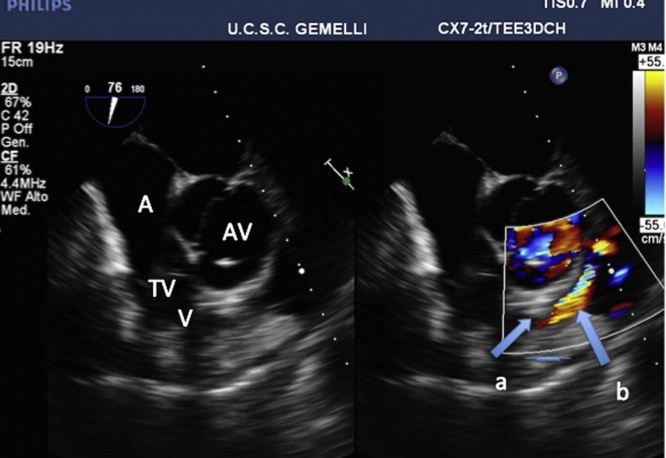
In the meantime, a cardiologist of the team performed a transesophageal echocardiogram (TEE)(PHILIPS, iE 33), which showed a hyperkinetic, small sized left ventricle and pointed out the presence of a severe dynamic obstruction of the right ventricle outflow tract with end systolic obliteration of the outflow tract and the presence of evident aliasing as a result of blood acceleration at color Doppler mode (see Fig. 1). It was impossible to measure blood speed for non-alignment.
Fig. 1.
Mid-esophageal short axis view showing severe dynamic obstruction of the right ventricle outflow tract. At color Doppler mode, end-systolic obstruction is pointed out by swan-necked obliteration(a) and evident aliasing (b).
A right atrium; V ventricle; TV tricuspid valve; AV aortic valve.
Ringer lactate, 1000 mL, was rapidly infused and systolic blood pressure rose to 120 mmHg; at the first hemodynamic assessment with thermodilution, the cardiac index was 2.3 L/min/m2. The peak pressure gradient between the right ventricle and the pulmonary artery was assessed again and was 22 mmHg. The subsequent course was uneventful. The patient received a triple bypass (the left internal mammary artery to the left anterior descending artery and two saphenous vein bypass grafts to the left marginal artery and to the posterior intraventricular artery). At the end of the procedure, he was transferred to the Cardiac Surgical Intensive Care Unit. Afterwards, he was moved to the cardiac surgical ward in the 4th postoperative day and discharged from the hospital in the 8th postoperative day.
3. Discussion
Dynamic RVOTO has been first described in some hypertrophic cardiomyopathies [4] and after lung transplantation [5]; successively, an incidence of 1–4% has been reported in patients who developed hemodynamic instability after cardiopulmonary bypass (CPB) [6]. To our knowledge, RVOTO has never been described as a cause of hemodynamic instability during anesthesia induction.
Dynamic RVOTO may originate from the higher susceptibility of the infundibulum to inotropic agents, perhaps as a mechanism to protect the pulmonary vasculature from high pressure [7], [8]. It may cause an acute heart failure and represents a challenging diagnosis. Echocardiography is needed to exclude the presence of other causes of arterial hypotension and to highlight RVOTO and its dynamic nature. The typical finding is the end-systolic obliteration of the right ventricular outflow tract, with evidence of aliasing. A diagnostic criterion is the presence of a peak pressure gradient greater than 25 mmHg between the right ventricle cavity before the obstruction and the pulmonary artery. A rough estimate of the gradient may be performed with echocardiogram by Doppler function, but its precise assessment requires right heart catheterization. A correct diagnosis is essential for a correct therapy that is based on fluid administration, decreasing heart rate, and maintaining or restoring an effective atrial contraction to improve right ventricle filling. Drugs that have inotropic properties are contraindicated.
In the case reported, RVOTO occurred after the arterial vasodilation induced by anesthetics; successively, inotrope drugs may have worsened the obstruction. As soon as the correct diagnosis was made, preload optimization and inotropic agents withdrawal were successful in restoring adequate hemodynamics. This is the first report of RVOTO during general anesthesia induction. The diagnosis was made by TEE and pulmonary catheterization; in non cardiac surgery, the occurrence of this condition may easily go undiagnosed.
4. Conclusions
-
•
To our knowledge, this is the first case report about RVOTO that occurs during general anesthesia induction.
-
•
RVOTO is an unusual, but possible cause of severe arterial hypotension during general anesthesia induction.
-
•
In absence of PAC monitoring, TEE is a useful tool for the evaluation of severely hypotensive patients who do not respond to routine treatment with fluids and vasoconstrictors.
Conflic of interest
The authors declare that they have not competing interest.
Funding
This case did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Not ethical approval required.
Consent
A written consent by the patient could not be obtained because the authors were not able to contact him. Therefore, any personal detail has been removed from the manuscript and the figure to protect anonymity.
Author contribution
Maria Enrica Antoniucci: writing the article, echocardiographic image review.
Christian Colizzi writing the article, echocardiographic image.
Gabriella Arlotta data collection.
Maria Calabrese literature research.
Michele Corrado literature review.
Sergio Guarneri literature review.
Lorenzo Martinelli echocardiographic image review.
Andrea Scapigliati data collection.
Roberto Zamparelli data collection.
Franco Cavaliere Review of final manuscript.
Guarantor
Franco Cavaliere.
References
- 1.Reich D.L. Predictors of hypotension after induction of general anesthesia. Anesth. Analg. 2005;101:622–628. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 2.Boccara G. Terlipressin versus norepinephrine to correct refractory arterial hypotension after general anesthesia in patients chronically treated with renin-angiotensin system inhibitors. Anesthesiology. 2003;98:1338–1344. doi: 10.1097/00000542-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The CARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;36(Pt A):180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Stierle U., Sheikhzadeh A., Shakibi J.G., Langbehn A.F., Diederich K.W. Right ventricular obstruction in various types of hypertrophic cardiomyopathy. Jpn. Heart J. 1987;28:115–125. doi: 10.1536/ihj.28.115. [DOI] [PubMed] [Google Scholar]
- 5.Kirshbom P.M., Tapson V.F., Harrison J.K., Davis R.D., Gaynor J.W. Delayed right heart failure following lung transplantation. Chest. 1996;109:575–577. doi: 10.1378/chest.109.2.575. [DOI] [PubMed] [Google Scholar]
- 6.Denault A.Y. Dynamic right ventricular outflow tract obstruction in cardiac surgery. J. Thorac. Cardiovasc. Surg. 2006;132:43–49. doi: 10.1016/j.jtcvs.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Heerdt P.M., Pleimann B.E. The dose-dependent effects of halothane on right ventricular contraction pattern and regional inotropy in swine. Anesth. Analg. 1996;82:1152–1158. doi: 10.1097/00000539-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Stobierska-Dzierzek B., Awad H., Michler R.E. The evolving management of acute right-sided heart failure in cardiac transplant recipients. J. Am. Coll. Cardiol. 2001;38:923–931. doi: 10.1016/s0735-1097(01)01486-3. [DOI] [PubMed] [Google Scholar]