Abstract
Urinary tract infection is commoner in patients with spinal cord injuries because of incomplete bladder emptying and the use of catheters that can result in the introduction of bacteria into the bladder. 145 patients suffering from spinal cord injuries, admitted to the Institute for physical medicine and rehabilitation, Centre for paraplegia of the Clinical Centre of the University of Sarajevo, were included. The patients were divided in three groups according to the method of bladder drainage: Group A (n=61) consisted of patients on clean intermittent catheterization; Group B (n=54) consisted of patients with indwelling catheters; Group C (n=30) consisted of patients who had performed self-catheterization. From a total of 4539 urine samples, 3963 (87,3%) were positive and 576 (12,7%) were sterile. More than 90% of the infected patients were asymptomatic.
The overall rate of urinary infection amounted to about 2,1 episodes, and bacteriuria to 8,1 episodes per patient. 77% of infections (113/145) were acquired within seven days from catheterization.
Infection was usually polymicrobial; the greatest number of urine samples 1770/3943 (44,9%) included more than one bacterium.
The vast majority of cases of urinary tract infection and bacteriuria are caused by Gram-negative bacilli and enterococci, commensal organisms of the bowel and perineum, representative of those from the hospital environment. Providencia stuarti (18,9%) being the most common, followed by Proteus mirabilis (16,3%), Escherichia coli (11,8%), Pseudomonas aeruginosa (10,2%), Klebsiella pneumoniae (8,1%), Morganella morgani (5,4%), Acinetobacter baumannii (4,6%), Providencia rettgeri (3,5%). 15,7% of isolates were Gram-positive with Enterococcus faecalis (8,6%) as the most common. 55,3% of isolates were multidrug-resistant, and the highest rates of resistance were found among Acinetobacter baumannii (87,8%), Providencia rettgeri (86,7%), Pseudomonas aeruginosa (85,4%), Providencia stuarti (84,3%) and Morganella morgani (81,0%). Lower rates of resistance were found in Group C, i.e. patients on intermittent self- catheterisation. Eradication of organisms was achieved in only 53 (10,05%) of patients; hence, antibiotic therapy had no or very low effect.
Significant correlations were found between the method of catheterization and the frequency of bacteriuria and urinary tract infections. The analysis of Group C showed a rate of lower urinary tract infection and bacteriuria than the other two Groups of patients. The objective of this study is the update of etiology and antimicrobial susceptibility in urinary tract infections in this group of patients. In addition, possible correlations between UTI and the type of bladder management were examined.
Keywords: SCI-spinal cord injuries, catheterization, bacteriuria, urinary tract infection, antibiotic resistance
INTRODUCTION
Urinary tract infections in patients with spinal cord injury (SCI) are the most frequent complication due to vesical neurogenic alteration (1). Several factors may act to predispose patients with neurogenic bladder to UTI. The most important of these are high pressure voiding, large amounts of post-voiding residuals, bladder cath- eterization, vesicoureteral reflux, bladder overdisten- sion, stones in the urinary tract and outlet obstruction (2). Recurrent UTI requires multiple courses of antibi-otic therapy, thus markedly increasing the incidence of multidrug-resistant (MDR) bacteria (1). Bacteriuria - the presence of bacteria in the urine - is very common in patients with an indwelling catheter (3). Studies show that in patients with spinal cord injuries the incidence of bacteria in the bladder is 1-3% per catheterization, and 14 episodes of bacteriuria occur per 100 days of intermittent catheterization performed 4 times a day (4). Abnormal levels of pyuria are present in the great majority of people with SCI who have indwelling catheters and also in those using IC. Lack of pyuria rea-sonably predicts the absence of UTI in SCI patients (5).
The majority of organisms are from the patients’ own colonic flora and may be native inhabitants or new immigrants, that is, exogenous organisms from the hospital environment (6,7). Bacteria enter the urinary tract trough the meatus, migrate to the bladder, and proliferate in the urinary tract. Within 8 hours of insertion of a catheter, a biofilm can be found on the surface of the catheter, drainage bag and mucosa. This biofilm consists of the Tamm-Horsfall protein, stru-vite and apatite crystals, bacterial polysaccharides, glycocalyces and living bacteria. The presence of the biofilm is thought to be responsible for the persistence of bacteriuria (2, 8, and 9). Additionally, exog-enous organisms may colonize catheter equipment, if transferred via the hands of health care personnel (6). Among short-term catheterized patients, Escherichia coli are the most frequent species isolated. Other common organisms are Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis and enterococci. Particularly when antibiotics are in use, yeast may be isolated as well. Most bacteriuria in short-term catheterization is of single organisms. However, as much as 15% may be polymicrobial. Among long-term catheterized patients infections are mostly polymicrobial in up to 95% of urine specimens. Such specimens commonly have 3-4 bacterial species, each at concentrations of 105 CFU/ml or more; some may have up to 6-8 species at that concentration. As noted, these include common uropathogens such Escherichia coli, Pseudomonas aeruginosa and Proteus mirabilis, as well as less familiar species such as Providencia stuartii, Morganella morganii and Acinetobacter spp. This high prevalence of polymicrobial bac-teriuria and of unfamiliar uropathogens is sometimes not recognized by clinicians and laboratories (6,10). The use of intermittent catheterization has improved the care of these patients, but infections still arise, and the dilemma facing the urologist or physician is whether or not to administer antibiotic therapy (11).
MATERIAL AND METHODS
Patients
145 patients suffering from spinal cord injuries, admitted to the Institute for physical medicine and rehabilitation, Centre for Paraplegia Clinical Centre of the University of Sarajevo, were included. The patients were divided into the three groups according to the method of bladder drainage: Group A (n=61) consisted of patients on clean intermittent catheterization; Group B (n=54) consisted of patients with indwelling catheter; Group C (n=30) consisted of patients who had performed self-catheterization.
Patient data
Demographic information (age, sex, spinal cord injury and time of injury, time and frequency of hospitalization, intake of systemic antibiotic with activity against urinary pathogens during the study period, method of bladder drainage) were collected. The age distribution was comparable for all the three groups and ranged from 4 to 71 years with a mean age of 33,7 years. Male/female proportion was 113/32 or 77,9%/22,1%.
Specimen
A total of 4539 urine samples were examined. 1989 were from patients on clean intermittent catheterization, 729 of patients who had performed self-catheterization and 1821 from patients with indwelling catheter. Urine samples were collected by catheterization or using the clean catch technique for patients able to void spontaneously. Urine specimens collected by cath-eterization were obtained by aseptically aspirating the clamped and disinfected catheter with a sterile syringe.
Microscopic examination
Staining of the uncentrifuged urine with methylene blue was performed. The presence of bacteria and leukocytes has been examined. The presence of at least one bacterium per oil-immersion field in a midstream, clean-catch, uncentrifuged urine correlates with 103 bacteria or more per millilitre of urine. The absence of bacteria in several fields in stained un-centrifuged urine indicates the probability of fewer than 103 bacteria/ml. A finding > 5 leukocytes per high-power field is considered abnormal (pyuria).
Urine culture
Quantitative urine culture was done at the Institute of Microbiology, Immunology and Parasitology, Clinical Centre of the University of Sarajevo. The filter paper method (Leigh and Williams, “Basic Laboratory Procedures in Clinical Bacteriology,” WHO, Geneva, 1991) in which a given volume of urine is absorbed by a piece of filter paper and then put on a plate was performed. Filter paper strips were cut in the following dimensions: length 7,5 cm, width 0,6 cm, and in a length of 1,2 cm they were curved and then autoclaved. This curved part of the filter paper was put on a plate and held for 2-3 seconds. Blood agar plates (containing Columbia blood agar base by Becton Dickinson and 5% of sheep blood) were used for bacterial count and to facilitate the growth of fastidious microorganisms, particularly Gram-positive bacteria. Endo agar and Mac Conkey agar (Becton Dickinson) were used for selective isolation of Enterobacteriacea. These media are specially designed to distinguish lactose-fermenting (pink to red) from non-lactose-fermenting colonies (colorless or slightly beige). The plates were incubated overnight at 37°C (±1, 5°C) in bacteriological incubators under atmospheric conditions (Endo agar and Mac Conkey agar) and in an atmosphere enriched with 5% CO2 (blood agar); bacterial count was performed, and if judged to be significant, isolates were identified to the species level. According to the Medical Laboratory Manual (1993), Carbohydrate fermentation patterns and the activity of amino acid decarboxylases and other enzymes are used in biochemical differentiation (12). Significant bacteriuria was determined to start from the level of 105 or more colonies of bacteria forming units per cm3.
Antimicrobial susceptibility testing
Susceptibility testing was only performed on bacteria considered significant. For Gram negatives, the primary antibiotic sensitivity screening consisted of ampicillin, cotrimoxazole, nitrofurantoin, cephalexin, cefuroxime, gentamicin, and quinolones. For multiresistant strains (less than two susceptibilities left in the primary antibiotic sensitivity screening), amikacin, piperacillin+tazobactam, ceftriaxone, ceftazidime, and imipenem were tested. For Pseudomonas aeruginosa, we tested gentamicin, ami- kacin, ciprofloxacin, piperacillin+tazobactam, ceftazi-dime and imipenem. Penicillin, methicillin, gentamicin, nalidiksic acid, nitrofurantoin and trimethoprim-sul- phametoxazole were tested in the case of staphylococci. Streptococci were tested to penicillin, ampicillin, amoxi- cillin, nitrofurantoin and trimethoprim-sulphame- thoxazole. Disk diffusion was used according to the Kirby Bauer method and CLSI-criteria (Medical Laboratory Manual, Volume II: Microbiology 1993) (12).
Effectiveness ofantibiotic therapy
The effectiveness of antibiotic therapy was determined on the basis of the elimination of bacteria from urine (sterility of urine) 72 hour after the beginning of antibiotic therapy.
Statistical analysis
χ2 test, student t-test and Spearman’s Rank Correlation were used for statistical data processing. The significance of differences observed was assessed using Pearson’s chi-square test, with p<0,05 considered to be statistically significant. Test results are presented both graphically and in tabular form.
RESULTS
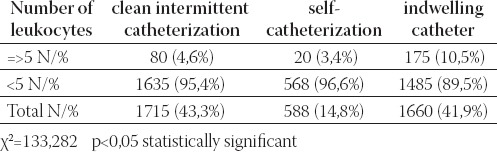
Of the 4539 urine samples 3963 (87,3%) were positive and 576 (12, 7%) sterile. Patients who had performed self-catheterization (Group C) had a significantly higher percentage of sterile urine culture versus Groups A and B (P<0,05) (Table 1). The frequency of significant bacteriuria (>104 CFU/ml) was calculated, giving the result of 88,9% (2606/2931). There were no significant differences between patients in respect of the percentage of significant bacteriuria (p>0,05) (Table 2). Pyuria was found only in 20/588 (3,4%) urine samples of patients on self-catheterization. This is a statistically significant difference (significantly less) in comparison with Groups A and B (p<0,05) (Table 3).
TABLE 1.
Positive and sterile urine culture in three groups of patients
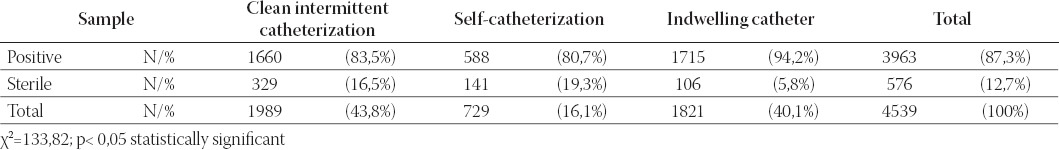
TABLE 2.
Total number of bacteria/ml accordingly of the method of bladder drainage
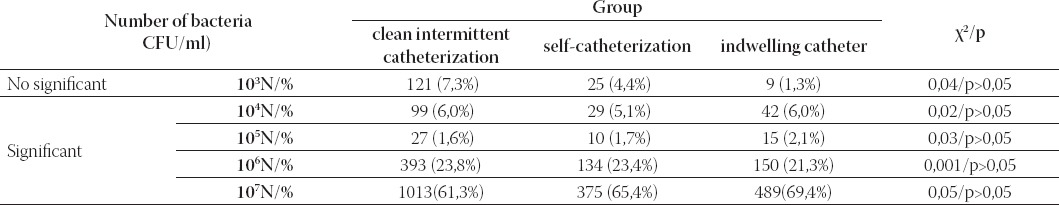
TABLE 3.
Pyuria in urine samples of three groups of patients
During hospitalisation there were significant differences in the number of episodes of bacteriuria and urinary tract infection between the three Groups. The median number of episodes of bacteriuria for Groups A, B and C amounted to 8,2 : 7,7 : 8,2, and urinary tract infection to 2,2 : 1,2 : 2,7 respectively. The average number of urinary tract infections was 2,1 episodes per patient with minimum 1, and maximum 4 episodes. Graph 1. shows the number of urinary tract infections related to the three methods of bladder drainage. 77,9% (113/145) of infections were acquired within seven days from catheterization. Patients on self-catheterization had a significantly smaller number of episodes of bacteriuria and urinary tract infection in comparison to patients on clean intermittent catheterization or with indwelling catheter (p<0,05).
GRAPH 1.
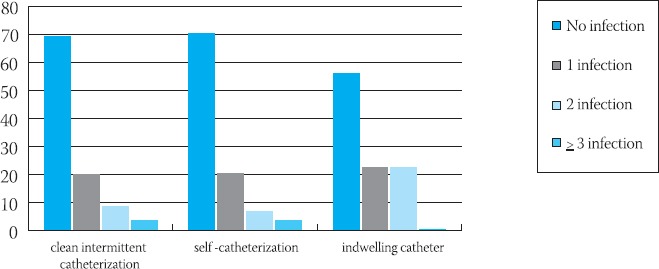
Number of urinary tract infection related to the three methods of bladder drainage
Number of isolates
44,9% (1770/3943) of urine samples had >1 isolates (polymicrobial bacteriuria/infection) (Table 4). The majority of the infections were caused by Gram negatives (overall 85,9%), mainly Providencia stuartii, Proteus mirabilis and Escherichia coli. 848/6052 (13,1%) of isolates were Gram-positive bacteria, mainly Enterococcus faecalis and Staphylococcus epidermidis (coagulase-negative staphylococci). The lowest number of both isolates was found in patients on self-catheterization (χ2=260,516; p<0,05) (Graph 2).
TABLE 4.
Number of isolates of three groups of patients
GRAPH 2.
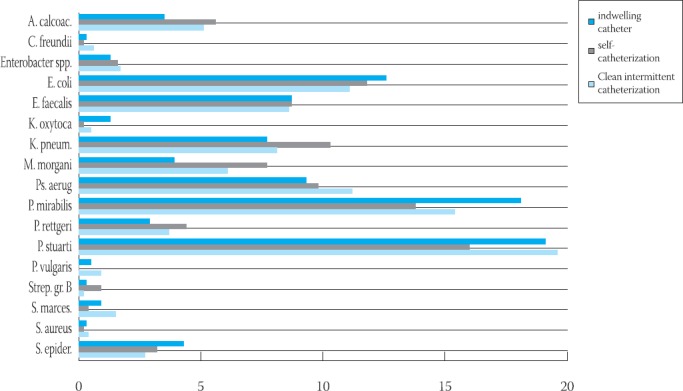
lhe most common isolates from urine samples of three group of patients
Resistance patterns
Of the 6052 isolates 3345 (55,3%) were multidrug- resistant. In the group of patients on clean intermittent catheterization 1254/3345 (37,4%) were multidrug-resistant, while 505/3345 (15,1%) in the group on self-catheterization, and 1586/3345 (47,5%) with indwelling catheter were multidrug-resistant. Patients on self-catheterization had a significantly lower number of multidrug-resistance strains versus patients on clean intermittent catheterization and indwelling catheter (p<0,05). Table 5. shows the most common multidrug-resistant strains.
TABLE 5.
Multidrug-resistance (strains resistant on >4 antimicrobials)
Effectiveness of antibiotic therapy
Antibiotic therapy was included in 527 of episodes of bacteriuria/urinary tract infection. The elimination of bacteria was achieved in only 53 (10,05%) of cases: 25 (4,7%) in patients on clean intermittent catheterization, 4 (0,75%) in those on self-catheterization, and 24 (4,5%) in patients with indwelling catheter. (Table 6).
TABLE 6.
Effectiveness of antibiotic therapy
DISCUSSION
Urinary tract infection is a serious complication of neu-rogenic bladder in SCI patients, related to high morbidity and mortality rates (3). Despite improved methods of treatment, urinary tract morbidity still ranks as the second leading cause of death in the SCI patient (13). The most important factor which may act to predispose the patients with neurogenic bladder to UTI is bladder catheterization (14). In our study only 12,7% of urine samples remained sterile, while in all other samples one or more etiological agents were isolated. Similarly, Betran et al, (24) and Ramić (15) in their studies on urine samples from patients with spinal cord injury found high percentages of positive urine culture (86,5% and 94 % respectively). The presence of significant bacteriuria in our study (>104 CFU/ml) was found in 88,9% of patients, and the majority of samples showed the amount of 107 CFU/ ml (65,4%). Billote-Domingo et al. (16) in their study conducted in Spain from April-November 1998 report that 86% of samples had significant bacteriuria. Abnormal levels of pyuria are present in the great majority of people with SCI who have indwelling catheters and also in those using IC. Lack of pyuria reasonably predicts the absence of UTI in SCI patients (17,18). In our study pyuria was present in only 6,9% of samples. Rather than infection, in most of the cases asymptomatic bacteriuria (colonisation) was found. Asymptomatic bacteriuria (colonisation of distal urethra) was found in many studies, mostly present in 80-90% of patients with spinal cord injury (19, 20, 21, and 22). Average number of urinary tract infection in our study was 2,1 episodes per patient with minimum 1, and maximum 4 episodes. The mean frequency of urinary tract infection in a study by Woodbury et al. was 2,6±2,6 for IC users (23). The vast majority of cases of UTI in SCI patients are caused by Gram-negative bacilli and enterococci, commensal organisms of the bowel and perineum representative of those from the hospital environment. Organisms such as Escherichia coli, Klebsiella spp., Pseudomonas spp., Serratia spp., Providencia stuartii, Acinetobacter spp., yeast and staphylococci are relatively more common in patients with catheter-associated UTI. Polymicrobial bacteriuria is the rule in patients with indwelling catheters, and occurred in 44% of culture-positive urine specimens. Concerning the types of organisms causing urinary tract infections in our three Groups of patients, figures are very similar to those found in the literature about patients with chronic indwelling catheters (22,23,24), only Providencia stuartii being more highly represented in our population. Providencia stuartii is an important urinary pathogen in SCI with a degree of isolation twenty-fold higher than in the rest of patients and with antimicrobial multiresistance (24). The reason for this frequent change in the pathological organism causing urinary infections needs to be further investigated. A possible explanation could be changes occurring in the urethral flora. The place of origin of these organisms causing infections needs to be clarified. Probably, they are present in the residential flora in the fosse navicularis and are introduced into the urinary tract, despite disinfection, whenever catheterization occurs. As to the catheterization technique, it is widely accepted that intermittent catheterization, when compared with indwelling catheters, reduces the risk of urinary tract infection in SCI patients and is the preferred method of bladder drainage in this patient population (13). Because of the disadvantages of indwelling urethral catheters it is the best to change to intermittent catheterization as soon as possible after injury. By doing this, the risk of bladder and kidney infection is reduced and the bladder will return to a more natural pattern of regular filling and emptying (25,26). Patients can perform intermittent catheterization themselves without increasing the risk for infections. The programme followed in our hospital to learn this technique, therefore, fulfils the standard requirements of hygiene. As soon as possible, patients are trained to assess self-catheterization. There is a trend towards more resistant isolates in all three groups of patients. Prolonged or repeated exposure to antimicrobial agents and the consequent antibiotic pressure increase the risk of colonization and infection with multiresistant bacteria (27, 28, 29 and 30). Waites et al. (17) have reported that 33% of isolates from SCI outpatients were multiresistant organisms.
Many patients with significant bacteriuria are considered to be colonized rather than infected, and treatment should be reserved for those with clinical symptoms or other signs of infection. Asymptomatic bacteriuria need not be treated with antibiotics (17,9). Prophylactic antibiotics are not routinely recommended either, because of their cost, potential adverse effects and the increased rate of isolation of resistant organisms (17).
CONCLUSION
The urinary tract of catheterized patients is highly susceptible to infection. Recurrent problems with these nosocomially acquired catheter-related urinary tract infections are the changes in the microbiological and antibiotic sensitivity pattern of the pathogens isolated. There is an emergence of antibiotic-resistant organisms. Close urological follow-up is crucial in ensuring that adequate bladder drainage is achieved, avoiding the use of long term indwelling urinary catheters if at all possible. For those patients who require long term urinary appliances, patient education and strict attention to hygiene and catheter care policies is important. The most highly recommended preventive strategies include proper hand washing, aseptic insertion technique, maintaining a closed sterile drainage system and maintaining an unobstructed urine flow. Bacteriuria is inevitable in patients with long-term catheterization, and in most cases, treatment should be started only in the presence of symptoms. Treatment of UTI in patients with urinary catheters requires replacement of the catheter and selection of antibiotics based on the extension of the infection and the results of the urine culture.
REFERENCES
- 1.Šumarac-Petrović Z. Neurogena disfunkcija mokraćne bešike uzrokovana povredom kičmene moždine. U: Urologija; Petković S. Medicinska knjiga Beograd-Zagreb. 1984:579–596. [Google Scholar]
- 2.Alcaide M.L, Lichtstein D.M. Management of urinary tract infections in patients with urinary catheters. Hospital Physician. 2004:29–33. [Google Scholar]
- 3.Sobel D.J, Kaye D. In: Urinary tract infections. In: Principles and Practice of Infectious Diseases. 4th edition. Mandel L.G, Bennet E.J, Dolin R, editors. Churchill Livingstone; 1995. pp. 662–690. [Google Scholar]
- 4.De Groat WC. Anatomy of the central neural pathways controlling the lower urinary tract. Eur Urol. 1998;34(suppl 1):2. doi: 10.1159/000052265. [DOI] [PubMed] [Google Scholar]
- 5.Tambyah P.A, Maki D.G. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch. Intern. Med. 2000;160(5):673–677. doi: 10.1001/archinte.160.5.673. [DOI] [PubMed] [Google Scholar]
- 6.Dimanovski J. Neurogeni mjehur. U: Odabrana poglavlja iz urologije. Novak R. i suradnici Zagreb. 1987:108–112. [Google Scholar]
- 7.Hukić M, Ljubović-Dedeić A. Bolničke i laboratorijske infekcije U: Bakteriologija. Sarajevo: Hukić i saradnici Jež; 2005. pp. 145–157. [Google Scholar]
- 8.Trautner B.W, Darouiche R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control. 2004;32(3):177–183. doi: 10.1016/j.ajic.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautner B.W, Darouiche R.O. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 2004;164(8):842–850. doi: 10.1001/archinte.164.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dedeić-Ljubović A, Hukić M, Šiširak M. Catheter-associated urinary tract infections. Case study of spinal cord injured patients. Fifth Congress of the International Federation of Infection control. Porec, Croatia. 2004:31, 32. Abstract book. [Google Scholar]
- 11.Reid G, Howard L. Effect on uropathogens of prophylaxis for urinary tract infection in spinal cord injured patients: preliminary study. Spinal Cord. 1997;35:605–607. doi: 10.1038/sj.sc.3100456. [DOI] [PubMed] [Google Scholar]
- 12.Cheesbrough M. Collection, transport, and examination of urine In: Medical Laboratory Manual, Volume II: Microbiology. Tropical Health Technology Oxford. 1993:146–160. [Google Scholar]
- 13.Syroky MB. Pathogenesis of bacteriuria and infection in spinal cord injured patient. AM. J. Med. 2002;113(1A):67, 79. doi: 10.1016/s0002-9343(02)01061-6. [DOI] [PubMed] [Google Scholar]
- 14.Biering-Sorensen F. Urinary tract infection in individuals with spinal cord lesion. Curr. Opin. Urol. 2002;12(1):45–49. doi: 10.1097/00042307-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ramić I. Uroinfekt kod osoba sa stanjem paraplegije. Med. Arh. Sarajevo, 2004;58(4):244–245. [PubMed] [Google Scholar]
- 16.Billote-Domingo K, Mendoza M.T, Tan Torres T. Catheter-related urinary tract infections: incidence, risk factors and microbi-ologic profile. Phil. J. Mibrobiol. Infect. Dis. 1999;28(4):133–138. [Google Scholar]
- 17.Waites K.B, Cheng Y.Y, DeVivo M.J, et al. Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch. Phys. Rehab. 2000;81:764–769. doi: 10.1016/s0003-9993(00)90108-4. [DOI] [PubMed] [Google Scholar]
- 18.Tambyah P.A, Maki D.G. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch. Intern. Med. 2000;160(5):673–677. doi: 10.1001/archinte.160.5.673. [DOI] [PubMed] [Google Scholar]
- 19.Urologic Complications and Management after SCI. Spinal cord injury information network. [accessed 27.09.2005]. http://www.spinalcord.uab.edu .
- 20.Fowler C.J. Investigation of the neurogenic bladder. J. Neurol. Neurosurg. Psychiatry. 1996;60:6. doi: 10.1136/jnnp.60.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tambyah P.A, Maki D.G. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheter-ized patients. Arch. Intern. Med. 2000;160(5):678–682. doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 22.Tantisiriwat N, Kittisomprayoonkul W, Sukonthamarn K, et al. Uropathogens and empiric antibiotics for the treatment of urinary tract infections in spinal cord injured patients at rehabilitation center, Thai Red Cross Society during 2001 to 2005. J Med Assoc Thai. 2007;90(11):2482–2486. [PubMed] [Google Scholar]
- 23.Woodbury M.G, Hayes K.C, Askes H.K. Intermittent catheter-ization practices following spinal cord injury: a national survey. Can J Urol. 2008;15(3):4065–4071. [PubMed] [Google Scholar]
- 24.Betran A, Marne C, Aisa M.L, Revillo M.J. Urinary tract infection in patients with spinal cord injury. Clin Microbiol Infect. 2005;11(2):680. [Google Scholar]
- 25.Cravens D, Zweig S. Urinary catheter management. Am Fam Physician. 2000;61(2):1–11. [PubMed] [Google Scholar]
- 26.Jamil F. Towards a catheter free status in neurogenic bladder dysfunction: a review of bladder management options in spinal cord injury (SCI) Spinal Cord. 2001;39:355–361. doi: 10.1038/sj.sc.3101132. [DOI] [PubMed] [Google Scholar]
- 27.Hernández González E, Zamora Pérez F, Martínez Arroyo M, et al. Epidemiologic, clinical and microbiological characteristics of nosocomial urinary infection in the spinal cord lesioned patient. Actas Urol Esp. 2007;31(7):764–770. doi: 10.1016/s0210-4806(07)73719-1. [DOI] [PubMed] [Google Scholar]
- 28.Gazi H, Surucuoglu S, Kurutepe A, et al. Bacterial profile and antimicrobial susceptibility pattern of catheter related infections. Clin Microbiol Infect. 2004;10(3):598–599. [Google Scholar]
- 29.Dedeić-Ljubović A, Hukic M, Šiširak M, Zvizdić A. Frequency of multidrug resistant bacteria from clinical samples. Clin Micro-biol Infect. 2004;10(3):387–388. [Google Scholar]
- 30.Salomon J, Denys P, Merle C, et al. Prevention of urinary tract infection in spinal cord-injured patients: safety and efficacy of a weekly oral cyclic antibiotic (WOCA) programme with a 2 year follow-up--an observational prospective study. J Antimicrob Chemother. 2006;57(4):784–788. doi: 10.1093/jac/dkl010. [DOI] [PubMed] [Google Scholar]


