Abstract
Betulae cortex, Betulapendula Roth., Betulaceae, comprise triterpene substances which are confirmed to posses very important pharmacological activities such as anti-inflammatory, anticancer and antiviral. In this study, extraction of triterpene substances from both, inner and external birch bark was carried out and after that qualitative analysis on betulin, betulinic acid, oleanolic acid and lupeol was performed by method of thin layer chromatography. By this separation method, applying system for development benzene-ethyl acetate-formic acid (36:12:5), is gained a good separation of examined triterpene substances from methanol extracts of inner and external birch bark as well as used standards. From obtained row triterpene mixtures, certain triterpene substances are isolated using method of dry column chromatography. To those substances infrared (IR) spectra were recorded and compared with IR spectra of adequate standards.
The study encloses all obtained IR spectra and interpretations on the basis of which can be concluded that triterpene substances, betulin, betulin acid and lupeol isolated from external birch bark give identical characteristic signals and absorbance as referent standards. Method of dry column chromatography has resulted as simple, efficient, repeatable and economical for laboratory conditions. Beside this, a sufficient quantity of examined triterpene substances is also obtained for continuation of their further analytical analysis.
Keywords: betulin, betulinic acid, lupeol, isolation, dry column chromatography, IR spectroscopy
INTRODUCTION
Phytochemical analysis of plant material primarily offers the possibility to data on chemical composition of determined drugs, obtained from certain plant materials. Specificity of pharmacologic activity and usage of each drug or isolated substances is determined on the basis of identified chemism. Phytochemical analysis is supported by the new technologies of separation and other analytical methodologies, but must include the following methods: extraction from the plant material, separation and isolation of constituents of interest, characterization of isolated substances, and investigation of biosynthetic pathway towards single components as well as qualitative evaluation (1-3). Chromatography belongs to the group of analytically separation methods used for identification, separation and determination of content within different extracts and mixtures. Separation of different components by using chroma-tography is based on dynamic distribution of melted substance between the two un-mixable phases, which are mobile one compared to another. Immobile phase slows mobility of melted substance and enable its separation under adequate conditions. In this way it is possible to separate substances having very small difference in chemical structure, therefore in physical-chemical characteristics, which chromatography distinguishes comparing to other methods. Investigation of natural row materials, drugs and isolation of substances in accordance with pharmacognostical principles is carried out by using methods of thin layer chromatography and column chromatography (4-7). Triterpene substances of different type are situated in determinate parts of plant specie Birch (8). Given the fact that is a very spared plant specie it is justify a need for a detailed phytochemical investigation of plant parts of birch on presence of different triterpene substances (9). Previous investigations were most usually linked to official drug Betulae folium, birch leaf (8). Nevertheless, investigation on birch bark resulted with data on content of triterpene derivates of β-amirin saponin type with hemolytic activity, furthermore presence of triterpene derivates of lupeol type like betulin and betulin acid (10). These substances are pharmacologically significant and as such investigated in samples of inner and external birch bark (11-13).
MATERIALS AND METHODS
Extraction procedure
Properly powdered herbal drug (external and inner birch bark, 50 g each) is extracted with 250 ml of 99,5% acetone (dimethyl ketone, Kemika, Zagreb) at water bath (60°C) with back-cooler for two hours. Acetone extract is eliminated and afterward drug is twice extracted with acetone (150 ml) applying same conditions, but with duration of 30 minutes. The remaining part of drug, after the extraction with acetone, is dried at water bath, in order to definitely eliminate used solvent. Such dried and elaborated drug is extracted with 250 ml of 80% methanol (Kemika, Zagreb) in the same way and under the same conditions as is described with acetone extraction (7-8). Gathered methanolic fractions were as such treated with water, what produces huge amount of blanc sediment which is separated with filtration under vacuum through Büchner’s funnel, using filter paper MN 640 m, white ribbon (Macherey-nagel, Germany). Sediment is than dried in dry-kiln at the temperature up to 60°C after what the finest dray blank powder remain, which than is conserved in fridge at +4°C in a closed glass bowl. Such purified extracts are used for thin layer chromatography and dry column chromatography.
Conditionsfor Thin layer chromatography (TLC)
Dried methanol extract (50 mg) of plant material is dissolved in 10 ml methanol and 20 μ of this solution is used for TLC. Standard substances (12,5 mg) are dissolved in methanol (10 ml) and solution (20 μ.) is used for chromatographic analysis. Adsorbent: silica gel GF254 “Merck” Dürmsttadt, Germany, finished plates; System for development: benzene (Kemika, Zagreb) - ethyl acetate (Kemika, Zagreb) - formic acid (Kemika, Zagreb) (36:12:5); Visualization: 4-methoxybenzaldehyd (Merck, Germany) - sulphuric acid (Kemika, Zagreb).
Conditionsfor Dry column chromatography (DCC)
DCC is a “non-elution” method of column chromatography, meaning, the separated substances remain in the column at the end of the chromatographic process. The use of nylon foil tubing allows for the removal of these separated substances by easily cutting the tubing into segments. In this work DCC method is performed with aim of further purifying of obtained methanolic extracts as well as isolation of triterpene substances.
Preparation of column
Naylon tube (Nylon Tubing for DCC, 2” x 100’, Sorbtech, USA) is closed in one of its two extremities with stringent ribbon and security clip. From the other side the tube is fulfilled with a small quantity of glassy wool at the bottom of column. At that part of column, with simple needle are perforated small holes, so that air which press solvent mixture could come out. Column than is fulfilled with sorbent (silica gel DCC, 63-200um, Merck, Germany) up to % of its overall height. Fulfilment is performed in small portions with a constant pressure of content by beating firm basis. In the end the column is so hard that it can bear itself and is fixed at porter.
Preparation ofsamplesfor DCC
Methanolic liquid extract (sample) which need to be separated, is mixed with the same sorbent (silica gel DCC, 63-200um, Merck, Germany) with which the column is fulfilled with in proportion 1:10. The solvent is then evaporated using a rotation evaporator until sample and sorbent form a free flowing powder. The adsorbate of the sample is applied as a level layer on the top of the column. This layer is covered with about 2 cm of pure sorbent and a peace of glass wool (Himedia, India) as a final layer.
Gathering of separate components
After solvent mixture, benzene (Kemika, Zagreb) - ethyl acetate (Kemika, Zagreb) - formic acid (Kemika, Zagreb) (36:12:5), has passed through entire length of column, it is settled in horizontal position and than 1/16 of column is cut vertically. Cut part is sprayed with reagent 4-methoxybenzaldehyd (Merck, Germany) - sulphuric acid (Kemika, Zagreb) in order to identify separated components. Sprayed part is settled in parallel with the rest of column in order to mark the separated components in the rest of the column. At those places the column is cut with knife (scalpel) and different fractions are gathered into laboratory glasses (50ml, Na- hita, Spain). Silica-gel (silica gel DCC, 63-200um, Merck, Germany) is separated with filtration and separated components are further elaborated with TLC method.
Infrared (IR) spectrophotometry
IR spectra of isolated substances are recorded by using apparatus Spectrophotometer Perkin-Elmer Model 1000 FT-IR, by method KBr triturate in infrared zone of waves length of do 400 cm-1. Firstly were observed spectra of standard substances examined in this study (betulinic acid, betulin, oleanolic acid, lupeol), saved in the data base and than spectra of isolated products.
RESULTS AND DISCUSSION
Extraction
As it is confirmed with experiments that investigated triterpene standards (betulin, betulinic acid, oleanolic acid and lupeol), are not soluble in water, this fact is used in order to obtain row triterpene products with water from methanolic extracts. Obtained quantities of dry row triterpene product are illustrated in Table 1.
TABLE 1.
Quantity (g) of dry, row triterpene product

Thin layer chromatography
For thin layer chromatography more systems for development are prepared, and it was tried to obtain a system in which triterpene substances would be separated in a best manner. In this sense, a chromato-graphic separation of prepared mixture of standard substances - betulin acid, oleanolic acid, betulin and lupeol, is done. The best system resulted to be benzene-ethyl acetate - formic acid (36:12:5), which was further used in experimental part (Figure 1). From chromatogram it can be noted a well separated all four substances from mixture of standards. Separate spots are colored in violet shades having different retention factor (Rf) values sprayed with reagents anisaldehyde sulphuric acid. Nevertheless, small differences in Rf values between oleanolic and betulinic acid enables however their identification, given the fact that oleanolic acid is colored in dark violet and betulin acid in light violet. Betulin has the lowest Rf value and is colored in violet and lupeol has the highest Rf value and is colored darker violet. These conditions of thin layer chromatography were used for the following investigation of mentioned substances within analyzed plant parts of birch, inner and external bark (Figure 2). On chromatographic plates with silica gel were applied methanolic extracts of tested samples (20 μl) and standards (betulin acid, oleanolic acid, betulin and lupeol as well as methanolic solution) with concentration of 12,5 mg of standard in 10 ml of methanol. Developed chromatographic plates presented in Figure 2 implicates at good separation both of standards and substances within investigated extracts in the system of benzene - ethyl acetate - formic acid (36:12:5). The presence of investigated standard substances was demonstrated in the samples of inner and external birch bark.
FIGURE 1.
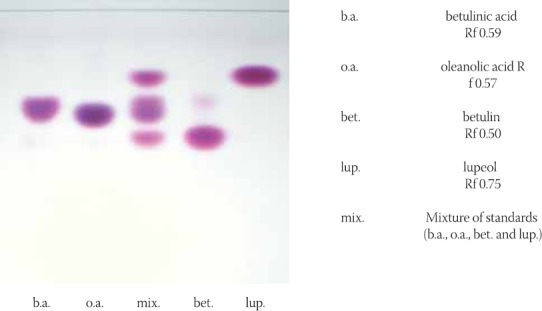
Chromatogram of separated triterpen standards
FIGURE 2.
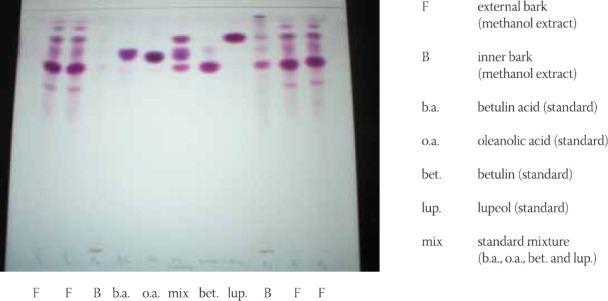
Chromatogram of separated components in samples of external and inner birch bark
Dry column chromatography (DCC)
Applying thin layer chromatography method was noted that methanolic extract of external bark is full of triterpene substances; Method of dry column chromatography of this extract was carried out aiming at isolation of these substances. Ten fractions were isolated, which were then investigated with method of thin layer chromatography with already described conditions, using betulin, betulin acid, lupeol and oleanolic acid as standard substances (Figure 3).
FIGURE 3.
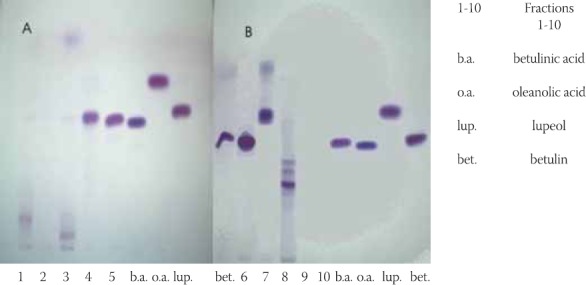
Chromatogram of separated fractions from 1 to 10, obtained by DCC
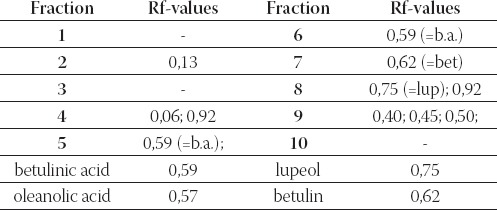
From the chromatogram illustrations (Figure 3.) and Table 2. it can be seen that fractions 5 and 6 give spots, which are in full conformity with betulinic acid standard, violet color of spots and the same Rf value (0,59). Fraction 7 has one separate spot (Rf 0,62), which in accordance with Rf value, with its shape and color is fully in conformity with betulin standard (Rf 0,62, violet color). Fraction 8 results in two separate spots where one is fully clear and conforms with lupeol (Rf 0,75). Another spot has Rf value 0,92 and blue-shadowed color with violet color of very weak intensity. Fractions which resulted in more chromatographically separated spots are further elaborated with method of preparatory thin layer chromatography in order to separate components, which would be in accordance only with standards and would be chromatographically clean. They would be further treated with IR spectros- copy. Fraction 9 resulted in 3 separate spots with Rf values lower than Rf values of standards in use, but violet color after spraying with anisaldehyde sulphuric acid is in conformity with triterpene substances. The results we obtained by using method of dry column chromatography implicate justification of usage of this method aiming at isolation of triterpene substances given the fact that it enables efficient separation and isolation of investigated substances. Beside this, sufficient quantity of examined tripertene substances (betulinic acid, lupeol and betulin) is obtained for continuation of their further analytical analysis.
TABLE 2.
Rf- values of separated fractions obtained with DCC method
Infrared spectroscopy
Method of infra-red spectroscopy, pharmacopoeia prescribed as method used for identification and determination of medicaments and investigation of their purity. With this method are investigated samples of triterpene substances isolated from external birch bark such as betulinic acid, betulin and lupeol as well as their standards and standard of oleanolic acid. Results we obtained with KBr pastille technique are illustrated at spectra shown in Figures 4, 5, 6 and 7.
FIGURE 4.
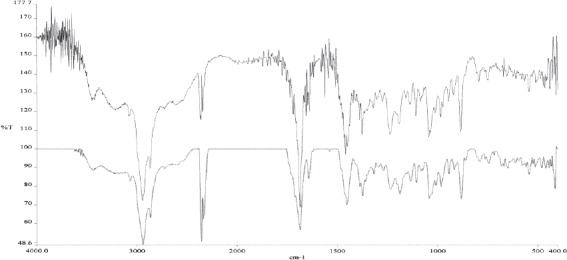
IC- betulinic acid spectrum; * lower line (blue color) betulin acid standard ** uper line (bleck color) isolated betulin acid
FIGURE 5.
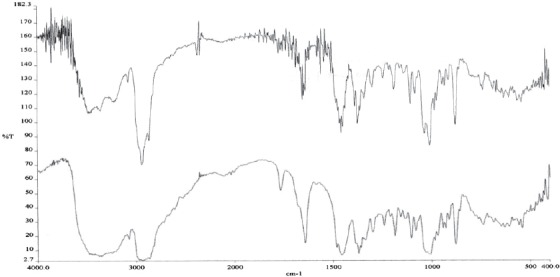
IR- betulin spectrum; * lower line (blue color) betulin standard; ** uper line (black color) isolated betulin
FIGURE 6.
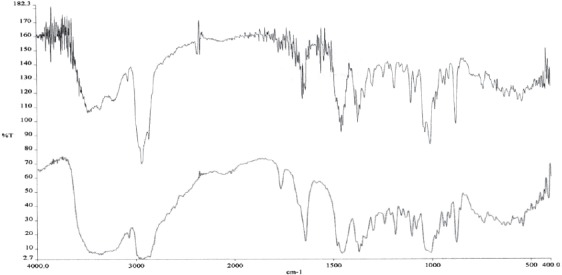
IR- lupeol spectrum; * lower line (blue color) lupeol standard; ** upper line (black color) isolated lupeol
FIGURE 7.
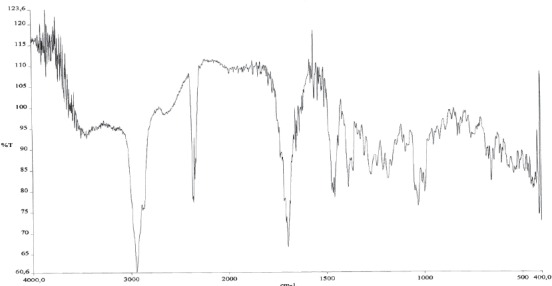
IR- oleanolic acid spectrum
Interpretation ofIR spectres ofisolated substances
Betulinic acid - (3P)-hidroksi-lup-20(29)-en-28-oic acid
IR (KBr) (ν, cm-1): 3484 (OH); 2870 (CH2); 1686 (C=O); 1432 (OH); 1362
(CH2=CH-CH3); 1156 (C-O).
Betulin - (lup-20(29)-en-(3P),28-diol)
IR (KBr) (ν, cm-1): 3448 (OH); 2868 (CH2 i CH3); 1638 (C=C); 1432 (OH); 1388
(CH2=CH-CH3).
Lupeol - Lup-20(29)-en-3-ol
IR (KBr) (ν, cm-1): 3308 (OH); 2872 (CH2); 1630 (C=C); 1468 (CH); 1380 (CH3)
“Umbrella”; 920 (CH alkenes).
Interpretation ofIR spectrum ofstandard of oleanolic acid
Oleanolic acid- (3P)-3-Hidroacid-12-en-28-oic acid
IR (KBr) (ν, cm-1): 3426 (OH); 2864 (CH2); 1698 (C=O); 1462 (OH); 1376 (CH3); 1108 (C-O).
IR spectra of betulin and betulin acid show a small difference in absorption ribbon, which derivate from carbonyl group linked in C28 position of heterocyclic 5-membered rink of betulinic acid (Figures 4 and 5). At IR spectra of betulinic acid (Figure 4.) appears very intensive absorption ribbon derived from OH group in the area of 3448 cm-1 and in the area of 1432 cm1, absorption ribbon OH vibration of planar bending. In the area of 2870 cm-1 appears very intensive absorption ribbon, which derivates from CH2 group, at 1686 cm-1 appears a characteristic ribbon of car-bonyl group (C=O) and at 1362 cm-1 appears absorption ribbon of isopril group (CH2=CH-CH3). At IR spectra of lupeol (Figure 6.) in the area of 3308 cm-1 appears a very intensive absorption ribbon vibration of stretching vibrations of OH group. A very inten-sive absorption ribbon in the area of 2872 cm-1 derives from symmetric vibrations CH2 cm-1 grope. In the area of 1630 cm-1 appears absorption ribbon which derivates from C=C group. At 1468 cm-1 appears absorption ribbon which derivates from CH vibrations. In the area of 1380 cm-1 appears a characteristic ribbon which derivates from distortion vibrations of CH3 group. At IR spectra of oleanolic acid (Figure 7.) appears a very intensive adsorption ribbon, which derivates from OH group in the area of 3426 cm1. A very inten-sive absorption ribbon in the area of 2846 cm-1 derives from symmetric vibrations of CH2 cm-1 group. In the area of 1698 cm-1 appears a characteristic ribbon of carbonyl group (C=O). At 1462 cm-1 appears absorption ribbon from OH vibrations of planar distortion. In the area of 1376 cm-1 appears a characteristic ribbon, which derives from CH3 group and at 1108 cm-1 stretching vibrations of C-O group of carbonic acid. In this study are obtained IR spectrums and interpretations of spectrum on the basis of which can be concluded that triterpene substances, betulin, betulinic acid and lupeol are isolated from external birch bark, give identical, characteristic signals and absorbance as well as reference standards. With mentioned procedures of extractions and isolation as well as with chromato-graphic methods are obtained pure isolated substances.
CONCLUSION
Triterpene substances were successfully extracted from external and inner birch bark using acetone-methanole Soxhlet extraction.
Experimentally it is confirmed that triterpene substances (betulin, betulinic acid, lupeol and oleanolic acid) practically are not soluble in water what is used for precipitation of triterpene substances from obtained methanolic extracts.
Using method of thin layer chromatography was obtained a good separation of investigated triterpene substances, applying a system for development, benzene - ethyl acetate - formic acid (36:12:5).
Reagents anisaldehyde sulphuric acid, coloured separated triterpene substances as well as triterpene standards (betu- lin, betulinic acid, lupeol and oleanolic acid) in shadows of violet colour and did not show fluorescency (UV lamp 254 and 366 nm).
Method of dry column chromatography has shown as a simple, efficient, repeatable and economical for laboratory conditions.
Beside this, sufficient quantities of examined triterpene substances (betulinic acid, lupeol and betulin) were obtained by method of dry column chromatography, for continuation of their further analytical analyses, as well as microbiological and pharmacological investigation.
All presented IR spectrums and their interpretations direct to conclusion that triterpene substances betulin, betulinic acid and lupeol, isolated from external birch bark give identical, characteristic signals and absorbance as reference standards.
With mentioned procedures of extraction and isolation as well as with chromatographic methods are obtained isolated substances of good purity.
REFERENCES
- 1.Vlietinck A.J, Apers S. Biological screening methods in the search for pharmacologically active natural products. In: Tringali C, editor. Bioactive compounds from natural sources: isolation, characterisation and biological properties. London: Taylor and Francis; 2000. pp. 1–29. [Google Scholar]
- 2.Bruneton J. Pharmacognosy, Phytochemistry medicinal plants. 2nd ed. Paris: Tec$dDoc; 1999. [Google Scholar]
- 3.Heinrich M, Barnes J, Gibbons S, Williamson E. M. Fundamentals of Pharmacognosy and Phytotherapy. Edinburgh-London: Churchill Livingston; 2004. [Google Scholar]
- 4.Nyiredy SZ. Planar Chromatography, a retrospective view for the third millennium. Budapest: Springer; 2001. [Google Scholar]
- 5.Wagner H. Pharmazeutische Biologie, Drogen und ihre Inhaltsst-offe. Stuttgart-New York: Gustav Fischer Verlag; 1993. [Google Scholar]
- 6.Kovač-Bešović E.E. Methods in pharmacognosy. Sarajevo: Sarajevo Publishing; 2001. [Google Scholar]
- 7.Zhao G, Yan W, Cao D. Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J Pharm Biomed Anal. 2007;43(3):959–962. doi: 10.1016/j.jpba.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. European Scientific Co-operative on Phytotherapy, ESCOP Monographs Betulae folium. 2nd ed. Exeter, England: Argyle House; 2003. [Google Scholar]
- 9.Krasutsky P.A. Birch bark research and development. Nat. Prod. Rep. 2006;23(6):919–942. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]
- 10.Tolstikova T.G, Sorokina I.V, Tolstikova T.A, Tolstikova A.G, Flekhter O.B. Biological activity and pharmacological prospects of lupane terpenoids: Natural lupane derivatives. Bioorg. Khim. 2006;32(1):42–55. doi: 10.1134/s1068162006010031. [DOI] [PubMed] [Google Scholar]
- 11.Jäger S, Laszczyk M.N, Scheffler A. A preliminary pharmacoki-netic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex) Molecules. 2008;13(12):3224–3235. doi: 10.3390/molecules13123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M, Biedermann D, Markova L, Urban M, Sarek J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006;23(3):394–411. doi: 10.1039/b515312n. [DOI] [PubMed] [Google Scholar]
- 13.Krasutsky PA. Birch bark research and development. Nat. Prod Rep. 2006;23(6):919–942. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]


