Abstract
Antimicrobial peptides (AMPs) are a pervasive and evolutionarily ancient component of innate host defense which is present in virtually all classes of life. In recent years, evidence has accumulated that parallel or de novo mechanisms by which AMPs curb infectious pathologies are also effective at restraining cancer cell proliferation and dissemination, and have consequently stimulated significant interest in their deployment as novel biologic and immunotherapeutic agents against human malignancies. In this review, we explicate the biochemical underpinnings of their tumor-selectivity, and discuss results of recent clinical trials (outside of oncologic indications) which substantiate their safety and tolerability profiles. Next, we present evidence for their preclinical antitumor activity, systematically organized by the major and minor classes of natural AMPs. Finally, we discuss the barriers to their clinical implementation and envision directions for further development.
Keywords: antimicrobial peptides, cancer immunotherapy, biopharmaceuticals, anticancer drugs, microbiome
Introduction
Cancer continues to take a toll on global public health systems, accounting for an estimated 8.7 million deaths annually (1, 2). In 2015, 17.5 million incident cases were diagnosed worldwide, and this is projected to spiral to 22.2 million by 2030 (1–3). Despite tremendous progress in reducing mortality rates from cancer and transformative shifts in therapeutic paradigms over the past few years, the development of novel therapeutic approaches remains an urgent priority, particularly in the setting of advanced, treatment-refractory malignancies.
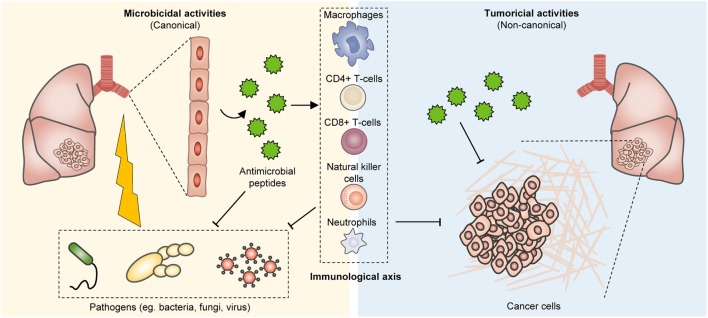
Owing to the clinical success of cancer immunotherapy, as exemplified by the broad efficacy of immune checkpoint inhibitors (e.g., pembrolizumab, ipilimumab, atezolizumab) across multiple tumor histologies, there has been renewed interest in the development of immunomodulatory strategies in oncology treatment (4). Antimicrobial peptides (AMPs) are structurally diverse, critical effector molecules of the innate immunity which rapidly act to inactivate invading microorganisms, especially at mucosal surfaces and epithelial barriers. Despite most translational studies involving AMPs being oriented toward their development as antibacterial and antifungal biopharmaceuticals, pioneering research has led to the identification of numerous AMPs with promising anticancer properties (5–14) (Figure 1). These include, but are not limited to various α-helical peptides, β-Sheet peptides, linear peptides, hybrid, and synthetic peptides (5, 15–18).
Figure 1.
Bioproduction of antimicrobial peptides (AMPs) and their tumor suppressive effects. In humans, AMPs are present at various tissues, including the epithelium of the skin, respiratory system, and gastrointestinal tract, as well as the immune system. These peptides can effectively impinge on a broad spectrum of microorganisms including fungi, bacteria, and viruses. However, they also restrain tumor growth and their immunostimulatory properties further co-opt anticancer immunity for enhanced tumor eradication.
Bioproduction, Physicochemical Properties, and Functions of AMPs
Antimicrobial peptides are small molecular weight oligopeptides, that is, they generally comprise 5–40 amino acid residues, with few exceptions. Both eukaryotes and prokaryotes are capable of producing these peptides. In humans, AMPs are present at various tissues, including the epithelium of the skin, respiratory system, and gastrointestinal tract, as well as the immune system (Figure 1). Depending on the site of synthesis, AMPs are broadly classified as non-ribosomal peptides (NRAMPs) if they are synthesized in the cytosol of bacteria and fungi, or ribosomal peptides (RAMPs) when they are synthesized in the ribosomes of both prokaryotes and eukaryotes (19, 20). AMPs are structurally heterogeneous and may assume linear (with amphipathic and hydrophobic α-helical residues [~30% or more]), β-sheet, cyclic, lipo, macrocyclic, or α-helical rod conformations (21, 22). It is worth noting that this diversity arises in part from post-translation modifications including glycosylation, phosphorylation, and amidation (23–27). AMPs are mostly cationic with a net charge at neutral pH ranging between +2 and +9 due to the presence of positively charged residues (typically, lysine [Lys] and arginine [Arg]), which endow these peptides with the ability to engage with and disrupt microorganismal membranes.
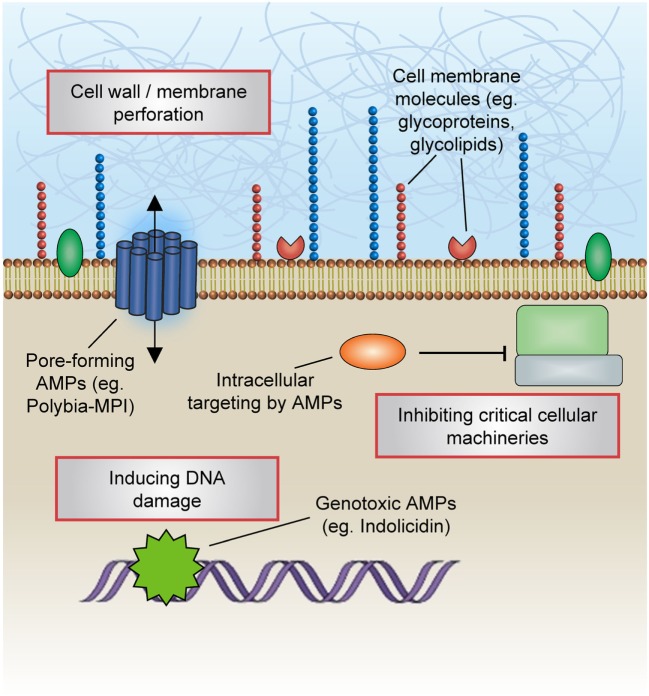
These peptides can effectively impinge on a broad spectrum of microorganisms including fungi, bacteria, and viruses (Figure 1). Notably, endogenous AMPs are recognized for being highly selective against pathogens, and for the most part spares untransformed mammalian cells. Classical mechanisms which mediate their antibiotic actions include their penetration of the plasma membrane or cell wall, thus resulting in lysis or disruption of ionic gradients; binding to and damaging nucleic acids (e.g., DNA); and blockade of enzymes essential for maintaining the integrity of microorganisms’ cell walls (28–37) (Figure 2). Amphipathicity (i.e., the spatial segregation of cationic and hydrophobic residues) in particular appears to be a major determinant of function. Other physicochemical considerations that may impact structure–activity relationships include the amino acid sequence, net charge, hydrophobicity, structural folding (i.e., secondary structure, dynamics, and orientation) in membranes, oligomerization, peptide concentration, and membrane composition (38).
Figure 2.
Mechanisms of antimicrobial actions. Classical mechanisms which mediate the antibiotic actions of antimicrobial peptides (AMPs) include their penetration of the plasma membrane or cell wall, thus resulting in lysis or disruption of ionic gradients; binding to and damaging nucleic acids (e.g., DNA); and blockade of enzymes essential for maintaining the integrity of microorganisms’ cell walls.
Immunomodulatory Effects of AMPs
Innate immunological responses to infectious agents, including the secretion of AMPs, are foremost initiated and orchestrated by the precise interaction between pathogen-derived ligands and immune receptors. Besides their canonical functions in carrying out microbicidal actions, AMPs are increasingly recognized to interact with host cells to influence diverse signaling cascades which may enhance the resolution of infections. For instance, β-defensins, a peptide active against many Gram-negative and -positive bacteria and viruses, also serves as a ligand for the CCR6 chemokine receptor that is expressed on T lymphocytes and dendritic cells, hence serving as a bridge between the innate and adaptive arms of host immunity. Other hitherto-unappreciated consequences on host immune cells have been described, including altering host gene expression; inducing chemokine secretion; modulating the activation or death of neutrophils, T lymphocytes, and dendritic cells; regulating cellular differentiation pathways; and promoting immune-mediated wound healing (39–41).
The detailed spatiotemporal control of these interactions between AMPs and host cells are hitherto less well known. Several models have been proposed, including the “alternate ligand model,” wherein AMPs transduce intracellular signaling cascades by acting as ligands for cell surface receptors, and the “membrane disruption model,” in which AMPs focally modify a part of the receptor to alter their functions. Furthermore, the “trans-activation model” posits that the indirect action of AMPs may lead to the production of membrane-bound factors that are capable of inducing receptor activation. It has also been shown that the scavenging of the endotoxin lipopolysaccharide (LPS) by AMPs may impede LPS binding with toll-like receptor 4, thus suppressing a proinflammatory process (42).
AMPs and Cancer Treatment
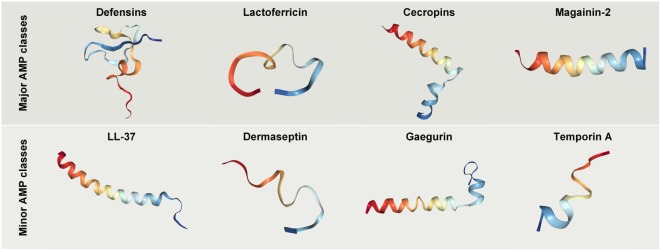
Henceforth in this review, we have defined classes of AMPs as “major” or “minor,” respectively, depending on whether their anticancer properties have been the subject of intensive study or are less well described (Figure 3).
Figure 3.
Crystal structures of selected antimicrobial peptides (AMPs) with anticancer potential. Protein databank accession codes: human beta-Defensin-3, IKJ6; cecropin B derivative CB1a, 2IGR; lactoferricin B, 1Y58; magainin 2, 2MAG; LL-37, 2K6O; Dermaseptin analog NC12-K4S4(1–13)a, 2DCX; Gaegurin 4, 2G9L; Temporin-1 Ta, 2MAA.
Major Therapeutically Relevant Classes of AMPs
Defensins
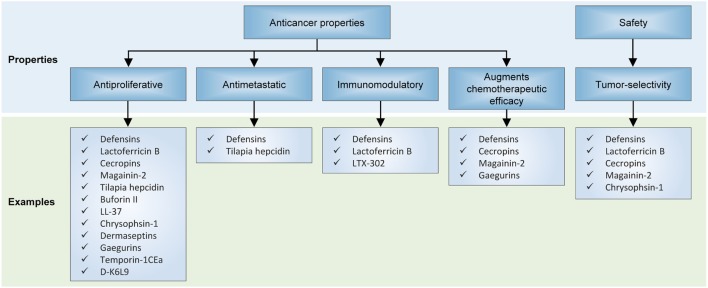
The defensins are cationic peptides produced by eukaryotes and comprise two superfamilies which have undergone divergent evolution in terms of sequence, structure, and function. The anticancer properties of human defensins are featured in a rapidly growing body of findings, and encouraging preclinical results have been obtained with the treatment of cancer cells or xenograft models with various natural or synthetic defensins. For instance, it has been shown that natural human β-defensin-3 (hBD-3) is capable of suppressing VEGF-induced cancer cell migration capabilities (43–46). In another study, hBD-3 were shown to be produced by tumor-infiltrating monocytes and inhibited the invasiveness and motility of colon cancer cells in a dose-dependent and paracrine fashion (45). Considering that these in vitro malignant phenotypic traits correlate with a primary tumor’s propensity to establish life-threatening metastatic outgrowths, the finding that defensins are effective at repressing cancer cell motility suggests that they can be developed as potential antimetastatic agents (Figure 4).
Figure 4.
Effects of antimicrobial peptides on in preclinical models of neoplasia. Several recurrent themes have emerged from unfolding research on their anticancer properties, including their antiproliferative and antimetastatic capabilities, invigoration of antitumor immunity, activity against multidrug-resistant cancer cells, and selectivity for cancer cells but not normal cells.
Furthermore, natural defensins appear to exert antiproliferative and proapoptotic effects on cancer cells and to induce cell cycle arrest (44, 47–51), which are evidenced by increases in the levels of phosphorylated retinoblastoma protein, suppressed activities of transcriptional and cell cycle cyclin-dependent kinases and their catalytic cyclin partners (52), and enhanced expression of caspase 7 and 9 and other markers of apoptosis. Interestingly, human beta-defensin-2 (hBD-2) have been shown to also reduce the viability of melanoma cells through the downregulation of BRAF (52). Besides their natural derivatives, synthetic defensin analogs may be designed for greater anticancer efficacy: Du et al. demonstrated that recombinant tailored defensin (DF-HSA) comprising human β-defensin-2 (DF) and human serum albumin (HSA) was more effective than natural β-defensin at curbing the proliferation of K-Ras-mutant MIA PaCa-2 cells and suppressing the growth of a pancreatic carcinoma xenograft (53).
Two additional facets of human defensins warrant discussion: first, it is notable that they appear to have an impressive level of specificity for tumor cells, yet do not appear to exert palpable cytotoxic or cytostatic effects against normal untransformed cells (48, 50, 51, 54). It has been shown that defensins induce apoptosis in MCF-7 cells via the intrinsic pathway, enhanced MAPK p38 phosphorylation, as well as increased expression of cytochrome c, Apaf-1, caspase 7 and 9, but did not affect the membrane potential and calcium flow (48). Another study indicated that Laterosporulin10, a defensin-like anticancer bacteriocin, results in apoptotic and necrotic death of MCF-7, HEK293T, HT1080, HeLa, and H1299 cells (50).
This observation is arguably consistent with the fact that human AMPs are endogenously derived, and therefore are designed to avoid causing overt collateral toxicity to normal healthy tissues during an inflammatory response. Second, antimicrobial defensins may present novel opportunities to address unmet clinical issues such as chemotherapeutic resistance. For instance, defensins have been shown to potentiate cancer cell-kill in combination with cytotoxic chemotherapeutic agents such as doxorubicin in multidrug-resistant cancer cells (51, 54).
Another compelling application for defensins is their significant potential to augment the effectiveness of cancer immunotherapy. Li et al. for instance employed a recombinant plasmid which expresses beta-defensin 2 and evaluated its potential as both cancer gene therapy and immunotherapy (55). In vitro and in vivo results indicated that physiological changes occurred in immature dendritic cells in a fashion which is likely to enhance adaptive anticancer immunosurveillance (55).
Lactoferricin B
Lactoferrin is an 80 kDa iron-sequestering glycoprotein present in exocrine secretions such as milk and in the granules of polymorphonuclear leukocytes, and represents another class of AMPs which have also been at the focal point of research into their anticancer properties. Lactoferricin B possess antitumor capabilities as it is capable of exerting lethal, selective destabilizing effects on cancer cell cytoplasmic and mitochondrial membranes (11), and has been shown to be effective against colorectal, neuroblastoma, and melanoma cancer cells (11, 56). As alluded to earlier in the article, AMPs may assume various conformations. A recent study demonstrated that bovine lactoferrin (bLf), cyclic LfcinB, and linear LfcinB were all capable of activating multiple antineoplastic signaling cascades, including p53 induction, apoptosis, and anti-angiogenic pathways as revealed by transcriptomic analyses (57). Furthermore, both bLf and LfcinB led to the induction of proapoptotic pathways mediated by caspase-8, p53, and p21 in colorectal carcinoma cells (57). Like the defensin proteins, lactoferricin appears to have immunomodulatory effects, and have been shown to orient lymphocytes toward eradicating cancer cells (58).
Cecropins A and B
Cecropins represent a class of small and basic peptides prototypically ranging between 31 and 39 amino acid residues with a strongly basic N-terminus and hydrophobic C-terminus and were initially isolated from the silk moth Hyalophora cecropia. Again as with a recurring theme regarding AMPs, cecropins A and B have been shown to have selective cytotoxic and cytostatic effects on bladder neoplasms but not on human fibroblast cell lines (59), while ABP-dHC-Cecropin A and its analog ABP-dHC-Cecropin A-K are cytotoxic against leukemic but not non-cancerous cell lines (60). Cecropin A has been shown to induce apoptosis in the promyelocytic cell line HL-60 through a ROS signaling mechanism (61), and in human hepatocellular carcinoma cells via expression of Fas, Fas-L, caspase-3, and -8 (12). CecropinXJ induces growth inhibition, S-phase arrest and apoptosis in hepatocellular carcinoma cells through expression of caspase-3 and poly(ADP-ribose) polymerase, and downregulation of B cell lymphoma 2 (Bcl-2) (62). One particularly enticing finding has been the result that in vitro, cecropin A enhances the anticancer effects of common chemotherapeutic agents against squamous skin cancer cells, which could open up new possibilities for rational combination strategies consisting of these two modalities (63).
Magainin II (MG2)
Magainin II (MGN-II) is an AMP isolated from the skin of the African clawed frog Xenopus laevis which has demonstrated potent anticancer effects in various hematopoietic and solid malignancies. As an ionophoric peptide with a helical structure, it has been shown to perforate cancer cell membranes to act as ion channels, causing cytolysis of cancerous cells (64). MGN-II greatly enhances the tumoricidal effects of cytotoxic chemotherapeutic agents. For instance, magainin A (MAG A) and magainin G (MAG G) have been shown to have synergistic effects when used with chemotherapy against non-small cell lung cancer cell lines (65). MGN-II also displays tumor-selectivity; for instance, they have been demonstrated to lyse various hematopoietic tumor and solid tumor cells but have little or no effect on normal human fibroblasts and peripheral blood lymphocytes (8, 10, 12).
Derivatives of MGN-II have also been synthesized to enhance their cancer-specific cytotoxic properties (18, 64, 66). For instance, a magainin II-bombesin conjugate (MG2B) has demonstrated enhanced activity at a lower dose against a wide range of human cancer cells, without adverse effects on normal cells, as well as potent antitumor activity in a murine model of breast cancer (66). In yet another example, the fusion peptide MG2A, which was synthesized by conjugating MGN-II to the NH2-terminal of the cell-penetrating peptide penetratin (Antp), exhibited augmented cytotoxicity against a variety of human cancer cells and rat glioma cells, while having very limited off-target effects on normal cells (18).
Minor Therapeutic Classes of AMPs
Structurally, LTX-302 is a 9-mer peptide derived from its parental peptide LfcinB, and features an α-helical secondary structure optimized to exert greater antitumor activity (67–69). The effects of LTX-302 have been examined in vivo against A20 B cell lymphomas in BALB/c mice (69). Interestingly, antitumor activity hinged on the mobilization and activation of CD4 and CD8 T-lymphocytes, but ultimately LTX-302 administration induced long-lived cellular immunity directed against lymphoma cells and mediated complete regression of tumors in the majority of xenograft mice (69).
Tilapia hepcidin (TH) 1–5 represent three hepcidin-like AMPs (TH1–5, TH2–2, and TH2–3) extracted from tilapia (Oreochromis mossambicus). They were previously shown by Chen et al. to specifically inhibit the growth of human fibrosarcoma HT1080 cells via cell membrane-perforating mechanisms, and to also diminish cell migration capabilities (10). These findings were corroborated by Chang et al. who found that TH1–5 decreased colony formation and induced rapid cell death in various human cancer cell lines (70).
Buforin II is a 21-residue α-helical AMP with sequence similarity to the N-terminal region of histone H2A. Buforin IIb is a synthetically derived analog of buforin II, modified to contain a proline residue in between two α-helices (71). Buforin IIb was found in a study to have activity against breast cancer cells MX-1 and MCF-7, and to suppress tumor growth in a mouse xenograft through anti-vasculogenic and anti-angiogenic mechanisms (13). It is postulated that the glycosylation of breast cancer cells plays a significant role in enabling interaction with this AMP, thereby allowing it to exert anticancer effects (13).
LL-37 is a derivative of human cathelicidin-derived α-helical AMPs. Its precursor hCAP18 is found in body fluids and functions as a peptide antibiotic and signaling molecule (72). In a prior study, human colon cancer cells (HCT116 and LoVo) treated with FK-16, a 16-residue fragment of LL-37 underwent caspase-independent apoptosis and autophagy as a result of activation of the p53-Bcl-2/Bax cascade (16).
Chrysophsin-1 is an α-helical AMP found in the gill cells of red sea bream, which is distinct due to its hemolytic properties, in addition its antibiotic functions (73). In 2011, Hsu et al. tested the effects of chrysophsin-1 on a wide panel of cancer cell lines (74), and showed that low-dose chrysophsin-1 selectively culls tumor cells via a membrane-depolarizing lytic mechanism (74).
Dermaseptins B1–B6 are six related peptides of 24–33 residues in length which are constitutively expressed in the skin secretions of the South American frog Phyllomedusa bicolor. Zoggel and colleagues demonstrated that the B2 peptide had antiproliferative and anti-colony-forming effects on a wide range of human cancer cells as well as xenograft model of prostate adenocarcinoma (15).
Gaegurins are a family of six AMPs isolated from Rana rugosa and are broadly divided into two subfamilies based on their length and sequence. The family II peptide GGN6, as well as its derivative PTP7, has been shown in a previous experiment by Kim et al. to have potent anticancer effects against multiple human cancer cell lines, including A549, PC-3, and MCF-7 (17). Intriguingly, as has also been demonstrated in aforementioned AMPs, GGN6 derivatives potently induced cell cycle arrest and apoptosis in multidrug-resistant cancer cells, suggesting its potential to be deployed in the treatment of refractory malignancies (17).
Polybia-MPI is a cationic peptide extracted from the social wasp Polybia paulista. Polybia-MPI is characterized by potent bactericidal (against both Gram-positive and Gram-negative bacteria), fungicidal, and tumoricidal biochemical properties, while being relatively non-toxic to human red blood cells and normal fibroblasts (75, 76). Polybia-MPI and its derivatives have been shown to induce pore formation leading to the death of prostate cancer, bladder cancer, and drug-resistant myelogenous leukemic cells (75, 76).
Temporin-1CEa is an amphipathic AMP secreted by the skin of the Chinese brown frog Rana chensinensis. Studies have demonstrated that the treatment of a range of human cancer cell lines with temporin-1CEa rapidly induces tumor cell death by disrupting their cell membrane and mitochondria (77, 78). Melanoma cells seem particularly susceptible, perhaps because of their overexpression of phosphatidylserine, which has high affinity for temporin-1CEa (78).
D-K6L9 is a peptide is bound by phosphatidylserine which is capable of inducing tumor necrosis. Its administration to B16-F10 murine melanoma tumors inhibited its growth, whereas therapeutic cessation led to tumor relapse (79). Combinations comprising D-K6L9 with glycyrrhizin (an inhibitor of HMGB1 protein), BP1 peptide, and interleukin (IL)-12 exhibit antitumor efficacy. When glycyrrhizin or BP1 is combined with D-K6L9, the growth of tumors was suppressed during the period of their administration. Long-lasting tumor growth suppression effect was achieved by combining D-K6L9 plus IL-12. Two months after therapeutic cessation, a remarkable 60% of animals remained alive. Significantly prolonged survival was observed in both mice bearing B16-F10 tumors as well as in mice bearing C26 colon carcinoma tumors (79).
AMPs in Clinical Trials
Some cationic AMPs may exert their microbicidal effects chiefly through the potent induction of host immunoreactivity, rather than through direct modes of action. It is for this reason that their potential for use as adjuncts to current anticancer modalities has galvanized significant interest over the past few years, boosted by tantalizing efficacy results from recent clinical trials of cancer immunotherapies.
The safety profile of AMPs deserves mention, if only briefly. Whereas in the context of oncologic indications, these biologics remain experimental (Table 1), it should be noted that several AMPs have transitioned to phase II clinical trial evaluation or have even obtained U.S. Food and Drug Administration approval for use in the treatment of various infectious diseases (80, 81), such as pexiganan acetate (MSI-78) (82), hLF1–11 (83), omiganan (MBI-226) (81), CZEN-002, and novexatin (NP-213) (84) (Table 2).
Table 1.
Antimicrobial peptides for oncologic indications in ongoing clinical trials.
| Phase | Peptide name | Identifier number | Condition | Administration route |
|---|---|---|---|---|
| I | LL37 | NCT02225366 | Metastatic melanoma | Intratumoral |
| GRN-1201 | NCT02696356 | Solid tumors | Intravenous | |
| LTX-315 | NCT01058616 | Solid tumors | Intravenous | |
| WT-2725 | NCT01621542 | Hematological malignancy and solid tumors | Intravenous | |
| II | SGX942 | NCT02013050 | Head and neck cancer | Intravenous |
| ANG-1005 | NCT02048059 | Breast and brain metastasis | Intravenous | |
| III | ITK-1 | UMIN000011308 | Glioblastoma and prostate cancer | Intravenous |
Table 2.
Antimicrobial peptides for infectious diseases indications in ongoing clinical trials.
| Phase | Peptide name | Identifier numbers | Condition | Administration route |
|---|---|---|---|---|
| II | NP213 (Novexatin) | NCT02933879 | Fungal nail infection | Topical |
| PAC-113 | NCT00659971 | Oral candidiasis in HIV patients | Topical | |
| MBI-226 | NCT00211523 | Acne vulgaris and acne | Topical | |
| Dalbavancin | NCT02685033 | Acute hematogenous osteomyelitis | Intravenous | |
| Brilacidin | NCT02052388 | Skin and bacterial infection | Topical | |
| CLS001 (Omiganan) | NCT02596074 | Acne vulgaris | Topical | |
| III | Pexiganan (MSI-78) | NCT01594762 | Diabetic foot infection | Topical |
| Surotomycin | NCT01597505 | Clostridium difficile associated diarrhea | Oral | |
| CLS001 (Omiganan) | NCT02576847 | Papulopustular rosacea | Topical | |
| P2TA | NCT01417780 | Necrotizing soft tissue infection | Intravenous | |
The first AMP developed commercially was pexiganan acetate (MSI-78) (82). A number of AMPs are being developed for systemic applications. For instance, hLF1-11, a cationic fragment comprised N-terminal amino acids 1–11 of human lactoferricin, is intravenously administered for the treatment of severe bacterial and fungal infections in immunosuppressed stem cell transplant recipients (83). Another AMP that is at an advanced stage of clinical trialing is omiganan (MBI-226), a derivative of indolicidin which was purified from bovine neutrophils, being tested as a topical gel for the prophylaxis of contamination of central venous catheters (81). Additional examples abound: Fopical pexiganan might be an effective alternative to oral antibiotic therapy compared to ofloxacin for treatment of mildly infected foot ulcer in diabetic patients (85). Novexatin (NP-213), a cyclic and highly cationic peptide based on human α- and β-defensins, has shown promise in treating recalcitrant fungal infections in toenails while CZEN-002, a dimeric peptide sequentially derived from α-melanocyte-stimulating hormone, is targeting vaginal candidiasis (84).
Antimicrobial peptides are only beginning to encroach into the oncological sphere, and therefore efficacy data are relatively limited (Table 1). However, the safety data in infectious diseases trials, albeit indirectly, substantiate the notion that AMPs could also be well tolerated in cancer patients. An example of an ongoing oncology trial is NCT02225366, wherein the optimal biological dose and therapeutic activity of LL37 against metastatic melanoma is being evaluated in a phase I setting. LL37 is being administered intratumorally in patients with documented metastatic melanoma and at least three cutaneous lesions measuring with stage IIIB, IIIC, or IV or nodal lesions.
Conclusion and Future Directions
Therapeutic resistance and metastatic dissemination are some of the most clinically challenging aspects of cancer. There is now an abundance of evidence that AMPs hold substantial potential for filling these therapeutic voids in clinical oncology. In this review, we have discussed evidence for their antiproliferative, proapoptotic, and antimetastatic effects on cancer cells. Crucially, AMPs have also shown activity against multidrug-resistant cancer cell lines, and therefore could prove valuable for the treatment of advanced, refractory cancers. Hence, we envision that in the future, combination strategies involving this novel therapeutic class with conventional cancer treatments (targeted therapies, immunotherapies, and chemotherapy) may improve treatment outcomes.
Nevertheless, significant challenges lie ahead in the path toward their clinical development and deployment. Toxicity continues to feature as a prominent concern, especially with regardsto the administration of non-human natural or synthetic AMPs. However, lessons can be learnt from the field of infectious diseases, where several AMPs have transitioned to clinical trials or have even gained a foothold in clinical care. One area in which the therapeutic index of these peptides can be improved is through the development of innovative formulations and drug delivery systems. Structure–activity relationship, lead identification, and optimization studies are also crucial to improving the anticancer efficacy of AMPs (Figure 5). Yet, these will also require greater understanding of their underlying anti-tumorigenic mechanisms, which hitherto remain somewhat speculative. It is also worth noting that the vast majority of AMPs that exist in nature have yet to be characterized, and given their potential for therapeutic applications, there are certainly compelling reasons to conduct more comprehensive surveys to identify drug candidates. However, with focused efforts to overcome these limitations and obstacles, and a rigorous commitment to translate—at a reasonable cost—these promising peptides into cancer care, the future for this emerging therapeutic class looks exceptionally bright.
Figure 5.
Physicochemical considerations that may impact structure–activity. A number of biochemical factors need to be considered when predicting the anticancer activity of natural or synthetic antimicrobial peptides, including but not limited to the amino acid sequence, net charge, hydrophobicity, structural folding (i.e., secondary structure, dynamics, and orientation) in membranes, oligomerization, peptide concentration, and membrane composition.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. NS is supported by Wong Hock Boon Society funds from the Yong Loo Lin School of Medicine, National University of Singapore.
References
- 1.Antoni S, Soerjomataram I, Møller B, Bray F, Ferlay J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull World Health Organ (2016) 94:174–84. 10.2471/BLT.15.164384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65(1):5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol (2017) 3(4):524–48. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syn NL, Yong WP, Goh BC, Lee SC. Evolving landscape of tumor molecular profiling for personalized cancer therapy: a comprehensive review. Expert Opin Drug Metab Toxicol (2016) 12(8):911–22. 10.1080/17425255.2016.1196187 [DOI] [PubMed] [Google Scholar]
- 5.Huang TC, Lee JF, Chen JY. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar Drugs (2011) 9(10):1995–2009. 10.3390/md9101995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SP, Huang TC, Lin CC, Hui CF, Lin CH, Chen JY. Pardaxin, a fish antimicrobial peptide, exhibits antitumor activity toward murine fibrosarcoma in vitro and in vivo. Mar Drugs (2012) 10(8):1852–72. 10.3390/md10081852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papo N, Shahar M, Eisenbach L, Shai Y. A novel lytic peptide composed of dl-amino acids selectively kills cancer cells in culture and in mice. J Biol Chem (2003) 278(23):21018–23. 10.1074/jbc.M211204200 [DOI] [PubMed] [Google Scholar]
- 8.Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, et al. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol (2006) 50(1):141–7. 10.1016/j.eururo.2005.12.043 [DOI] [PubMed] [Google Scholar]
- 9.Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A (1991) 88(9):3792–6. 10.1073/pnas.88.9.3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JY, Lin WJ, Lin TL. A fish antimicrobial peptide, tilapia hepcidin TH2-3, shows potent antitumor activity against human fibrosarcoma cells. Peptides (2009) 30(9):1636–42. 10.1016/j.peptides.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 11.Eliassen LT, Berge G, Leknessund A, Wikman M, Lindin I, Løkke C, et al. The antimicrobial peptide, lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. Int J Cancer (2006) 119(3):493–500. 10.1002/ijc.21886 [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Mei H, Li X, Ma Y, Zeng A-H, Wang Y, et al. Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim Biophys Sin (Shanghai) (2010) 42(4):259–65. 10.1093/abbs/gmq021 [DOI] [PubMed] [Google Scholar]
- 13.Han YY, Liu HY, Han DJ, Zong XC, Zhang SQ, Chen YQ. Role of glycosylation in the anticancer activity of antibacterial peptides against breast cancer cells. Biochem Pharmacol (2013) 86(9):1254–62. 10.1016/j.bcp.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 14.Heilborn JD, Nilsson MF, Jimenez CIC, Sandstedt B, Borregaard N, Tham E, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer (2005) 114(5):713–9. 10.1002/ijc.20795 [DOI] [PubMed] [Google Scholar]
- 15.van Zoggel H, Carpentier G, Dos Santos C, Hamma-Kourbali Y, Courty J, Amiche M, et al. Antitumor and angiostatic activities of the antimicrobial peptide dermaseptin B2. PLoS One (2012) 7(9):e44351. 10.1371/journal.pone.0044351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One (2013) 8(5):e63641. 10.1371/journal.pone.0063641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Kim SS, Bang Y-J, Kim S-J, Lee BJ. In vitro activities of native and designed peptide antibiotics against drug sensitive and resistant tumor cell lines. Peptides (2003) 24(7):945–53. 10.1016/S0196-9781(03)00194-3 [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Yang H, Wan L, Cheng J, Lu X. Penetratin-mediated delivery enhances the antitumor activity of the cationic antimicrobial peptide magainin II. Cancer Biother Radiopharm (2013) 28(4):289–97. 10.1089/cbr.2012.1328 [DOI] [PubMed] [Google Scholar]
- 19.Grünewald J, Marahiel MA. Chemoenzymatic and template-directed synthesis of bioactive macrocyclic peptides. Microbiol Mol Biol Rev (2006) 70(1):121–46. 10.1128/MMBR.70.1.121-146.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papagianni M. Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv (2003) 21(6):465–99. 10.1016/S0734-9750(03)00077-6 [DOI] [PubMed] [Google Scholar]
- 21.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta (1999) 1462(1):11–28. 10.1016/S0005-2736(99)00198-4 [DOI] [PubMed] [Google Scholar]
- 22.Cornut I, Thiaudiere E, Dufourcq J. The amphipathic helix in cytotoxic peptides. In: Epand RM, editor. The Amphipathic Helix. Boca Raton, FL: CRC Press; (1993). p. 173–219. [Google Scholar]
- 23.Kuipers OP, Bierbaum G, Ottenwälder B, Dodd HM, Horn N, Metzger J, et al. Protein engineering of lantibiotics. Antonie Van Leeuwenhoek (1996) 69(2):161–9. 10.1007/BF00399421 [DOI] [PubMed] [Google Scholar]
- 24.Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Biopolymers (1998) 47(6):415–33. [DOI] [PubMed] [Google Scholar]
- 25.Cheigh CI, Pyun YR. Nisin biosynthesis and its properties. Biotechnol Lett (2005) 27(21):1641–8. 10.1007/s10529-005-2721-x [DOI] [PubMed] [Google Scholar]
- 26.Jack RW, Jung G. Lantibiotics and microcins: polypeptides with unusual chemical diversity. Curr Opin Chem Biol (2000) 4(3):310–7. 10.1016/S1367-5931(00)00094-6 [DOI] [PubMed] [Google Scholar]
- 27.Xie L, Van Der Donk WA. Post-translational modifications during antibiotic biosynthesis. Curr Opin Chem Biol (2004) 8(5):498–507. 10.1016/j.cbpa.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz R. Phylogenetic perspectives in innate immunity. Science (1999) 284(5418):1313–8. 10.1126/science.284.5418.1313 [DOI] [PubMed] [Google Scholar]
- 29.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature (2002) 415(6870):389–95. 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- 30.Broekaert WF, Terras F, Cammue B, Osborn RW. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol (1995) 108(4):1353–8. 10.1104/pp.108.4.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulet P, Stöcklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev (2004) 198(1):169–84. 10.1111/j.0105-2896.2004.0124.x [DOI] [PubMed] [Google Scholar]
- 32.Otvos L, Bokonyi K, Varga I, Ertl HC, Hoffmann R, Bulet P, et al. Insect peptides with improved protease-resistance protect mice against bacterial infection. Protein Sci (2000) 9(4):742–9. 10.1110/ps.9.4.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganz T, Lehrer RI. Antimicrobial peptides of leukocytes. Curr Opin Hematol (1997) 4(1):53–8. 10.1097/00062752-199704010-00009 [DOI] [PubMed] [Google Scholar]
- 34.Thevissen K, Terras FR, Broekaert WF. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol (1999) 65(12):5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thevissen K, Osborn RW, Acland DP, Broekaert WF. Specific binding sites for an antifungal plant defensin from Dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol Plant Microbe Interact (2000) 13(1):54–61. 10.1094/MPMI.2000.13.1.54 [DOI] [PubMed] [Google Scholar]
- 36.Jigami Y, Odani T. Mannosylphosphate transfer to yeast mannan. Biochim Biophys Acta (1999) 1426(2):335–45. 10.1016/S0304-4165(98)00134-2 [DOI] [PubMed] [Google Scholar]
- 37.Tang Y-Q, Yuan J, Ösapay G, Ösapay K, Tran D, Miller CJ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science (1999) 286(5439):498–502. 10.1126/science.286.5439.498 [DOI] [PubMed] [Google Scholar]
- 38.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta (1999) 1462(1):55–70. 10.1016/S0005-2736(99)00200-X [DOI] [PubMed] [Google Scholar]
- 39.Territo M, Ganz T, Selsted M, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest (1989) 84(6):2017–20. 10.1172/JCI114394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol (2002) 23(6):291–6. 10.1016/S1471-4906(02)02246-9 [DOI] [PubMed] [Google Scholar]
- 41.Heilborn JD, Nilsson MF, Sørensen O, Ståhle-Bäckdahl M, Kratz G, Weber G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol (2003) 120(3):379–89. 10.1046/j.1523-1747.2003.12069.x [DOI] [PubMed] [Google Scholar]
- 42.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol (2009) 30(3):131–41. 10.1016/j.it.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Wang JH, Baskaran H, Wang R, Jurevic R. Effect of human beta-defensin-3 on head and neck cancer cell migration using micro-fabricated cell islands. Head Neck Oncol (2012) 4(1):41. 10.1186/1758-3284-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerashchenko O, Zhuravel E, Skachkova O, Khranovska N, Filonenko V, Pogrebnoy P, et al. Biologic activities of recombinant human-beta-defensin-4 toward cultured human cancer cells. Exp Oncol (2013) 35(2):76–82. [PubMed] [Google Scholar]
- 45.Uraki S, Sugimoto K, Shiraki K, Tameda M, Inagaki Y, Ogura S, et al. Human β-defensin-3 inhibits migration of colon cancer cells via downregulation of metastasis-associated 1 family, member 2 expression. Int J Oncol (2014) 45(3):1059–64. 10.3892/ijo.2014.2507 [DOI] [PubMed] [Google Scholar]
- 46.Han Q, Wang R, Sun C, Jin X, Liu D, Zhao X, et al. Human beta-defensin-1 suppresses tumor migration and invasion and is an independent predictor for survival of oral squamous cell carcinoma patients. PLoS One (2014) 9(3):e91867. 10.1371/journal.pone.0091867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong JH, Ng TB. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides (2005) 26(7):1120–6. 10.1016/j.peptides.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 48.Guzmán-Rodríguez JJ, López-Gómez R, Salgado-Garciglia R, Ochoa-Zarzosa A, López-Meza JE. The defensin from avocado (Persea americana var. drymifolia) PaDef induces apoptosis in the human breast cancer cell line MCF-7. Biomed Pharmacother (2016) 82:620–7. 10.1016/j.biopha.2016.05.048 [DOI] [PubMed] [Google Scholar]
- 49.Winter J, Kraus D, Reckenbeil J, Probstmeier R. Oncogenic relevant defensins: expression pattern and proliferation characteristics of human tumor cell lines. Tumour Biol (2016) 37(6):7959–66. 10.1007/s13277-015-4701-7 [DOI] [PubMed] [Google Scholar]
- 50.Baindara P, Gautam A, Raghava G, Korpole S. Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci Rep (2017) 7:46541. 10.1038/srep46541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strzelecka P, Czaplinska D, Sadej R, Wardowska A, Pikula M, Lesner A. Simplified, serine-rich theta-defensin analogues as antitumour peptides. Chem Biol Drug Des (2017) 90(1):52–63. 10.1111/cbdd.12927 [DOI] [PubMed] [Google Scholar]
- 52.Gerashchenko O, Zhuravel E, Skachkova O, Khranovska N, Pushkarev V, Pogrebnoy P, et al. Involvement of human beta-defensin-2 in regulation of malignant potential of cultured human melanoma cells. Exp Oncol (2014) 36(1):17–23. [PubMed] [Google Scholar]
- 53.Du Y, Shang B-Y, Sheng W-J, Zhang S-H, Li Y, Miao Q-F, et al. A recombinantly tailored β-defensin that displays intensive macropinocytosis-mediated uptake exerting potent efficacy against K-Ras mutant pancreatic cancer. Oncotarget (2016) 7(36):58418–34. 10.18632/oncotarget.11170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnstone SA, Gelmon K, Mayer LD, Hancock RE, Bally MB. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des (2000) 15(2):151–60. [PubMed] [Google Scholar]
- 55.Li D, Wang W, Shi HS, Fu YJ, Chen X, Chen XC, et al. Gene therapy with beta-defensin 2 induces antitumor immunity and enhances local antitumor effects. Hum Gene Ther (2014) 25(1):63–72. 10.1089/hum.2013.161 [DOI] [PubMed] [Google Scholar]
- 56.Fadnes B, Rekdal Ø, Uhlin-Hansen L. The anticancer activity of lytic peptides is inhibited by heparan sulfate on the surface of the tumor cells. BMC Cancer (2009) 9(1):183. 10.1186/1471-2407-9-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang R, Lönnerdal B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem Cell Biol (2016) 95(1):99–109. 10.1139/bcb-2016-0094 [DOI] [PubMed] [Google Scholar]
- 58.Ganz T, Lehrer RI. Defensins. Pharmacol Ther (1995) 66(2):191–205. 10.1016/0163-7258(94)00076-F [DOI] [PubMed] [Google Scholar]
- 59.Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, et al. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol (2008) 8(1):5. 10.1186/1471-2490-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sang M, Zhang J, Zhuge Q. Selective cytotoxicity of the antibacterial peptide ABP-dHC-Cecropin A and its analog towards leukemia cells. Eur J Pharmacol (2017) 803:138–47. 10.1016/j.ejphar.2017.03.054 [DOI] [PubMed] [Google Scholar]
- 61.Cerón JM, Contreras-Moreno J, Puertollano E, De Cienfuegos GA, Puertollano MA, De Pablo MA. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides (2010) 31(8):1494–503. 10.1016/j.peptides.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 62.Xia L, Wu Y, Ma J, Yang J, Zhang F. The antibacterial peptide from Bombyx mori cecropinXJ induced growth arrest and apoptosis in human hepatocellular carcinoma cells. Oncol Lett (2016) 12(1):57–62. 10.3892/ol.2016.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Do N, Weindl G, Grohmann L, Salwiczek M, Koksch B, Korting HC, et al. Cationic membrane-active peptides-anticancer and antifungal activity as well as penetration into human skin. Exp Dermatol (2014) 23(5):326–31. 10.1111/exd.12384 [DOI] [PubMed] [Google Scholar]
- 64.Yang D, Zou R, Zhu Y, Liu B, Yao D, Jiang J, et al. Magainin II modified polydiacetylene micelles for cancer therapy. Nanoscale (2014) 6(24):14772–83. 10.1039/c4nr04405c [DOI] [PubMed] [Google Scholar]
- 65.Ohsaki Y, Gazdar AF, Chen H-C, Johnson BE. Antitumor activity of magainin analogues against human lung cancer cell lines. Cancer Res (1992) 52(13):3534–8. [PubMed] [Google Scholar]
- 66.Liu S, Yang H, Wan L, Cai H-W, Li S-F, Li Y-P, et al. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol Sin (2011) 32(1):79–88. 10.1038/aps.2010.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haug BE, Camilio KA, Eliassen LT, Stensen W, Svendsen JS, Berg K, et al. Discovery of a 9-mer cationic peptide (LTX-315) as a potential first in class oncolytic peptide. J Med Chem (2016) 59(7):2918–27. 10.1021/acs.jmedchem.5b02025 [DOI] [PubMed] [Google Scholar]
- 68.Eksteen JJ, Ausbacher D, Simon-Santamaria J, Stiberg T, Cavalcanti-Jacobsen C, Wushur I, et al. Iterative design and in vivo evaluation of an oncolytic antilymphoma peptide. J Med Chem (2017) 60(1):146–56. 10.1021/acs.jmedchem.6b00839 [DOI] [PubMed] [Google Scholar]
- 69.Berge G, Eliassen LT, Camilio KA, Bartnes K, Sveinbjørnsson B, Rekdal Ø. Therapeutic vaccination against a murine lymphoma by intratumoral injection of a cationic anticancer peptide. Cancer Immunol Immunother (2010) 59(8):1285–94. 10.1007/s00262-010-0857-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang W-T, Pan C-Y, Rajanbabu V, Cheng C-W, Chen J-Y. Tilapia (Oreochromis mossambicus) antimicrobial peptide, hepcidin 1–5, shows antitumor activity in cancer cells. Peptides (2011) 32(2):342–52. 10.1016/j.peptides.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 71.Lee HS, Park CB, Kim JM, Jang SA, Park IY, Kim MS, et al. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett (2008) 271(1):47–55. 10.1016/j.canlet.2008.05.041 [DOI] [PubMed] [Google Scholar]
- 72.Dürr UH, Sudheendra U, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta (2006) 1758(9):1408–25. 10.1016/j.bbamem.2006.03.030 [DOI] [PubMed] [Google Scholar]
- 73.Mason AJ, Bertani P, Moulay G, Marquette A, Perrone B, Drake AF, et al. Membrane interaction of chrysophsin-1, a histidine-rich antimicrobial peptide from red sea bream. Biochemistry (2007) 46(51):15175–87. 10.1021/bi701344m [DOI] [PubMed] [Google Scholar]
- 74.Hsu JC, Lin LC, Tzen JT, Chen JY. Characteristics of the antitumor activities in tumor cells and modulation of the inflammatory response in RAW264. 7 cells of a novel antimicrobial peptide, chrysophsin-1, from the red sea bream (Chrysophrys major). Peptides (2011) 32(5):900–10. 10.1016/j.peptides.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 75.Wang KR, Yan JX, Zhang BZ, Song JJ, Jia PF, Wang R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett (2009) 278(1):65–72. 10.1016/j.canlet.2008.12.027 [DOI] [PubMed] [Google Scholar]
- 76.Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides (2008) 29(6):963–8. 10.1016/j.peptides.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Zhou Y, Li S, Li H, Tian L, Wang H, et al. Anticancer mechanisms of temporin-1CEa, an amphipathic α-helical antimicrobial peptide, in Bcap-37 human breast cancer cells. Life Sci (2013) 92(20):1004–14. 10.1016/j.lfs.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 78.Wang C, Chen Y-W, Zhang L, Gong X-G, Zhou Y, Shang D-J. Melanoma cell surface-expressed phosphatidylserine as a therapeutic target for cationic anticancer peptide, temporin-1CEa. J Drug Target (2016) 24(6):548–56. 10.3109/1061186X.2015.1113539 [DOI] [PubMed] [Google Scholar]
- 79.Cichon T, Smolarczyk R, Matuszczak S, Barczyk M, Jarosz M, Szala S. D-K6L 9 peptide combination with IL-12 inhibits the recurrence of tumors in mice. Arch Immunol Ther Exp (Warsz) (2014) 62(4):341–51. 10.1007/s00005-014-0268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andres E, Dimarcq J. Cationic antimicrobial peptides: update of clinical development. J Intern Med (2004) 255(4):519–20. 10.1046/j.1365-2796.2003.01278.x [DOI] [PubMed] [Google Scholar]
- 81.Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res (2005) 30(7):505–15. 10.1080/02713680590968637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore A. The big and small of drug discovery. EMBO Rep (2003) 4(2):114–7. 10.1038/sj.embor.embor748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Velden WJ, van Iersel TM, Blijlevens NM, Donnelly JP. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med (2009) 7(1):44. 10.1186/1741-7015-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov (2012) 11(1):37–51. 10.1038/nrd3591 [DOI] [PubMed] [Google Scholar]
- 85.Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis (2008) 47(12):1537–45. 10.1086/593185 [DOI] [PubMed] [Google Scholar]