ABSTRACT
Introduction: Livestock husbandry is critical for food security and poverty reduction in a low-income country like Tanzania. Infectious disease is one of the major constraints reducing the productivity in this sector. Peste des petits ruminants (PPR) is one of the most important diseases affecting small ruminants, but other infectious diseases may also be present.
Objective: The objective of this study was to determine the seroprevalence and risk factors for exposure to PPR, contagious caprine pleuropneumonia (CCPP), foot-and-mouth disease (FMD), bluetongue (BT), and bovine viral diarrhoea (BVD) in sheep and goats in Tanzania.
Methods: Serum samples were collected in 2014 and 2015, and analysed using enzyme-linked immunosorbent assays to detect antibodies to the five pathogens.
Results and discussion: This is the first description of seroprevalence of FMD and BT among small ruminants in Tanzania. Risk factor analysis identified sex (female) (OR for 2014: PPR: 2.49, CCPP: 3.11, FMD: 2.98, BT: 12.4, OR for 2015: PPR: 14.1, CCPP: 1.10, FMD: 2.67, BT: 1.90, BVD: 4.73) and increasing age (>2 years) (OR for 2014: PPR: 14.9, CCPP: 2.34, FMD: 7.52, BT: 126, OR for 2015: PPR: 8.13, CCPP: 1.11, FMD: 2.98, BT: 7.83, BVD: 4.74) as risk factors for exposure to these diseases.
KEYWORDS: peste des petits ruminants, contagious caprine pleuropneumonia, foot-and-mouth disease, bluetongue, bovine viral diarrhoea, Tanzania
Introduction
Small ruminants play an important role in food security and livelihood resilience in many parts of the world [1], but there are several constraints reducing the productivity in this sector [2,3]. Infectious disease is considered a major restriction causing direct losses, such as death and decreased production, and indirect losses, such as export constraints [3].
Peste des petits ruminants (PPR) is one of the most important diseases affecting small ruminants worldwide [4,5]. PPR is caused by peste des petits ruminants virus (PPRV), a highly contagious virus that gives rise to disease in sheep, goats, and camels and has also been reported in wild ruminants [6]. Clinical signs of PPR include pyrexia (40–41°C), ocular and nasal discharges, lesions in the oral and nasal mucus membranes, dyspnoea, cough, pneumonia, diarrhoea, and severe dehydration [7]. Morbidity and case fatality rates vary and, depending on factors such as immune status, age, species, and presence of other co-infections, they can be as high as 90–100% [8].
Clinical presentation of PPR can be difficult to differentiate from other diseases affecting small ruminants [7]. Differential diagnoses include contagious caprine pleuropneumonia (CCPP), foot-and-mouth disease (FMD), and bluetongue (BT) [9]. CCPP is caused by the bacterium Mycoplasma capricolum subsp. capripneumoniae (Mccp) [10], FMD is caused by foot-and-mouth disease virus (FMDV) [11], and BT is caused by bluetongue virus (BTV) and is spread by the vector Culicoides mosquitos [12]. Infection with bovine viral diarrhoea virus (BVDV), or the closely related border disease virus (BDV), is generally not considered a differential diagnosis of PPR as these viruses mostly cause reproductive disease in small ruminants [13–15]. However, co-infections with PPRV and BVDV, BDV, or BTV are believed to exacerbate the clinical signs of PPR [16,17].
PPR, CCPP, FMD, and BT are among the 10 most important diseases in sheep and goats worldwide in terms of lost livestock units [5]. For PPR, 6 of the 10 most affected countries during 2006–2009 were African countries [5]. Tanzania, located on the east coast of Africa, is a low-income country with 28.2% of the population living below the national poverty line [18]. Of the total population, 68.4% live in rural areas and three of five rural households earn, on average, 22% of their income from livestock husbandry [19]. Poorer households tend to keep small livestock, such as chicken, sheep and goats, whereas wealthier households keep large livestock [19]. Small ruminants are kept by 52% of Tanzanian households, with an estimated number of 15 million goats and 6 million sheep [20]. PPR was first confirmed in Tanzania in 2008 [21], but a retrospective study on samples collected in the northern districts found antibodies to PPRV were probably already present in 2004 [22]. The disease has since spread to the southern parts of the country and is now considered endemic in the domestic, small ruminant population in the whole country [23–25]. CCPP, FMD, BT, and BVD are endemic in Tanzania [24], however studies on FMD and BT have only been performed on large ruminants.
All of the diseases in question (PPR, CCPP, FMD, BT, and BVD) have been described in wildlife [26–30]. Wild ruminants have been shown to carry PPRV and several species can develop clinical signs of PPR [28,31,32]. Whether interaction or proximity between livestock and wildlife in general, and wild ruminants in particular, is an important risk factor for exposure to PPRV has not yet been determined.
The objective of this study was to estimate the seroprevalence of, and determine possible risk factors for exposure to, PPR, CCPP, FMD, BT, and BVD in small domestic ruminants in selected areas of Tanzania.
Materials and methods
Study area and study design
This study was carried out with the aim to understand the epidemiology of PPR at the wildlife–livestock interface in Tanzania. Thus, the study area was in parts of the country with such an interface (shared pastures, shared water, and regular proximity) and in regions where PPR had previously been described [21,33]. Tanzania is divided into 26 administrative regions, subdivided into districts, and further into wards [34]. Four districts were purposively selected for this study: Ngorongoro in the northern Arusha region, and Ulanga, Kilombero, and Mvomero in the south-eastern Morogoro region (Figure 1). Wards in the districts, located outside, bordering, or within parks or reserves (with a wildlife–livestock interface) were purposively selected, after which 50% were then randomly assigned to the study. In collaboration with local extension officers, wards were replaced with neighbouring wards when those selected did not have enough animals or were inaccessible.
Figure 1.
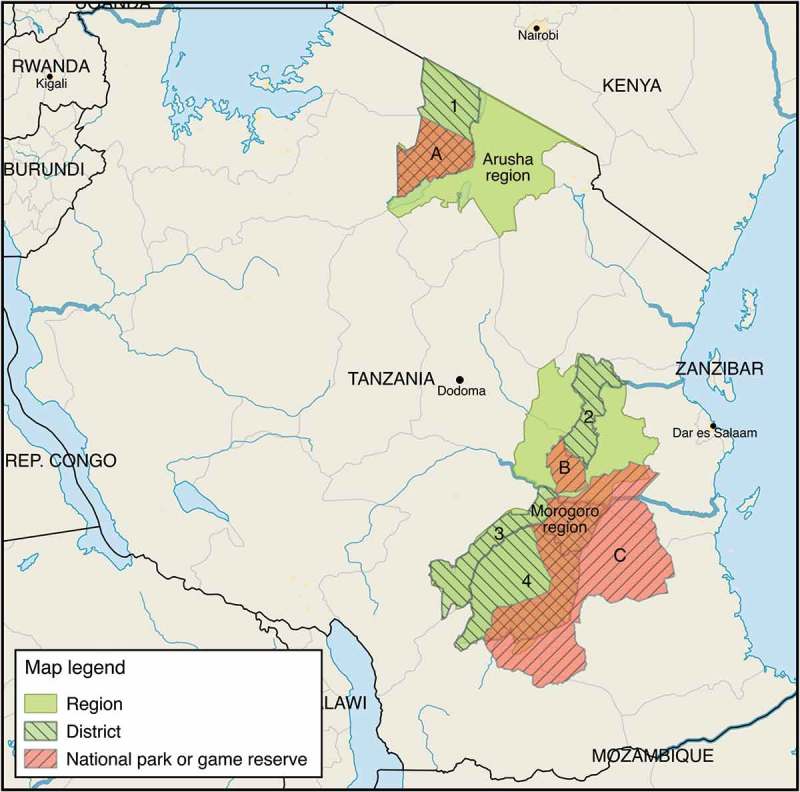
Geographical map of sampling area. Striped green areas indicate visited districts (1 = Ngorongoro, 2 = Mvomero, 3 = Kilombero, 4 = Ulanga). Striped red areas indicate parks or game reserves, i.e. areas with a higher concentration of wildlife (A = Ngorongoro Conservation Area, including Ngorongoro National Park, B = Mikumi National Park, C = Selous Game Reserve).
A confidence interval of 95%, a margin of error of 5%, an infinite population, an assumed true overall prevalence of 50% to obtain maximum sample size, and the sensitivity (94.5%) and specificity (99.4%) of the PPR competitive enzyme-linked immunosorbent assay (cELISA) [35] were used [36,37] in calculations of the sample size (PPR cELISA was used to calculate sample size as PPR was the main focus of the study). This gave the needed sample size of 435 samples for each of the two years when herds (containing sheep and/or goats) were visited. To reach the estimated sample size and to assure an even dispersion of the samples in the selected area, we aimed to sample 3 villages in each ward, 2–3 herds per village and 12–20 animals per herd, depending on herd size. If herds were smaller than 12 animals, all of the animals in the herd were sampled.
The study was conducted during two successive years: 2014 (Ngorongoro, Mvomero, and Ulanga) and 2015 (Ulanga and Kilombero). No herd was visited and sampled in both 2014 and 2015. Animals of all ages (2014) were sampled as previously described [38]. However, according to the interview study from this first visit, 43.7% of the sampled animals had been vaccinated against PPRV, possibly resulting in biased prevalence estimates. Therefore, in 2015, young animals (3–12 months) were selected to avoid false positive results due to vaccination or maternal antibodies. If herds did not include enough animals within this age range, older animals were sampled to reach the goal of 12–20 animals per herd.
Ethical consideration
Sampling was done in collaboration with Tanzania District Veterinary Office, and a local veterinarian or veterinary assistant was present at all sampling sites. Ethical approval was sought and received from the Swedish University of Agricultural Sciences Research Animal Council (SLU ua 2017.1.1.1–1881).
Sample and data collection
Herds of pastoralists or traditional farmers were visited during September–October 2014 and June–July 2015. Oral consent to sample animals was obtained from the herd owners prior to sample collection. Blood was collected from the jugular vein using sterile needles and vacutainer tubes without additives (BD vacutainer, Plymouth, UK). Blood samples were left to coagulate and separate in a vertical position in a cool box. After separation, the serum was transferred to 2-ml cryotubes and stored at −45°C until analysis.
A pre-prepared questionnaire in English was used for epidemiological data collection at the sampling sites. Interviews were performed in Swahili by a local translator. The questionnaires differed between the two years, due to preliminary results from 2014 and extension of the study for the sample collection in 2015. The 2014 questionnaire included open-ended questions regarding the size of herd, type of animals in the herd, if and when animals were vaccinated against PPR, if the animals interacted with wildlife, and if so, which wildlife species. Interaction with wildlife was specified as physical proximity or shared pastures. The 2015 questionnaire was modified to include information about vaccinations against PPR, CCPP, and FMD, and interaction of the herd with other domestic herds of sheep, goats, or cattle and wildlife. This questionnaire included open-ended questions regarding size of the herd, type of animals in it, if and when animals were vaccinated against PPR, CCPP, and FMD, how often the herd interacted with other domestic herds, latest introduction of new animals into the herd, and how often the herd interacted with wildlife.
Laboratory analysis
Commercial enzyme-linked immunosorbent assay (ELISA) kits were used to analyse the presence of antibodies to the selected pathogens: ID screen PPR competition ELISA (detects anti-PPRV nucleoprotein antibodies [35], sensitivity 94.5%, specificity 99.4%; ID. Vet, Grabels, France), IDEXX CCPP Ab test (uses monoclonal antibody ‘4.52’ against Mycoplasma sp. Type F38 [39], no information for sensitivity, specificity 99.6%; IDEXX, Hoofddorp, The Netherlands), ID screen FMD NSP competition (detects anti-FMDV 3ABC non-structural protein antibodies, sensitivity 100%, specificity 99.4%; ID. Vet, Grabels, France), Bluetongue Virus (BTV) Antibody Test Kit (detects anti-BTV VP7 protein antibodies, sensitivity 83%, specificity 100%; IDEXX, Hoofddorp, The Netherlands), and BVDV p80 Ab Test Kit (detects anti-BVDV p80 antibodies sensitivity 100%, specificity 99.2%; IDEXX, Hoofddorp, The Netherlands). The BVDV p80 Ab Test Kit detects antibodies to both BVDV and BDV, without the ability to differentiate between the two. All kits were used and interpreted according to the manufacturers’ instructions. For PPR, BT, and BVD, there were three different outcomes for the ELISA: positive, negative, or doubtful. In the statistical analysis a doubtful result was considered as negative.
Statistical analysis
The true prevalence was calculated based on the apparent prevalence and the sensitivity and specificity of the diagnostic tests used, in accordance with [40]. Individual animal results were analysed for possible risk factors for seropositivity, as an indirect measure of exposure: sex, species, age group, and, in the case of PPR and CCPP, vaccination was also included in the analysis, given that the diagnostic test used could not differentiate between infected and vaccinated animals. Age of animal and date of when vaccination had been performed according to the owners were taken into consideration when classifying animals as vaccinated or not. A confidence interval (95%) for the positive proportion was calculated using the score method with continuity correction [41]. To minimize vaccination as a confounder, the animals that owners reported to be vaccinated were excluded from the results in the univariable analysis. All statistical analyses were performed in R, Version 3.2.2 [42], and each of the pathogens was analysed separately. Association between risk factors and outcome (i.e. seropositivity to one of the pathogens) was analysed using the command oddsratio from the fmsb package, with corrections for difference in proportions. A p-value < .05 was considered as significant. Risk factors with a p-value < .2 in the univariable analysis were analysed in a generalized linear mixed-effect model, using the glmer command from the lme4 package [43]. Risk factors were included as fixed effects, and herd was included as a random effect to account for potential clustering. At herd level in 2014, the risk factors included the district and whether owners reported their animals being in proximity to wildlife, whereas in 2015 they included district, reports of proximity to wildlife, interaction with other domestic herds, and introduction of new animals in the last 12 months. The proportion of positive animals in herds and all risk factors were added to the generalized linear mixed-effect model without previous univaribale analysis. Again, herd was included as a random effect. Interactions between risk factors were tested for in all models.
Results
Descriptive analysis
Of 957 animals, 476 animals (from 39 different herds) were sampled in 2014 and 481 animals (from 46 different herds) in 2015. In 2014, 50% of the animals were goats and in 2015 67.2% of the animals were goats. The remainder were sheep. The sex distribution was 73.5% female (including both goats and sheep) in 2014 and 64.9% were female in 2015. In 2014, 17.9% of sampled animals were < 1 year (52.3% females), 26.1% were 1–2 years (58.9% females), and 56% were > 2 years (87.1% females). In 2015, the age distribution was: 53.3% < 1 year (54.3% females), 26.4% 1–2 years (71.4% females), and 20.1% > 2 years (83.3% females).
Seroprevalence
Antibodies to the pathogens were detected in all visited districts, with some exceptions. BVDV was not detected in the 2014 Ulanga samples, nor was CCPP detected either year in this district. No samples from Ngorongoro were analysed for BT or BVD.
The true prevalence for PPR was estimated at 49.3% (95% CI 44.5;54.0) in 2014 and 10.0% (95% CI 7.1;12.8) in 2015. The true prevalence of FMD was 39.0% (95% CI 33.8;44.3) in 2014 and 14.1% (95% CI 10.9;17.2) in 2015. The highest seroprevalence was for BT for both years: 98.9% (95% CI 90.1;100) in 2014 and 74.5 % (95% CI 68.4;80.6) in 2015. The lowest was for BVD: 3.9% (95% CI 0;8.0) in 2014 and 1.7% (95% CI 0.1;3.4) in 2015. It was not possible to calculate the true prevalence of CCPP because there was no information for sensitivity of the ELISA kit [44]. Observed prevalence for the pathogens is given in Tables 1 and 2.
Table 1.
Seroprevalence at individual animal level according to sex, species, age group, and vaccination status (PPR) from the first round of a repeated cross-sectional study of small ruminants carried out in Tanzania in 2014.
| PPR |
CCPP |
FMD |
BT |
BVD |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | |
| Total | 476 (223) | 46.8 (42.8; 51.4) | 323 (47) | 14.6 (11.0; 19.0) | 340 (134) | 39.4 (34.2; 44.8) | 106 (87) | 82.1 (73.2; 88.6) | 106 (5) | 4.7 (1.7; 11.2) | |
| Sex | Female | 350 (190) | 54.3 (48.9; 59.6) | 230 (41) | 17.8 (13.2; 23.5) | 264 (118) | 44.7 (38.6; 50.9) | 76 (71) | 93.4 (84.6; 97.5) | 76 (5) | 6.6 (2.5; 15.4) |
| Male | 125 (32) | 25.6 (18.4; 34.3) | 92 (6) | 6.5 (2.7; 14.2) | 75 (16) | 21.3 (13.0; 32.6) | 30 (16) | 53.3 (34.6; 71.2) | 30 (0) | 0 (0; 14.1) | |
| Species | Sheep | 238 (108) | 45.5 (39.0; 52.0) | 159 (2) | 1.3 (0.2; 5.0) | 182 (64) | 35.2 (28.4; 42.7) | 60 (48) | 80.0 (67.3; 88.8) | 60 (2) | 3.3 (0.6; 12.5) |
| Goat | 238 (115) | 48.3 (41.8; 54.8) | 164 (45) | 27.4 (20.9; 35.0) | 158 (70) | 44.3 (36.5; 52.4) | 46 (39) | 84.8 (70.5; 93.2) | 46 (3) | 6.5 (1.7; 18.9) | |
| Age group | < 1 year | 85 (15) | 17.6 (10.5; 27.7) | 60 (0) | 0 (0; 7.5) | 60 (8) | 13.3 (6.3; 25.1) | 17 (6) | 35.3 (15.3; 61.4) | 17 (0) | 0 (0; 22.9) |
| 1–2 years | 124 (24) | 19.4 (13.1; 27.7) | 82 (13) | 15.9 (9.1; 26.0) | 69 (13) | 18.8 (10.8; 30.4) | 17 (10) | 58.8 (33.4; 80.6) | 17 (0) | 0 (0; 22.9) | |
| > 2 years | 262 (180) | 68.7 (62.7; 74.2) | 178 (34) | 19.1 (13.8; 25.8) | 207 (111) | 53.6 (46.6; 60.5) | 70 (69) | 98.6 (91.3; 99.9) | 70 (5) | 7.1 (2.6; 16.5) | |
| Vaccination | Yes | 208 (131) | 63.0 (56.0; 69.5) | – | – | – | – | – | – | – | – |
| No | 253 (86) | 34.0 (28.3; 40.2) | – | – | – | – | – | – | – | – | |
Table 2.
Seroprevalence at individual animal level according to sex, species, age group, and vaccination status (PPR and CCPP) from the second round of a repeated cross-sectional study of small ruminants carried out in Tanzania in 2015.
| PPR |
CCPP |
FMD |
BT |
BVD |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | Analysed (positive) | % Positive (95% CI) | |
| Total | 481 (48) | 10.0 (7.5; 13.1) | 340 (64) | 18.8 (14.9; 23.5) | 480 (70) | 14.6 (11.6; 18.2) | 359 (222) | 61.8 (56.5; 66.8) | 357 (9) | 2.5 (1.2; 4.9) | |
| Sex | Female | 312 (45) | 14.4 (10.8; 18.9) | 217 (42) | 19.4 (14.5; 25.4) | 312 (57) | 18.3 (14.3; 23.1) | 223 (156) | 70.0 (63.5; 75.8) | 223 (8) | 3.6 (1.7; 7.2) |
| Male | 169 (3) | 1.8 (0.5; 5.5) | 123 (22) | 17.9 (11.8; 26.1) | 168 (13) | 7.7 (4.3; 13.1) | 120 (66) | 55.0 (45.7; 64.0) | 128 (1) | 0.8 (0.04; 4.9) | |
| Species | Sheep | 158 (13) | 8.2 (4.6; 13.9) | 92 (3) | 3.3 (0.9; 10.0) | 157 (15) | 9.6 (5.7; 15.6) | 96 (51) | 53.1 (42.7; 63.3) | 97 (4) | 4.1 (1.3; 10.8) |
| Goat | 323 (35) | 10.8 (7.7; 14.8) | 248 (61) | 24.6 (19.5; 30.5) | 379 (57) | 15.0 (11.6; 19.1) | 263 (171) | 65.0 (58.9; 70.7) | 260 (5) | 1.9 (0.7; 4.7) | |
| Age group | < 1 year | 255 (9) | 3.5 (1.7; 6.8) | 180 (32) | 17.8 (12.7; 24.3) | 255 (30) | 11.8 (8.2; 16.6) | 187 (89) | 47.6 (40.3; 55.0) | 188 (3) | 1.6 (0.4; 5.0) |
| 1–2 years | 126 (16) | 12.7 (7.6; 20.1) | 90 (19) | 21.1 (13.5; 31.2) | 126 (13) | 10.3 (5.8; 17.3) | 96 (66) | 68.8 (58.4; 77.7) | 96 (1) | 1.0 (0.05; 6.4) | |
| > 2 years | 96 (22) | 22.9 (15.2; 32.8) | 67 (13) | 19.4 (11.1; 31.2) | 95 (27) | 28.4 (19.9; 38.7) | 73 (64) | 87.7 (77.4; 93.9) | 70 (5) | 7.1 (2.6; 16.5) | |
| Vaccination | Yes | 81 (19) | 23.5 (15.1; 34.5) | 42 (0) | 0 (0; 10.4) | – | – | – | – | – | – |
| No | 372 (21) | 5.6 (3.6; 8.6) | 268 (61) | 22.8 (18.0; 28.4) | – | – | – | – | – | – | |
Risk factor analysis
Univariable analysis showed a significant difference between male and female animals, with females at higher risk of being seropositive for the tested pathogens, except BVD and CCPP in 2015 (Tables 3-7). Goats were found to be at higher risk than the sheep for seropositivity against CCPP in both years (OR 57.2 in 2014 and OR 9.68 in 2015), and FMD (OR 1.94) and BT (OR 1.64) in 2015 (Tables 4-6). Multivariate analysis identified sex (female) as a significant risk factor for all pathogens, except CCPP and BT in 2015 (Tables 3-7). Increased seropositivity in animals older than 2 years was significant for all pathogens in both years, except for CCPP in 2015.
Table 3.
Univariable and multivariable analyses for risk factors associated with PPR seropositivity at individual animal level and herd level.
| Univariable | 2014 |
2015 |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Sex | Male | 2.49 | 1.37;4.54 | .002 | Male | 14.1 | 1.87;106 | <.001 |
| Female | Female | |||||||
| Species | Sheep | 1.01 | 0.60;1.70 | .964 | Sheep | 1.38 | 0.49;3.88 | .535 |
| Goat | Goat | |||||||
| Vaccination | No | 3.30 | 2.25;4.85 | <.001 | No | 5.12 | 2.69;10.0 | <.001 |
| Yes | Yes | |||||||
| Age group | < 1 year | Baseline | <.001 | < 1 year | Baseline | <.001 | ||
| 1–2 years | 1.12 | 0.56;2.29 | 1–2 years | 3.98 | 1.70;9.27 | |||
| > 2 years | 14.9 | 8.10;27.5 | > 2 years | 8.13 | 3.59;18.4 | |||
| Multivariate | ||||||||
| Sex | Male | Baseline | .002 | Male | Baseline | .006 | ||
| Female | 2.78 | 1.48;5.40 | Female | 6.18 | 1.95;28.2 | |||
| Vaccination | No | Baseline | .774 | |||||
| Yes | 1.22 | 0.31;4.93 | ||||||
| Age group | < 1 year | Baseline | ||||||
| 1–2 years | 3.51 | 1.11;12.4 | .037 | |||||
| > 2 years | 17.6 | 3.78;113 | <.001 | |||||
| Vaccination *Age group | Yes*< 1 year | 5.14 | 1.43;19.3 | .010 | ||||
| Yes*1–2 years | – | – | – | |||||
| Yes*> 2 years | 0.86 | 0.08;10.2 | .010 | |||||
| Herd level | ||||||||
| District | Ulanga | Baseline | Kilombero | Baseline | ||||
| Mvomero | 4.68 | 2.34;10.5 | <.001 | Ulanga | 1.10 | 0.33;3.72 | .874 | |
| Ngorongoro | 2.21 | 0.74;7.27 | .155 | |||||
| Interaction with wildlife | 0.59 | 0.21;1.59 | .285 | Interaction with wildlife | 0.94 | 0.29;2.75 | .910 | |
| Interaction with domestic herds | 1.65 | 0.42;7.71 | .476 | |||||
| Introduction of new animals | 1.24 | 0.31;4.90 | .740 | |||||
Serological results from a repeated cross-sectional study of small ruminants carried out in Tanzania. Factors with p < .2 in univariabale analysis were used in multivariate analysis. p-Values <.05 were considered significant and are in bold. Interaction between vaccination and age group in samples from 2014 are marked with *.
Table 7.
Univariable analysis for risk factor associated with BVD seropositivity at individual animal level.
| Univariable | 2014 |
2015 |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Sex | Male | – | – | .150 | Male | 4.73 | 0.58;38.2 | .110 |
| Female | Female | |||||||
| Species | Sheep | 2.02 | 0.32;12.6 | .445 | Sheep | 0.46 | 0.12;1.73 | .239 |
| Goat | Goat | |||||||
| Age group | < 1 year | – | – | .279 | < 1 year | Baseline | .023 | |
| 1–2 years | 1–2 years | 0.65 | 0.07;6.33 | |||||
| > 2 years | > 2 years | 4.74 | 1.10;20.4 | |||||
Serological results from a repeated cross-sectional study of small ruminants carried out in Tanzania. There were no positive male animals or age groups <1 and 1–2 years in samples from 2014, so it was not possible to obtain OR for the risk factor ‘age group’ or ‘sex’. Multivariate analysis was not possible due to an insufficient number of seropositive animals. p-Values <.05 were considered significant and are in bold text.
Table 4.
Univariable and multivariable analyses for risk factors associated with CCPP seropositivity at individual animal level and herd level.
| Univariable | 2014 |
2015 |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Sex | Male | 3.11 | 1.22;7.60 | .010 | Male | 1.10 | 0.62;1.95 | .740 |
| Female | Female | |||||||
| Species | Sheep | 57.2 | 7.78;420 | <.001 | Sheep | 9.68 | 2.96;31.7 | <.001 |
| Goat | Goat | |||||||
| Age group | < 2 years | 2.34 | 1.18;4.63 | .013 | < 1 year | Baseline | .801 | |
| > 2 years | 1–2 years | 1.24 | 0.66;2.33 | |||||
| > 2 years | 1.11 | 0.54;2.28 | ||||||
| Multivariate | ||||||||
| Sex | Male | Baseline | .029 | |||||
| Female | 4.46 | 1.24;19.0 | ||||||
| Species | Sheep | Baseline | – | <.001 | Sheep | Baseline | .021 | |
| Goat | 81.9 | 17.4;726 | Goat | 9.21 | 1.70;84.0 | |||
| Age group | < 2 years | Baseline | – | .012 | ||||
| > 2 years | 5.17 | 1.54;21.1 | ||||||
| Herd level | ||||||||
| Interaction with wildlife | 0.60 | 0.06;4.44 | .598 | Interaction with wildlife | 0.008 | <0.01;0.16 | .006 | |
| Interaction with domestic herds | 0.045 | <0.01;0.48 | .016 | |||||
| Introduction of new animals | 4.24 | 0.14;303 | .414 | |||||
Serological results from a repeated cross-sectional study of small ruminants carried out in Tanzania. Factors with p < .2 in univariabale analysis were used in multivariate analysis. p-Values <.05 were considered significant and are in bold text.
Table 6.
Univariable and multivariable analyses for risk factors associated with BT seropositivity at individual animal level and herd level.
| Univariable | 2014 |
2015 |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Sex | Male | 12.4 | 3.90;39.5 | <.001 | Male | 1.90 | 1.22;2.95 | .004 |
| Female | Female | |||||||
| Species | Sheep | 1.39 | 0.50;3.88 | .527 | Sheep | 1.64 | 1.02;2.64 | .040 |
| Goat | Goat | |||||||
| Age group | < 1 year | Baseline | <.001 | < 1 year | Baseline | <.001 | ||
| 1–2 years | 2.62 | 0.65;10.5 | 1–2 years | 2.42 | 1.44;4.07 | |||
| > 2 years | 126 | 13.9;1153 | > 2 years | 7.83 | 3.68;16.7 | |||
| Multivariate | ||||||||
| Sex | Male | Baseline | – | .030 | Male | Baseline | .437 | |
| Female | 7.49 | 1.29;63.9 | Female | 1.26 | 0.70;2.27 | |||
| Species | Sheep | Baseline | .023 | |||||
| Goat | 2.32 | 1.14;4.93 | ||||||
| Age group | < 1 year | Baseline | – | – | < 1 year | Baseline | ||
| 1–2 years | 3.34 | 0.42;155 | .319 | 1–2 years | 3.04 | 1.53;6.32 | .002 | |
| > 2 years | 183 | 15.2;23,216 | .001 | > 2 years | 18.4 | 6.61;61.4 | <.001 | |
| Herd level | ||||||||
| District | Ulanga | Baseline | .356 | |||||
| Mvomero | 2.42 | 0.30;21.9 | ||||||
| Interaction with wildlife | 0.57 | 0.03;7.61 | .636 | Interaction with wildlife | 1.18 | 0.48;2.94 | .698 | |
| Interaction with domestic herds | 3.85 | 1.55;10.5 | .004 | |||||
| Introduction of new animals | 0.99 | 0.26;3.65 | .983 | |||||
Serological results from a repeated cross-sectional study of small ruminants carried out in Tanzania. Factors with p < .2 in univariabale analysis were used in multivariate analysis. p-Values <.05 were considered significant and are in bold text.
Analysis at herd level showed a significant association between the Mvomero region (visited in 2014) and seropositivity for PPR and FMD. For FMD, an association with the region Kilombero was significant in 2015 (Tables 3 and 5). Proximity to wildlife was not identified as a risk factor for any of the pathogens for either of the years (Tables 3-7). Rather, proximity to wildlife was identified in 2015 to have a negative association with seropositivity for CCPP. Interaction with other domestic herds was identified to have the same association for CCPP (Table 4). Interaction with other domestic herds was a significant risk factor for being seropositive for FMD and BT in 2015 (Tables 5 and 6).
Table 5.
Univariable and multivariable analysis for risk factors associated with FMD seropositivity at individual animal level and herd level.
| Univariable | 2014 |
2015 |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Sex | Male | 2.98 | 1.63;5.45 | <.001 | Male | 2.67 | 1.41;5.03 | .002 |
| Female | Female | |||||||
| Species | Sheep | 1.47 | 0.95;2.27 | .086 | Sheep | 1.94 | 1.06;3.56 | .030 |
| Goat | Goat | |||||||
| Age group | < 1 year | Baseline | <.001 | < 1 year | Baseline | <.001 | ||
| 1–2 years | 1.51 | 0.58;3.93 | 1–2 years | 0.86 | 0.43:1.72 | |||
| > 2 years | 7.52 | 3.40;16.6 | > 2 years | 2.98 | 1.66;5.35 | |||
| Multivariate | ||||||||
| Sex | Male | Baseline | .003 | Male | Baseline | .001 | ||
| Female | 3.77 | 1.58;9.48 | Female | 4.70 | 1.91;13.1 | |||
| Species | Sheep | Baseline | – | .099 | Sheep | Baseline | .008 | |
| Goat | 1.81 | 0.90;3.68 | Goat | 4.19 | 1.54;13.0 | |||
| Age group | < 1 year | Baseline | < 1 year | Baseline | ||||
| 1–2 years | 1.51 | 0.4;5.56 | .534 | 1–2 years | 1.21 | 0.46;3.20 | .698 | |
| > 2 years | 8.73 | 2.82;30.5 | <.001 | > 2 years | 9.10 | 3.10;30.8 | <.001 | |
| Herd level | ||||||||
| District | Ulanga | Baseline | Ulanga | Baseline | .044 | |||
| Mvomero | 25.1 | 11.1;73.8 | <.001 | Kilombero | 6.15 | 1.02;44.7 | ||
| Ngorongoro | 2.36 | 0.43;12.2 | .298 | |||||
| Interaction with wildlife | 1.13 | 0.36;3.47 | .816 | Interaction with wildlife | 1.52 | 0.28;8.97 | .612 | |
| Interaction with domestic herds | 20.7 | 3.10;262 | .005 | |||||
| Introduction of new animals | 0.13 | 0.01;1.18 | .067 | |||||
Serological results from a repeated cross-sectional study of small ruminants carried out in Tanzania. Factors with p < .2 in univariabale analysis were used in multivariate analysis. p-Values <.05 were considered significant and are in bold text.
An interaction was found between the variables age group and vaccination against PPR in the samples from 2014. Effect of vaccination against PPR differed among the age groups.
Discussion
In this study, we investigated the seroprevalence of PPR and some of its differential diagnoses in selected areas in Tanzania. Commercial ELISA tests were used to detect antibodies in serum samples from sheep and goats. The serological results were used further to calculate risk factors for exposure to PPRV, Mccp, FMDV, BTV, and BVDV. In Tanzania, and other east African countries, small ruminant production is an important livelihood for a significant proportion of the population [19]. This important position of small ruminants is one of the reasons behind the joint Food and Agriculture Organization (FAO) and World Organization for Animal Health programme to control and eradicate PPR and control small ruminant diseases [4]. PPRV is quickly increasing its spread across the world and is now threatening the most southern countries of Africa, with Tanzania currently being its southern border on the east coast [25]. To stop the spread further south, it is important to understand the prevalence and epidemiology of both PPR and its most common differential diagnosis, as the clinical presentation can be difficult to diagnose [4].
The calculated true seroprevalence for PPR was 49.3% in 2014 and 10.0% in 2015. A vaccination campaign had been carried out in the Morogoro and Mtwara region prior to our sample collection [45], which may have influenced the 2014 results. Therefore, we aimed to sample animals aged 3–12 months in 2015, as animals in this age group would not have been alive during the vaccination campaign. As expected for an endemic disease, where survival of infection results in lifelong immunity, age was identified as a risk factor for exposure (Table 3). Age and vaccination bias of sampled animals could be the reasons for the difference in seroprevalence in 2014 and 2015. In addition, we did not visit the same areas both years; the differences in seroprevalence could therefore have been due to geographical differences. Previous studies in northern Tanzania found an overall seroprevalence of 45.5% in 2008 [46] and 22.1% in 2008–2009 [21]. In southern Tanzania, in the Mtwara region bordering Mozambique, 31% of sampled small ruminants had antibodies to PPRV [47]. A recent study analysing samples from 14 different regions of Tanzania described an overall seroprevalence of 27.1%, with regions varying from 2.4% (Kagera) to 72.8% (Morogoro), demonstrating the varying level of seroprevalence within the country [23].
Sex has previously been described as a risk factor for PPR; mostly females are identified to be at higher risk [21,48,49]. However, some studies found the opposite association [50–52]. Our results suggest that females had a higher risk of being seropositive for PPRV in both 2014 and 2015. Previous studies on risk factors for PPR have suggested that females are kept longer by their owners (to be used in reproduction), and therefore have a longer risk period for PPRV exposure [48]. In addition, females are more likely to be vaccinated, which may bias the results. The stress associated with pregnancy and milk production may also predispose females to infection [48,49]. Differences between the studies, such as management systems or breed of sheep and goats, may also influence the results. In our study, we found that the age group >2 years was mainly composed of females. This age group had the highest proportion of seropositive individuals; the result might be due to a selection bias. However, the multivariable analysis did not find an interaction between these two variables, indicating that this cannot be the entire explanation.
The true prevalence for CCPP was not possible to calculate because there was no available information on sensitivity for the ELISA test used [44]; however, the apparent prevalence was 14.6% in 2014 and 18.8% in 2015. Previously, a prevalence of 51.2% (in 2007) and 33.7% (in 2009) had been described in southern Tanzania [51].
Goats were identified to be at higher risk than sheep for seropositivity towards CCPP in both years, due to CCPP having a higher affinity for goats. Sheep can develop clinical signs following infection by CCPP, but the infection can also be subclinical [53].
The calculated true prevalence for FMD was 39.0% in 2014 and 14.1% in 2015 for both sheep and goats. To the best of our knowledge, no previous reports of seroprevalence of FMD in small ruminants in Tanzania are available. FMDV causes a less severe disease in small ruminants compared with large ruminants [54]; however, the oral lesions sometimes seen even in small ruminants make FMD an important differential diagnosis of PPR, especially in light of the attempt to eradicate PPR [7]. A study in neighbouring Uganda found a seroprevalence of 14% in goats and 22% in sheep [55]. Seroprevalence of FMD in buffalo and cattle in Tanzania is high. Mkama et al. [56] found an overall prevalence of 76.3% (248 of 330) for buffalo and cattle, with the buffalos from western Tanzania having a 100% seroprevalence (29 of 29). Antibodies to FMDV decrease faster in sheep than cattle [57], which could be one explanation for the difference in seroprevalence between small and large ruminants. As for PPR, our study identified age as a risk factor for FMDV exposure. Age is a documented risk factor for FMDV exposure in cattle, both in endemic and epidemic settings [58,59]. A higher age gives a longer time to be exposed in the endemic setting, and the higher mortality seen in younger animals leaves the older seropositive animals to be sampled [59,60]. Also in line with the results for PPR, female animals were identified to be at a higher risk than males for FMDV exposure. Similar explanations in PPR can be applied to FMD as well, with the exception of vaccination. None of the owners reported that their animals had been vaccinated against FMD.
The calculated true prevalence for BT was 98.9% in 2014 and 74.5% in 2015. No previous studies have been done on seroprevalence of BT in domestic animals in Tanzania. As with FMD, possible oral lesions caused by BTV makes it an important differential diagnosis of PPR [12]. Free-living wild buffalos from eight different areas in Tanzania were sampled between 1987 and 1989 and analysed for antibodies to a selection of pathogens, including BT [61]. An overall prevalence of 91.6% was found, with six of eight areas having a 100% prevalence [61]. A similar study was performed on wildlife in Zimbabwe with samples collected between 1989 and 1995 [62]. Most samples came from buffaloes, followed by different species of antelopes, and also white and black rhinoceroses. An overall prevalence of antibodies to BTV of 44.1% was found [62]. Domestic cattle were sampled in western Sudan and serological evidence of BTV infection was found in 19.4% of them (58 of 299) [63]. Our results are more in line with those from [59] and [60]. A high seroprevalence is expected from a virus that often gives a subclinical or unapparent disease in ruminants and is spread very efficiently by its vector [12]. Risk factors identified for exposure to BTV included age and sex (Table 6), as for the other pathogens in this study. Age as a risk factor for exposure to BTV is in agreement with a risk factor analysis in cattle in western Sudan [63]. In 2015, multivariate analysis identified goats as being at higher risk for exposure to BTV than sheep. In 2015, 73% of samples analysed came from goats, which may have biased the result.
The calculated true prevalence for BVD was 3.9% in 2014 and 1.7% in 2015. This is lower than what has previously been described for domestic animals in Tanzania. In Tanzanian samples collected between 1985 and 1987 from cattle, sheep, and goats, evidence of BVD exposure was described in 34.0% of cattle, 32.1% of sheep, and 24.9% of goats [64]. In wild buffaloes, mainly from northern parts of Tanzania, 16.9% had antibodies to BVDV [61]. Five cattle herds in the Kafue flats of Zambia were tested for antibodies to a selection of pathogens, and 76.2% were positive for BVDV [65]. A more recent study was performed in western Kenya; calves aged 3–7 days were tested for antibodies to BVDV and an adjusted seroprevalence of 19.8% was identified [66]. Seroprevalence for BVD varies significantly between the different studies, with our study having the lowest prevalence. Dissimilarities in the studies include differences in production of animals sampled, method of analysis, year of sampling, and study design, which makes comparisons difficult. Univariable analysis of our serological results from 2015 identified age (> 2 years) as a risk factor for exposure to BVD. Because of the low number of seropositive animals (9 out of 357), further studies are warranted before making any definite conclusions on risk factors for exposure. Multivariate analysis could not be performed with the BVD results due to too few positive samples.
Correlation between seropositivity for the studied pathogens, except BVD, was analysed at herd level; a generalized linear mixed-effect model was used to identify risk factors affecting the entire herd. In this study no difference was found, for any of the pathogens, between herds with proximity to wildlife and those without. PPRV has long been known to cause disease in wildlife [28]. Clinical signs are yet to be described in wild ruminants in sub-Saharan Africa, but have been reported in wild ruminants in Asia and in the Middle East [67]. Antibodies have been described in wild buffaloes, Grant’s gazelle, wildebeest, and impala in Tanzania [32,68]. Recently, a Grant’s gazelle without clinical signs of PPR in northern Tanzania tested positive on real-time reverse transcription polymerase chain reaction [32]. The gazelle was sampled in an area with an ongoing outbreak of PPR among domestic animals. The same study found a 63% seroprevalence in 46 sampled wild ruminants [32]. Although it is probable that PPR transmits between domestic and wild animals [32,69,70], our results do not support the hypothesis of wildlife as an important risk factor for exposure for domestic animals in an endemic setting. For the closely related rinderpest virus, the well-accepted hypothesis was that infection in wildlife was not self-sustaining, but rather a case of spillover from domestic animals [71,72]. The same hypothesis has been suggested for PPRV [32,73], and our results seem to be in agreement with this.
For FMD, contact with wildlife has been described as an important risk factor for infection in domestic animals in sub-Saharan Africa [74]. However, among wildlife species, only the African buffalo has been identified as a long-term maintenance host [74]. Small ruminants are highly susceptible to FMDV infection, but they are not as efficient as cattle in maintaining the infection within the population [11]. Years of experience with FMD in southern Africa have been unable to reveal small ruminants as an important part of the maintenance or transmission of the disease [75]. Our results did not identify proximity to wildlife as a risk factor for FMD in domestic small ruminants in these areas of Tanzania.
Bluetongue virus is endemic in both the domestic and wild populations of many African countries [12]. Various wildlife species, both in Africa and in Europe, have been discussed as possible reservoirs [62,76,77]. The epidemiology of BTV differs from the other viruses studied here, as it is spread through its vector, the Culicoides mosquito, not through direct contact. In parts of Europe where BT is endemic, studies suggest that wild ruminants, mainly red deer, play a role in the epidemiology [76]. Our results did not indicate proximity to wildlife as an important risk factor for small ruminants to be exposed to BTV in the studied area. However, we did identify interaction with other domestic herds as a risk factor, in agreement with a previous study of sheep and goats in Iran [78]. Possibly the vector is attracted by the increased number of animals in the same location.
For CCPP in 2015, proximity to wildlife had a statistically significant negative association, as did interaction with other domestic herds (Table 4). The ELISA used for detection of CCPP is specific for antibodies against Mccp [44]; however, cross-protection between different subspecies of mycoplasmas cannot be excluded [53,79,80]. It is possible that other members of the Mycoplasma mycoides cluster are circulating in the studied areas and producing cross-protection against CCPP.
Limitations of this study include none of the ELISAs used being able to differentiate between vaccinated and naturally infected animals, and all questionnaire data being collected by a local translator. Information regarding vaccination status of the animals was acquired from the owners. Owners could, for a variety of reasons, provide incorrect information; for example, they do not remember, or a previous owner had the animals vaccinated. To minimize this bias during the sample collection in 2015, we targeted animals 3–12 months of age, animals the owners were more likely to have the correct information about. Further, we used the information from the questionnaires to study whether interaction with wildlife was a possible risk factor for exposure to the studied pathogens. Owners were asked how often the animals had contact with wildlife. The question could, however, have been misunderstood or interpreted in a different way than what we intended. Answers given to the question were, for example: ‘never’, ‘during dry season’, and ‘everyday’. The interaction between wildlife and livestock can be measured using several methods, with the usage of a questionnaire and local knowledge being a fast and practical method to get preliminary data [81]. The method is not, however, as precise as others, and this insecurity should be considered when interpreting the results of the risk factor analysis.
Conclusion
This study confirmed the presence of antibodies to PPRV, CCPP, FMDV, BTV, and BVDV in sheep and goats in northern and south-eastern Tanzania, indicating a continuous circulation of these pathogens. This is the first description of the presence of antibodies for FMD and BT in small ruminants in Tanzania. Risk factor analysis at individual animal level identified sex (female) and increasing age as two important factors influencing level of exposure to infection. Proximity to wildlife was not identified as a risk factor for any of the pathogens studied.
Acknowledgments
The authors acknowledge: Nica Wachtmeister, Lovisa Levin, and Miriam Makange for invaluable help in collecting samples; Professor Paul Gwakisa and Professor Christopher J. Kasanga for sharing their knowledge on infectious diseases in Tanzania; Dr Mikael Andersson Franko for help with statistical analysis; and the Tanzania District Veterinary Office and Ngorongoro Conservation Area for help with field trips. The authors are also greatly thankful to the animal owners for participating in the study.
Biographies
Emeli Torsson DVM from Swedish University of Agricultural Sciences. Currently holds position as PhD student at Swedish University of Agricultural Sciences.
Mikael Berg BSc from Uppsala University. PhD from Swedish University of Agricultural Sciences. Now Professor in Veterinary Virology at Swedish University of Agricultural Sciences.
Gerald Misinzo BVM from Sokoine University of Agriculture, MSc from Katholieke Universiteit Leuven and PhD from Universiteit Gent. Currently holds a position as Associate Professor of Virology and Head of Department at Sokoine University of Agriculture and Centre Leader of SACIDS – African Center of Excellence for Infectious Diseases of Humans and Animals in Southern and Eastern Africa (SACIDS-ACE).
Ida Herbe DVM from Swedish University of Agricultural Sciences. Currently works as a veterinarian.
Tebogo Kgotlele MSc from Sokoine University of Agriculture. Currently PhD student at Sokoine University of Agriculture.
Malin Päärni DVM from Swedish University of Agricultural Sciences. Currently works as a veterinarian.
Nils Roos DVM from Swedish University of Agricultural Sciences. Currently works as a veterinarian.
Anne-Lie Blomström PhD from the Swedish University of Agricultural Sciences. Currently a researcher and Associate Professor in molecular virology at the Swedish University of Agricultural Sciences.
Karl Ståhl DVM and PhD from Swedish University of Agricultural Sciences. Currently holds a position as researcher and Deputy State Epizootlogist at National Veterinary Institute, Sweden.
Jonas Johansson Wensman DVM and PhD from Swedish University of Agricultural Sciences. Currently holds a position as researcher in Ruminant Medicine and Associate Professor in Veterinary Science with focus on Infectious Diseases at Swedish University of Agricultural Sciences.
Funding Statement
This study was funded by the Swedish Research Council [grant no. 348-2013-6402 and 348-2014-4293].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1]. FAO Supporting livelihoods and building resilience through Peste des petits ruminants (PPR) and small ruminant disease control. Animal Production and Health Position Paper. Rome; 2013. [Google Scholar]
- [2]. FAO Livestock sector development for poverty reduction: an economic and policy perspective – Livestock’s many virtues, by J. Otte, A. Costales, J. Dijkman, U. Pica-Ciamarra, T. Robinson, V. Ahuja, C. Ly and D. Roland-Holst. Rome: 2012; pp. 161. [Google Scholar]
- [3]. Pradère J. Improving animal health and livestock productivity to reduce poverty. Rev Sci Tech. 2014;33:723–13. [PubMed] [Google Scholar]
- [4]. FAO and OIE. Global Strategy for the control and eradication of PPR. Rome; 2015. [Google Scholar]
- [5]. World Bank World livestock disease atlas: a quantitative analysis of global animal health data (2006-2009). Washington (DC): World Bank; 2011. [Google Scholar]
- [6]. Banyard AC, Parida S, Batten C, et al. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91:2885–2897. Epub 2010 September 17. [DOI] [PubMed] [Google Scholar]
- [7]. Roeder P, Obi T. Recognizing PPR a field manual. Rome: Food and Agriculture Organization; 1999. [Google Scholar]
- [8]. Munir M, Zohari S, Berg M. Pathophysiology and Clinical Assessment of Peste des Petits Ruminants. Molecular Biology and Pathogenesis of Peste Des Petits Ruminants Virus. 2013;33–48.Springer. [Google Scholar]
- [9]. Balamurugan V, Hemadri D, ccvGajendragad M, et al. Diagnosis and control of peste des petits ruminants: a comprehensive review. Virusdisease. 2014;25:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Swai ES, Kaaya JE, Noah EY. Antibody response to Mycoplasma capricolum subsp. capripneumoniae bacterium in small holder dairy goats in Tanzania. Trop Anim Health Prod. 2013;45:1603–1608. Epub 2013 April 6. [DOI] [PubMed] [Google Scholar]
- [11]. Kitching R, Hughes G. Clinical variation in foot and mouth disease: sheep and goats. Revue Scientifique Et Technique-Office International Des Epizooties. 2002;21:505–510. [DOI] [PubMed] [Google Scholar]
- [12]. Maclachlan NJ. Bluetongue: history, global epidemiology, and pathogenesis. Prev Vet Med. 2011;102:107–111. [DOI] [PubMed] [Google Scholar]
- [13]. Nettleton PF, Gilray JA, Russo P, et al. Border disease of sheep and goats. Vet Res. 1998;29:327–340. [PubMed] [Google Scholar]
- [14]. Paniagua J, Garcia-Bocanegra I, Arenas-Montes A, et al. Absence of circulation of Pestivirus between wild and domestic ruminants in southern Spain. Vet Rec. 2016;178:215 Epub 2016 February 13. [DOI] [PubMed] [Google Scholar]
- [15]. Passler T, Riddell KP, Edmondson MA, et al. Walz PH. Experimental infection of pregnant goats with bovine viral diarrhea virus (BVDV) 1 or 2.. Veterinary Res. 2014;45:38 Epub 2014 April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Mondal B, Sen A, Chand K, et al. Evidence of mixed infection of peste des petits ruminants virus and bluetongue virus in a flock of goats as confirmed by detection of antigen, antibody and nucleic acid of both the viruses. Trop Anim Health Prod. 2009;41:1661–1667. Epub 2009 May 12. [DOI] [PubMed] [Google Scholar]
- [17]. Saeed IK, Ali YH, AbdulRahman MB, et al. Mixed infection of peste des petits ruminants virus (PPRV) and other respiratory viruses in dromedary camels in Sudan, an abattoir study. Trop Anim Health Prod. 2015;47:995–998. [DOI] [PubMed] [Google Scholar]
- [18]. World Bank 2016. [cited 2016 September 7]. Available from: http://data.worldbank.org/country/tanzania.
- [19]. Covarrubias K, Nsiima L, Zezza A. Livestock and livelihoods in rural Tanzania, A descriptive analysis of the 2009 National Panel Survey. Rome: FAO; 2012. [Google Scholar]
- [20]. United Republic of Tanzania (URT) National sample census of agriculture, Small holder agriculture, Volume III: Livestock sector - National Report. 2012. [Google Scholar]
- [21]. Kivaria FM, Kwiatek O, Kapaga AM, et al. The incursion, persistence and spread of peste des petits ruminants in Tanzania: epidemiological patterns and predictions. Onderstepoort J Vet Res. 2013;80:1–10. [DOI] [PubMed] [Google Scholar]
- [22]. Karimuribo E, Loomu P, Mellau L, et al. Retrospective study on sero-epidemiology of peste des petits ruminants before its official confirmation in northern Tanzania in 2008. Res Opinions Anim Vet Sci. 2011;1(3): 184–187. [Google Scholar]
- [23]. Kgotlele T, Torsson E, Kasanga C, et al. Seroprevalence of Peste Des Petits Ruminants virus from samples collected in different regions of Tanzania in 2013 and 2015. J Vet Sci Technol. 2016;7:2. [Google Scholar]
- [24]. OIE 2016. [cited 2016 September 14]. Available from: http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home.
- [25]. Torsson E, Kgotlele T, Berg M, et al. History and current status of peste des petits ruminants virus in Tanzania. Infection Ecology & Epidemiology. 2016 ;6: doi: 10.3402/iee.v6.32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Allepuz A, Stevenson M, Kivaria F, et al. Risk factors for foot‐and‐mouth disease in Tanzania, 2001–2006. Transbound Emerg Dis. 2015;62:127–136. [DOI] [PubMed] [Google Scholar]
- [27]. Arif A, Schulz J, Thiaucourt F, et al. Contagious caprine pleuropneumonia outbreak in captive wild ungulates at Al Wabra Wildlife Preservation, State of Qatar. J Zoo Wildl Med. 2007;38:93–96. [DOI] [PubMed] [Google Scholar]
- [28]. Hamdy F, Dardiri A. Response of white-tailed deer to infection with peste des petits ruminants virus. J Wildl Dis. 1976;12:516–522. [DOI] [PubMed] [Google Scholar]
- [29]. Nelson DD, Duprau JL, Wolff PL, et al. Persistent bovine viral diarrhea virus infection in domestic and wild small ruminants and camelids including the mountain goat (Oreamnos americanus). Front Microbiol. 2015;6: doi: 10.3389/fmicb.2015.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Van Vuuren M, Penzhorn BL. Geographic range of vector-borne infections and their vectors: the role of African wildlife. Rev Sci Tech. 2015;34:139–149. [DOI] [PubMed] [Google Scholar]
- [31]. Abubakar M, Rajput ZI, Arshed MJ, et al. Evidence of peste des petits ruminants virus (PPRV) infection in Sindh Ibex (Capra aegagrus blythi) in Pakistan as confirmed by detection of antigen and antibody. Trop Anim Health Prod. 2011;43:745–747. [DOI] [PubMed] [Google Scholar]
- [32]. Mahapatra M, Sayalel K, Muniraju M, et al. Spillover of Peste des Petits ruminants virus from domestic to wild ruminants in the serengeti ecosystem, Tanzania. Emerg Infect Dis. 2015;21:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Kgotlele T, Macha E, Kasanga C, et al. Partial genetic characterization of peste des petits ruminants virus from goats in northern and eastern Tanzania. Transbound Emerg Dis. 2014;61:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. United Republic of Tanzania (URT) Tanzania in figures 2014. Dar es Salaam: National Bureau of Statistics MoF; 2015. editor. [Google Scholar]
- [35]. Libeau G, Prehaud C, Lancelot R, et al. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleobrotein. Res Vet Sci. 1995;58:50–55. [DOI] [PubMed] [Google Scholar]
- [36]. AusVet Animal Health Services EpiTools. Available from: http://epitools.ausvet.com.au/
- [37]. Fosgate GT. Practical sample size calculations for surveillance and diagnostic investigations. J Vet Diagn Invest. 2009;21:3–14. [DOI] [PubMed] [Google Scholar]
- [38]. Wensman JJ, Lindahl J, Wachtmeister N, et al. A study of Rift Valley fever virus in Morogoro and Arusha regions of Tanzania–serology and farmers’ perceptions. Infect Ecol Epidemiol. 2015;5: doi: 10.3402/iee.v5.30025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Thiaucourt F, Bölske G, Libeau G, et al. The use of monoclonal antibodies in the diagnosis of contagious caprine pleuropneumonia (CCPP). Vet Microbiol. 1994;41:191–203. [DOI] [PubMed] [Google Scholar]
- [40]. Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol. 1978;107:71–76. [DOI] [PubMed] [Google Scholar]
- [41]. Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. [DOI] [PubMed] [Google Scholar]
- [42]. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. 2015. Available from: https://www.R-project.org/
- [43]. Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using {lme4}. J Stat Softw. 2015;67:1–48. [Google Scholar]
- [44]. Peyraud A, Poumarat F, Tardy F, et al. An international collaborative study to determine the prevalence of contagious caprine pleuropneumonia by monoclonal antibody-based cELISA. BMC Vet Res. 2014;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. United Republic of Tanzania (URT) Peste Des Petites Ruminants (PPR): assessment of socio economic impacts in the Republic of Tanzania. Working document, NO 1. Nairobi: FAO Animal Health and Production, ECTAD; 2013. [Google Scholar]
- [46]. Swai ES, Kapaga A, Kivaria F, et al. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Vet Res Commun. 2009;33:927–936. [DOI] [PubMed] [Google Scholar]
- [47]. Muse EA, Karimuribo ED, Gitao GC, et al. Epidemiological investigation into the introduction and factors for spread of Peste des Petits Ruminants, southern Tanzania. Onderstepoort J Vet Res. 2012;79:49–54. [DOI] [PubMed] [Google Scholar]
- [48]. Ul R A, Abubakar M, Rasool MH, et al. Evaluation of Risk Factors for Peste des Petits Ruminants virus in sheep and goats at the wildlife-livestock interface in Punjab Province, Pakistan. Biomed Res Int. 2016;2016:7826245 Epub 2016 June 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Kihu SM, Gachohi JM, Ndungu EK, et al. Sero-epidemiology of Peste des petits ruminants virus infection in Turkana County, Kenya. BMC Vet Res. 2015;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Mahajan S, Agrawal R, Kumar M, et al. Risk of seroconversion to peste des petits ruminants (PPR) and its association with species, sex, age and migration. Small Ruminant Res. 2012;104:195–200. [Google Scholar]
- [51]. Mbyuzi AO, Komba EV, Kimera SI, et al. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuro-pneumonia in goats and sheep in the Southern Zone of Tanzania. Prev Vet Med. 2014;116:138–144. [DOI] [PubMed] [Google Scholar]
- [52]. Sarker S, Islam MH. Prevalence and risk factor assessment of Peste des petits ruminants in goats in Rajshahi, Bangladesh. Vet World. 2011;4:546–549. [Google Scholar]
- [53]. OIE OIE Terrestrial Manual 2014; 2.7.6; Contagious Caprine Pleuropneumonia. 2014. [cited 2016 April 28]. Available from: http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/2.07.06_CCPP.pdf
- [54]. Barnett P, Cox S. The role of small ruminants in the epidemiology and transmission of foot-and-mouth disease. Vet J. 1999;158:6–13. [DOI] [PubMed] [Google Scholar]
- [55]. Balinda SN, Tjørnehøj K, Muwanika VB, et al. Prevalence estimates of antibodies towards foot‐and‐mouth disease virus in small ruminants in Uganda. Transbound Emerg Dis. 2009;56:362–371. [DOI] [PubMed] [Google Scholar]
- [56]. Mkama M, Kasanga CJ, Sallu R, et al. Serosurveillance of foot-and-mouth disease virus in selected livestock-wildlife interface areas of Tanzania. Onderstepoort J Vet Res. 2014;81:1–4. [DOI] [PubMed] [Google Scholar]
- [57]. Dellers RW, Hyde JL. Response of sheep to experimental infection with foot-and-mouth disease virus. Am J Vet Res. 1964;25:469–473. Epub 1964 March 1. [PubMed] [Google Scholar]
- [58]. Elnekave E, Van Maanen K, Shilo H, et al. Prevalence and risk factors for foot and mouth disease infection in small ruminants in Israel. Prev Vet Med. 2016;125:82–88. [DOI] [PubMed] [Google Scholar]
- [59]. Megersa B, Beyene B, Abunna F, et al. Risk factors for foot and mouth disease seroprevalence in indigenous cattle in Southern Ethiopia: the effect of production system. Trop Anim Health Prod. 2009;41:891–898. [DOI] [PubMed] [Google Scholar]
- [60]. Rufael T, Catley A, Bogale A, et al. Foot and mouth disease in the Borana pastoral system, southern Ethiopia and implications for livelihoods and international trade. Trop Anim Health Prod. 2008;40:29–38. [DOI] [PubMed] [Google Scholar]
- [61]. Hamblin C, Anderson E, Jago M, et al. Antibodies to some pathogenic agents in free-living wild species in Tanzania. Epidemiol Infect. 1990;105:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Anderson E, Rowe L. The prevalence of antibody to the viruses of bovine virus diarrhoea, bovine herpes virus 1, rift valley fever, ephemeral fever and bluetongue and to Leptospira sp in free-ranging wildlife in Zimbabwe. Epidemiol Infect. 1998;121:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Adam IA, Abdalla MA, Mohamed ME, et al. Prevalence of bluetongue virus infection and associated risk factors among cattle in North Kordufan State, Western Sudan. BMC Vet Res. 2014;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Hyera J, Liess B, Frey H-R. Bovine viral diarrhoea virus infection in cattle, sheep and goats in northern Tanzania. Trop Anim Health Prod. 1991;23:83–94. [DOI] [PubMed] [Google Scholar]
- [65]. Ghirotti M, Semproni G, De Meneghi D, et al. Sero-prevalences of selected cattle diseases in the Kafue flats of Zambia. Vet Res Commun. 1991;15:25–36. Epub 1991 January 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Callaby R, Toye P, Jennings A, et al. Seroprevalence of respiratory viral pathogens of indigenous calves in Western Kenya. Res Vet Sci. 2016;108:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Parida S, Muniraju M, Mahapatra M, et al. Peste des petits ruminants. Vet Microbiol. 2015;181:90–106. Epub 2015 October 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Lembo T, Oura C, Parida S, et al. Peste des petits ruminants infection among cattle and wildlife in northern Tanzania. Emerg Infect Dis. 2013;19:2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Elzein E, Housawi F, Bashareek Y, et al. Severe PPR Infection in Gazelles kept under semi‐free range conditions. J Vet Medicine, Ser B. 2004;51:68–71. [DOI] [PubMed] [Google Scholar]
- [70]. Kinne J, Kreutzer R, Kreutzer M, et al. Peste des petits ruminants in Arabian wildlife. Epidemiol Infect. 2010;138:1211–1214. [DOI] [PubMed] [Google Scholar]
- [71]. Kock R, Wamwayi H, Rossiter P, et al. Re-infection of wildlife populations with rinderpest virus on the periphery of the Somali ecosystem in East Africa. Prev Vet Med. 2006;75:63–80. [DOI] [PubMed] [Google Scholar]
- [72]. Munir M. Role of wild small ruminants in the epidemiology of peste des petits ruminants. Transbound Emerg Dis. 2014;61:411–424. [DOI] [PubMed] [Google Scholar]
- [73]. Couacy-Hymann E, Bodjo C, Danho T, et al. Surveillance of wildlife as a tool for monitoring rinderpest and peste des petits ruminants in West Africa. Rev Sci Tech. 2005;24:869–877. Epub 2006 April 29. [PubMed] [Google Scholar]
- [74]. Thomson G, Vosloo W, Bastos A. Foot and mouth disease in wildlife. Virus Res. 2003;91:145–161. [DOI] [PubMed] [Google Scholar]
- [75]. Sutmoller P, Thomson GR, Hargreaves SK, et al. The foot-and-mouth disease risk posed by African buffalo within wildlife conservancies to the cattle industry of Zimbabwe. Prev Vet Med. 2000;44:43–60. [DOI] [PubMed] [Google Scholar]
- [76]. Chatzopoulos D, Valiakos G, Giannakopoulos A, et al. Bluetongue Virus in wild ruminants in Europe: concerns and facts, with a brief reference to bluetongue in cervids in Greece during the 2014 outbreak. Small Ruminant Res. 2015;128:79–87. [Google Scholar]
- [77]. Gerdes GH, South A. African overview of the virus, vectors, surveillance and unique features of bluetongue. Vet Ital. 2004;40:39–42. Epub 2004 July 1. [PubMed] [Google Scholar]
- [78]. Mohammadi A, Tanzifi P, Nemati Y. Seroepidemiology of bluetongue disease and risk factors in small ruminants of Shiraz suburb, Fars province, Iran. Trop Biomed. 2012;29:632–637. [PubMed] [Google Scholar]
- [79]. Kanyi Kibe M, Smith GR. A study of F38-type and related mycoplasmas by mycoplasmaemia and cross-immunization tests in mice. J Hyg (Lond). 1984;93:465–473. Epub 1984 December 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Smith GR, Oliphant JC. The ability of Mycoplasma mycoides subspecies mycoides and closely related strains from goats and sheep to immunize mice against subspecies capri. J Hyg (Lond). 1981;87:321–329. Epub 1981 October 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Brook RK, McLachlan SM. Trends and prospects for local knowledge in ecological and conservation research and monitoring. Biodivers Conserv. 2008;17:3501–3512. [Google Scholar]


