Abstract
Purpose
Observational studies suggest that menopausal hormone therapy protects against sleep-disordered breathing, but such findings may be biased by a “healthy-user effect.” When the Women’s Health Initiative Study reported in 2002 that estrogen-progestin therapy increases heart disease risk, many women discontinued hormone therapy. We investigate healthy-user bias in the association of hormone therapy with sleep-disordered breathing in the Sleep in Midlife Women Study.
Methods
228 women age 38–62 were recruited from the Wisconsin Sleep Cohort Study. They underwent polysomnography to measure apnea-hypopnea index, at home semiannually from 1997–2006, and in the sleep laboratory every four years (N=1,828 studies). Hormone therapy was recorded monthly. Linear models with empirical standard errors regressed logarithm of apnea-hypopnea index on hormone use with a pre/post-July 2002 interaction, adjusting for menopause and age.
Results
The association of hormone therapy and sleep-disordered breathing was heterogeneous (p<0.01); apnea-hypopnea index among users was 15% lower in the early period (95% confidence interval: −27%, −1%), but similar to non-users in the late.
Conclusions
Hormone therapy was negatively associated with sleep-disordered breathing only until the Women’s Health Initiative results were publicized. Hormone therapy may have been a marker for healthfulness in the early period, creating a spurious association with sleep-disordered breathing.
Keywords: Sleep Apnea Syndromes, Sleep Apnea, Obstructive, Women’s Health, Menopause, Estrogen Replacement Therapy, Bias (Epidemiology)
INTRODUCTION
Before the halting of the Women’s Health Initiative Study’s estrogen-progesterone trial was announced in July 2002 [1], the association of hormone therapy with sleep-disordered breathing was an active area of investigation, in which several observational studies found that midlife women using hormone therapy had less sleep-disordered breathing than nonusers. Estrogen therapies have since become a widely-cited example of an exposure whose effects appear different when investigated by observational studies or by randomized controlled trials. The most famous results from the Women’s Health Initiative suggested that observational studies linking hormone therapy use to reduced risk of coronary heart disease had been biased by a healthy-user effect, in which healthier subjects self-selected into the user group or sicker subjects selectively dropped out. However, some of the study’s other findings, including that hormone therapy reduced risk of hip fracture [2] and colorectal cancer [3], were consistent with the observational literature. Sleep outcomes were not measured in detail in the Women’s Health Initiative Study or any other large randomized trial, so there are no results with which to compare observational study estimates. Therefore it is unclear whether the seeming protective effect of hormone therapy on sleep-disordered breathing can be explained by a healthy-user bias.
The most common manifestation of sleep-disordered breathing, obstructive sleep apnea, is a disorder in which the airway repeatedly narrows or closes during sleep, leading to decreased airflow and a drop in oxyhemoglobin saturation. Typically, the brain responds by arousing the sleeper, allowing the airway to reopen. Breathing is intermittently impaired and sleep is fragmented, throughout the night. Obstructive sleep apnea has health consequences that include greater risk of hypertension, coronary heart disease, stroke, depression, cognitive impairment and motor vehicle accidents, as well as greater mortality [4–9].
Among younger adults, the prevalence of sleep-disordered breathing in men is roughly twice the prevalence in women [10,11], but among older adults, women’s prevalence approaches men’s [12]. Lower sex hormone levels among older women could explain this pattern, and as a corollary it has been suggested that exogenous estrogen could prevent or treat sleep-disordered breathing. Since coronary heart disease is an observed outcome of obstructive sleep apnea, for women predisposed to sleep-disordered breathing the benefit of preventing or treating the disorder could in theory outweigh the harm of hormone therapy. Several clinical trials have attempted to test this hypothesis directly, but all were small, several studied male subjects, the quality of the study design was variable, and results were conflicting [13–21]. The strongest evidence supporting the hormone hypothesis has come from population-based observational studies [22–24].
As the findings from the Women’s Health Initiative received widespread publicity, many clinicians stopped prescribing hormone therapy for preventive indications in the months following July 2002, and many women abruptly discontinued their medications [25,26]. Thus behavioral correlates of hormone therapy use likely changed from healthy, to neutral or unhealthy. The present study compares data from before and after July 2002 from the Sleep in Midlife Women Study to investigate whether a healthy-user bias could explain the negative association of hormone therapy use with sleep-disordered breathing severity.
MATERIALS AND METHODS
Study design and population
The sample for the Sleep in Midlife Women Study was recruited from women participating in the Wisconsin Sleep Cohort Study. Full details of the Wisconsin Sleep Cohort Study design are described elsewhere [12]. Briefly, from 1989–1993 a random sample of state workers was recruited to participate in a mailed survey. A stratified random subsample of survey responders was chosen, with sampling weights based on self-report of snoring and other factors chosen to enrich the sample with subjects with sleep apnea. From 1989–2003 these responders were invited to participate in overnight polysomnography studies in the sleep lab. Participants were invited to return for in-laboratory studies approximately every four years through the present.
Beginning in 1996, all female Wisconsin Sleep Cohort Study participants who were over 47 years old or who had begun perimenopause were invited to participate in the Sleep in Midlife Women Study. The substudy was designed specifically to measure data relating to sleep health and the menopausal transition. Response rate was approximately 80%. Every six months on average through 2007, subjects underwent sleep studies in their own homes. During this time they also completed daily diaries. Data from the same subjects’ lab visits for the parent study were also used for this analysis; any lab visit dates for which hormone therapy use and confounding factors were known were used. Protocols and informed consent documents for the Sleep in Midlife Women Study and Wisconsin Sleep Cohort Study were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board.
Assessment of exposure, outcome, and covariates
Hormone therapy use was recorded in diaries, where subjects reported any hormonal medications they had used that month, along with daily menstrual bleeding and sleep symptoms [27]. Details of type of medications reported are available in the Appendix (Table A.1). When diary data was missing, hormone therapy use (including current use, past use, and start and stop dates) was assessed by questionnaire at the time of the sleep study. For this analysis, subjects with any hormone therapy use since their last sleep study were classified as current hormone therapy users.
Sleep-disordered breathing was assessed by measuring the apnea-hypopnea index to indicate the rate of breathing pauses during sleep. Polysomnography was used to measure arterial oxyhemoglobin saturation, oral and nasal airflow, nasal air pressure (in-laboratory only), and rib cage and abdominal respiratory motion. Apnea-hypopnea index was calculated by summing the number of apneas (air flow cessation ≥10 seconds) and hypopneas (discernable decrease in airflow or nasal pressure for ≥ 10 seconds, with oxygen desaturation of ≥ 4%), divided by objectively measured total sleep time.
In-home studies used a polysomnography monitor (P-series, Compumedics USA, Inc., Fridley, MN), including piezoelectric chest and abdominal bands to record breathing effort, nasal-oral thermistry to detect airflow, and finger-pulse oximetry to record arterial oxygen saturation. For in-lab studies, a 20-channel polysomnography digital sleep system (Telefactor Heritage, Grass Instruments, Warwick, RI) was used. Oxyhemoglobin saturation was recorded by pulse oximetry (Datex-Ohmeda 3740, Madison, WI), airflow was recorded by thermocouples (Dymedix, Shoreview, MN) and a nasal pressure transducer (Protec, Andover, MA), and rib cage and abdominal excursions were recorded by respiratory inductance plethysmography (Respitrace, Ambulatory Monitoring, Ardsley, NY).
Wherever possible, menopausal status was categorized based on diary-reported menstrual bleeding pattern, an approach consistent with the Stages of Reproductive Aging Workshop criteria for research [28]. Different criteria were used for subjects taking medication that could prolong menstrual bleeding, including hormone therapy and hormonal contraceptives. Subjects who had undergone hysterectomy were categorized based on date and type of surgery in conjunction with circulating levels of follicle-stimulating hormone. Complete details of menopausal staging are available in the Appendix (Figure A.1).
Subjects were weighed and underwent neck girth measurement at every sleep study visit; details of the protocol are publicly available. [29] measured height was taken from most recent lab visits to calculate BMI (kg/m2). Number of alcoholic drinks per week and smoking history were assessed by interview.
Statistical analysis
Descriptive statistics of the sample were obtained by averaging values for each subject, and then obtaining the mean and standard deviation across all subjects.
Because most subjects were healthy, the distribution of the apnea-hypopnea index was expected to be skewed toward zero. To produce a more normal distribution of outcome values, we transformed the apnea-hypopnea index by taking a natural logarithm, adding one to allow use of zero values. That is, transformed apnea-hypopnea index is equal to ln(apnea-hypopnea index + 1).
Transformed apnea-hypopnea index was used as the outcome in mixed linear regression models, with random intercepts for each subject to account for repeated measures, and empirical standard errors. Regression parameters were then exponentiated, so that results may be interpreted as the ratio of (apnea-hypopnea index + 1) compared to the relevant reference. Potential confounders were chosen a priori based on previous literature and available data, including menopausal status, age, BMI, neck girth, alcohol, and smoking.
To examine whether the association of hormone therapy use with sleep-disordered breathing differed before and after July 2002, models were fit on data from all years, including as independent variables hormone therapy use, a pre/post indicator, and their product term. Other potential modifiers of the association between hormone therapy use and sleep-disordered breathing were examined by the same method, including age, menopausal status, time in postmenopause, and duration of hormone therapy use.
Apnea-hypopnea index values calculated from in-home measurements were systematically higher than values calculated from in-laboratory measurements. There is a linear relationship between apnea-hypopnea index measured in the laboratory and apnea-hypopnea index measured at the closest home visit in time (Pearson correlation 0.65, p<0.01). To account for the systematic difference, our regression models also adjusted for study venue. Further models excluded in-lab values, as a check against information bias.
To investigate whether July 2002 was in fact a demarcation point between two different paradigms in the use of hormone therapy, and not merely an indicator for unrelated secular trends, we conducted a further sensitivity analysis. Mixed linear models were fitted using time points other than July 2002 to mark different Before and After periods. These models were adjusted for menopausal status and age, and were limited to the in-home data. We compared the estimated magnitude of the product term from each model (interpretable as the difference between the association of sleep-disordered breathing with hormone use before the cutoff date and the association with hormone use after that date). All dates for which 30 or more observations were available in each category of hormone use were used.
All statistical analysis was conducted using SAS v9.3 software (SAS Institute; Cary, NC). PROC MIXED was used for linear regression models.
RESULTS
Participant characteristics and hormone therapy use
239 subjects participated in the Sleep in Midlife Women Study. Subjects underwent 1–14 home visits each (median number of visits was 7), for a total of 1,949 in-home sleep studies. Eleven subjects were excluded from this analysis: two did not yield usable sleep studies, seven could not be categorized with respect to menopausal status at the time of their sleep studies, and two were concurrently participating in the Women’s Health Initiative study so their hormone therapy use was unknown. 203 subjects had one or more sleep studies before July 2002, and 228 subjects had one or more sleep studies after July 2002. Characterization of hormone therapy use and all confounders was possible for 1,502 in-home sleep studies, plus an additional 326 sleep studies conducted in-laboratory (1–4 per subject), yielding a total sample of 1,828 studies. Characteristics of the 228 included study participants are shown at baseline in Table 1 and across all visits in Table 2.
Table 1.
Baseline Characteristics of 228 Participants in the Sleep in Midlife Women Study, Madison, WI, 1990–2009
| Mean | (SD) | |
|---|---|---|
| Apnea-Hypopnea Index | 8.8 | (12.9) |
| Age (years) | 48.5 | (6.9) |
| BMI | 31.0 | (7.8) |
| Neck girth (cm) | 35.6 | (3.5) |
| Alcoholic drinks per week | 2.3 | (3.2) |
| N | % | |
| Date of first sleep study | ||
| Before July 2002 | 195 | (86%) |
| After July 2002 | 33 | (14%) |
| Hormone Therapy | ||
| Nonuser | 181 | (79%) |
| Usera | 47 | (21%) |
| Menopausal stage | ||
| Premenopause or Early Perimenopause | 105 | (46%) |
| Late Perimenopause | 36 | (16%) |
| Postmenopause | 87 | (38%) |
| Smoking history | ||
| Never smoker | 117 | (51%) |
| Past smoker | 75 | (33%) |
| Current smoker | 36 | (16%) |
| Venue | ||
| Home | 172 | (75%) |
| Lab | 56 | (25%) |
| All | 228 | |
One of the subjects using hormone therapy at baseline entered the study after July 2002. All other baseline users entered the study in the early period.
Table 2.
Characteristics of Sleep in Midlife Women Study Participants, Madison, WI, Over 1–18 Sleep Studies from 1990–2009.
| Before July 2002 | After July 2002 | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hormone Therapy Nonuser | Hormone Therapy User | Hormone Therapy Nonuser | Hormone Therapy User | |||||||
| Meana | (SD) | Meana | (SD) | Meana | (SD) | Meana | (SD) | Meana | (SD) | |
| Apnea-Hypopnea Index | 10.1 | (14.2) | 8.9 | (9.3) | 11.9 | (13.2) | 12.3 | (11.4) | 10.9 | (13.0) |
| Age (years) | 48.9 | (7.3) | 53.2 | (3.9) | 54.3 | (4.7) | 54.1 | (3.5) | 52.2 | (6.1) |
| BMI | 31.3 | (8.3) | 30.5 | (8.1) | 32.2 | (8.8) | 32.0 | (8.5) | 31.6 | (8.5) |
| Neck girth (cm) | 35.5 | (3.7) | 35.4 | (3.5) | 35.8 | (3.4) | 35.6 | (3.4) | 35.6 | (3.5) |
| Alcoholic drinks per week | 2.4 | (3.2) | 1.8 | (2.4) | 2.2 | (3.0) | 2.0 | (2.9) | 2.2 | (3.0) |
| N | (%) | N | (%) | N | (%) | N | (%) | N | ||
| Menopausal stage | ||||||||||
| Premenopause or Early Perimenopause | 253 | (70%) | 1 | (0%) | 108 | (30%) | 0 | (0%) | 362 | |
| Late Perimenopause | 128 | (42%) | 21 | (7%) | 142 | (47%) | 14 | (5%) | 305 | |
| Postmenopause | 214 | (18%) | 201 | (17%) | 648 | (56%) | 98 | (8%) | 1161 | |
| Smoking history | ||||||||||
| Never smoker | 299 | (30%) | 126 | (13%) | 485 | (49%) | 72 | (7%) | 982 | |
| Past smoker | 199 | (34%) | 78 | (13%) | 289 | (49%) | 28 | (5%) | 594 | |
| Current smoker | 97 | (38%) | 19 | (8%) | 124 | (49%) | 12 | (5%) | 252 | |
| Venue | ||||||||||
| Home | 472 | (31%) | 202 | (13%) | 736 | (49%) | 92 | (6%) | 1502 | |
| Lab | 123 | (38%) | 21 | (6%) | 162 | (50%) | 20 | (6%) | 326 | |
| Total Observations | 595 | (33%) | 223 | (12%) | 898 | (49%) | 112 | (6%) | 1828 | |
| Total Subjectsb | 159 | 60 | 182 | 47 | 228 | |||||
Mean values are the mean of each individual’s mean; standard deviations represent the variation across individuals.
Some subjects contributed observations as both users and non-users, and/or in both periods, thus the total number of subjects in each category sums to more than 228.
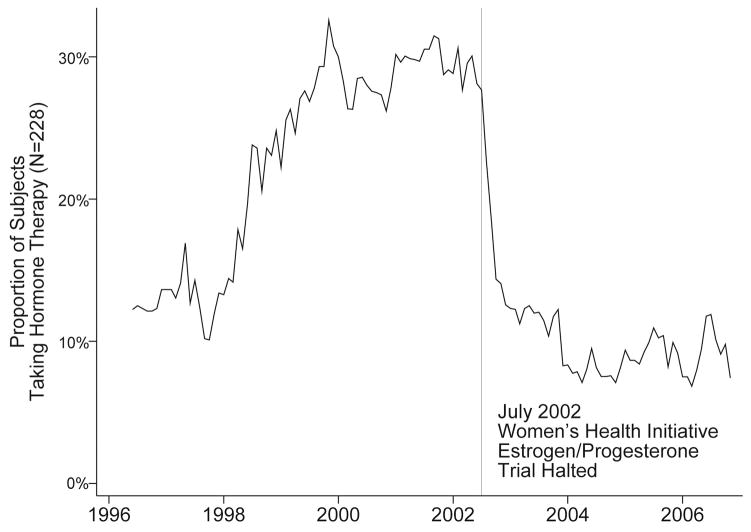
Hormone therapy use showed a temporal pattern, rising from 1990–1999, remaining relatively flat until July 2002, then sharply declining, and remaining low thereafter. Figure 1 shows hormone therapy use over the course of the Sleep in Women’s Health substudy as reported in monthly diaries.
Figure 1.
Hormone therapy use among 228 participants in the Sleep in Midlife Women Study, Madison WI, as reported monthly in diaries. Figure excludes May 1996 due to small sample size: N=15, 5 hormone therapy users (33%).
Seven subjects (3%) used hormone therapy throughout their study visits, 69 subjects (30%) had at least one sleep study using and at least one not using, and 152 subjects (67%) never used hormone therapy. Of the 69 subjects who used hormone therapy over the course of the study, 47 were prevalent users at baseline (Table 1). In the early period, mean duration of hormone therapy use was 17.9 months (SD 18.5); in the later period mean duration was 15.6 months (SD 19.4).
Mean apnea-hypopnea index from home visits was 11.8 (SD 13.4). As a result of using different equipment, mean apnea-hypopnea index from lab visits was lower, at 5.2 (7.5). Overall mean apnea-hypopnea index was 10.9 (13.0).
Association of hormone therapy and sleep-disordered breathing
Hormone therapy users had lower apnea-hypopnea index at baseline (Table 1). Across all observations, hormone therapy users had lower mean apnea-hypopnea index before July 2002, but after July 2002 apnea-hypopnea index was similar between users and nonusers (Table 2). Hormone users were also younger in the early period, whereas in the later period users and nonusers were the same age on average.
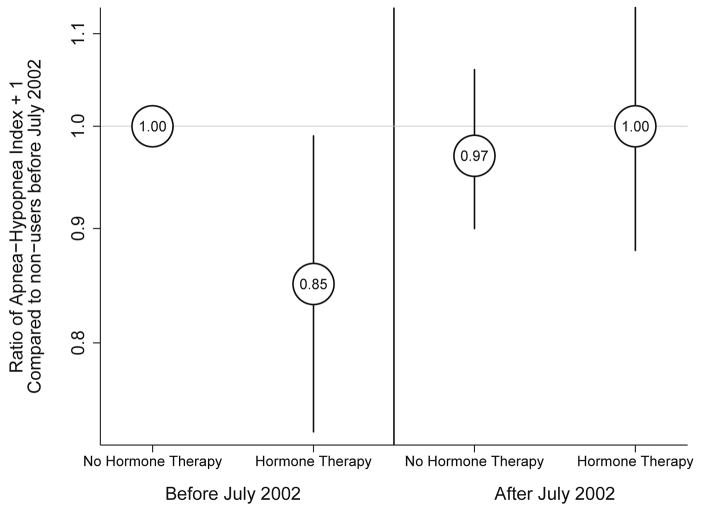
In all regression models, the association of hormone therapy with sleep-disordered breathing was heterogeneous between the two time periods, showing hormone therapy associated with lower apnea-hypopnea index before July 2002, but no meaningful association after July 2002 (Figure 2). The product term P-value was equal to or below 0.01 in all models. Table 3 shows results from a multivariable model regressing transformed apnea-hypopnea index on hormone therapy use before and after July 2002, adjusting for menopausal status, age, BMI, neck girth, alcohol use and smoking history. The relationship of hormone therapy to apnea-hypopnea index was not modified by age, menopausal status, time in postmenopause, or duration of hormone therapy use.
Figure 2.
Estimated ratio of apnea-hypopnea index plus one in each group compared to hormone therapy non-users before July 2002, among participants in the Sleep in Midlife Women Study (N= 1,828 observations from 228 subjects), from mixed multivariable regression model with empirical standard errors, adjusting for menopausal stage, age, BMI, neck girth, smoking history, and alcohol use. Product term between hormone therapy use and time period: p<0.01. Madison, WI, 1990–2009.
Table 3.
Ratios of (Apnea-Hypopnea Index + 1) Among 228 Participants (1,828 Observations) in the Sleep in Midlife Women Study, Madison, WI, 1990–2009, from Mixed Multivariable Regressions Modeling ln(apnea-hypopnea index+1) with Random Intercepts and Empirical Standard Errors. Models are adjusted for all covariates shown, including age.
| Ratio | 95% CI | Percent Difference | 95% CI | |
|---|---|---|---|---|
| Hormone Therapy Usea,b | ||||
| Before July 2002 | ||||
| No Use | 1.00 | 0 | ||
| Use | 0.85 | 0.73, 0.99 | −15 | −27, −1 |
| After July 2002 | ||||
| No Use | 0.97 | 0.90, 1.06 | −3 | −10, 6 |
| Use | 1.00 | 0.88, 1.13 | 0 | 12, 13 |
| Menopausal status | ||||
| Premenopause or Early Perimenopause | 1.00 | 0 | ||
| Late Perimenopause | 1.07 | 0.97, 1.20 | 7 | −3, 20 |
| Postmenopause | 1.15 | 1.02, 1.28 | 15 | 2, 28 |
| Age (years) | 1.05 | 1.03, 1.06 | 5 | 3, 6 |
| BMI | 1.05 | 1.02, 1.07 | 5 | 2, 7 |
| Neck girth (cm) | 1.06 | 1.03, 1.09 | 6 | 3, 9 |
| Alcoholic drinks per week | 1.01 | 1.00, 1.02 | 1 | 0, 2 |
| Smoking history | ||||
| Never smoker | 1.00 | 0 | ||
| Past smoker | 0.89 | 0.74, 1.07 | −11 | −26, 7 |
| Current smoker | 1.01 | 0.84, 1.22 | 1 | −16, 22 |
| Venue | ||||
| Home | 1.00 | 0 | ||
| Lab | 0.68 | 0.64, 0.73 | −32 | −36, −27 |
Of the 195 subjects that contributed one or more sleep studies in the early period, 60 (31%) were using hormone therapy at one or more of those visits. Of the 228 subjects contributing sleep studies in the late period, 47 (21%) were using hormone therapy at one or more visits.
Estimated association of SDB with hormone therapy use after July 2002, compared to no use after July 2002, is ratio 1.03 (95% CI: 0.93, 1.14), percent difference 3 (95% CI: −7, 14)
Before July 2002, hormone therapy use was associated with 15% lower apnea-hypopnea index (95% confidence interval [−27%, −1%]) as estimated by the fully adjusted model. After July 2002, apnea-hypopnea index was similar among users and nonusers. Exclusion of data obtained at in-lab studies reduced power but did not change the pattern of association between hormone therapy use and apnea-hypopnea index across time periods.
Sensitivity analysis
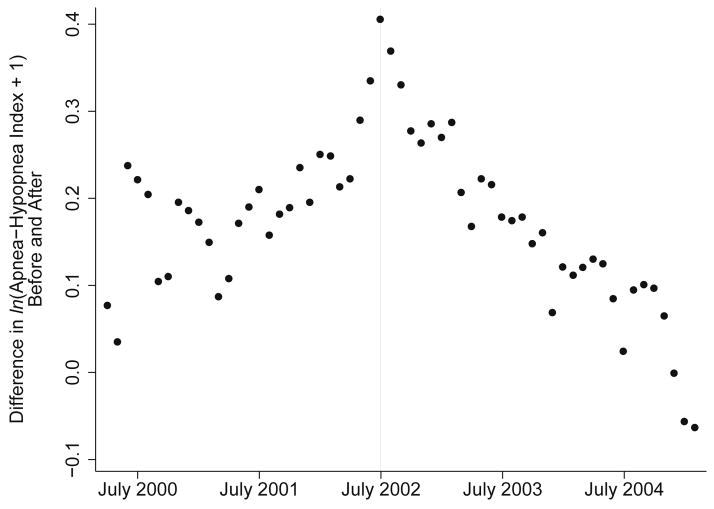
There were 58 dates (April 2000–February 2005) before and after which there were at least 30 in-home sleep studies among both hormone users and non-users in both time periods. The date chosen a priori, July 2002, showed the strongest contrast between the association of hormone therapy use with sleep-disordered breathing before and after (Figure 3). Time points closest to July 2002 showed greater positive contrasts, and earlier and later time points showed smaller or negative contrasts, suggesting a systematic pattern.
Figure 3.
Results of sensitivity analysis, in which 58 linear mixed models regressed ln(Apnea-Hypopnea Index+1) on hormone therapy use before and after a cutoff date, among 228 subjects in the Sleep in Midlife Women Study, Madison, WI, 1990–2009. Each model used a different date. Each data point is the estimated difference between the effect of hormone therapy use before and after the date shown. Models were adjusted for menopausal stage and age. In-home visits only were used (N=1,519 observations).
DISCUSSION
Our findings suggest that hormone therapy use was associated with less severe sleep-disordered breathing before July 2002, and was no longer associated with sleep-disordered breathing after that date. Were there a true biological mechanism for the effect of hormone therapy on breathing during sleep, that association would be expected to persist irrespective of time period. A healthy-user bias, however, could explain the period effect. Before the halting of the Women’s Health Initiative trial, hormone therapy use may have been a marker for overall healthfulness, confounding its relationship with sleep health. After the risks of hormone therapy were made public, when hormone therapy use was no longer perceived as a healthy behavior, it was no longer associated with sleep-disordered breathing.
Hormone therapy use in our sample comprised many different types of medication, including synthetic estrogens, progesterones, and combination therapies, as well as over-the-counter remedies such as black cohosh which have no known relationship to sleep-disordered breathing and whose effectiveness at treating the symptoms of menopause is debated [30]. Though sample size did not allow us to examine effect modification by medication type, the finding that these substances with different mechanisms of action are on average associated with lower sleep-disordered breathing only until July 2002 makes a biological effect less plausible and a bias effect more so.
Several mechanisms for a healthy-user effect are possible. Previous studies using data from before 2002 have suggested that healthier women select into the hormone therapy user category [31–33], while women who get sick stop using hormone therapy [34]. While development of sleep-disordered breathing itself is unlikely to be a reason for stopping hormone therapy use, some of its sequelae could be. Furthermore, socioeconomic status, exercise, and alcohol use have been shown to be predictors of hormone therapy use [33,35–37]. Our measures of socioeconomic status and exercise were too limited to allow us to model whether they were associated with hormone therapy initiation or maintenance, but alcohol and smoking were weak predictors of hormone therapy use.
Three previous population-based studies have found evidence of varying strength that hormone therapy use is associated with healthier breathing during sleep [22–24]. However, they all used data collected before 2002, when hormone therapy use was more common among healthy women. Our study suggests that a healthy-user effect may explain those findings. Though the question remains relevant, few studies have been conducted since the halting of the Women’s Health Initiative trials. Our data is unique in its timespan, bridging the years before and after prescription of hormone therapy underwent a paradigm shift away from preventive indications.
Alternative secular trends could explain the difference between the Before and After periods rather than a healthy-user effect. Prescribing practices changed in response to the Women’s Health Initiative findings, including reducing the dose of hormone therapy [38]. It is possible that hormone therapy is effective at lowering the apnea-hypopnea index only at high doses. It is also possible that hormone therapy is most effective at preventing sleep-disordered breathing in women who are otherwise healthy, and that as the pool of hormone therapy users become on average less healthy overall, the medications were less effective. A chance association is also possible.
This study’s generalizability is also limited. The Wisconsin Sleep Cohort sample included almost exclusively Caucasians, and thus it is not possible to investigate whether race or ethnicity would affect the relationships observed here.
The relationship of hormone therapy to sleep-disordered breathing may represent an interesting case study of healthy-user bias. The conversation sparked by the Women’s Health Initiative results brought the concept of a healthy-user bias to renewed prominence. Competing explanations for the discrepancy between trial results and observational results have also been advanced, however, including non-representativeness of the Women’s Health Initiative study population [39], and the sampling of prevalent users among the observational studies [40]. Our findings support the theory that hormone therapy was once a marker for healthfulness, potentially biasing studies of its preventive indications.
The apparent lack of association between hormone therapy and sleep-disordered breathing since July 2002 is also relevant because the evidence base to guide prescription of hormone therapy for sleep complaints in menopausal women remains weak. Several studies have suggested that sleep complaints may be symptoms of underlying sleep-disordered breathing in female sleep clinic patients [41,42]. When a gynecology or primary care clinician misdiagnoses sleep-disordered breathing as a temporary disruption caused by menopausal discomforts, a patient may receive a prescription for a hormonal medication. Our findings suggest that these drugs are unlikely to benefit such a patient’s sleep health.
HIGHLIGHTS.
We studied bias in menopausal hormone therapy’s effect on sleep-disordered breathing.
When the Women’s Health Initiative ended in July 2002, many women quit these drugs.
Before July 2002, hormone users had lower apnea-hypopnea index.
After July 2002, the association disappeared.
A healthy-user effect may have caused a spurious association in the early period.
Acknowledgments
FUNDING
This work was supported by the National Institute of Aging (R01AG14124), National Heart, Lung, and Blood Institute (R01HL62252) and the National Center for Research Resources (1UL1RR025011) at the National Institutes of Health. Anna G. Mirer is in the University of Wisconsin Medical Scientist Training Program (T32GM008692).
We thank Laurel A. Finn, Jodi H. Barnet, and Rachel Steidl for their help preparing this article. We thank Diane Austin, Diane Demonoco Dowd, Mari Dresner, Crystal Halvorson, Kathryn Hoffman, Angie Kujak, Jennifer Kujak, Andrea Peterson, Angela Slattery, and Rebecca Swain-Eng for their work on the Sleep in Midlife Women Study.
ABBREVIATIONS
- BMI
Body-Mass Index
APPENDIX A
Table A1.
Type of menopausal hormone therapy used before and after July 2002 in the Sleep in Midlife Women Study, Madison, WI, 1990–2009. N=3,899 out of 17,010 diaries
| Before July 2002 | After July 2002 | |
|---|---|---|
| Estrogen, conjugated (Cenestin, Premarin) | 464 | 186 |
| Estrogen, esterified (Estratab, Menest) | 48 | 0 |
| Estradiol (Alora, Climara, Esclim, Estrace, Estraderm, Estring, FemPatch, Gynodiol, Innofem, Vagifem, Vivel le, Vivelle-dot) | 430 | 322 |
| Estradiol & Norethindrone (Activelle, Combipatch, Femhrt) | 29 | 34 |
| Estrogen & Medroxyprogesterone (Premphase, Prempro) | 795 | 115 |
| Estrone/Estradiol/Progesterone compound | 25 | 17 |
| Medroxyprogesterone acetate (Amen, Androderm, Curretab, Cycrin, Depo-Provera, Provera) | 435 | 182 |
| Estrogen & Methyltestosterone (Estratest, Menogen) | 99 | 49 |
| Selective Estrogen-receptor modulators (Droloxifene, Raloxifene(Evista), Tamoxifen, Tiboline(Livial)) | 27 | 34 |
| Herbal and OTC preparations (Black Cohosh, Promensil, flax seed, yam extracts) | 167 | 101 |
| Estrogen (Version 1 diary onlya) | 192 | N/A |
| Progesterone (Version 1 diary onlya) | 148 | N/A |
Diaries used through May 1998 offered only two options to describe hormonal medications, “Estrogen” or “Progesterone.” Later versions of the diary offered more categories of medication, as well as a blank space to specify those not on the list.
Table A.2.
Criteria used to define menopausal stage in the Sleep in Midlife Women Study, Madison, WI, 1990–2009. For the present analysis, subjects who had not met criteria for late perimenopause were classified as premenopause/early perimenopause. Subjects meeting the criteria for late perimenopause were classified as being in late perimenopause until meeting criteria for postmenopause. Hormonal medications in this context include menopausal therapies and hormonal contraceptives.
| Criteria | Start of Late Perimenopause | Start of Postmenopause | |
|---|---|---|---|
| Menstrual Bleeding | No Hormonal Medication Hormonal Medication |
Beginning of 3 months of no flow | Beginning of 12 months of no flow Beginning of 6 months of no flow |
| Surgical History and FSH Level | Ovary-Sparing Surgery | Hot flashes or night sweats, where FSH>10 for 6 months or more FSH>40 prior to surgery | FSH>40 any time after surgery |
| Ovary-Removing Surgery | Surgery <6 months ago | Surgery ≥6 months ago | |
| Medication History | Initiation of menopausal hormone therapy | 12 months of menopausal hormone therapy | |
| Age | No Hormonal Medication Hormonal Medication Any Medication History |
60th birthday 55th birthday 573 days past start of late perimenopause, where no other criteria are available |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg CL, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus Progestin and Colorectal Cancer in Postmenopausal Women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. Wis Med J. 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179:1159–64. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 12.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cistulli PA, Barnes DJ, Grunstein RR, Sullivan CE. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699–702. doi: 10.1136/thx.49.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin K, Lundgren R, Rabben T. Sleep apnoea syndrome treated with oestradiol and cyclic medroxyprogesterone. Lancet. 1991;338:251–2. doi: 10.1016/0140-6736(91)90389-7. [DOI] [PubMed] [Google Scholar]
- 15.Hensley MJ, Saunders NA, Strohl KP. Medroxyprogesterone treatment of obstructive sleep apnea. Sleep. 1980;3:441–6. doi: 10.1093/sleep/3.3-4.441. [DOI] [PubMed] [Google Scholar]
- 16.Keefe DL, Watson R, Naftolin F. Hormone replacement therapy may alleviate sleep apnea in menopausal women: a pilot study. Menopause. 1999;6:196–200. doi: 10.1097/00042192-199906030-00004. [DOI] [PubMed] [Google Scholar]
- 17.Manber R, Kuo TF, Cataldo N, Colrain IM. The effects of hormone replacement therapy on sleep-disordered breathing in postmenopausal women: a pilot study. Sleep. 2003;26:163–8. [PubMed] [Google Scholar]
- 18.Pickett CK, Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Moore LG. Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women. J Appl Physiol. 1989;66:1656–61. doi: 10.1152/jappl.1989.66.4.1656. [DOI] [PubMed] [Google Scholar]
- 19.Polo-Kantola P, Rauhala E, Helenius H, Erkkola R, Irjala K, Polo O. Breathing during sleep in menopause: a randomized, controlled, crossover trial with estrogen therapy. Obstet Gynecol. 2003;102:68–75. doi: 10.1016/s0029-7844(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 20.Saletu-Zyhlarz G, Anderer P, Gruber G, Mandl M, Gruber D, Metka M, et al. Insomnia related to postmenopausal syndrome and hormone replacement therapy: sleep laboratory studies on baseline differences between patients and controls and double-blind, placebo-controlled investigations on the effects of a novel estrogen-progestogen combination (Climodien, Lafamme) versus estrogen alone. J Sleep Res. 2003;12:239–54. doi: 10.1046/j.1365-2869.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- 21.Wesström J, Ulfberg J, Nilsson S. Sleep apnea and hormone replacement therapy: a pilot study and a literature review. Acta Obstet Gynecol Scand. 2005;84:54–7. doi: 10.1111/j.0001-6349.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 22.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–5. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 23.Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, et al. Hormone Replacement Therapy and Sleep-disordered Breathing. Am J Respir Crit Care Med. 2003;167:1186–92. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 24.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 25.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Watkins ES. The Estrogen Elixir: A History of Hormone Replacement Therapy in America. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- 27. [accessed 13.3.14];Wisconsin Sleep Cohort Study Manual of Operations. http://sleepcohort.wisc.edu/operations/womens_study/diaries/
- 28.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric J Int Menopause Soc. 2012;15:105–14. doi: 10.3109/13697137.2011.650656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Women’s Study Protocol Instruction Sheet. Wisconsin Sleep Cohort Study; [accessed 12.6.15]. https://sleepcohort.wisc.edu:9027/operations/womens_study/in_home/In-Home_Protocolr.htm. [Google Scholar]
- 30.Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD007244.pub2. Art. No.: CD007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egeland GM, Kuller LH, Matthews KA, Kelsey SF, Cauley J, Guzick D. Premenopausal determinants of menopausal estrogen use. Prev Med. 1991;20:343–9. doi: 10.1016/0091-7435(91)90033-z. [DOI] [PubMed] [Google Scholar]
- 32.Posthuma WF, Westendorp RG, Vandenbroucke JP. Cardioprotective effect of hormone replacement therapy in postmenopausal women: is the evidence biased? BMJ. 1994;308:1268–9. doi: 10.1136/bmj.308.6939.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to Use of Estrogen Replacement Therapy, Are Users Healthier than Nonusers? Am J Epidemiol. 1996;143:971–8. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 34.Sturgeon SR, Schairer C, Brinton LA, Pearson T, Hoover RN. Evidence of a healthy estrogen user survivor effect. Epidemiology. 1995;6:227–31. doi: 10.1097/00001648-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Humphrey LL, Chan BKS, Sox HC. Postmenopausal Hormone Replacement Therapy and the Primary Prevention of Cardiovascular Disease. Ann Intern Med. 2002;137:273–84. doi: 10.7326/0003-4819-137-4-200208200-00012. [DOI] [PubMed] [Google Scholar]
- 36.Lawlor DA, Davey Smith G, Ebrahim S. Socioeconomic Position and Hormone Replacement Therapy Use: Explaining the Discrepancy in Evidence From Observational and Randomized Controlled Trials. Am J Public Health. 2004;94:2149–54. doi: 10.2105/ajph.94.12.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha-Vinh P, Clavaud H, Sauze L. Caractéristiques individuelles et mortalité associées au traitement hormonal substitutif de la ménopause: étude d’une cohorte française de femmes d’âge compris entre 60 et 69 ans. J Gynécologie Obstétrique Biol Reprod. 2010;39:453–65. doi: 10.1016/j.jgyn.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118:64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 39.Michels KB. Hormone replacement therapy in epidemiologic studies and randomized clinical trials - are we checkmate? Epidemiology. 2003;14:3–5. doi: 10.1097/00001648-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–79. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7:377–89. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 42.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–14. [PubMed] [Google Scholar]