Abstract
Serological monitoring is a feature of surveillance programmes for the detection of the circulation of notifiable low pathogenic avian influenza (LPAI) viruses in commercial poultry holdings. Commercial multispecies nucleoprotein (NP) enzyme‐linked immunosorbent assays (ELISAs) have been replacing the haemagglutination inhibition (HI) test as pre‐screening tools. Few comparative studies have been conducted to test sera from domestic ducks for diagnostic purposes. Therefore, we evaluated the correlation between commercial NP ELISAs and the HI test. Anti‐NP and anti‐haemagglutinin (HA) antibodies were measured in sera from domestic ducks that had undergone serological screening and from juvenile domestic Pekin ducks that were experimentally infected with LPAI viruses. The findings highlight an absence of a correlation between NP ELISA and HI results with both field and experimental duck sera. Dissimilar kinetics of the antibodies detected during the follow‐up evaluation of the humoral immune responses in experimentally infected ducks may explain this lack of correlation. Indeed, anti‐NP titres decreased over time, whereas anti‐HA titres remained unchanged after inoculation with the H3N1 LPAI virus isolated from domestic duck or the H7N1 LPAI virus isolated from chicken. Despite these differences, the NP ELISA may serve as a valid pre‐screening tool to detect circulating LPAI viruses in domestic duck populations at the flock level.
Keywords: HI test, NP ELISA, duck immune response, notifiable avian influenza, Pekin duck, serological diagnosis
Introduction
Avian influenza (AI) viruses belong to the influenza A genus of the Orthomyxoviridae family and are divided into two pathotypes based on the severity of the disease caused in chickens. Highly pathogenic avian influenza (HPAI) viruses are extremely virulent, inducing systemic disease with high morbidity and mortality. In contrast, low pathogenic avian influenza (LPAI) viruses may be associated with mild respiratory diseases, reduced egg production and moderate increases in mortality (Alexander & Brown 2009; Webster et al. 1992). To date, only the H5 and H7 subtypes of AI viruses have been found to possess the potential to mutate into highly pathogenic viruses during circulation in poultry (Bean et al. 1985; Bowes et al. 2004; Capua et al. 2000; Elbers et al. 2004; Garcia et al. 1996; Rojas et al. 2002). All H5 and H7 AI viruses have been classified as notifiable viruses by the World Animal Health Organisation (OIE) since 2005 (2005/94/CE).
To prevent extensive circulation of notifiable LPAI viruses in poultry populations in European Member States, mandated serological surveillance and survey programmes have been implemented in accordance with the European Commission Decision (2007/268/EC). In Belgium, serological surveillance is required for commercial breeder and laying hen holdings of all species of domestic poultry, including chickens, turkeys, ducks, geese and other poultry such as guinea fowl and partridges (2005/734/EC and 2007/268/EC). Surveillance of notifiable LPAI virus circulation in domestic duck populations is important because ducks are likely to play an important role in the transmission of LPAI viruses from the natural wildlife reservoir to other poultry (Koch & Elbers 2006). Moreover, ducks bred in captivity are domesticated counterparts of the natural reservoir for LPAI viruses and they are commonly infected with AI viruses (Annual Reports on Surveillance for Avian Influenza in Poultry in Member States of the European Union on the Europa site: http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/eu_resp_surveillance_en.htm; accessed 29 January 2015). Thus, the presence of ducks in professional holdings is considered to be a risk factor for AI viral transmission (Koch & Elbers 2006).
Among the 10 proteins encoded by the AI virus, two are particularly used for serological diagnosis: haemagglutinin (HA) and nucleoprotein (NP). HA is a homotrimeric glycoprotein that is inserted into the viral envelope and is the most abundant surface antigen of the influenza virus. HA is responsible for the binding of virions to host cell receptors and plays a major role in the pathogenicity of AI viruses (Lee & Saif 2009; Webster et al. 1992). NP is an internal protein that binds to and encapsidates viral RNA. NP is the second most abundant protein of the influenza virus after the matrix (M) protein (Webster et al. 1992).
Historically, sera for serological surveillance were tested by the haemagglutination inhibition (HI) test, considered to be the reference test by the OIE. Enzyme‐linked immunosorbent assays (ELISA) were developed to detect antibodies directed against NP (type A), and indirect ELISAs were initially used to detect anti‐NP antibodies mainly in chicken sera (Snyder et al. 1985). Thereafter, commercial blocking/competition NP ELISAs were developed for broader use in multiple avian species. NP ELISAs are easier and faster to conduct than the HI test, and can be used in a screening test to perform AI serological monitoring in poultry populations. The use of a multispecies blocking NP ELISA from IDVet as a screening tool for sera of chicken, turkey, geese and duck was implemented in Belgium in 2010 (Marche & van den Berg 2010). All positive findings are confirmed by the HI test to detect the H5 and H7 subtypes specifically.
Despite the development of the blocking/competition ELISAs for use in multiple species, the test is generally validated using chicken sera. Few validation studies have been performed using sera from other avian domestic species, such as ducks. Some evaluation studies have been conducted with sera from wild ducks (Brown et al. 2009, 2010; Fereidouni et al. 2010; Jourdain et al. 2010; Perez‐Ramirez et al. 2010; Claes et al. 2012), but very few were conducted with domestic ducks in the context of the annual EU serological screening (Spackman et al. 2009). Therefore, the aim of this study was to compare multispecies NP ELISAs with HI tests, using field sera provided by the annual AI serological screening performed in duck holdings or sera from domestic Pekin ducks that had been experimentally infected with several LPAI viruses isolated from domestic duck, wild goose or chicken.
Materials and methods
Field sera
Duck sera tested in this study were collected in 2013 and early 2014. For sera collected in 2013, the duck holdings in this study represent all duck holdings showing sera positive by the NP ELISA IDVet and HI tests (with H5 or H7 antigens). For sera collected in 2014, a subset of the positive duck farms among all tested holdings is represented. For all holdings, sera were collected on the basis of 50 sera per flock, and no AI virus was isolated in the tested flocks.
Viruses
All LPAI viruses used in this study were propagated in 10‐day‐old embryonated specific pathogen‐free (SPF) chicken eggs (Valo Biomedia, Osterholz‐Scharmbeck, Germany) and stored at −80°C. The laboratory of Dr. I. Capua kindly provided the chicken‐origin H7N1 A/ck/It/1067v99 (hereinafter, H7N1/chicken). This virus was received as the third passage. The fifth passage was used to inoculate animals. The H3N1 virus A/duck/Belgium/02216/06 (hereinafter, H3N1/domestic duck) was isolated from a cloacal swab of domestic duck in Belgium (Marche et al. 2010). The second passage was used to infect animals. The H7N7 A/Branta canadenis/Belgium/13000‐9‐2/10 virus (hereinafter, H7N7/Canada goose) was isolated from a cloacal swab of a wild Canada goose in 2010 (Marche et al. 2010), and the second passage was used to inoculate animals.
Experimental infections
One‐day‐old Pekin ducks (Anas platyrhynchos) were purchased from a local provider (Wijverkens, Halle, Belgium) and raised in biosecurity level 3 isolators with ad libitum access to feed and water. Each animal experiment was conducted under the authorisation and supervision of the Biosafety and Bioethics Committees at the VAR (authorisation no. 110124‐01), following national and European regulations. Before infection, blood samples were collected from ducks and tested for AIV NP‐specific antibodies by IDVet NP ELISA. All samples from the ducks were negative (data not shown).
For each inoculated group, seven to eight 3‐week‐old Pekin ducks were inoculated via the oculonasal route with a dose of 106 egg infective dose 50 of diluted viral stock in sterile phosphate‐buffered saline. Ducks from the group inoculated with H3N1/domestic duck virus were inoculated a second time with the homologous strain at 21 days post infection (dpi). Sera were collected at different days after infection, depending on the inoculated group: 0, 7, 10, 14, 21 and 35 dpi for the H3N1/domestic duck virus‐inoculated group; 0, 7, 9, 14, 21 and 28 dpi for the H7N1/chicken virus‐inoculated group and 0, 14 and 21 dpi for the H7N7/Canada goose virus‐inoculated group. All sera were stored at −20°C until they were used for serological tests. Cloacal and buccal swabs were sampled at 1, 3, 6 and 10 dpi and directly stored in 1.5 mL of brain heart infusion medium (BHI) with antibiotics at −80°C until they were used for real‐time RT‐PCR (RRT‐PCR) detection.
HI tests
The HI tests were performed essentially according to official procedures (OIE, 2015; Terrestrial Manual). Briefly, the HA titres of different viruses were standardised to a concentration of four units of haemagglutination activity per 25 μL. Sera titres were defined by the last dilution to inhibit haemagglutination completely and were expressed as log2 values. A titre below 3 log2 was considered negative. A titre above or equal to 4 log2 was considered positive.
Field sera were tested by using reference H5 or H7 antigens (European APHS reference laboratory, Weybridge, UK) referred as H5N3W for the low pathogenic H5N3 (A/teal/7394‐2805/06) and H7N7W for the low pathogenic H7N7 (A/tky/Eng/6477/77). Sera obtained from experimental infections of Pekin ducks were tested by using the homologous virus used for infection.
NP ELISA kits
Two commercial multispecies competitive ELISA kits, both targeting influenza A NP antibodies, were used to test the sera: the IDScreen Influenza A Antibody Competition ELISA kit (IDVet, Montpellier, France) and the Influenza A Virus Antibody Test kit (Idexx, Westbrook, ME, USA), hereinafter referred to as IDVet NP ELISA and Idexx NP ELISA, respectively. All NP ELISAs were performed and results calculated according to the manufacturers’ instructions. For the IDVet NP ELISA, the short protocol was selected (i.e. sera were incubated for 1 h).
For both the IDVet and Idexx NP ELISAs, a percentage of inhibition ratio greater or sample‐to‐negative ratio equal to 50% or 0.5%, respectively, was considered negative. All doubtful results (% inhibition = 49–46%) with the IDVet NP ELISA are considered positive as is done when this kit is used as a screening tool for AI serological monitoring. However, for a more comprehensive understanding of the figures, the results for both ELISAs were expressed as 100% of inhibition.
Detection of M RNA by RRT‐PCR
Viral RNA was extracted from 50 μL of BHI‐immersed swabs by using the Magmax AI/ND 96 Viral RNA kit (Ambion Inc., Austin, TX, USA), which was adapted for semi‐automated extraction using a Kingfisher magnetic particle processor (Thermo Fisher Scientific, Erembodegem, Belgium). Purified RNA was eluted in a final volume of 50 μL. Two microlitres of purified RNA were used as a template for the RRT‐PCR. The Quantitect Probe RRT‐PCR kit (QiagenGmBH, Hilden, Germany) was used for amplification using a Biosystems 7500 Real‐Time PCR Cycler (Applied Biosystems, Lennik, Belgium). RRT‐PCR was performed to amplify the viral M gene, which allowed for detection of all AI virus subtypes (Steensels et al. 2007; Van Borm et al. 2007). RRT‐PCR was run on known dilutions of in vitro synthetised M RNA to establish a standard curve (Van Borm et al. 2007). Quantification of M RNA copy number in each sample was based on the standard curve, and the M RNA copy number per millilitre of BHI‐immersed swabs was calculated.
Results
Correlation between the IDVet NP ELISA and HI test with field duck sera
In Belgium, only commercial poultry holdings with more than 200 birds are considered for the AI serological survey, thus excluding backyard poultry (Welby et al. 2010). Each year, 50 sera from each of about 20 duck holdings, mostly of fattening ducks, are tested, representing about 1000 sera tested annually. Sera collected during 2013 and early 2014 were from H5‐ or H7‐positive duck flocks, representing 350 serum samples corresponding to seven holdings. These sera were used to evaluate the correlation between the IDVet NP ELISA and the HI test. AI virus was not isolated from any of these seven holdings, although sero‐positive sera revealed a current or older circulation of notifiable AI viruses.
The correlation between the IDVet NP ELISA and HI results was variable, ranging from 34% to 68% (Table 1). Sera testing positive by ELISA alone or HI alone were apparent in some holdings. For example, among the 50 serum samples analysed from holding 5, 31 sera tested positive by IDVet NP ELISA but not by HI, and only 6 sera tested positive by both assays. Conversely, in holdings 3 and 7, a substantial number of sera tested positive with HI (3: 28, 7:14) but not with the IDVet NP ELISA, and a small number of sera tested positive with both screening methods (3: 11, 7:2) among the 50 tested.
Table 1.
Comparison of results between ID‐vet NP ELISA and HI test for field sera from different ducks holdings
| ID‐vet NP ELISA/HIa | |||||
|---|---|---|---|---|---|
| Posb/pos | Posc/neg | Negd/pos | Nege/neg | % agreement | |
| Holding 1 | 13 | 6 | 10 | 21 | 68 |
| Holding 2 | 20 | 16 | 4 | 10 | 60 |
| Holding 3 | 11 | 2 | 28 | 9 | 40 |
| Holding 4 | 15 | 4 | 13 | 18 | 66 |
| Holding 5 | 6 | 31 | 2 | 11 | 34 |
| Holding 6 | 20 | 24 | 0 | 3 | 49 |
| Holding 7 | 2 | 1 | 14 | 29 | 67 |
Sera from seven different holdings were tested by IDVet NP ELISA and HI test using reference antigens H5N3W and H7N7W.
Sera were tested using H5 or H7 antigens recommended by the reference European laboratory.
Number of sera tested positive with ID‐vet NP ELISA and HI test.
Number of sera tested positive with ID‐vet NP ELISA, but negative with HI tests.
Number of sera tested negative with ID‐vet NP ELISA, but positive with HI tests.
Number of sera tested negative with ID‐vet NP ELISA and with HI tests.
Correlation between commercial ELISAs and HI tests with experimentally infected Pekin duck sera
Three‐week‐old Pekin ducks were infected with LPAI viruses originating from poultry or wild birds. During infection, serological responses were followed using two commercial NP ELISAs (from IDVet‐Vet and Idexx) and by the HI test using the homologous virus. Comparisons between the commercial IDVet‐Vet NP ELISA and the HI test were performed with the 98 serum samples collected during the experimental infections. The percentage of agreement between the two serological methods was relatively similar to that obtained using field sera, with 65% agreement for sera from ducks experimentally infected with H7N1/chicken virus and 62% agreement for sera from ducks infected with H3N1/domestic duck virus. Agreement between the commercial IDVet NP ELISA and the HI test was 100% for sera collected from ducks inoculated with the H7N7/Canada goose virus, as all animals tested positive with both assays. Very similar results were obtained when comparing the HI test with the Idexx ELISA (Table 2). Similar differences between the HI test and ELISAs were observed with experimental as well as field sera.
Table 2.
Comparison of the results of ID‐vet NP ELISA and HI test with sera from experimentally infected ducks
| NP ELISA tests/HIa | |||||
|---|---|---|---|---|---|
| Posb/pos | Posc/neg | Negd/pos | Nege/neg | % agreement | |
| Ducks inoculated with H7N1/chicken virus | |||||
| ID‐vet NP ELISA | 18 | 2 | 12 | 8 | 65 |
| Idexx NP ELISA | 20 | 2 | 10 | 8 | 70 |
| Ducks inoculated with H3N1/domestic duck virus | |||||
| ID‐vet NP ELISA | 12 | 5 | 8 | 9 | 62 |
| Idexx NP ELISAf | 12 | 5 | 6 | 9 | 64 |
| Ducks inoculated with H7N7/Canada goose virus | |||||
| ID‐vet NP ELISA | 16 | 0 | 0 | 8 | 100 |
| Idexx NP ELISA | 16 | 0 | 0 | 8 | 100 |
Sera from different experimental infections were tested by IDVet NP ELISA and HI tests using homologous antigens.
Sera were tested using homologous antigens.
Number of sera tested positive with ID‐vet NP ELISA and HI test.
Number of sera tested positive with ID‐vet NP ELISA, but negative with HI tests.
Number of sera tested negative with ID‐vet NP ELISA, but positive with HI tests.
Number of sera tested negative with ID‐vet NP ELISA and with HI tests.
Three sera from ducks infected with the H3N1/domestic virus were not in sufficient quantity to be tested with the Idexx NP ELISA.
Results of the two NP ELISAs were also compared by using data from three experimental infections. Very high agreement between the two methods was observed (95% for H7N1/chicken, 91% for H3N1/domestic duck and 100% for H7N7/Canada goose). Scatterplots illustrate that the non‐correlated results between the two NP ELISAs were not far from the threshold value for both tests, with only a few strong outliers (Fig. 1).
Figure 1.
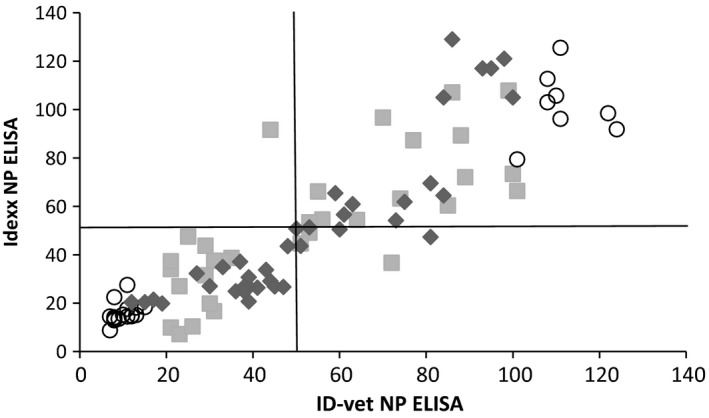
Comparison of sample‐to‐negative ratios between IDVet and Idexx NP ELISAs. Sample‐to‐negative ratios are expressed as percentages. Symbols represent the results of sera from ducks inoculated with H7N1/chicken virus (diamonds), H3N1/domestic duck virus (squares) or H7N7/Canada goose virus (circles). Threshold values, equal to 50% for both NP ELISAs, are indicated by solid lines.
Kinetics of the anti‐HA and anti‐NP responses after LPAI experimental infections of Pekin ducks
Inoculation of ducks with H7N1/chicken virus
The kinetics of anti‐NP antibodies detected by the two ELISAs were similar during follow‐up of the infection period (28 days). Anti‐NP antibodies peaked at 9 dpi, and then gradually decreased up to 28 dpi (Fig. 2a,b). Conversely, anti‐HA antibodies detected by the HI test remained steady and stable from 10 to 28 dpi (Fig. 2c). Altogether, the HI titres were relatively low, averaging 5.1 (±1.1) at 21 dpi.
Figure 2.
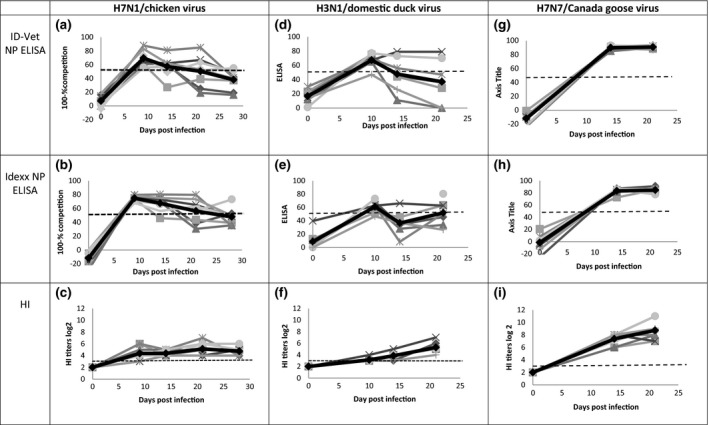
Serological results of experimentally inoculated Pekin ducks. Kinetics of antibodies detected by IDVet NP ELISA, Idexx NP ELISA and HI test were determined for Pekin ducks inoculated with the H7N1/chicken virus (a, b, c), with the H3N1/domestic duck virus (d, e, f) or with the H7N7/Canada goose virus (g, h, i). Each symbol represents one specific animal. The black, bold line represents the average value for each test.
When the results of the different tests were quantified individually, the most divergent results were observed at 28 dpi, when only 12.5% and 25% of sera tested positive by the IDVet and the Idexx NP ELISA, respectively, whereas 100% of the sera tested positive by HI. This finding accounts for the low agreement between the HI test and IDVet NP ELISA (65%) and Idexx NP ELISA (70%) (Table 2).
Inoculation of ducks with H3N1/domestic duck virus
For ducks inoculated with the H3N1/domestic duck LPAI virus, the average results for each ELISA indicated that anti‐NP antibodies peaked at 10 dpi and then decreased until 21 dpi (Fig. 2d,e). A slight difference was observed between the two NP ELISAs at 21 dpi. Mean sample‐to‐negative ratios obtained with the Idexx NP ELISA tended to increase from 14 to 21 dpi, whereas those of the IDVet NP ELISA continued to decrease. When tested by HI using the homologous antigen, the observed mean serological response was delayed compared to the NP‐specific response, which peaked at 21 instead of 10 dpi (Fig. 2f). Overall, the HI titres were relatively low (5.3 ± 0.88) at 21 dpi.
As a consequence of these different anti‐NP and anti‐HA kinetics, the most divergent results appeared at 10 and 21 dpi. At 10 dpi, 100% of the tested sera were positive with both NP ELISAs, but only 14% were positive with HI. However, at 21 dpi, 29% and 57% of sera tested positive with the IDVet and the Idexx NP ELISA, respectively, whereas 100% tested positive with HI. Owing to these divergent results, there was only a 62% agreement between the IDVet NP ELISA and HI test and a 64% agreement between the Idexx NP ELISA and HI test (Table 2).
After a second homologous infection of the ducks 21 dpi, the mean level of anti‐NP increased markedly, with almost all sera testing positive and with comparable sample‐to‐negative ratios by 35 dpi (14 day after the second infection) regardless of the NP ELISA used. Conversely, the average anti‐HA titre at 35 dpi (6 ± 1.6) remained similar to that at 21 dpi (5.3 ± 0.88). In contrast to the results of both ELISA assays, HI titres were still variable when serum samples were considered individually.
Inoculation of ducks with H7N7/Canada goose virus
After H7N7/Canada goose viral inoculation, the kinetics of NP and HA antibodies were very similar during the 21‐day infection period (Fig. 2g–i). Anti‐NP and anti‐HA titres were high at 14 dpi and remained constant until 21 dpi, with a mean HI titre of 8.8 (±1.6). When analysed individually, no divergence between the serological tests was observed. All sera tested positive by the three assays as early as 14 dpi; therefore, the agreement between all tests was 100% (Table 2).
Viral shedding after LPAI experimental infections of Pekin ducks
All inoculated ducks from the three different experimental infections excreted virus at least once by the buccal or cloacal route (Fig. 3). Ducks inoculated with the H3N1/domestic duck virus shed for a shorter time. Excretion occurred primarily 6 dpi, and a high amount of M RNA was detected by the cloacal route (mean 2 × 108 M viral RNA copy number per mL). Only one duck out of eight excreted by the tracheal route, at 3 and 10 dpi (Fig. 3c–d). Ducks inoculated with the H7N1/chicken or H7N7/Canada goose virus had similar excretion patterns lasting up to 10 dpi. However, cloacal shedding demonstrated that excretion peaked at 10 dpi for the H7N1/chicken virus (Fig. 3a–b), but at 3 dpi for the H7N7/Canada goose virus (Fig. 3e–f). None of the ducks showed clinical signs or symptoms during the entire infection period.
Figure 3.
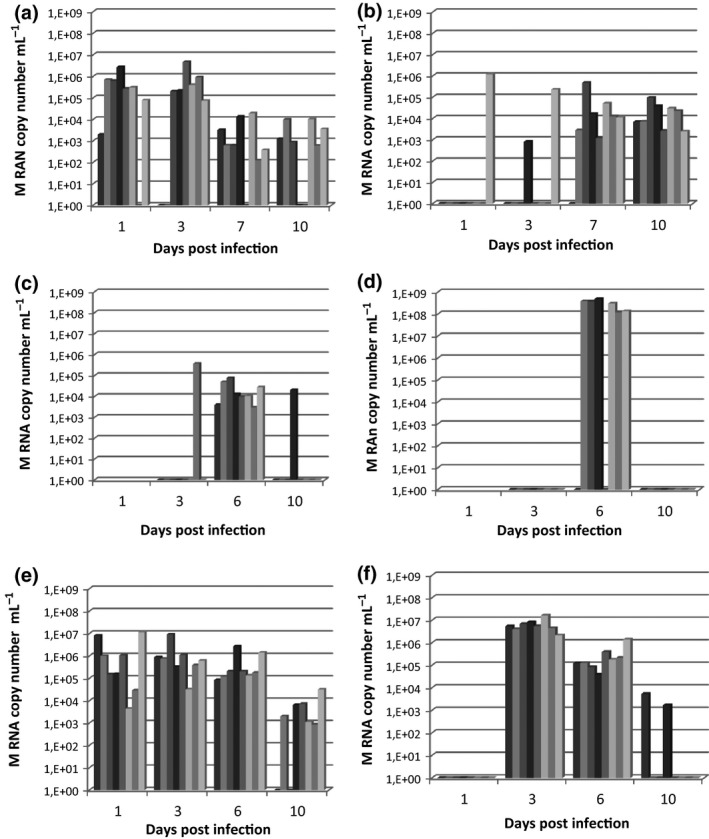
Viral excretion of experimentally inoculated ducks. The M viral RNA was detected by RRT‐PCR. Results are expressed as the M RNA copy number per ml. Vertical bars represent the results of individual inoculated Pekin ducks. Graphs represent tracheal or cloacal excretion of virus by ducks inoculated with H7N1/chicken virus (a, b), H3N1/domestic duck virus (c, d) or H7N7/Canada goose virus (e, f). No swabs (tracheal or cloacal) were collected at 1 dpi for H3N1/domestic duck virus‐inoculated ducks.
Discussion
Indirect or competition/blocking NP ELISAs are useful tools for assessing the serological response against AI viruses and are frequently replacing the HI test for research or diagnostic purposes. However, few reports are available regarding domestic duck sera (Spackman et al. 2009), despite the primary use of competition/blocking NP ELISAs in estimating seroprevalence in wild birds (Brown et al. 2009, 2010; Fereidouni et al. 2010; Jourdain et al. 2010; Perez‐Ramirez et al. 2010; Claes et al. 2012).
In this study, we carried out a comparative evaluation of commercial NP ELISAs with HI tests using sera from field and experimentally infected ducks. The H3N1/domestic duck virus is an example of an LPAI virus isolated from a domestic duck during the annual sero‐surveillance in Belgium. Previous experimental infections of SPF chickens showed that this viral isolate replicates poorly in this host (Marche et al. 2010). The H7N7/Canada goose virus, isolated from a wild Canada goose in Belgium, demonstrated low infectivity of SPF chickens and a variable ability to replicate in this host (Marche et al. 2012). These strains are also good representatives of LPAI subtypes isolated from wild bird as H3 and H7 viruses are among the most represented in wild birds (Fouchier & Munster 2009) or mallards in Europe (Munster et al. 2007). The third strain included in our study was the H7N1/chicken. This chicken‐adapted strain was isolated in Italy during the 1999–2000 outbreak. This strain readily replicates in SPF chickens (Marche et al. 2010).
No correlation between the NP ELISA and HI test results was observed with duck sera from commercial holdings or from experimental infections. Sera testing positive with both commercial ELISAs were observed in commercial holdings and early experimental infections (≤10 dpi) with the H7N1/chicken and H3N1/domestic duck viruses. This discrepancy was previously observed by others for chicken and duck sera tested by commercialised NP ELISAs, and was attributed to the higher sensitivity of ELISAs compared to HI tests (Starick et al. 2006; Perez‐Ramirez et al. 2010). Although this possibility might be true for chicken sera, as the IDVet NP ELISA has a higher detection limit than the HI test for these sera, duck sera may be an exception as the detection limits of these tests are quite similar for this species (S. Marché, personal communication). This divergence could be linked to differences in the kinetics of anti‐NP and anti‐HA antibodies and may indicate a recent infection. Sera of holdings 2, 5 and 6 may reflect this point, as it is possible that the LPAI virus was recently introduced into these flocks.
Sera testing positive solely with the HI test were observed late in duck experimental infections (around 14–21 dpi) with the H7N1/chicken and H3N1/domestic duck viruses. This observation might be the consequence of difference in the kinetics of anti‐NP and anti‐HA antibodies. Few studies have described the kinetics of anti‐NP antibody responses in infected ducks (Spackman et al. 2009; Fereidouni et al. 2010; Globig et al. 2013; Tolf et al. 2013). However, the kinetics of anti‐NP antibodies, decreasing at 21 dpi, were previously described after primary infection of naïve ducks (Spackman et al. 2009). Failed detection of anti‐NP antibodies in HI‐positive sera was reported in different studies, such as for sera collected at 21 dpi in domestic ducks inoculated with LPAI H5N2 and tested by Idexx NP ELISA (Spackman et al. 2009), for sera sampled from ducks and geese from a surveillance programme in Germany and tested by in‐house NP ELISA (Starick et al. 2006), or for sera sampled from sentinel ducks in Spain tested by the IDVet NP ELISA (Perez‐Ramirez et al. 2010). Therefore, most sera testing positive exclusively with the HI test could indicate an older infection, such as observed in holding 7, and thus, a lower probability of isolating the virus in the flock where this type of profile is observed.
The transient NP antibody production observed for some ducks after experimental infections with the H3N1/domestic duck and H7N1/chicken viruses may reflect the induction of an IgM response instead of an IgM response followed by an IgG response. Indeed, ducks have a weak humoral response to LPAI viral infection (Kida et al. 1980), characterised by the induction of local immunity only and short‐lived memory (Magor 2011). Moreover, MHC class II is down‐regulated during LPAI infections of ducks, suggesting a lower antibody response than in chickens (Adams et al. 2009). This down‐regulation could enhance the difference between the induction of immune responses against these two viral antigens, because NP is a major target of the host cytotoxic T‐cell immune response, whereas HA is considered to be an efficient B‐cell inducer (Webster et al. 1992). Interestingly, re‐infection of ducks inoculated with the H3N1/domestic duck virus led to a stronger anti‐NP humoral response, potentially indicative of an IgG response, as well as a positive correlation between the results of the NP ELISAs and HI test. A very high correlation between the IDVet ELISA and HI results had similarly been observed with field duck sera (Marche et al. 2013). Further analyses of the immune response of ducks infected with LPAI viruses are needed to support this hypothesis.
In contrast, transient anti‐NP responses were not observed during experimental infection with the H7N7/Canada goose virus, indicating that duck humoral responses might be strong depending on the LPAI virus. After this infection, anti‐NP and anti‐HA responses were high, with a close correlation between the two commercial NP ELISAs and HI test. Moreover, individual variability in humoral responses was not observed as in the experimental infections with H3N1/domestic duck and H7N1/chicken viruses (data not shown) or in another study with sentinel ducks (Tolf et al. 2013). This difference in the humoral response in ducks inoculated with the H7N7/Canada goose virus may be attributed to differences between the replication profiles of the distinct viruses examined in this study. Indeed, more ducks were excreting virus after infection (1–10 dpi) with the H7N7/Canada goose virus with slightly higher titres of virus than ducks inoculated with the two other viruses.
In conclusion, this study showed that the serological responses against NP and HA proteins were variable in the same bird when the immune response was low. The intensity of the immune response might depend on the LPAI virus and its replicative properties, in addition to other factors such as age, genetic variability, environmental factors or diet, as described previously (Fereidouni et al. 2010; van Gils et al. 2007). Owing to these factors, divergence between the results of the two diagnostic methods, NP ELISAs and the HI test, may occasionally be observed. However, because this diagnosis is based on an entire flock rather than a single individual, NP ELISA can still be used as a pre‐screening tool to detect circulation of LPAI viruses in domestic duck populations. However, the use of both NP ELISAs and the HI test is preferable for evaluating the anti‐AI immune status if individual ducks, and for potentially identifying recent infections.
Source of funding
This study was funded by the Federal Agency for the Safety of the Food Chain.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
Sylvie marché = study conception, design, acquisition and analysis of data, drafting of manuscript Thierry van den berg = critical revision Bénédicte Lambrecht = strategic and scientific discusion and critical revision
Acknowledgements
We thank A. Nadi for his excellent technical assistance, and C. Delgrange and M. Vandenbroeck for their excellent animal assistance.
References
- Adams S.C., Xing Z., Li J. & Cardona C.J. (2009) Immune‐related gene expression in response to H11N9 low pathogenic avian influenza virus infection in chicken and Pekin duck peripheral blood mononuclear cells. Molecular Immunology 46, 1744–1749. [DOI] [PubMed] [Google Scholar]
- Alexander D.J. & Brown I.H. (2009) History of highly pathogenic avian influenza. Revue Scientifique et Technique 28, 19–38. [DOI] [PubMed] [Google Scholar]
- Bean W.J., Kawaoka Y., Wood J.M., Pearson J.E. & Webster R.G. (1985) Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. Journal of Virology 54, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes V.A., Ritchie S.J., Byrne S., Sojonky K., Bidulka J.J. & Robinson J.H. (2004) Virus characterization, clinical presentation, and pathology associated with H7N3 avian influenza in British Columbia broiler breeder chickens in 2004. Avian Diseases 48, 928–934. [DOI] [PubMed] [Google Scholar]
- Brown J.D., Stallknecht D.E., Berghaus R.D., Luttrell M.P., Velek K., Kistler W. et al (2009) Evaluation of a commercial blocking enzyme‐linked immunosorbent assay to detect avian influenza virus antibodies in multiple experimentally infected avian species. Clinical and Vaccine Immunology 16, 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.D., Luttrell M.P., Berghaus R.D., Kistler W., Keeler S.P., Howey A. et al (2010) Prevalence of antibodies to type a influenza virus in wild avian species using two serologic assays. Journal of Wildlife Diseases 46, 896–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua I., Mutinelli F., Marangon S. & Alexander D.J. (2000) H7N1 avian influenza in Italy (1999 to 2000) in intensively reared chickens and turkeys. Avian Pathology 29, 537–543. [DOI] [PubMed] [Google Scholar]
- Claes G., Vangeluwe D., Van der Stede Y., van den Berg T., Lambrecht B. & Marche S. (2012) Evaluation of four enzyme‐linked immunosorbent assays for the serologic survey of avian influenza in wild bird species. Avian Diseases 56, 949–954. [DOI] [PubMed] [Google Scholar]
- Elbers A.R., Fabri T.H., de Vries T.S., de Wit J.J., Pijpers A. & Koch G. (2004) The highly pathogenic avian influenza A (H7N7) virus epidemic in The Netherlands in 2003–lessons learned from the first five outbreaks. Avian Diseases 48, 691–705. [DOI] [PubMed] [Google Scholar]
- Fereidouni S.R., Grund C., Hauslaigner R., Lange E., Wilking H., Harder T.C. et al (2010) Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenicity avian influenza viruses. Avian Diseases 54, 79–85. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A. & Munster V.J. (2009) Epidemiology of low pathogenic avian influenza viruses in wild birds. Revue Scientifique et Technique 28, 49–58. [DOI] [PubMed] [Google Scholar]
- Garcia M., Crawford J.M., Latimer J.W., Rivera‐Cruz E. & Perdue M.L. (1996) Heterogeneity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. Journal of General Virology 77 (Pt. 7), 1493–1504. [DOI] [PubMed] [Google Scholar]
- van Gils J.A., Munster V.J., Radersma R., Liefhebber D., Fouchier R.A. & Klaassen M. (2007) Hampered foraging and migratory performance in swans infected with low‐pathogenic avian influenza A virus. PLoS ONE 2, e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globig A., Fereidouni S.R., Harder T.C., Grund C., Beer M., Mettenleiter T.C. & Starick E. (2013) Consecutive natural influenza a virus infections in sentinel mallards in the evident absence of subtype‐specific hemagglutination inhibiting antibodies. Transboundary and Emerging Diseases 60, 395–402. [DOI] [PubMed] [Google Scholar]
- Jourdain E., Gunnarsson G., Wahlgren J., Latorre‐Margalef N., Brojer C., Sahlin S. et al (2010) Influenza virus in a natural host, the mallard: experimental infection data. PLoS ONE 5, e8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida H., Yanagawa R. & Matsuoka Y. (1980) Duck influenza lacking evidence of disease signs and immune response. Infection and Immunity 30, 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. & Elbers A.R.W. (2006) Outdoor ranging of poultry: a major risk factor for the introduction and development of High‐Pathogenicity Avian Influenza. NJAS ‐ Wageningen Journal of Life Sciences 54, 179–194. [Google Scholar]
- Lee C.W. & Saif Y.M. (2009) Avian influenza virus. Comparative Immunology, Microbiology and Infectious Diseases 32, 301–310. [DOI] [PubMed] [Google Scholar]
- Magor K.E. (2011) Immunoglobulin genetics and antibody responses to influenza in ducks. Developmental and Comparative Immunology 35, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Marche S. & van den Berg T. (2010) Evaluation of different strategies for the use of ELISA tests as first screening tools for serologic surveillance of low pathogenic avian influenza in the Belgian poultry sector. Avian Diseases 54, 627–631. [DOI] [PubMed] [Google Scholar]
- Marche S., Lambrecht B. & van den Berg T. (2010) Evaluation of different serologic markers for the early detection of avian influenza infection in chickens. Avian Diseases 54, 690–698. [DOI] [PubMed] [Google Scholar]
- Marche S., Claes G., Van Borm S., Vangeluwe D., van den Berg T. & Lambrecht B. (2012) Different replication profiles in specific‐pathogen‐free chickens of two H7 low pathogenic avian influenza viruses isolated from wild birds. Avian Diseases 56, 959–965. [DOI] [PubMed] [Google Scholar]
- Marche S., Van Borm S., Lambrecht B., Houdart P. & van den Berg T. (2013) Chasing notifiable avian influenza in domestic poultry: a case report of low‐pathogenic avian influenza H5 viruses in two Belgian holdings. Transboundary and Emerging Diseases 6, 526–536. [DOI] [PubMed] [Google Scholar]
- Munster V.J., Baas C., Lexmond P., Waldenstrom J., Wallensten A., Fransson T. et al (2007) Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathogens 3, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health) (2015) Terrestrial Manual, Avian Influenza. (version adopted in May 2009). Available at: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.04_AI.pdf (Accessed 8 January 2016).
- Perez‐Ramirez E., Rodriguez V., Sommer D., Blanco J.M., Acevedo P., Heffels‐Redmann U. & Hofle U. (2010) Serologic testing for avian influenza viruses in wild birds: comparison of two commercial competition enzyme‐linked immunosorbent assays. Avian Diseases 54, 729–733. [DOI] [PubMed] [Google Scholar]
- Rojas H., Moreira R., Avalos P., Capua I. & Marangon S. (2002) Avian influenza in poultry in Chile. The Veterinary Record 151, 188. [PubMed] [Google Scholar]
- Snyder D.B., Marquardt W.W., Yancey F.S. & Savage P.K. (1985) An enzyme‐linked immunosorbent assay for the detection of antibody against avian influenza virus. Avian Diseases 29, 136–144. [PubMed] [Google Scholar]
- Spackman E., Pantin‐Jackwood M.J., Swayne D.E. & Suarez D.L. (2009) An evaluation of avian influenza diagnostic methods with domestic duck specimens. Avian Diseases 53, 276–280. [DOI] [PubMed] [Google Scholar]
- Starick E., Werner O., Schirrmeier H., Kollner B., Riebe R. & Mundt E. (2006) Establishment of a competitive ELISA (cELISA) system for the detection of influenza A virus nucleoprotein antibodies and its application to field sera from different species. Journal of Veterinary Medicine B, Infectious Diseases and Veterinary Public Health 53, 370–375. [DOI] [PubMed] [Google Scholar]
- Steensels M., Van Borm S., Lambrecht B., De Vriese J., Le Gros F.X., Bublot M. & van den Berg T. (2007) Efficacy of an inactivated and a fowlpox‐vectored vaccine in Muscovy ducks against an Asian H5N1 highly pathogenic avian influenza viral challenge. Avian Diseases 51, 325–331. [DOI] [PubMed] [Google Scholar]
- Tolf C., Latorre‐Margalef N., Wille M., Bengtsson D., Gunnarsson G., Grosbois V. et al (2013) Individual variation in influenza A virus infection histories and long‐term immune responses in Mallards. PLoS ONE 8, e61201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Borm S., Steensels M., Ferreira H.L., Boschmans M., De Vriese J., Lambrecht B. & van den Berg T. (2007) A universal avian endogenous real‐time reverse transcriptase‐polymerase chain reaction control and its application to avian influenza diagnosis and quantification. Avian Diseases 51, 213–220. [DOI] [PubMed] [Google Scholar]
- Webster R.G., Bean W.J., Gorman O.T., Chambers T.M. & Kawaoka Y. (1992) Evolution and ecology of influenza A viruses. Microbiological Reviews 56, 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welby S., van den Berg T., Marche S., Houdart P., Hooyberghs J. & Mintiens K. (2010) Redesigning the serological surveillance program for notifiable avian influenza in Belgian professional poultry holdings. Avian Diseases 54, 597–605. [DOI] [PubMed] [Google Scholar]


