Abstract
Spontaneous canine malignant melanoma provides an excellent pre‐clinical model to study DNA vaccines for melanoma immunotherapy. A USDA‐approved xenogeneic human tyrosinase (huTYR) plasmid DNA vaccine delivered intramuscularly induces detectable immune responses and has clinical activity in some dogs with melanoma. The objective of this pilot study was to evaluate the feasibility, safety and immunogenicity of huTYR plasmid DNA administered to the skin via microseeding in dogs with spontaneous melanoma. DNA microseeding utilizes a modified tattooing device as an alternate and potentially more potent delivery method for DNA immunization. DNA was delivered to shaved inner thigh skin of six companion dogs with melanoma approximately every 14 days for a planned total of four vaccination time points. An anti‐huTYR ELISA was used to test pre‐ and post‐treatment sera. Biopsies of treated skin were obtained for detection of huTYR transgene expression. DNA microseeding was well tolerated with no significant toxicity detected beyond local site irritation, and there were no signs of autoimmunity. huTYR‐expressing cells were observed in biopsies of huTYR DNA microseeding sites. Increased humoral anti‐huTYR antibodies were seen in two of five evaluable dogs following microseeding compared to baseline. DNA microseeding is well tolerated in companion dogs with melanoma. Further investigation is needed to determine if combining DNA microseeding with other immunotherapy regimens potentiates this delivery platform for cancer immunotherapy.
Keywords: canine melanoma, comparative oncology, DNA vaccine, microseeding, tattooing
Introduction
Malignant melanoma is among the most common canine oral malignancy and is associated with high rates of metastasis occurring via lymphatics or blood vessels to regional lymph nodes, lungs, liver, brain and kidney (Paoloni & Khanna 2008; Gordon & Khanna 2010; Vail & Thamm 2011). Oral malignant melanoma in dogs has similar biologic behaviour to that of cutaneous melanoma in people and most closely resembles the acral lentiginous form (Rogers & Gibson 1997; Piliang 2011). Our laboratory and others have detected the expression of canine analogs of the human melanoma‐associated antigens (MAAs) gp100, MART‐1, and tyrosinase in canine melanomas (Alexander et al. 2006; Ramos‐Vara & Miller 2011; Phillips et al. 2012), affording shared targets for the development of immunotherapeutic approaches for both human and canine melanoma. Thus, strategies involving DNA vaccines to activate an immune response to a MAA have potential for significant clinical impact in dogs and humans with melanoma.
Early studies demonstrated safety and immunogenicity following intramuscular or intravenous delivery of DNA in pre‐clinical animal models (Nabel et al. 1992; Ulmer et al. 1993). However, generation of robust immune responses remains a challenge for practical implementation of DNA vaccines in large animals and humans (Wahren & Liu 2014). Although no human DNA vaccines have been approved, four DNA biologics have been licensed for animal use, including Oncept®, an immunotherapeutic canine melanoma vaccine (Wahren & Liu 2014).
Oncept®, a xenogeneic human tyrosinase (huTYR) plasmid DNA (pDNA) vaccine delivered intramuscularly, prolonged survival of dogs with melanoma compared with historical, stage‐matched controls and stimulated detectable immune responses in some dogs (Liao et al. 2006; Grosenbaugh et al. 2011). Tyrosinase, a self‐antigen, catalyses the biosynthesis of melanin and is expressed in many human and canine melanomas (Cormier et al. 1998; Ramos‐Vara & Miller 2011). Canine and human tyrosinase proteins share approximately 90% sequence identity (Bergman et al. 2003). Thus, the regions of dissimilarity and similarity between human and canine tyrosinase may be sufficient to overcome self‐tolerance through xeno‐recognition and to induce an immune response against the heterologous melanoma, respectively. The use of a cross‐species xenogeneic DNA vaccine therefore presents an opportunity to break self‐tolerance, making tyrosinase an attractive vaccine candidate for melanoma immunotherapy in both dogs and humans with melanoma.
Intradermal delivery of DNA vaccines by various technologies has been demonstrated to induce antigen‐specific immune responses in animal models of infectious disease, wound healing and cancer (Eriksson et al. 1998; Bergman et al. 2003; Bins et al. 2005; Liao et al. 2006; Goubier et al. 2008). In DNA microseeding, DNA solution is applied to the skin at a controlled rate via a pump and is intradermally deposited in micropunctures of the skin created by solid microneedles of a conventional tattoo device (Eriksson et al. 1998). A similar technique, ‘DNA tattooing’, also uses a tattoo device, but rather than a controlled delivery, administers the total DNA volume to the skin at once (Bins et al. 2005).
A potential advantage of DNA microseeding is vaccine distribution over a large surface area, which could facilitate the transfection of a broad cell population (Kis et al. 2012). Additional advantages include: (1) natural production of antigen in vivo (as opposed to delivery of purified protein/peptides); (2) no need for specific formulation of the DNA vaccine; (3) relatively simple equipment; (4) linear scale up from pre‐clinical models; (5) procedure‐related skin damage that could function as a vaccine adjuvant; and (6) demonstration of promise in small and large animal models (Eriksson et al. 1998; Oosterhuis et al. 2012). In addition, DNA microseeding can be readily translated to humans (van den Berg et al. 2009).
In this study, we evaluated microseeding of pDNA encoding huTYR into the skin of companion dogs with spontaneous melanoma as a potentially potent delivery method. The primary endpoint of this study was to assess the safety and feasibility of DNA microseeding in the dog. Secondary objectives were to demonstrate in vivo expression and immunogenicity of huTYR pDNA delivered to the skin by DNA microseeding. Although its role in tumour immunology is incompletely understood, we monitored humoral immunity as a ‘biomarker’ of efficient delivery and in vivo antigen presentation of pDNA. Moreover, in the light of conflicting data in the literature, we focused our immune monitoring on the ability of intradermal delivery of huTYR pDNA by microseeding to induce humoral responses in dogs (Liao et al. 2006; Goubier et al. 2008). The clinical outcome of dogs treated in this study was also monitored.
Materials and methods
The study was approved by the University of Wisconsin Institutional Animal Care and Use Committee and the Office of Biological Safety. Owners provided written, informed consent prior to enrolling their pet dog in the study. Treatment was under the care of the veterinary oncology service at UW Veterinary Care, University of Wisconsin School of Veterinary Medicine.
Patient selection
Client‐owned dogs with histologically confirmed malignant melanoma were eligible for treatment in this pilot study. Dermal melanomas were confirmed to be malignant by histology. Client‐owned healthy dogs without melanoma were eligible to donate blood samples for this study and did not receive treatment.
Vaccine preparation
This was a pilot study to evaluate feasibility of the novel microseeding tattoo device to deliver a plasmid encoding human tyrosinase, pING/Tyrosinase(Human), to client‐owned dogs with melanoma. This plasmid was obtained from Dr. Jedd Wolchok (Memorial Sloan‐Kettering Cancer Center) and was propagated in E. coli, and purified endotoxin‐free with Qiagen EndoFree Plasmid Giga Kit (Valencia, CA). The huTYR pDNA preparation met or surpassed all release criteria (e.g. concentration, endotoxin level, supercoil, DNA sequence and absence of E. coli genomic DNA or RNA). Vaccine aliquots were stored at −80°C until needed and concentration adjusted with sterile distilled water.
Study design
A complete physical examination, complete blood count (CBC) and serum biochemistry profile was obtained before initial anaesthesia, and thoracic radiographs were performed prior to initial treatment. A complete physical examination was performed at each study visit. Limited biochemistry profiles were obtained prior to anaesthesia for subsequent vaccine administrations. Dogs were anaesthetized before receiving huTYR pDNA scheduled to be delivered by microseeding to the inner thigh every 14 days (± 3 days) for a total of four vaccination time points. Tumour measurements were obtained from dogs with measurable disease at the time of each vaccination and through routine follow‐up exams. Thoracic radiographs were repeated following the last vaccine administration. Whole blood was scheduled to be collected at the following time points: pre‐treatment; Day 29 before the third vaccination; Day 42 before the fourth vaccination; and Day 57 (2 weeks after the fourth vaccination).
Study design and treatment protocol
Each vaccination time point included two microseeding treatments on shaved, uninvolved skin of the inner thigh of the hind legs, each using a different area (i.e. Site A and Site B). The area of treatment and needle depth was 2.25 cm2 and 1.5 mm, respectively. The volume of pDNA solution was delivered to solid microneedles (Revolution Needle Bars, 2000GG ‐ 6 Needle Flat Shader Bar, Spaulding & Rogers Mfg., Inc.) via fine tubing connected to a 3 mL syringe and 30‐gauge blunted needle assembly controlled by a Syringe Infusion Pump. Rate of delivery was adjusted via the syringe pump to deliver the required volume in 60 s.
The first four dogs enrolled were randomized to one of two dosing regimens. The DNA vaccine schedule was selected to be comparable to that of the recommendation for delivery of Oncept® (Bergman et al. 2003). The dosing regimen was selected to include the recommended dose of Oncept® (100 μg), as well as a twofold decrease (50 μg) and twofold increases (200 and 400 μg). For the first and second vaccinations of Dogs 1‐4, each treatment site received microseeding with 0.2 mL or 0.4 mL of huTYR pDNA over a period of 1 min. For the third and fourth vaccinations, the vaccine was concentrated twofold and half of the original volume (i.e. 0.1 mL or 0.2 mL) was delivered to Dogs 1, 3 and 4 maintaining the previous huTYR pDNA doses (i.e. 50, 100, 200, or 400 μg). Dog 5 received ONCEPT® at Site A and in‐house huTYR pDNA at Site B; the amount of DNA delivered (83 μg) to both sites was normalized based on ONCEPT® delivery via microseeding. Dog six received in‐house huTYR pDNA at Site A (700 μg delivered in 0.2 mL) and Aldevron gWiz™ green fluorescent protein (GFP) plasmid at Site B, delivered by microseeding for all four treatments. Doses of gWiz™ GFP plasmid for treatments 1–4 were 100 μg, 1 mg, 200 μg, or 400 μg, respectively. Vaccinated area was covered with a moist dressing (DuoDerm, ConvaTec, Inc.) for 7–10 days.
Vaccine site biopsies were performed under local anaesthetic 24 h after the first and third vaccination time points, and 48 h after the second and fourth vaccination time points. A control biopsy of unvaccinated skin was collected concurrently at 24 h after the first vaccination. Biopsies were obtained with an 8‐mm punch biopsy device.
Clinical response
Disease‐free interval, starting the day of the first vaccination, was recorded for dogs that had no evidence of melanoma at the time of initiation of vaccine treatment. Progression‐free interval, starting the day of the first vaccination, was recorded for dogs that had local or metastatic melanoma at the time of initiation of vaccine treatment. For dogs with gross disease, response was recorded according to Veterinary Cooperative Oncology Group (VCOG) criteria as follows: Complete response (CR, disappearance of all measurable lesions without the development of new lesions); Partial response (PR, >30% reduction in the diameter of the tumour without the development of new lesions); Progressive disease (PD, >20% increase in the diameter of the tumour and/or the development of new lesions); Stable disease (SD, change in tumour diameters meeting the criteria for neither PR nor PD) (Nguyen et al. 2015).
Assessment of adverse events
Presence of adverse events was based on physical examination and owner observation at each study visit. In addition, biochemistry profiles were performed prior to each vaccine treatment. Adverse events were characterized and graded according to VCOG‐Common Terminology Criteria for Adverse Events (CTCAE) version 1.1 (Veterinary cooperative oncology group, 2011).
Preparation of serum and peripheral blood mononuclear cell (PBMC)
A total of 13 mL whole blood was collected for isolation of PBMC (10 mL) and serum (3 mL). Whole blood was drawn into tubes containing EDTA and PBMC separated by density gradient centrifugation as described previously (Pellin et al. 2016). Whole blood was drawn into tubes without anticoagulant and serum separated by centrifugation following clot formation. PBMC and sera were stored at −140°C and −80°C, respectively, until needed.
Histology
Punch biopsies were bisected, half kept frozen in OCT compound for further potential studies, and half fixed in 10% buffered formalin and processed for H&E staining and immunohistochemistry studies. Transgene expression was assessed by immunohistochemistry using a mouse anti‐human tyrosinase antibody (clone 3C11, NeoMarkers Inc., Freemont, CA) performed on a Ventana staining device. Human skin and pre‐treatment biopsies from each dog's untreated skin served as positive and negative experimental controls, respectively. Mouse isotype control was used as a negative staining control.
Anti‐huTYR ELISA
Anti‐huTYR antibody responses were measured at baseline and after vaccination by ELISA. Briefly, 96‐well Nunc Polysorp plates were coated with recombinant huTYR protein (1 μg mL−1, 50 μL per well) (Enzo Life Sciences, Farmingdale, NY) in Antigen Coating Buffer (ImmunoChemistry Technologies (ICT), Bloomington, MN), or with Neptune Block (ICT) overnight at room temperature (RT). Plates were washed three times with 1× PBS containing 0.05% Tween‐20 (PBST), and blocked with 300 μL per well Neptune Block for 1 h at RT. Neptune Block was removed, sera diluted in Neptune Sample Diluent (ICT) added in triplicate (50 μL per well) and plates incubated for 1 h at RT. Following washing 3× with PBST, horseradish peroxidase (HRP)‐labelled Protein A/G (Thermo Scientific, Rockford, IL) was added (50 μL per well) for 1 h at RT. Plates were washed 3× with PBST and 3,3′,5,5′‐ tetramethylbenzidine (TMB) 1‐Step™ Ultra (Thermo Scientific) (50 μL per well) added to detect HRP activity. TMB Stop (KPL, Gaithersburg, IL) (50 μL per well) was added and absorbance measured at 450 nm on a SpectraMax M3 plate reader running SoftMax Pro 6.2.2 software (Molecular Devices, Sunnyvale, CA). Clone T311, a mouse monoclonal antibody against huTYR (Thermo Scientific), was used as a positive control. Serum from a healthy control dog was used as negative control.
Statistical methods
Freely available R program was used to initially randomize the dogs to receive DNA vaccinations at one of two pDNA concentrations. In similar pilot studies, we have found that this number (n = 6) is sufficient to determine intervention feasibility. The data collected were descriptive and formal statistical analysis was not performed on progression‐free or overall‐survival. Histology, immune monitoring and clinical response analyses were exploratory. Anti‐huTYR humoral response pre‐treatment versus post‐treatment in a single dog was compared using the non‐parametric Mann–Whitney test.
Results
Patient population and vaccine dosing
A total of six dogs were enrolled between February 2015 and April 2016, and all six dogs were evaluable for antitumor response (Table 1). This study represents the first time a DNA vaccine has been delivered to dogs by microseeding. Therefore, we wanted to study the delivery parameters of DNA concentration and volume. The intent was for each dog to receive four immunizations; however, due to disease progression, one dog (Dog 2) received only the initial treatment and a second dog (Dog 1) received three treatments. Treatment was then discontinued upon the owner's request. Four of the six dogs received all four planned immunizations. One of those four dogs (Dog 6) also received a second vaccine component, GFP, in addition to huTYR. The remaining five dogs were immunized with huTYR alone.
Table 1.
Vaccine dosing details and clinical summary
| Dog # | Baseline Disease Status | # of Tx | Vaccine Site | pDNA Dose (μg) | Day 57 Disease Status* | PFI (days from 1st Tx.) | Survival (days from 1st Tx.)† |
|---|---|---|---|---|---|---|---|
| 1 | Stage IV; Oral + lung mets‡ | 3 | A | 50 | PD | 0 | 101 |
| B | 100 | ||||||
| 2 | Stage IV; Oral/skin/lung mets§ | 1 | A | 200 | PD | 0 | 14 |
| B | 400 | ||||||
| 3 | Stage II; Oral¶ | 4 | A | 200 | NED | 412 | 412+ |
| B | 400 | ||||||
| 4 | Stage III; Oral** | 4 | A | 50 | PD | 57 | 367 |
| B | 100 | ||||||
| 5 | Stage I; Dermal†† | 4 | A | 83 (ONCEPT®)‡‡ | NED | 433+ | 433+ |
| B | 83 (in‐house) | ||||||
| 6 | Stage II; Dermal§§ | 4 | A | 700 | NED | 238+ | 238+ |
| B | GFP¶¶ |
Tx, treatment; PFI, progression‐free interval; mets, metastasis; PD, progressive disease; NED, no evidence of disease; GFP, green fluorescent protein. *Day 57 is the final scheduled study time point, 2 weeks after the fourth microseeding treatment. †Bolded and italicized values indicate dog was alive as of last update. ‡3 cm oral mass resected with incomplete surgical margins. §3 cm mandibular lymph node, 2–14 mm skin and lung metastases. ¶3.1 cm oral mass resected with incomplete surgical margins. **Oral primary melanoma measured 9.1 × 6.1 × 3.8 cm. Primary tumour irradiated weekly, for 4 weeks, final radiation Tx delivered concurrently with microseeding Tx. ††5 mm dermal melanoma resected with narrow surgical margins. ‡‡Commercial ONCEPT® pDNA delivered by microseeding to Site A. §§3.3 cm dermal melanoma resected with complete surgical margins. ¶¶Aldevron gWiz™ pDNA 100μg, 1 mg, 200 μg and 400 μg was delivered to Site B on the first, second, third, and fourth microseeding treatments, respectively.
Two dogs required modifications of the planned vaccine dosing and immune monitoring. Dog 1 received only three treatments with a 2‐week delay between the second and third treatments; blood was drawn at the time of the third vaccination. Dog 2 did not have follow‐up immune or biopsy monitoring as only the initial microseeding vaccination was given due to disease progression and subsequent euthanasia of the dog. The remaining four dogs completed the study as planned (Fig. 1), and a total of five dogs were evaluable for immune and biopsy monitoring pre‐ and post‐microseeding (Table 1). The three dogs with known disease at study entry received 3, 1 and 4 treatments before discontinuation of treatment due to progressive disease. The three dogs without evidence of disease at study entry received all four planned treatments and remained without evidence of recurrence for the duration of the study (6 weeks).
Figure 1.
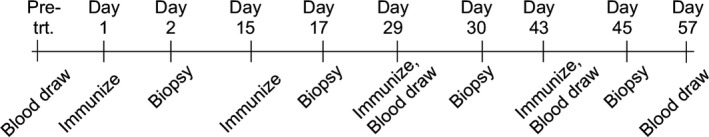
Time points (± 3 days) for blood samples, DNA microseeding vaccinations and vaccine site biopsies were at pre‐treatment (Pre‐trt.) or at specified times.
Safety evaluation
No toxicity, beyond local site irritation related to the vaccine administration, was observed in any of the dogs in this study. The local skin irritation was observed within 24 h of vaccine administration and presented as an inflamed local skin reaction at the site of vaccine administration. When dogs returned 2 weeks following each vaccine treatment, the inflammation was completely resolved. There were no clinical signs of autoimmunity in any of the dogs in this study.
Clinical response
Three of six dogs were clinically free of disease at the start of the study. Dog 3 (diagnosed with oral melanoma) developed a local recurrence at 412 days. Dogs 5 and 6 (both diagnosed with dermal melanoma) remained free of disease at 433 and 238 days, respectively. The remaining three dogs had gross disease diagnosed as oral melanoma at the start of the study. Dogs 1 and 2 were euthanized due to progressive disease at day 101 and 14, respectively. Dog 4 had stable disease for 57 days at which time the dog developed a lymph node metastasis and was euthanized on day 367 for progression of pulmonary metastasis.
Transgene expression
Increasing dose of huTYR was not associated with increased transgene expression in the sections examined. However, rare huTYR+ cells with macrophage‐like morphology were observed in some vaccine site biopsies; e.g. Dog 3 had a single cell positive for huTYR expression in the 24 h post‐treatment #3 Site A (200 μg dose) biopsy. Of note, the expression was observed in a cell with fibroblast‐ or dendritic cell‐like morphology (Fig. 2). In all biopsies, when found, huTYR+ cells were located in the upper dermis. Cells with similar dendrite extensions, but negative for huTYR, were found in both control (pre‐treatment) and vaccinated biopsies (data not shown). Microseeding‐induced physical damage was not visible in the sections. There was minimal to no lymphoid inflammation. However, in some biopsies, there was superficial ulceration of the epidermis with associated acute, neutrophil‐rich inflammatory infiltrate (data not shown). There was no significant GFP expression in Dog 6.
Figure 2.
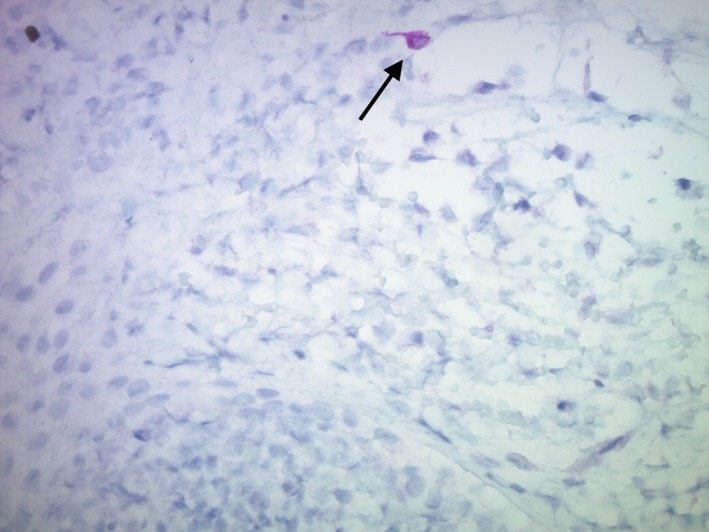
huTYR + expression noted in a dendritic cell‐like or fibroblast‐like cell (black arrow) after three DNA microseeding doses. Photomicrograph from Site A (200 μg dose) of Dog 3, 24 h post third vaccine (40× mag.).
Humoral response
Humoral response to huTYR was measured using indirect ELISA for the five dogs receiving at least three DNA microseeding treatments. Dog 2 was euthanized due to progressive disease and did not receive the second treatment; therefore, only pre‐treatment serum was available and was not analysed. Increased anti‐huTYR antibody levels post‐treatment compared to pre‐treatment was noted for Dogs 3 and 4 (Fig. 3a–b). Sera from a single healthy control dog demonstrated anti‐huTYR responses that were either lower or comparable to responses in vaccinated Dog 3 or Dog 4, respectively (data not shown). A confirmatory anti‐huTYR ELISA for Dog 3 detected a significantly increased anti‐huTYR response at Day 43 (2 weeks after the third vaccine) and at Day 57 (2 weeks after the fourth administration) compared to pre‐treatment (P = 0.03) (data not shown). The humoral response to an irrelevant non‐mammalian protein was negligible over the course of the study in Dogs 3 and 4, as well as the control dog (data not shown). Preliminary results from the other treated dogs (1, 4 and 6) were negative for anti‐huTYR responses (data not shown).
Figure 3.
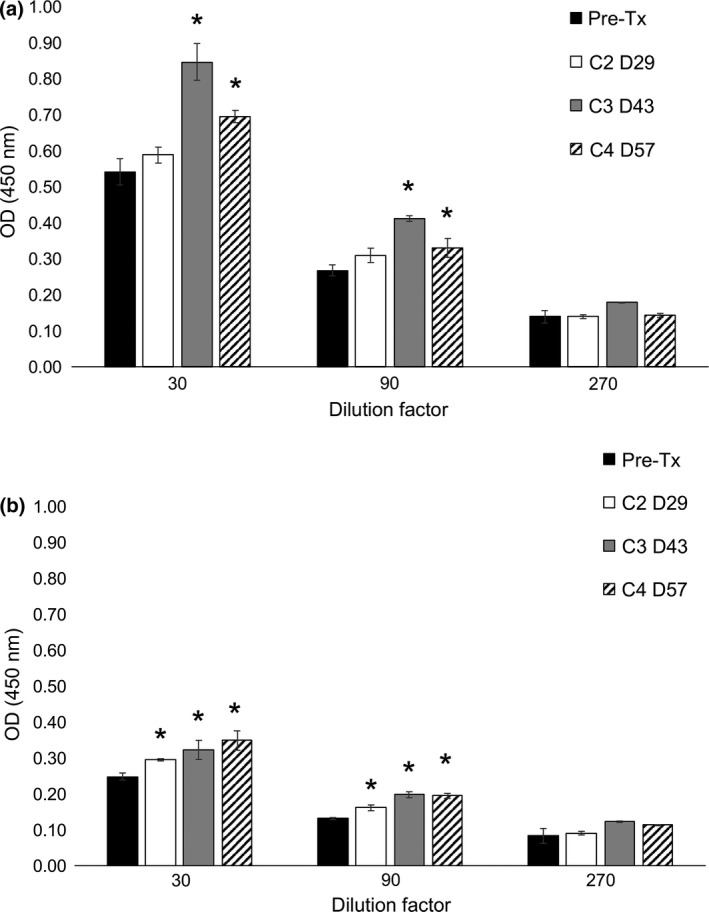
Increasing anti‐huTYR humoral response at Day 43 and Day 57 in Dog 3 (a) and Dog 4 (b) after microseeding with huTYR pDNA. Sera were serially diluted threefold and analysed in triplicate via indirect ELISA for anti‐huTYR antibodies. Mean of triplicate wells ± standard deviation (SD). * Post‐treatment mean greater than pre‐treatment + 3SD. Pre‐Tx, pre‐treatment; C2 D29, course 2, Day 29; C3 D43, course 3, Day 43; C4 D57, course 4, Day 57.
Discussion
Numerous gene therapy clinical trials for cancer have been conducted in companion animals (Glikin & Finocchiaro 2014). Similar to the current report, much of the previous work focuses on spontaneous tumours in client‐owned pets. In this pilot study, we report for the first time (to the best of our knowledge) delivery of a DNA vaccine to dogs by microseeding. The lack of significant adverse events during the course of study indicates the safety of microseeding administration in the canine. The change in administration route of huTYR pDNA did not alter the previously established safety profile of this vaccine (Bergman et al. 2003; Ottnod et al. 2013). DNA microseeding is a relatively straightforward process with simple equipment, facile administration and low vaccine production cost. The tattoo site dimensions can be scaled to target more antigen presenting cells. Moreover, the resultant skin injury and inflammation can function to provide collateral immune response stimulation. The spontaneous nature of canine melanoma, its resistance to chemotherapy and radiotherapy (Grosenbaugh et al. 2011), the histologic and immunologic characteristics of dog skin diseases (Hill & Olivry 2001; Nuttall et al. 2002; Marsella & Olivry 2003; Tobin et al. 2003; Olivry et al. 2006) and the outbred genetically diverse background of the dog parallel the human situation, thus providing a suitable translational model for human melanoma.
Dog skin is physiologically different from that of other mammalian species previously treated via DNA microseeding or tattooing (Eriksson et al. 1998; Bins et al. 2005; Verstrepen et al. 2008). We, therefore, assessed feasibility and delivery parameters for DNA microseeding in the dog. Our results document the induction of antibody responses against the xenogeneic target (human tyrosinase) in two of five dogs with malignant melanoma vaccinated via microseeding. This is similar to the response rate observed when huTYR pDNA was delivered intramuscularly (Liao et al. 2006). The two dogs in this report with increased anti‐huTYR antibodies were clinically Stage II and III (Dog 3 and Dog 4, respectively). At the start of treatment, Dog 3 had local tumour control, while Dog 4 had an oral melanoma but no lung or lymph node involvement. Studies leading to the USDA approval of ONCEPT® had a substantially longer follow‐up with the greatest level of anti‐tyrosinase IgG detected 9–12 months after the last scheduled vaccination (Liao et al. 2006). An extended follow‐up of the humoral response in this study's dogs would be of great interest. Unfortunately, extended follow‐up blood samples were not available from these client‐owned dogs, and some dogs had been euthanized due to disease progression.
We anticipated that DNA microseeding would result in high levels of huTYR transgene expression. Since high huTYR transgene expression was not seen in the first five dogs in this study, our plan with Dog 6 was to determine whether a well‐characterized commercial plasmid (gWiz™ GFP) would result in robust transgene expression following delivery by microseeding. As there was no significant GFP expression detected in Dog 6 following pDNA delivery by microseeding, we conclude that pDNA delivery with the microseeding device requires additional optimization. Importantly, detection of rare huTYR+ cells indicates that transgene uptake and expression is possible with this system. It should also be noted that stains were performed on a single 5–7 μm section of a large (2.25 cm2) injected area. Therefore, if extrapolated, this suggests many more huTYR positive cells across the whole injected area.
The ability of intradermal vaccination with huTYR pDNA to generate cellular responses has been demonstrated in healthy animals of the same age and breed (Goubier et al. 2008). In contrast, the dogs in our study had spontaneous malignant melanoma and were of various age, gender, breed and stage of disease, thus more faithfully recapitulating the human condition. Although not formally investigated, our data and those of others, suggest a lack of correlation between pDNA dose and body weight or size (Lembcke et al.; Bergman et al. 2003; Liao et al. 2006).
We have recently obtained several canine melanoma cell lines from collaborators (Helfand et al. 1994, 1996; Alexander et al. 2006). In the absence of available recombinant canine tyrosinase, these reagents can be used in subsequent studies to determine if huTYR DNA microseeding induces canine tyrosinase‐specific antibodies. Moreover, in the absence of autologous tumour cells, canine and human melanoma cell lines can be used to examine if the antibodies recognize endogenous as well as exogenous protein (both canine and human) (Liao et al. 2006). Such assessments could further develop the DNA microseeding platform.
The role humoral immunity plays in tumour immunology and tumour vaccine is incompletely understood. However, antibodies generated against tumour antigens have been demonstrated to mediate cytotoxicity (Frost et al. 1997; Patel et al. 2007; Morris et al. 2016). Additional assessment of tumour‐infiltrating lymphocytes, delayed‐type hypersensitivity and other cellular activity is of great interest and can be investigated in subsequent studies. The primary endpoint for this small pilot study was to determine the feasibility of delivering pDNA vaccine with the microseeding device (e.g. monitoring toxicity, transgene expression and humoral responses). The dogs in this study had undergone surgical resection of their melanoma prior to enrolling in the study. Therefore, these tissues were not available for analysis. Future studies of DNA microseeding can incorporate prospective collection of biopsy tissue for such analyses.
Our study lacks sufficient power to evaluate antitumor effects or dose–response of DNA microseeding. However, we suggest further investigation of pDNA delivery parameters to include analysis of transgene expression and immunogenicity before proceeding with a study to evaluate antitumor activity following DNA microseeding. A larger study could then compare anti‐melanoma activity of the huTYR vaccine delivered either by microseeding or by the current Oncept® delivery method of intramuscular injection. The number of ongoing DNA vaccine clinical trials (ClinicalTrials.gov 2016), the need for development of novel DNA vaccine delivery platforms (DeMuth et al. 2013; Piras et al. 2016) and formulations (Kutzler et al. 2016) and the need for a vaccine to mediate a rapid immune response to a recent global health concern (Larocca et al. 2016; Morrison 2016), suggest that improvements in DNA vaccine delivery strategies are relevant for today's healthcare. The advent of additional canine‐specific reagents for testing in dogs, including anti‐PD1 antibody, will allow for study of combination therapies with DNA microseeding. Moreover, microseeding pDNA encoding non‐synonymous mutations resulting in neoantigens, effectively transforming microseeding into a ‘personalized’ medicine approach, would be of great interest.
Source of funding
Supported in part by Grant P30 CA014520 from the National Cancer Institute, by the University of Wisconsin (UW) Dermatology Department & The Skin Disease Research Center (SDRC) Core Grant 1P30AR066524 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, by the UW Institute for Clinical and Translational Research (ICTR) through the NIH National Center for Advancing Translational Sciences (NCATS) Grant UL1TR000427, and by resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the views of the Dept. of Veterans Affairs or the United States Government.
Conflict of interest
The authors have the following financial or other conflicts of interest to disclose related to this publication: JDW is a patent holder on the xenogenic DNA vaccine technology and receives royalties for the vaccine from Merial; DMV is a paid consultant for Merial. All other authors declare no financial or other conflicts of interest related to this publication.
Ethics statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate review committee approval has been received. The University of Wisconsin School of Veterinary Medicine's guidelines for Animal Care and Use were followed (protocol ID: V005031).
Contributions
Study design and sample collection: CLZ, IDK, MDM, JDW, DMV, EE, MRA. Sample testing: CLZ, CK, EAR. Statistical analysis: MAN. Manuscript draft: CLZ, IDK, MDM, MRA. Revision and manuscript approval: CLZ, CK, EAR, IDK, MDM, MAN, JDW, DMV, EE, MRA.
Acknowledgements
The authors thank the UW Translational Research Initiatives in Pathology laboratory, in part supported by the UW Department of Pathology and Laboratory Medicine and the UW Carbone Cancer Center, for use of its facilities and services.
References
- Alexander A.N., Huelsmeyer M.K., Mitzey A., Dubielzig R.R., Kurzman I.D., Macewen E.G. et al (2006) Development of an allogeneic whole‐cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunology, Immunotherapy 55, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg J.H., Nujien B., Beijnen J.H., Vincent A., van Tinteren H., Kluge J. et al (2009) Optimization of intradermal vaccination by DNA tattooing in human skin. Human Gene Therapy 20, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman P.J., McKnight J., Novosad A., Charney S., Farrelly J., Craft D. et al (2003) Long‐term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clinical Cancer Research 9, 1284–1290. [PubMed] [Google Scholar]
- Bins A.D., Jorritsma A., Wolkers M.C., Hung C.F., Wu T.C., Schumacher T.N. et al (2005) A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nature Medicine 11, 899–904. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov . (2016). A service of the U.S. National Institutes of Health. Available online. https://clinicaltrials.gov/. Accessed on 21 Sep 2016.
- Cormier J.N., Abati A., Fetsch P., Hijazi Y.M., Rosenberg S.A., Marincola F.M. et al (1998) Comparative analysis of the in vivo expression of tyrosinase, MART‐1/Melan‐A, and gp100 in metastatic melanoma lesions: implications for immunotherapy. Journal of Immunotherapy 21, 27–31. [DOI] [PubMed] [Google Scholar]
- DeMuth P.C., Min Y., Huang B., Kramer J.A., Miller A.D., Barouch D.H. et al (2013) Polymer multilayer tattooing for enhanced DNA vaccination. Nature Materials 12, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson E., Yao F., Svensjo T., Winkler T., Slama J., Macklin M.D. et al (1998) In vivo gene transfer to skin and wound by microseeding. Journal of Surgical Research 78, 85–91. [DOI] [PubMed] [Google Scholar]
- Frost J.D., Hank J.A., Reaman G.H., Frierdich S., Seeger R.C., Gan J. et al (1997) A phase I/IB trial of murine monoclonal anti‐GD2 antibody 14.G2a plus interleukin‐2 in children with refractory neuroblastoma: a report of the Children's Cancer Group. Cancer 80, 317–333. [DOI] [PubMed] [Google Scholar]
- Glikin G.C. & Finocchiaro L.M. (2014) Clinical trials of immunogene therapy for spontaneous tumors in companion animals. Scientific World Journal 2014, 718520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I.K. & Khanna C. (2010) Modeling opportunities in comparative oncology for drug development. ILAR Journal 51, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubier A., Fuhrmann L., Forest L., Cachet N., Evrad‐Blanchard M., Juillard V. et al (2008) Superiority of needle‐free transdermal plasmid delivery for the induction of antigen‐specific IFNgamma T cell responses in the dog. Vaccine 26, 2186–2190. [DOI] [PubMed] [Google Scholar]
- Grosenbaugh D.A., Leard A.T., Bergman P.J., Klein M.K., Meleo K., Susaneck S. et al (2011) Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. American Journal of Veterinary Research 72, 1631–1638. [DOI] [PubMed] [Google Scholar]
- Helfand S.C., Soergel S.A., Donner R.L., Gan J., Hank J.A., Lindstrom M.J. et al (1994) Potential to involve multiple effector cells with human recombinant interleukin‐2 and antiganglioside monoclonal antibodies in a canine malignant melanoma immunotherapy model. Journal of Immunotherapy with Emphasis on Tumor Immunology 16, 188–197. [DOI] [PubMed] [Google Scholar]
- Helfand S.C., Hank J.A., Gan J. & Sondel P.M. (1996) Lysis of human tumor cell lines by canine complement plus monoclonal antiganglioside antibodies or natural canine xenoantibodies. Cellular Immunology 167, 99–107. [DOI] [PubMed] [Google Scholar]
- Hill P.B. & Olivry T. (2001) The ACVD task force on canine atopic dermatitis (V): biology and role of inflammatory cells in cutaneous allergic reactions. Veterinary Immunology and Immunopathology 81, 187–198. [DOI] [PubMed] [Google Scholar]
- Kis E.E., Winter G. & Myschik J. (2012) Devices for intradermal vaccination. Vaccine 30, 523–538. [DOI] [PubMed] [Google Scholar]
- Kutzler M.A., Wise M.C., Hutnick N.A., Moldoveanu Z., Hunter M., Reuter M.A. et al (2016) Chemokine‐adjuvanted electroporated DNA vaccine induces substantial protection from simian immunodeficiency virus vaginal challenge. Mucosal Immunology 9, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca R.A., Abbink P., Peron J.P., Zanotto P.M., Iampietro M.J., Badamchi‐Zadeh A. et al (2016) Vaccine protection against Zika virus from Brazil. Nature 536, 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembcke L.M., Kania S.A., Blackford J.T., Trent D.J., Odoi A., Grosenbaugh D.A. et al (2012). Development of Immunologic Assays to Measure Response in Horses Vaccinated with Xenogeneic Plasmid DNA Encoding Human Tyrosinase. Journal of Equine Veterinary Science 32, 607–615. https://doi.org/10.1016/j.jevs.2012.02.011. [Google Scholar]
- Liao J.C., Gregor P., Wolchok J.D., Orlandi F., Craft D., Leung C. et al (2006) Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma. Cancer Immunity 6, 8. [PMC free article] [PubMed] [Google Scholar]
- Marsella R. & Olivry T. (2003) Animal models of atopic dermatitis. Clinics in Dermatology 21, 122–133. [DOI] [PubMed] [Google Scholar]
- Morris Z.S., Guy E.I., Francis D.M., Gressett M.M., Werner L.R., Carmichael L.L. et al (2016) In Situ Tumor Vaccination by Combining Local Radiation and Tumor‐Specific Antibody or Immunocytokine Treatments. Cancer Research 76, 3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. (2016) DNA vaccines against Zika virus speed into clinical trials. Nature Reviews. Drug Discovery 15, 521–522. [DOI] [PubMed] [Google Scholar]
- Nabel E.G., Gordon D., Yang Z.Y., Xu L., San H., Plautz G.E. et al (1992) Gene transfer in vivo with DNA‐liposome complexes: lack of autoimmunity and gonadal localization. Human Gene Therapy 3, 649–656. [DOI] [PubMed] [Google Scholar]
- Nguyen S.M., Thamm D.H., Vail D.M. & London C.A. (2015) Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Veterinary and Comparative Oncology 13, 176–183. [DOI] [PubMed] [Google Scholar]
- Nuttall T.J., Knight P.A., McAleese S.M., Lamb J.R. & Hill P.B. (2002) Expression of Th1, Th2 and immunosuppressive cytokine gene transcripts in canine atopic dermatitis. Clinical and Experimental Allergy 32, 789–795. [DOI] [PubMed] [Google Scholar]
- Olivry T., Deangelo K.B., Dunston S.M., Clarke K.B. & McCall C.A. (2006) Patch testing of experimentally sensitized beagle dogs: development of a model for skin lesions of atopic dermatitis. Veterinary Dermatology 17, 95–102. [DOI] [PubMed] [Google Scholar]
- Oosterhuis K., van den Berg J.H., Schumacher T.N. & Haanen J.B. (2012) DNA vaccines and intradermal vaccination by DNA tattooing. Current Topics in Microbiology and Immunology 351, 221–250. [DOI] [PubMed] [Google Scholar]
- Ottnod J.M., Smedley R.C., Walshaw R., Hauptman J.G., Kiupel M. & Obradovich J.E. (2013) A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Veterinary and Comparative Oncology 11, 219–229. [DOI] [PubMed] [Google Scholar]
- Paoloni M. & Khanna C. (2008) Translation of new cancer treatments from pet dogs to humans. Nature Reviews: Cancer 8, 147–156. [DOI] [PubMed] [Google Scholar]
- Patel D., Balderes P., Lahiji A., Melchior M., Ng S., Bassi R. et al (2007) Generation and characterization of a therapeutic human antibody to melanoma antigen TYRP1. Human Antibodies 16, 127–136. [PubMed] [Google Scholar]
- Pellin M.A., Wouda R.M., Robinson K., Tsimbas K., Kurzman I.D., Biller B.J. et al (2016) Safety evaluation of combination doxorubicin and toceranib phosphate (Palladia(R)) in tumour bearing dogs: a phase I dose‐finding study. Veterinary and Comparative Oncology, https://doi.org/10.1111/vco.12232. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Phillips J.C., Lembcke L.M., Noltenius C.E., Newman S.J., Blackford J.T., Grosenbaugh D.A. et al (2012) Evaluation of tyrosinase expression in canine and equine melanocytic tumors. American Journal of Veterinary Research 73, 272–278. [DOI] [PubMed] [Google Scholar]
- Piliang M.P. (2011) Acral lentiginous melanoma. Clinics in Laboratory Medicine 31, 281–288. [DOI] [PubMed] [Google Scholar]
- Piras L.A., Riccardo F., Iussich S., Maniscalco L., Gattino F., Martano M. et al (2016) Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4‐antigen electrovaccination. Veterinary and Comparative Oncology https://doi.org/10.1111/vco.12239. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos‐Vara J.A. & Miller M.A. (2011) Immunohistochemical identification of canine melanocytic neoplasms with antibodies to melanocytic antigen PNL2 and tyrosinase: comparison with Melan A. Veterinary Pathology 48, 443–450. [DOI] [PubMed] [Google Scholar]
- Rogers R.S. 3rd & Gibson L.E. (1997) Mucosal, genital, and unusual clinical variants of melanoma. Mayo Clinic Proceedings 72, 362–366. [DOI] [PubMed] [Google Scholar]
- Tobin D.J., Gardner S.H., Luther P.B., Dunston S.M., Lindsey N.J. & Olivry T. (2003) A natural canine homologue of alopecia areata in humans. British Journal of Dermatology 149, 938–950. [DOI] [PubMed] [Google Scholar]
- Ulmer J.B., Donnelly J.J., Parker S.E., Rhodes G.H., Felgner P.L., Dwarki V.J. et al (1993) Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259, 1745–1749. [DOI] [PubMed] [Google Scholar]
- Vail D.M., Thamm D.H. (2011). Spontaneous companion animal (pet) cancers In Tumor Models in Cancer Research 2nd Ed (eds B.A. Teicher). Humana Press: New York, NY. [Google Scholar]
- Verstrepen B.E., Bins A.D., Rollier C.S., Mooij P., Koopman G., Sheppard N.C. et al (2008) Improved HIV‐1 specific T‐cell responses by short‐interval DNA tattooing as compared to intramuscular immunization in non‐human primates. Vaccine 26, 3346–3351. [DOI] [PubMed] [Google Scholar]
- Veterinary cooperative oncology group (2011) common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Veterinary and Comparative Oncology, https://doi.org/10.1111/j.1476-5829.2011.00283.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wahren B. & Liu M. (2014) DNA Vaccines: recent developments and the future. Vaccines 2, 785. [DOI] [PubMed] [Google Scholar]


