Abstract
Iron deficiency is a major problem in both developing and developed countries, and much of this can be attributed to insufficient dietary intake. Over the past decades several measures, such as supplementation and food fortification, have helped to alleviate this problem. However, their associated costs limit their accessibility and effectiveness, particularly amongst the financially constrained. A more affordable and sustainable option that can be implemented alongside existing measures is biofortification. To date, much work has been invested into staples like cereals and root crops—this has culminated in the successful generation of high iron-accumulating lines in rice and pearl millet. More recently, pulses have gained attention as targets for biofortification. Being secondary staples rich in protein, they are a nutritional complement to the traditional starchy staples. Despite the relative youth of this interest, considerable advances have already been made concerning the biofortification of pulses. Several studies have been conducted in bean, chickpea, lentil, and pea to assess existing germplasm for high iron-accumulating traits. However, little is known about the molecular workings behind these traits, particularly in a leguminous context, and biofortification via genetic modification (GM) remains to be attempted. This review examines the current state of the iron biofortification in pulses, particularly chickpea. The challenges concerning biofortification in pulses are also discussed. Specifically, the potential application of transgenic technology is explored, with focus on the genes that have been successfully used in biofortification efforts in rice.
Keywords: pulse biofortification, iron, genetic modification, crop improvement, chickpea
Introduction
The current world population stands at an estimated 7.3 billion (United Nations, 2015) and is projected to increase by 2 billion over the next four decades. Concomitant to this growth is the challenge of providing sustenance amidst dwindling resources. Currently food production is adequate at approximately four billion metric tons per annum, yet in spite of this, about 870 million people still suffer from chronic malnutrition due to factors like unequal distribution, wastage and poor diets (FAO, 2012; IMECHE, 2013).
Malnutrition, as defined by the World Health Organization (WHO), is “the cellular disparity amid the supply of energy, nutrients and the body's demand for them to ascertain maintenance, growth and specific functions” (Batool et al., 2013). It refers to both the insufficient and excessive intake of nutrients (both macro and micro) and as such covers not only food shortage but also obesity. Undernourishment can be classified categories: protein-energy malnutrition and micronutrient deficiency. As the names suggest, the former refers to inadequate calorie or protein intake while the latter to the lack of essential micronutrients such as vitamin A, iodine, zinc, and iron (Batool et al., 2013).
While both pose significant risks to health and negatively affect overall productivity and quality of life, micronutrient deficiency, also known “hidden hunger,” is perhaps the more pervasive and lethal due to the lack of visible effects. It is consequently more difficult to identify and tackle, and afflicts both developing and developed nations.
Among the various kinds of micronutrient deficiencies, iron deficiency is the most prevalent, afflicting more than two billion individuals worldwide (WHO, 2008). It has been identified as the greatest contributor to anemia, accounting for 66.2% of cases globally (Alvarez-Uria et al., 2014). The extent of its impact is such that the terms are used interchangeably and the prevalence of anemia is used as a measure for the more specific iron deficiency anemia (IDA) (WHO, 2001). IDA can be attributed to three main factors—increased iron requirement (e.g., growth and pregnancy), poor absorption, and inadequate dietary intake. The recommended values for daily iron intake varies depending on the gender and developmental stage (Table 1), and insufficient intake impedes the formation of biologically important compounds, most notably heme, resulting in anemia. Symptoms include fatigue, loss of energy, and dizziness, all of which diminish the work capacity of the individual. Iron deficiency also results in poor pregnancy outcomes and impediment of physical and cognitive development, thereby increasing the risk of morbidity in children (WHO, 2008).
Table 1.
Recommended Dietary Allowances (RDAs) for iron (Trumbo et al., 2001).
| Age | Male (mg) | Female (mg) | Pregnancy (mg) | Lactation (mg) |
|---|---|---|---|---|
| Birth to 6 months | 0.27 | |||
| 7–12 months | 11 | |||
| 1–3 years | 7 | |||
| 4–8 years | 10 | |||
| 9–13 years | 8 | |||
| 14–18 years | 11 | 15 | 27 | 10 |
| 19–50 years | 8 | 18 | 27 | 9 |
| 51+ years | 8 | |||
This presents a problem of great economic and social significance, particularly in developing countries where approximately 50% of pregnant women and 40% of preschool children suffer from IDA (WHO, 2008). The consequences manifest not only in the form of lives lost, but also in a rising generation of individuals afflicted with developmental complications. The significance of this issue has been understood by various governments, and through both nutrition and non-nutrition based interventions, considerable progress has been made in reducing IDA, particularly in South Asia, East Asia, Southeast Asia, and Eastern sub-Saharan Africa (Kassebaum, 2016). However, while a global decrease in the prevalence of severe anemia cases was observed between 1990 and 2013, the number of mild to moderate cases has increased (Table 2), and a prevalence rate below 10% has yet to be seen in any country (Kassebaum, 2016).
Table 2.
Prevalence of anemia between 1990 and 2013 (Kassebaum, 2016).
| Prevalent cases of anemia | ||||
|---|---|---|---|---|
| Severity of anemia | 1990 | 2013 | Difference between 1990 and 2013 (%) | |
| Global | Mild | 839,101,225 | 950,135,191 | 13.23 |
| Moderate | 901,120,023 | 905,501,751 | 0.49 | |
| Severe | 88,720,928 | 75,565,628 | −14.83 | |
| Developing countries | Mild | 717,671,655 | 816,531,244 | 13.78 |
| Moderate | 803,792,125 | 809,200,194 | 0.67 | |
| Severe | 82,149,100 | 69,480,441 | −15.42 | |
| Developed countries | Mild | 121,429,570 | 133,603,946 | 10.03 |
| Moderate | 97,327,897.80 | 96,301,556.30 | −1.05 | |
| Severe | 6,571,827.50 | 6,085,186.70 | −7.40 | |
Out of the three major risk factors contributing to IDA, the issue of dietary intake is the most feasible to address on a large scale. Efforts to remedy the problem include changes in policy, education, and food-based strategies. The latter can, in turn, come in various forms such as dietary diversification, food fortification, and supplementation, the definitions and examples of which are illustrated in Table 3. More specific details and an overview of the strategies can be found in the published guidelines by the WHO and FAO (2006).
Table 3.
The main food-based strategies to combat iron deficiency (WHO and FAO, 2006).
| Dietary diversification | Food fortification | Supplementation | |
|---|---|---|---|
| Definition | Inclusion and increased intake of foods rich in the target nutrient | Improvement of food nutritional quality via enhancing target nutrient content | Intake of moderately large doses of target nutrient. Can be done via oral (e.g., pills, capsules, or syrups) or intravenous routes |
| Examples |
|
|
|
To summarize, each strategy has its own unique advantages and several studies have proven their effectiveness in alleviating IDA (Baltussen et al., 2004; Gera et al., 2012; Rao et al., 2013). While that efficacy may vary across different temporal and spatial scales, the strategies can be used in concert to greater effect. For instance, dietary diversification can still be used where supplementation or food fortification strategies may not due to lack of suitable infrastructure or distribution networks. However, it in turn, is subject to local environmental conditions and resource availability.
Concerning the aforementioned strategies and their application, the issue of accessibility has been noted to be a major limitation (WHO and FAO, 2006). With the food fortification and supplementation schemes in particular, the recurring costs associated with processing and distribution can be prohibitive and beneficiaries are limited to those who can afford it. Such measures are therefore unfeasible for the low-income demographics that, incidentally, have the greatest need. The challenge then is to develop a sustainable, cost-effective means to deliver the required nutrients to the vulnerable parties.
One such means is biofortification, which can generally be defined as the enhancement of nutritional quality in the edible portions of food crops during plant growth (HarvestPlus, 2015b; WHO, 2016). Given that the process of plant nutrient accumulation is a complex interplay between genetics, environmental and management factors, the precise definition of the term “biofortification” may vary depending on the scope of the means (HarvestPlus, 2015b; WHO, 2016). For the purpose of this review which focuses on the genetic aspect, the term “biofortification” shall be used to refer solely to the generation of self-fortifying plants, to the exclusion of agronomic interventions. Such agronomic interventions include fertilizer application or bacterial inoculation, which can be used in conjunction with biofortified crops. Fertilizer application has been demonstrated to increase iron accumulation, though the degree of which varies between studies (Pahlavan-Rad and Pessarakli, 2009; Cakmak et al., 2010; Zhang et al., 2010; Aciksoz et al., 2011). The use of bacterial inoculation on the other hand, has been met with some success, though the choice of strains used may depend on the environmental conditions (Mishra et al., 2011; Rana et al., 2012; Sharma et al., 2013).
Biofortification as a means of alleviating global iron deficiency
Biofortification emerged within the last two decades as an approach to combat micronutrient deficiency. While it cannot be considered a cure-all to micronutrient deficiency, it alleviates the problem by complementing existing strategies like the aforementioned ones of dietary diversification, fortification, and supplementation. With the one-time cost of development thoroughly compensated by the long term benefits, biofortification presents a sustainable means of delivering the needed micronutrients across large spatial and temporal scales (Nestel et al., 2006; Horton et al., 2008; De Moura et al., 2014; HarvestPlus, 2015b). Biofortified crops are typically generated via selection of micronutrient accumulating traits, and there are a few means through which this can be achieved. Amongst these, one that has existed since the advent of agriculture is conventional breeding. Traditionally a long-term process requiring much investment of time and effort, advances in technology and molecular biology has since shortened the process and increased its precision when targeting specific traits. Several quantitative trait loci (QTLs) for iron accumulation have been identified in rice (Norton et al., 2010; Anuradha et al., 2012), wheat (Xu et al., 2012), maize (Jin et al., 2013), pearl millet (Kumar et al., 2016), cowpea (Santos and Boiteux, 2015), and bean (Blair et al., 2009, 2010, 2016). Already, several crops have been developed through conventional breeding under the HarvestPlus program, the most notable of which are iron biofortified pearl millet, rice and beans. The success of these biofortified crops has been demonstrated in several feeding trials. Consumption of biofortified pearl millet improved iron adsorption and iron stores in women and children (Cercamondi et al., 2013; Kodkany et al., 2013; Finkelstein et al., 2015), while biofortified rice have been found to help maintain the iron stores of non-anemic women (Haas et al., 2005). Increased iron absorption was also observed in biofortified bean meals (Petry et al., 2014, 2016). Collectively, meta-analysis of these trials indicated such biofortified crops to be particularly beneficial to iron deficient individuals (Finkelstein et al., 2017).
Despite its effectiveness, the extent to which biofortification can be done through conventional breeding is limited to the diversity in the gene pool and fertility of the species. In cases where such limitations prevail, genetic modification (GM) provides an alternative pathway. In this method, the genetic material of the host is altered in a manner that does not occur naturally. This may take the form of overexpression of a native gene, such as the OsNAS gene family in rice (Johnson et al., 2011), or expression of a foreign gene from an external source, such as the algal FEA1 gene in cassava (Ihemere et al., 2012). A major advantage of GM is its specificity—select genes, and thus related traits, can be introduced without linkage drag that is associated with unfavorable agronomic traits. Depending on the gene combinations used, increases in iron content of up to 7.5-fold have been reported using GM technology (Trijatmiko et al., 2016).
Even with this advantage however, the release of GM food crops is controversial due to public and political concerns for environmental and human safety. Many of such concerns are directed toward herbicide-resistant GM crops. Conversely, the current direction of GM focuses more on functional foods and is more subtle, leaning toward a cis-genic rather than transgenic approach. Recent years have also seen the rapid rise of genome editing techniques—these are more specific than GM, being capable of targeting specific genome locations for modification whilst also being integration-free. In genome editing, artificial nucleases are used for targeted gene integration or deletion. Four systems have developed—meganucleases, zinc finger nucleases (ZFN), transcription factor nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR-CAS) (Sander and Joung, 2014; Bortesi and Fischer, 2015). These have been tested in a wide range of species including crops like rice and wheat, though it has yet to be applied for biofortification purposes.
To date, starchy staples that contain little micronutrients like cereals, root crops, and banana have the primary targets for iron biofortification (Namanya, 2011; HarvestPlus, 2015a; Banana21, 2016). The advantages of such targets is that they form the bulk of local diets and given proper processing, have a long shelf-life, allowing for efficient delivery of the biofortified micronutrient over a large spatial and temporal scale. A wealth of information has been generated concerning these crops as a consequence of extensive focus. Biofortification works using GM in particular, have largely concentrated on major graminaceous crops like rice, wheat, and maize. In contrast, existing studies in non-graminaceous plants were conducted mainly in model species like tobacco or Arabidopsis for characterization purposes. While not as extensive, some work has also been conducted in crop species like banana (Matovu, 2016), cassava (Narayanan et al., 2015) lettuce (Goto et al., 2000), and soybean (Vasconcelos et al., 2006). Given the physiological differences between the non-graminaceous and graminaceous plants, it is difficult to extrapolate the effectiveness of iron biofortification approaches in the latter to the former. As it stands, there remains much to be explored in terms of iron biofortification of non-graminaceous crops.
Iron metabolism in plants
Prior to attempting any biofortification strategies, the significance of iron in the plants and the underlying mechanisms governing its metabolism must first be understood. Iron is the fourth most common element in the Earth's crust and can exist in a wide range of oxidation states, of which the most common are the ferrous (Fe2+) and ferric (Fe3+) forms. By virtue of its high redox potential, it forms a key component of biological processes involving electron exchange such as DNA synthesis, oxygen transport, cellular respiration, and photosynthesis, where it participates in the form of a cofactor in iron complexes. Examples of such complexes include hemoglobin, DNA helicases, and catalase.
For all its biological significance however, iron metabolic pathways can be summarized with the imagery of a precarious transfer of a radioactive material between containment facilities. Biologically, free iron may result from iron overload and/or insufficient sequestration capacity of the organism (Pietrangelo, 2003). Left alone, free Fe2+ catalyzes the formation of hydroxyl (·OH) radicals through the Fenton reaction, and the process repeats when Fe2+ is regenerated from the resultant Fe3+ through reduction by the superoxide radical () (Haber and Weiss, 1934). The summation of this self-perpetuating reaction is known as the Haber-Weiss reaction:
Reactive oxygen species (ROS) generated as a consequence of this reaction can react with cellular components to cause oxidative damage (Kehrer, 2000; Aisen et al., 2001; Papanikolaou and Pantopoulos, 2005; Jeong and Guerinot, 2009; Kobayashi and Nishizawa, 2012); however on the other hand, they also serve as important signaling molecules and are an integral part of the stress response (Apel and Hirt, 2004). The fine line between cytotoxicity and biological function, and the intimate association between iron and ROS production, highlights the significance of proper regulation of iron metabolic pathways.
Given that iron nutrition and metabolism in plants has been extensively reviewed over the years (see Table 5), this review will provide only a brief overview of the topic. Iron metabolic pathways can be divided into three main processes: uptake, translocation and storage. Despite its abundance iron has poor solubility under aerobic conditions, particularly in high pH and calcareous soils, necessitating its solubilization before uptake can occur. This process is mostly accomplished via root exudates, the composition of which varies in response to the plant's physiological state and needs. In response to iron deficiency, the plant triggers the production of factors that directly or indirectly aid iron solubilization. Enhanced concentration of glutamate, ribitol, and glucose were observed in the root exudates of iron-deficient maize, which were suggested to attract and support siderophore-producing bacterial communities to aid iron solubilization (Carvalhais et al., 2011). Notable increases were also observed in the production of organic acids like malate and citrate, which increase the availability of iron through dissolution of insoluble iron compounds (Jones et al., 1996; Sánchez-Rodríguez et al., 2014).
Table 5.
List of general reviews on iron nutrition and metabolism.
In addition to the aforementioned means, different plant species have adopted specific approaches toward solubilize and acquire iron. These were first categorized as Strategy I and Strategy II by Römheld and Marschner (1986) and later studies have served to cement this grouping. As the topic of iron metabolism has been extensively reviewed over the years (see Thomine and Lanquar, 2011; Kobayashi and Nishizawa, 2012; Brumbarova et al., 2015), the following sub-sections will only provide a brief overview of the process.
Strategy I uptake mechanism
Strategy I is a reduction-based strategy used predominantly by non-graminaceous species, which includes all plants except grasses. Under iron deficiency, proton extrusion occurs through the action of H+-ATPases (HA), resulting in the acidification of the rhizosphere and reduction of insoluble iron (Rabotti and Zocchi, 1994; Dell'Orto et al., 2000; Santi et al., 2005; Santi and Schmidt, 2009). Phenolic compounds may also be secreted to facilitate iron uptake (Rodríguez-Celma et al., 2013). Iron and iron chelates are then reduced at the root surface by ferric-chelate reductase oxidase (FRO), which reduces Fe3+ chelates to Fe2+ by transferring electrons across the plasma membrane (Robinson et al., 1999; Waters et al., 2002). FRO is a family of membrane-bound metalloreductases that transfer electrons from cytosolic NADPH across membranes to electron-accepting substrates on the other side. In addition to facilitating acquisition of iron from the soil, this capability is also utilized in localizations where iron reduction is required for transport and/or assimilation, such as in the mesophyll (Brüggemann et al., 1993), reproductive tissues (Waters et al., 2002; Li et al., 2004a), and chloroplast membranes (Jeong and Connolly, 2009).
Following reduction at the root surface, the resulting Fe(II) ions are absorbed across the plasma membrane via the iron-regulated transporter (IRT) (Eide et al., 1996; Vert et al., 2002). IRT is a member of the zinc-regulated transporter, iron-regulated transporter-like protein (ZIP) family that functions as membrane-bound uptake transporter for Zn and Fe (Lin et al., 2009). In Arabidopsis and tomato, IRT1 has been identified as responsible for uptake from the soil (Bereczky et al., 2003; Vert et al., 2009), with loss of function producing a severely stunted and chlorotic phenotype (Varotto et al., 2002; Vert et al., 2002). Co-regulated with IRT1 is the AtIRT2 homolog, which facilitates subcellular transport of iron and localizes to vesicle membranes instead of plasma membranes (Vert et al., 2009).
Both FRO and IRT activity is regulated in response to iron concentrations and increases in response to iron deficiency (Robinson et al., 1999; Connolly et al., 2003; Vert et al., 2003). Enhanced ferric reduction in particular, has been considered a hallmark indicator of iron deficiency (Römheld and Marschner, 1981; Higuchi et al., 1995) and increased capacity for FRO activity confers increased tolerance to low iron (Connolly et al., 2003; Peng et al., 2015).
Strategy II uptake mechanism
Unlike Strategy I, Strategy II is used by graminaceous plants (grasses) and revolves around the use of the mugineic acid (MA) family phytosiderophores in iron acquisition. The MA biosynthetic pathway starts with the conversion of three units of S-adenosylmethionine (SAM) into nicotianamine (NA) by nicotianamine synthase (NAS) (Higuchi et al., 1994, 1999). NA is converted to a 3′ keto-intermediate by nicotianamine aminotransferase (NAAT), before being reduced to deoxymugeneic acid (DMA) by deoxymugeneic acid synthase (DMAS) (Kanazawa et al., 1994; Bashir et al., 2006). DMA can subsequently be converted to other MAs through a series of hydroxylations (Mori and Nishizawa, 1989; Ma and Nomoto, 1993), increasing levels of which improves the affinity for Fe3+ and chelate stability under acidic conditions (von Wirén et al., 2000). Synthesized MAs are secreted into the soil through the phytosiderophore efflux transporter TOM1 (Nozoye et al., 2011) and the resulting ferric complexes are then taken up by the roots through specialized transporters like YELLOW STRIPE 1 (YS1) and YELLOW STRIPE 1-like (YSL) (Curie et al., 2001; Murata et al., 2006; Inoue et al., 2009; Kobayashi and Nishizawa, 2012). The resulting Fe(III)-phytosiderophore complex is subsequently taken up via specialized transporters and transported throughout the plant (Kawai et al., 2001). Unlike the reduction-based approach used in Strategy I, phytosiderophore uptake is not limited by high pH, thereby conferring an advantage where such conditions are present (Römheld and Marschner, 1986).
Translocation and storage
Following acquisition into the root symplast, iron is transported across the root to the vascular tissue and subsequently to the rest of the plant. The translocation process itself is a multi-step process, involving symplastic movement across the Casparian strip and to the desired site; the loading, unloading and transport through the vascular tissue; as well as remobilization from source tissue (Kim and Guerinot, 2007).
During transport, iron is maintained as a chelated complex with ascorbate, citrate or NA (Brown and Chaney, 1971; Stephan and Scholz, 1993; Pich et al., 1994; Grillet et al., 2014). With graminaceous plants, iron may also be complexed to DMA or MAs for transport (Koike et al., 2004; Ishimaru et al., 2010). Such complexes are pH-dependent, as are their interactions with other iron chelators (von Wirén et al., 1999). NA for instance, chelates both Fe(II) and Fe(III) at a higher pH but will preferentially bind for the former at pH 7.0. When bound to Fe(III) at equilibrium, the NA complex dominates at pH 7.0–9.0 while the structurally similar DMA complex dominates at pH 3.0–6.0. Citrate removes iron from NA at pH 5.5; and it must be converted to Fe(III)-citrate even if Fe(II) is the major form in which Fe is loaded into xylem.
As with the uptake process, non-graminaceous plants seem to rely on reduction-based strategy while graminaceous plants utilize a chelation-based one in which Fe(III) undergoes little or no change in redox state. The use of both strategies may be present in a single species, of which the only known example is rice (Ishimaru et al., 2006). This combination may represent an adaptation to the submerged conditions in which rice and its wild relatives grow, where iron is more readily available in ferrous than ferric form. Whether a similar occurrence may be found in other species remains to be seen, though orthologs of genes associated with Strategy I have also been found in other graminaceous species. An example of this is ZmIRT1 from maize, which was purported to be involved in both uptake and translocation, particularly to the seeds (Li et al., 2015). It should be noted that while differences between both strategies primarily affect the uptake process, the involvement of molecular components in the translocation process has further implications for the overall physiology of the plant.
Upon reaching the sink tissue, iron is reallocated as a cofactor in various complexes, or bound to the iron storage molecule ferritin and stored in the apoplastic space and vacuoles (Briat and Lobréaux, 1997). Subsequent translocation and remobilization may occur in response to developmental and physiological needs, such as during iron deficiency (Waters and Troupe, 2012), seed filling (Hocking and Pate, 1977; Burton et al., 1998; Garnett and Graham, 2005), senescence (Shi et al., 2012; Maillard et al., 2015), and nodulation (Strozycki et al., 2007).
Pulses as a vehicle for biofortification
Aside from the starchy staples, another group of crops has been targeted for biofortification, albeit to a lesser degree. Pulses, as defined by the FAO (1994), are leguminous crops harvested solely for dry grain. While this term encompasses most grain legumes, soybean and peanut are excluded from this classification, because they are traditionally viewed as oilseed crops (Pulse Australia, 2016b).
Like cereals, pulses have a long history of cultivation and have been a significant constituent in human diets since around 10,000 BC (Fuller et al., 2001; Caracuta et al., 2015). As a crop, pulses present two main benefits, both of which are complementary to cereals. The first is their agronomic characteristics. By virtue of their nitrogen fixing properties, pulses are often grown as an intercrop or as a mixed crop to replenish soil nitrogen levels, thereby reducing the need for fertilizers. Cultivation with pulse crops have also been shown to increase the uptake of nitrogen, sulfur, and phosphorus by cereals, resulting in an enhanced yield and grain quality (Li et al., 2003, 2004b; Agegnehu et al., 2006; Banik et al., 2006; Gooding et al., 2007). Yield stability is also increased (Rao and Willey, 1980).
The second benefit of pulses is their nutritional density. Pulses are a rich source of carbohydrates and fiber. Their most prominent feature however, is their high protein content of 21–26% and an amino acid profile complementary to that of cereals, being rich in lysine, leucine, and arginine (Phillips, 1993; Iqbal et al., 2006; Pulse Canada, 2016). Their excellence as a vegetarian source of protein and affordability in contrast to livestock products has earned them the famous moniker of “poor man's meat.” Pulses are also rich in micronutrients like folate, thiamine, riboflavin, niacin, calcium, magnesium, iron, and zinc (Phillips, 1993; Iqbal et al., 2006; Jukanti et al., 2012). Other than contributing to macro- and micronutritional needs, several health benefits have been associated with inclusion of pulses in the diet. Their low glycemic index (GI) has been linked to the management of diabetes and diabetes-related diseases (Rizkalla et al., 2002; Sievenpiper et al., 2009) while bioactive components have been investigated for their health potential—e.g., lectins for their immunomodulatory effect, protease inhibitors for anti-inflammatory effects, and angiotensin I-converting enzyme (ACE) inhibitory peptides for their anti-hypertensive properties (Rochfort and Panozzo, 2007; Roy et al., 2010).
Despite their agronomic and nutritional benefits, pulses have not received the same amount of attention or development as the main starchy staples. Between 1961 and 2014, pulse yield and production values increased by 42.3 and 90.4%, respectively, a small fraction compared to the increase of 187.2 and 219.4% in cereals (FAO, 2016b). Much of this disparity can be attributed to developments made during the Green Revolution, in which the focus on productivity and protein-calorie malnutrition led to the shift from cultivation of traditional micronutrient-rich crops to the more productive and profitable starchy cereals (Pinstrup-Andersen and Hazell, 1985; Pingali, 2012). Poor policy and diversion of land to cereal cultivation has led to a reduction in pulse supply, effectively driving prices up and decreasing consumption per capita (Kennedy and Bouis, 1993; Kataki, 2002; Akibode and Maredia, 2012).
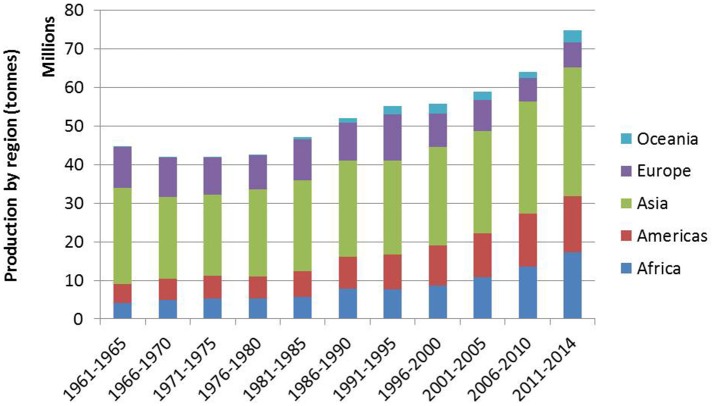
As highlighted in the special feature on pulses in the 2014 Food Outlook (FAO, 2014), recent years have seen several key changes in pulse production and trade. Asia remains the region with the highest pulse production, with India continuing as the largest pulse producing country, contributing at least 20% toward global pulse production (Figure 1 and Table 4). Production in other regions except Europe has also increased, fueled by domestic and international demand. In contrast to these countries is China, whose production has decreased due to a number of factors such as population increase and decreasing availability of arable land. Despite the shift in preference for animal-based products and protein that accompanies growing affluence, India and China remain major importers, consuming approximately 40% of the world's pulse production as food, and 30% as feed. Much of this is provided by major exporters like Canada and Australia. With other major producers like Myanmar and Brazil, pulse consumption is primarily domestic.
Figure 1.
Average pulse production by region (FAO, 2016a).
Table 4.
| Production (mmt) | |||||
|---|---|---|---|---|---|
| Country | 1961 | 1981 | 2001 | 2014 | Principal pulse crops |
| India | 12.9 | 10.8 | 12.2 | 19.98 | Chickpeas, beans, pigeon peas |
| Myanmar | 0.2 | 0.4 | 2.0 | 5.0 | Beans, pigeon peas, chickpeas |
| Canada | 0.1 | 0.2 | 3.4 | 5.8 | Peas, lentils |
| China | 8.5 | 6.4 | 5.1 | 4.5 | Beans, broad beans, peas |
| Brazil | 1.8 | 2.4 | 2.5 | 3.3 | Beans |
| Nigeria | 5 | 0.6 | 2.3 | 2.2 | Cowpeas |
| Ethiopia | 0.6 | 0.9 | 1.2 | 2.6 | Broad beans, beans, chickpeas, peas |
| Australia | 0 | 0.3 | 2.7 | 3.0 | Lupines, lentils, chickpeas |
| USA | 1.1 | 1.7 | 1.3 | 2.4 | Beans, peas |
| Tanzania, U. Rep. | 0.1 | 0.3 | 0.8 | 1.8 | Beans |
| Rest of the world | 15.0 | 17.5 | 22.6 | 27.02 | |
| Total | 40.8 | 41.6 | 55.9 | 77.6 | |
Pulse production, consumption, and trade are expected to increase alongside population growth, particularly with increasing promotion from government campaigns (Akibode and Maredia, 2012) and the declaration of 2016 as the “International Year of Pulses” by the UN General Assembly. Increasing awareness and concern over nutritional composition of food, particularly by food manufacturers, has attracted greater interest in pulses, which will likely translate into further support and development of the pulse industry (FAO, 2014).
Challenges of pulse biofortification
Concerning the biofortification of pulses however, there are some challenges. While pulses are a diverse group featuring a wide variety of species and cultivars, this has presented itself as a double-edged sword. The genetic richness is a treasure trove that lends itself to crop improvement, but has simultaneously resulted in a lack of a concerted global effort at development. Development has also thus far has been focused on yield, disease tolerance, and macronutrient quality, with little to no emphasis on other nutritional aspects. However, this has changed over the past decade with the growing interest in micronutrient content. Increasing numbers of genotypes and cultivars being assayed for their iron and zinc composition (Ray et al., 2014; Thavarajah et al., 2014; Santos and Boiteux, 2015; Blair et al., 2016). Concerning outputs however, there has been little published work on pulse biofortification, with even fewer examples of biofortified pulses. Currently the only known example of a biofortified pulse is the high iron common bean generated from the HarvestPlus breeding program; to date, several varieties have been produced with improvements in iron content ranging from 47 to 94% (Katsvairo, 2015).
Another challenge with pulse biofortification is the bioavailability of iron. Bioavailability, as defined by Carpenter and Mahoney (1992), is the “proportion of a nutrient present in food that the body is able to absorb and utilize by incorporation into physiologically functional pools.” It is a complex and multifaceted issue subject to a range of physiological and physiochemical factors such as those illustrated in Table 6. In humans, iron uptake occurs in the duodenum and upper jejunum in the intestinal tract. Regulation is done via absorption rather than excretion, and occurs in response to iron status and rate of erythropoiesis (Wheby et al., 1964; Bothwell, 1995). Under normal conditions, absorption is inversely correlated to the state of the body's iron stores with increased uptake by the intestinal mucosal cells as iron stores are depleted (Walters et al., 1975).
Table 6.
Factors affecting bioavailability of some trace elements (House, 1999).
| Host factors | Dietary factors | |
|---|---|---|
| Dietary composition | Food preparation | |
|
|
|
For uptake to occur however, the mineral must be in a form absorbable by the mucosal cells. Dietary iron can be classified as heme or non-heme based on its attachment to heme proteins or lack thereof. Both form separate pools in the gastrointestinal tract and undergo different absorption pathways (Björn-Rasmussen et al., 1974). Compared to its heme counterpart, non-heme iron is the predominant form found in plants and has a lower bioavailability and is particularly susceptible to the influence of dietary factors (Björn-Rasmussen et al., 1974). This is of especial significance in pulses due to the abundance of naturally occurring inhibitors which bind to iron and prevent uptake. Examples of such inhibitors include phytic acid, polyphenols, tannins, and fiber (Sandberg, 2002; Ghavidel and Prakash, 2007; Thavarajah et al., 2014).
Phytic acid (PA), also known as inositol hexaphosphate or IP6, has been identified as a one of the major inhibitors of iron bioavailability. PA serves as the principal form of phosphorus storage in seeds, where it is present as a phytate salt of mineral cations like potassium, magnesium, calcium, manganese, and zinc. Depending on the species, cultivar and conditions of growth, it may constitute 40–84% of total seed phosphorus (Lolas et al., 1976; Griffiths and Thomas, 1981; Ravindran et al., 1994). Amongst the inositol polyphosphates, the lower inositol phosphates IP2, IP3, and IP4 play a minor role in inhibiting iron bioavailability (Sandberg et al., 1989, 1999). The main inhibitors are IP6 and IP5, which are capable of reducing iron solubility by 38.8 and 33%, respectively, through the formation of insoluble complexes (Sandberg et al., 1989).
The impact of PA on biofortification efforts can be illustrated using the example of the biofortified beans. Despite their higher iron content, feeding trials conducted in Rwanda have indicated iron bioavailability of biofortified beans was similar, if not lower, than that of the unfortified beans (Petry et al., 2012, 2014). As a result, while the total of iron absorbed from the biofortified beans was higher than the unfortified beans, it was considerably less than expected. As a means to improve the effectiveness of biofortification, the reduction in PA concentration was recommended by the authors (Petry et al., 2012, 2014).
This recommendation has been applied in several cereal crops (Larson et al., 1998, 2000; Raboy et al., 2000; Guttieri et al., 2004) and more recently in bean (Campion et al., 2009). The effectiveness of the low-phytic acid bean lines is currently inconclusive however, as bioavailability assessments have yielded conflicting results due to differences in experimental design (Petry et al., 2013, 2016). Poor cooking quality was also observed in the low-phytic acid seeds, which may have contributed the adverse gastrointestinal side-effects in the participants in one of the studies (Petry et al., 2016). The relationship between phytic acid and cooking quality have been alluded to in other studies on lentil and bean (Kon and Sanchuck, 1981; Bhatty and Slinkard, 1989). Interestingly, no such effect was reported in low-phytic acid maize lines (Mendoza et al., 1998); whether this is a legume-specific issue remains to be confirmed. Aside from influencing cooking quality, phytic acid is also known to have antioxidant properties and protective effects against heart disease and cancer (Sharma, 1986; Nelson et al., 1988; Vucenik and Shamsuddin, 2003). It is unknown if reduction in phytic acid content would affect such properties. Similarly, the subsequent long-term effect on human health is unknown.
For all the challenges presented in this section however, there is much potential to be explored in pulse biofortification. Much of the existing knowledge concerning this area is limited to the work done on the iron biofortified bean. That has proven to be a successful means of alleviating iron deficiency, promising much for other pulses.
Chickpea as a target for biofortification
Chickpea (Cicer arietinum) is an important pulse crop that has been cultivated by humans since the Stone Age. As of 2009, it is the second most important pulse crop in the world after the common bean, having overtaken peas as the pulse crop with the second highest global production values. Global production has climbed steadily since 2008 to exceed 14.2 million tons in 2014, of which approximately 96% is grown in developing countries (FAO, 2016b). India in particular, has historically been the largest producer and consumer of chickpea; in 2013 alone it contributed approximately 65 and 33% to total chickpea production and import, respectively (FAO, 2016b). In terms of consumption, it is difficult to obtain precise statistics due to the lack of available data. However, based on calculations using production and trade values, the global average for chickpea consumption was estimated to be around 1.3 kg/year per person between 2006 and 2008, with South Asia and the Middle East-North Africa regions being the biggest consumers at 4.25 kg/person and 2.11 kg/person per year, respectively (Akibode and Maredia, 2012). The demand is predicted to grow, particularly in Africa and Asia, due to population increase and increasing support from the governments in encouraging pulse consumption (Rao et al., 2010; Akibode and Maredia, 2012). This increase in demand is not limited to those regions; in the USA for instance, net domestic use of chickpea nearly doubled from 199.6 g in 2010 to 322.1 g in 2014 (Wells, 2016).
Most of the chickpea in the global market can be classified into two main types which are primarily distinguishable by their seed morphology, specific aspects of which influence their end-use. The first type is the kabuli, also known as garbanzos. Kabuli seeds are large and round, weighing approximately 400 mg per seed (Pulse Australia, 2016a). The seed coat is thin and light-colored, ranging from shades of white to cream and the seeds are typically consumed whole or made into hummus (Gaur et al., 2015; Pulse Australia, 2016a). Kabuli cultivation areas are mostly located in Southern Europe, Northern Africa, Afghanistan, Pakistan, Chile, and India (Gaur et al., 2015).
The second type is the desi, which forms the bulk of the international export market (Rao et al., 2010). Desi seeds are small, wrinkled, and angular, with an approximate weight of 120 mg per seed (Pulse Australia, 2016a). The seed coat is also 1.2 to 3 times thicker than the kabuli (Umaid et al., 1984; Wood et al., 2011) and can be found in a greater variety of colors ranging from brown to yellow, as well as orange, black and green. Desi seeds are commonly dehulled and split to obtain the cotyledons, which are then known as chana dhal and can in turn be milled to flour, known as besan or gram flour.
As a food crop, chickpea can be utilized in a variety of ways. Green pods, immature seeds and young leaves can be consumed as a vegetable while the stover and pod husks can be used as animal feed (Ibrikci et al., 2003; Yadav et al., 2007). The primary commodity however, is the dried mature seed which can be used as animal feed or for human consumption. With the latter, the long history of consumption in various regions such as India, the Middle East, and Europe has given rise to a diversity of dishes in which chickpea can be utilized. Chickpeas are consumed on their own or with other foods; seeds may be eaten whole, hulled, or ground into flour from which other products may be derived. Preparation for consumption can be by various processing methods such as soaking, sprouting, fermenting, boiling, steaming, roasting, extrusion, and puffing (Yadav et al., 2007), all of which exert different effects on the overall nutritional quality (Poltronieri et al., 2000; Sebastiá et al., 2001; Ghavidel and Prakash, 2007; Hemalatha et al., 2007a).
Much like other pulses, the nutritional qualities of chickpea have long been recognized and documented. In addition to high protein content (20–22%), chickpeas are also rich in micronutrients like folate, magnesium, zinc, and iron (USDA, 2013). Studies conducted by different authors have found iron content to range from 2.4 to 11 mg/100 g (e.g., USDA1; Meiners et al., 1976; Wood and Grusak, 2007). Likewise, various studies have reported differing values for phytic acid and other antinutrients (e.g., Chitra et al., 1995; Ghavidel and Prakash, 2007; Hemalatha et al., 2007b), indicating a possible effect of genotype and environmental factors on overall iron bioavailability. When measured as dialyzable iron generated from a simulated gastrointestinal digest, bioavailability has been found to vary widely across different studies, ranging from about 6 to 25% (Chitra et al., 1997; Ghavidel and Prakash, 2007; Hemalatha et al., 2007b). The reason behind this disparity is as yet unclear, though analytical procedures and variations in samples have been suggested as a possible cause (Platel and Srinivasan, 2016). Given the multifaceted nature of nutrient bioavailability, the values obtained are at best relative.
Regardless of processing methods and culinary adjustments, the composition of the starting material is vital. As demonstrated by Petry et al. (2012), enhancement of iron content alone does not necessarily translate into an improved iron status of the consumer, particularly when there is a concurrent enhancement in phytate content. Both the iron content and overall composition of the grain itself should therefore be considered in biofortification strategies. However, in light of the lack of information concerning bioavailability, it would be prudent for biofortification efforts to first target total seed iron content before progressing to bioavailability. Considerable progress has been made to that end, particularly with the growing interest in chickpea as a target for iron biofortification. While a concerted global effort has yet to materialize, pockets of development have emerged with India and Canada at the forefront. To date, the chickpea genome has been sequenced (Varshney et al., 2013). Chickpea populations in those countries have also been screened for genetic diversity and iron accumulation traits, allowing for identification of the associated QTLs (Diapari et al., 2014; Upadhyaya et al., 2016). In terms of biofortification via GM, no work has been done yet. It is however, a viable option—while chickpea can be considered a recalcitrant species, successful transformation protocols have been established (Sarmah et al., 2004; Indurker et al., 2010). Such work would also provide insight into the physiological workings; this in turn can inform later biofortification efforts by identifying specific traits or mechanisms which can be targeted by breeding or GM.
Given the relative youth of this endeavor to biofortify chickpea for iron, no biofortification targets have yet been set. As stated by Bouis and Welch (2010), several variables need be considered in the setting of such targets. The challenge lies primarily in the lack of information concerning the different variables in chickpea. Unlike the common bean which serves as a staple, chickpea is a secondary staple and depending on the type and cultivar, may be processed into various forms for consumption (Yadav et al., 2007). This would in turn affect iron content and bioavailability. Consequently, the consumption profile for chickpea is expected to be lower and potentially more varied compared to the common bean, particularly across different age and cultural demographics. Until more detailed and specific information concerning chickpea is obtained, only general assumptions may be made. In the interim however, efforts can be made to understand and engineer for increased iron content.
Potential approaches to engineering for enhanced iron content in chickpea
In the interest of iron biofortification, five rate-limiting steps to grain iron accumulation have been identified by Sperotto et al. (2012): (1) uptake from soil, (2) xylem loading in roots, (3) phloem transport from leaves, (4) unloading for grain filling, and (5) grain sink strength. These steps can be classified into the three main processes of uptake, translocation, and storage. Genes associated with these processes have been identified as promising candidates for iron biofortification, and over the past decade several of them have been applied to different plant species.
Rice—a case study
Amongst these, rice can be considered the flagship for transgenic iron biofortification. Since rice is a grain crop, the lessons learnt may, in part, be transferrable to chickpea. As illustrated in the review by Masuda et al. (2013a), several gene combinations targeting the uptake, translocation, storage or any combination of the three processes have been attempted to differing levels of success. Of the combinations investigated thus far, those containing NAS and ferritin (FER) have yielded the most promising results. Individually, NAS and FER have been demonstrated to enhance iron accumulation. Overexpression of the former for instance, increased iron content in polished grains by four-fold (Johnson et al., 2011), while overexpression of a soy homolog of the latter produced up to a 3.7-fold increase (Vasconcelos et al., 2003). Similar results have been obtained when combined with other genes involved in iron transport or MAs synthesis. Approximately three-fold increase was obtained from overexpression of the rice yellow stripe like-2 (YSL2), barley NAS1, and soybean FER genes (Masuda et al., 2012). A four-fold increase was obtained from overexpressing soybean FER in conjunction with barley NAS1, two NAAT genes and a mugineic acid synthase gene (Masuda et al., 2013b).
The best results however, were achieved simply by using just the NAS-FER combination. Constitutive expression of Arabidopsis NAS1 together with barley FER and a fungal phytase, both under the regulation of a rice seed storage globulin promoter, enhanced iron accumulation in rice endosperm by up to six times (Wirth et al., 2009). More recently, up to 7.5-fold increase in iron content was obtained from transgenic rice through constitutive overexpression of OsNAS2 and seed-specific expression of soybean FER (Trijatmiko et al., 2016). The enhancement was not limited to total iron content alone, as iron bioavailability was similarly improved. This was demonstrated in studies with Caco-2 cell cultures, where the amount of iron absorbed from the transgenic lines was more than double that of the controls, even in the absence of any bioavailability enhancers (Trijatmiko et al., 2016).
Tailoring a GM approach to chickpea
Whether a similar feat may be emulated in other species is yet unknown as the NAS-FER gene combination has only been applied in rice. In this case, the success of the NAS-FER gene combination may be attributed to the unique role of NA in the non-graminaceous system. In it, NA, by virtue of its in role in the synthesis of DMA and MAs, is directly involved in the uptake and translocation processes (Wang et al., 2013). Through combination with FER, it allows for the simultaneous targeting of all three major processes of iron metabolism, thereby overcoming the rate-limiting steps listed by Sperotto et al. (2012). Incidentally, the issue of bioavailability is also resolved—NA is a known enhancer of iron bioavailability (Zheng et al., 2010), while ferritin has a bioavailability equivalent to that of ferrous sulfate which is used in iron supplements (Davila-Hicks et al., 2004; Lönnerdal et al., 2006).
Based on these observations, there is reason to believe that application of the NAS-FER approach to other graminaceous species will yield similar outcomes to rice. The effect however, may be limited in non-graminaceous crops like chickpea due to the absence of the MAs biosynthetic pathway, which diminishes the contribution of NAS to the uptake process. Such is evident when comparing the results of NAS overexpression in graminaceous and non-graminaceous species, where greater enhancement in iron content was observed in the former (Masuda et al., 2009; Johnson et al., 2011) than the latter (Douchkov et al., 2005; Cassin et al., 2009). In any case, prior studies in model species like Arabidopsis and tobacco have confirmed the individual effect of each gene (Van Wuytswinkel et al., 1999; Douchkov et al., 2005; Cassin et al., 2009); should the NAS-FER approach be applied to chickpea, some enhancement of seed iron content can still be expected. Similarly, iron bioavailability can increase, though the extent is difficult to predict given the higher levels of inhibitors in chickpeas compared to rice (Hemalatha et al., 2007b).
At the moment these are speculations—with the existing transgenic research concentrated on cereals, there is little precedent for reliable extrapolation to a leguminous crop. Nonetheless, two main principles can be drawn from the success of the NAS-FER strategy in rice, and that is (1) the simultaneous targeting of multiple rate-limiting steps, and 2) targeting of bioavailability in addition to iron content.
Targeting multiple rate-limiting steps
One of the main challenges to engineering for iron accumulation in chickpea is the lack of specific knowledge on iron homeostasis in chickpea. Even amongst closely related members of non-Gramineae, interspecies variation exists as different mechanisms or components may be favored. Such is evident when comparing studies on QTLS and iron accumulation traits—differing suites of associated genes have been found in soybean (Ning et al., 2015) and chickpea (Upadhyaya et al., 2016).
Examination of such genes may yield potential candidates for use in GM biofortification. Drawing from the example of NAS in rice, selection of such candidate genes can be based on their involvement in both uptake and translocation processes, though extra emphasis should be placed on the former. As mentioned by Sperotto et al. (2012), the ability to access soil iron under differing environmental conditions is the first major bottleneck to iron accumulation in plants, with poor uptake capacity typically associated with susceptibility to iron deficiency (Mahmoudi et al., 2007; Waters and Troupe, 2012).
Several genes fitting such criteria can be found in the list by Upadhyaya et al. (2016), and key examples include transporters like IRT, FRO, YSL, NRAMP (natural resistance-associated macrophage protein), and zinc-regulated transporter, iron-regulated transporter-like protein (ZIP). As these are involved in both uptake and translocation (Vert et al., 2002; Lanquar et al., 2005; Vasconcelos et al., 2006), targeting them may, theoretically, allow for two processes to be simultaneously enhanced. Constitutive overexpression of AtFRO2 in soybean for instance, was found to increase both iron uptake and leaf iron content (Vasconcelos et al., 2006). However, this effect may be dependent on the homolog used as each may serve specific functions. Within FRO family in Arabidopsis for instance, AtFRO2 is expressed in the roots and facilitates uptake during iron deficiency (Connolly et al., 2003), while FRO7 is expressed in the chloroplasts where it contributes to iron supply (Jeong et al., 2008).
A potential pitfall to such an approach is the inherent regulatory mechanisms. Unlike NAS, which appears to be amenable to manipulation with little to no side-effects (Pianelli et al., 2005; Johnson et al., 2011; Lee et al., 2012), transporters like IRT and FRO appear to be regulated by mechanisms which are less forgiving to interference. In Arabidopsis overexpressing AtIRT1 or AtFRO2, no increase in root reduction or protein levels was observed under iron-sufficient conditions due to post-transcriptional regulation (Connolly et al., 2002, 2003). However, no such impediment was noted when the maize homolog, ZmIRT1, was overexpressed, with transgenic lines having significantly higher seed iron contents compared to the wild-type (Li et al., 2015). This discrepancy was attributed to the low homology between the ZmIRT1 and the native IRT genes, which may in turn point to a means of bypassing post-transcriptional regulation.
Aside from enhancing uptake and translocation, sink strength may also be targeted. As far as GM biofortification efforts go, this has traditionally been done using FER. The application in chickpea appears to be highly feasible as amongst the fifteen genes associated with seed iron accumulation in chickpea, two were identified as ferritins. Strong constitutive FER overexpression however, carries the risk of excessive iron sequestration, resulting in manifestation iron deficiency symptoms (Van Wuytswinkel et al., 1999). This may be avoided through seed-specific expression, particularly in the cotyledons which are the main products. Much like the NAS-FER approach in rice, a multigenic approach combining FER with one of the aforementioned transporters may also be used. This will also allow for simultaneous targeting of all three major processes of iron metabolism, thereby overcoming the bottlenecks described by Sperotto et al. (2012). Theoretically, such a multigenic approach may also translate to higher levels of iron accumulation through synergy between the transgenes. Such was observed in rice, where the NAS-FER combination produced a synergistic effect (Wirth et al., 2009; Trijatmiko et al., 2016), resulting in higher seed iron contents compared to the monogenic NAS approach (Masuda et al., 2009; Johnson et al., 2011; Lee et al., 2011).
Targeting bioavailability
As previously mentioned, the use of FER has the added benefit of enhancing iron bioavailability in addition to total iron content. Concerning the enhancement of bioavailability however, the use of FER is but one means. Other options may include targeting the concentrations of inhibitors or enhancers. The former has already been attempted in maize and rice through the overexpression of phytase, and increases in bioavailability have been reported (Lucca et al., 2001; Drakakaki et al., 2005; Wirth et al., 2009). While promising, the actual effect on human health is unknown as the assays were done using in vitro methods. Given that no negative consequences were observed from the addition of exogenous phytase to food (Hurrell et al., 2003), it is likely that the detrimental effects observed with low-phytate beans (Petry et al., 2016) may be avoided.
Concerning bioavailability enhancers, some work has already been done in the form of NAS-overexpressing crops and the results discussed in the above sections (Johnson et al., 2011; Trijatmiko et al., 2016). An alternative candidate is ascorbic acid, a potent enhancer occurring naturally in plants which has been demonstrated to prevent the inhibitory effects of phytate and polyphenols (Hallberg et al., 1989; Siegenberg et al., 1991). However, while promising, ascorbic acid is also infamous for its thermal instability (Van den Broeck et al., 1998; Munyaka et al., 2010), with cooking generally resulting in degradation (Sood and Malhotra, 2002; Moriyama and Oba, 2008). It's effectiveness in a transgenic biofortification strategy is therefore questionable given the processing requirements of a grain crop like chickpea.
Summary and implications
In summary, iron deficiency is a global health problem which may be alleviated through the use of biofortified crops. Transgenic biofortification efforts, as well as most studies on iron metabolism, thus far have largely been directed at cereal crops like rice. As members of the Gramineae family, their molecular biology and physiology differ significantly from their non-graminaceous counterparts. Consequently, biofortification strategies successfully applied in a graminaceous species like rice may behave differently in a non-graminaceous species. The extent of this difference is currently unclear as studies have primarily been performed in model species like Arabidopsis and tobacco primarily for gene characterization. There is a need to tailor specific biofortification strategies for use in non-graminaceous species. This is particularly so for important secondary staples like pulses—population growth as well as environmental pressures has increased the demand for affordable, water-efficient sources of protein. As the second most important pulse crop in the world, chickpea stands in a unique position to meet this need. It is widely consumed in the Asian and African regions where population growth, as well as the incidence of iron deficiency, is highest. The iron biofortification of chickpea can therefore serve as a sustainable means to alleviate the public health burden where it is heaviest.
While some breeding work is currently underway, there has been no recorded attempts to biofortify chickpea via a GM approach. However, the avenue to do so is available, given the establishment of successful transformation protocols. Valuable lessons can be learnt from the success of the GM biofortified rice and applied to the formulation of biofortification strategies for pulse crops like chickpea. Existing QTL and trait analysis have identified several candidate genes which may be used to enhance iron content and/or bioavailability, opening up new doors for further exploration.
Author contributions
This review was part of a larger project designed and headed by SM, BW, AJ, and SD. The document was written by GT, TMLH and MRK. Drafts were edited by the other authors, and upon their approval, was submitted for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1USDA Basic Report: 16056, Chickpeas (Garbanzo Beans, Bengal Gram), Mature Seeds, Raw.
Funding. This review was written as part of the Tropical Pulses for Queensland, funded by the Queensland Government.
References
- Aciksoz S. B., Yazici A., Ozturk L., Cakmak I. (2011). Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 349, 215–225. 10.1007/s11104-011-0863-2 [DOI] [Google Scholar]
- Agegnehu G., Ghizaw A., Sinebo W. (2006). Yield performance and land-use efficiency of barley and faba bean mixed cropping in Ethiopian highlands. Eur. J. Agron. 25, 202–207. 10.1016/j.eja.2006.05.002 [DOI] [Google Scholar]
- Aisen P., Enns C., Wessling-Resnick M. (2001). Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 33, 940–959. 10.1016/S1357-2725(01)00063-2 [DOI] [PubMed] [Google Scholar]
- Akibode S., Maredia M. K. (2012). Global and Regional Trends in Production, Trade and Consumption of Food Legume Crops. Michigan State University, Michigan, USA.
- Alvarez-Uria G., Naik P. K., Midde M., Yalla P. S., Pakam R. (2014). Prevalence and severity of anaemia stratified by age and gender in rural India. Anemia 2014:176182. 10.1155/2014/176182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha K., Agarwal S., Rao Y. V., Rao K. V., Viraktamath B. C., Sarla N. (2012). Mapping QTLs and candidate genes for iron and zinc concentrations in unpolished rice of Madhukar × Swarna RILs. Gene 508, 233–240. 10.1016/j.gene.2012.07.054 [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Baltussen R., Knai C., Sharan M. (2004). Iron fortification and iron supplementation are cost-effective interventions to reduce iron deficiency in four subregions of the world. J. Nutr. 134, 2678–2684. [DOI] [PubMed] [Google Scholar]
- Banana21 (2016). Biofortification.
- Banik P., Midya A., Sarkar B. K., Ghose S. S. (2006). Wheat and chickpea intercropping systems in an additive series experiment: advantages and weed smothering. Eur. J. Agron. 24, 325–332. 10.1016/j.eja.2005.10.010 [DOI] [Google Scholar]
- Bashir K., Inoue H., Nagasaka S., Takahashi M., Nakanishi H., Mori S., et al. (2006). Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J. Biol. Chem. 281, 32395–32402. 10.1074/jbc.M604133200 [DOI] [PubMed] [Google Scholar]
- Batool R., Butt M. S., Sultan M. T., Saeed F., Naz R. (2013). Protein-energy malnutrition; a risk factor for various ailments. Crit. Rev. Food Sci. Nutr. 55, 242–253. 10.1080/10408398.2011.651543 [DOI] [PubMed] [Google Scholar]
- Bereczky Z., Wang H.-Y., Schubert V., Ganal M., Bauer P. (2003). Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J. Biol. Chem. 278, 24697–24704. 10.1074/jbc.M301365200 [DOI] [PubMed] [Google Scholar]
- Bhatty R. S., Slinkard A. E. (1989). Relationship between phytic acid and cooking quality in lentil. Can. Inst. Food Sci. Technol. J. 22, 137–142. 10.1016/S0315-5463(89)70349-7 [DOI] [Google Scholar]
- Björn-Rasmussen E., Hallberg L., Isaksson B., Arvidsson B. (1974). Food iron absorption in man. Applications of the two-pool extrinsic tag method to measure heme and nonheme iron absorption from the whole diet. J. Clin. Investig. 53:247. 10.1172/JCI107545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair M. W., Astudillo C., Grusak M. A., Graham R., Beebe S. E. (2009). Inheritance of seed iron and zinc concentrations in common bean (Phaseolus vulgaris L.). Mol. Breed. 23, 197–207. 10.1007/s11032-008-9225-z [DOI] [Google Scholar]
- Blair M. W., Knewtson S. J., Astudillo C., Li C.-M., Fernandez A. C., Grusak M. A. (2010). Variation and inheritance of iron reductase activity in the roots of common bean (Phaseolus vulgaris L.) and association with seed iron accumulation QTL. BMC Plant Biol. 10:215. 10.1186/1471-2229-10-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair M. W., Wu X., Bhandari D., Astudillo C. (2016). Genetic dissection of ICP-detected nutrient accumulation in the whole seed of common bean (Phaseolus vulgaris L.). Front. Plant Sci. 7:219. 10.3389/fpls.2016.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortesi L., Fischer R. (2015). The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 33, 41–52. 10.1016/j.biotechadv.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Bothwell T. H. (1995). Overview and mechanisms of iron regulation. Nutr. Rev. 53, 237–245. 10.1111/j.1753-4887.1995.tb05480.x [DOI] [PubMed] [Google Scholar]
- Bouis H. E., Welch R. M. (2010). Biofortification — a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 50, S20–S32. 10.2135/cropsci2009.09.0531 [DOI] [Google Scholar]
- Briat J.-F., Lobréaux S. (1997). Iron transport and storage in plants. Trends Plant Sci. 2, 187–193. 10.1016/S1360-1385(97)85225-9 [DOI] [Google Scholar]
- Briat J.-F., Dubos C., Gaymard F. (2015). Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 20, 33–40. 10.1016/j.tplants.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Brown J. C., Chaney R. L. (1971). Effect of iron on the transport of citrate into the xylem of soybeans and tomatoes. Plant Physiol. 47, 836–840. 10.1104/pp.47.6.836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann W., Maas-Kantel K., Moog P. R. (1993). Iron uptake by leaf mesophyll cells: the role of the plasma membrane-bound ferric-chelate reductase. Planta 190, 151–155. 10.1007/BF00196606 [DOI] [Google Scholar]
- Brumbarova T., Bauer P., Ivanov R. (2015). Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 20, 124–133. 10.1016/j.tplants.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Burton J. W., Harlow C., Theil E. C. (1998). Evidence for reutilization of nodule iron in soybean seed development. J. Plant Nutr. 21, 913–927. 10.1080/01904169809365453 [DOI] [Google Scholar]
- Cakmak I., Pfeiffer W. H., McClafferty B. (2010). REVIEW: biofortification of durum wheat with zinc and iron. Cereal Chem. J. 87, 10–20. 10.1094/CCHEM-87-1-0010 [DOI] [Google Scholar]
- Campion B., Sparvoli F., Doria E., Tagliabue G., Galasso I., Fileppi M., et al. (2009). Isolation and characterisation of an lpa (low phytic acid) mutant in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 118, 1211–1221. 10.1007/s00122-009-0975-8 [DOI] [PubMed] [Google Scholar]
- Caracuta V., Barzilai O., Khalaily H., Milevski I., Paz Y., Vardi J., et al. (2015). The onset of faba bean farming in the Southern Levant. Sci. Rep. 5:14370. 10.1038/srep14370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. E., Mahoney A. W. (1992). Contributions of heme and nonheme iron to human nutrition. Crit. Rev. Food Sci. Nutr. 31, 333–367. 10.1080/10408399209527576 [DOI] [PubMed] [Google Scholar]
- Carvalhais L. C., Dennis P. G., Fedoseyenko D., Hajirezaei M. R., Borriss R., von Wirén N. (2011). Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 174, 3–11. 10.1002/jpln.201000085 [DOI] [Google Scholar]
- Cassin G., Mari S., Curie C., Briat J.-F., Czernic P. (2009). Increased sensitivity to iron deficiency in Arabidopsis thaliana overaccumulating nicotianamine. J. Exp. Bot. 60, 1249–1259. 10.1093/jxb/erp007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercamondi C. I., Egli I. M., Mitchikpe E., Tossou F., Zeder C., Hounhouigan J. D., et al. (2013). Total iron absorption by young women from iron-biofortified pearl millet composite meals is double that from regular millet meals but less than that from post-harvest iron-fortified millet meals. J. Nutr. 143, 1376–1382. 10.3945/jn.113.176826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitra U., Singh U., Rao P. V. (1997). Effect of varieties and processing methods on the total and ionizable iron contents of grain legumes. J. Agric. Food Chem. 45, 3859–3862. 10.1021/jf970073a [DOI] [Google Scholar]
- Chitra U., Vimala V., Singh U., Geervani P. (1995). Variability in phytic acid content and protein digestibility of grain legumes. Plant Foods Hum. Nutr. 47, 163–172. 10.1007/BF01089266 [DOI] [PubMed] [Google Scholar]
- Connolly E. L., Campbell N. H., Grotz N., Prichard C. L., Guerinot M. L. (2003). Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 133, 1102–1110. 10.1104/pp.103.025122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly E. L., Fett J. P., Guerinot M. L. (2002). Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14, 1347–1357. 10.1105/tpc.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connorton J. M., Balk J., Rodríguez-Celma J. (2017). Iron homeostasis in plants – a brief overview. Metallomics 9, 813–823. 10.1039/C7MT00136C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C., Mari S. (2017). New routes for plant iron mining. New Phytol. 214, 521–525. 10.1111/nph.14364 [DOI] [PubMed] [Google Scholar]
- Curie C., Panaviene Z., Loulergue C., Dellaporta S. L., Briat J. F., Walker E. L. (2001). Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409, 346–349. 10.1038/35053080 [DOI] [PubMed] [Google Scholar]
- Davila-Hicks P., Theil E. C., Lönnerdal B. (2004). Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am. J. Clin. Nutr. 80, 936–940. [DOI] [PubMed] [Google Scholar]
- De Moura F. F., Palmer A. C., Finkelstein J. L., Haas J. D., Murray-Kolb L. E., Wenger M. J., et al. (2014). Are biofortified staple food crops improving Vitamin A and iron status in women and children? New evidence from efficacy trials. Adv. Nutr. 5, 568–570. 10.3945/an.114.006627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orto M., Santi S., De Nisi P., Cesco S., Varanini Z., Zocchi G., et al. (2000). Development of Fe-deficiency responses in cucumber (Cucumis sativus L.) roots: Involvement of plasma membrane H+-ATPase activity. J. Exp. Bot. 51, 695–701. 10.1093/jexbot/51.345.695 [DOI] [PubMed] [Google Scholar]
- Diapari M., Sindhu A., Bett K., Deokar A., Warkentin T. D., Tar'an B. (2014). Genetic diversity and association mapping of iron and zinc concentrations in chickpea (Cicer arietinum L.). Genome 57, 459–468. 10.1139/gen-2014-0108 [DOI] [PubMed] [Google Scholar]
- Douchkov D., Gryczka C., Stephan U. W., Hell R., Bäumlein H. (2005). Ectopic expression of nicotianamine synthase genes results in improved iron accumulation and increased nickel tolerance in transgenic tobacco. Plant Cell Environ. 28, 365–374. 10.1111/j.1365-3040.2005.01273.x [DOI] [Google Scholar]
- Drakakaki G., Marcel S., Glahn R., Lund E., Pariagh S., Fischer R., et al. (2005). Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol. Biol. 59, 869–880. 10.1007/s11103-005-1537-3 [DOI] [PubMed] [Google Scholar]
- Eide D., Broderius M., Fett J., Guerinot M. L. (1996). A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. U.S.A. 93, 5624–5628. 10.1073/pnas.93.11.5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (1994). Pulses and Derived Products.
- FAO (2012). The State of Food Insecurity in the World. FAO Economic and Social Development Department, Rome. 28140324
- FAO (2014). Food Outlook: Biannual Report on Global Food Markets. FAO.
- FAO (2016a). Annual Growth Rates of Total Pulse Production from 1961 to 2013. Available online at: http://faostat3.fao.org/browse/Q/QC/E
- FAO (2016b). FAO Statistical Database (FAOSTAT).
- Finkelstein J. L., Haas J. D., Mehta S. (2017). Iron-biofortified staple food crops for improving iron status: a review of the current evidence. Curr. Opin. Biotechnol. 44, 138–145. 10.1016/j.copbio.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. L., Mehta S., Udipi S. A., Ghugre P. S., Luna S. V., Wenger M. J., et al. (2015). A randomized trial of iron-biofortified pearl millet in school children in India. J. Nutr. 145, 1576–1581. 10.3945/jn.114.208009 [DOI] [PubMed] [Google Scholar]
- Fuller D. Q., Korisettar R., Venkatasubbaiah P. C. (2001). Southern Neolithic cultivation systems: a reconstruction based on archaeobotanical evidence. South Asian Stud. 17, 171–187. 10.1080/02666030.2001.9628599 [DOI] [Google Scholar]
- Garnett T. P., Graham R. D. (2005). Distribution and remobilization of iron and copper in wheat. Ann. Bot. 95, 817–826. 10.1093/aob/mci085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur P. M., Samineni S., Sajja S., Chibbar R. N. (2015). Achievements and challenges in improving nutritional quality of chickpea. Legume Perspect. 31–33. [Google Scholar]
- Gera T., Sachdev H. S., Boy E. (2012). Effect of iron-fortified foods on hematologic and biological outcomes: systematic review of randomized controlled trials. Am. J. Clin. Nutr. 96, 309–324. 10.3945/ajcn.111.031500 [DOI] [PubMed] [Google Scholar]
- Ghavidel R. A., Prakash J. (2007). The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci. Technol. 40, 1292–1299. 10.1016/j.lwt.2006.08.002 [DOI] [Google Scholar]
- Gooding M. J., Kasyanova E., Ruske R., Hauggaard-Nielsen H., Jensen E. S., Dahlmann C., et al. (2007). Intercropping with pulses to concentrate nitrogen and sulphur in wheat. J. Agric. Sci. 145, 469–479. 10.1017/S0021859607007241 [DOI] [Google Scholar]
- Goto F., Yoshihara T., Saiki H. (2000). Iron accumulation and enhanced growth in transgenic lettuce plants expressing the iron-binding protein ferritin. Theor. Appl. Genet. 100, 658–664. 10.1007/s001220051336 [DOI] [Google Scholar]
- Griffiths D. W., Thomas T. A. (1981). Phytate and total phosphorus content of field beans (Vicia faba L.). J. Sci. Food Agric. 32, 187–192. 10.1002/jsfa.2740320215 [DOI] [Google Scholar]
- Grillet L., Ouerdane L., Flis P., Hoang M. T. T., Isaure M.-P., Lobinski R., et al. (2014). Ascorbate efflux as a new strategy for iron reduction and transport in plants. J. Biol. Chem. 289, 2515–2525. 10.1074/jbc.M113.514828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttieri M., Bowen D., Dorsch J. A., Raboy V., Souza E. (2004). Identification and characterization of a low phytic acid wheat. Crop Sci. 44, 418–424. 10.2135/cropsci2004.4180 [DOI] [Google Scholar]
- Haas J. D., Beard J. L., Murray-Kolb L. E., del Mundo A. M., Felix A., Gregorio G. B. (2005). Iron-biofortified rice improves the iron stores of nonanemic Filipino women. J. Nutr. 135, 2823–2830. [DOI] [PubMed] [Google Scholar]
- Haber F., Weiss J. (1934). The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. A 147, 332–351. 10.1098/rspa.1934.0221 [DOI] [Google Scholar]
- Hallberg L., Brune M., Rossander L. (1989). Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 49, 140–144. [DOI] [PubMed] [Google Scholar]
- HarvestPlus (2015a). Crops. 28955035
- HarvestPlus (2015b). FAQ about Biofortification.
- Hell R., Stephan U. W. (2003). Iron uptake, trafficking and homeostasis in plants. Planta 216, 541–551. 10.1007/s00425-002-0920-4 [DOI] [PubMed] [Google Scholar]
- Hemalatha S., Platel K., Srinivasan K. (2007a). Influence of heat processing on the bioaccessibility of zinc and iron from cereals and pulses consumed in India. J. Trace Elem. Med. Biol. 21, 1–7. 10.1016/j.jtemb.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Hemalatha S., Platel K., Srinivasan K. (2007b). Zinc and iron contents and their bioaccessibility in cereals and pulses consumed in India. Food Chem. 102, 1328–1336. 10.1016/j.foodchem.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Higuchi K., Kanazawa K., Nishizawa N.-K., Chino M., Mori S. (1994). Purification and characterization of nicotianamine synthase from Fe-deficient barley roots. Plant Soil 165, 173–179. 10.1007/BF00008059 [DOI] [Google Scholar]
- Higuchi K., Nakanishi H., Suzuki K., Nishizawa N. K., Mori S. (1999). Presence of nicotianamine synthase isozymes and their homologues in the root of graminaceous plants. Soil Sci. Plant Nutr. 45, 681–691. 10.1080/00380768.1999.10415831 [DOI] [Google Scholar]
- Higuchi K., Nishizawa N.-K., Yamaguchi H., Römheld V., Marschner H., Mori S. (1995). SHORT COMMUNICATION: Response of nicotianamine synthase activity to Fe-deficiency in tobacco plants as compared with barley. J. Exp. Bot. 46, 1061–1063. 10.1093/jxb/46.8.1061 [DOI] [Google Scholar]
- Hocking P. J., Pate J. S. (1977). Mobilization of minerals to developing seeds of legumes. Ann. Bot. 41, 1259–1278. 10.1093/oxfordjournals.aob.a085415 [DOI] [Google Scholar]
- Horton S., Mannar V., Wesley A. (2008). Micronutrient fortification (iron and salt iodization), in Copenhagen Consensus 2008 (Denmark: ). [Google Scholar]
- House W. A. (1999). Trace element bioavailability as exemplified by iron and zinc. Field Crops Res. 60, 115–141. 10.1016/S0378-4290(98)00136-1 [DOI] [Google Scholar]
- Hurrell R. F., Reddy M. B., Juillerat M.-A., Cook J. D. (2003). Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 77, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Ibrikci H., Knewtson S. J. B., Grusak M. A. (2003). Chickpea leaves as a vegetable green for humans: evaluation of mineral composition. J. Sci. Food Agric. 83, 945–950. 10.1002/jsfa.1427 [DOI] [Google Scholar]
- Ihemere U. E., Narayanan N. N., Sayre R. T. (2012). Iron biofortification and homeostasis in transgenic cassava roots expressing the algal iron assimilatory gene, FEA1. Front. Plant Sci. 3:71. 10.3389/fpls.2012.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMECHE (2013). Global Food - Waste Not, Want Not. Wales. [Google Scholar]
- Indurker S., Misra H. S., Eapen S. (2010). Agrobacterium-mediated transformation in chickpea (Cicer arietinum L.) with an insecticidal protein gene: optimisation of different factors. Physiol. Mol. Biol. Plants 16, 273–284. 10.1007/s12298-010-0030-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Kobayashi T., Nozoye T., Takahashi M., Kakei Y., Suzuki K., et al. (2009). Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 284, 3470–3479. 10.1074/jbc.M806042200 [DOI] [PubMed] [Google Scholar]
- Iqbal A., Khalil I. A., Ateeq N., Sayyar Khan M. (2006). Nutritional quality of important food legumes. Food Chem. 97, 331–335. 10.1016/j.foodchem.2005.05.011 [DOI] [Google Scholar]
- Ishimaru Y., Masuda H., Bashir K., Inoue H., Tsukamoto T., Takahashi M., et al. (2010). Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 62, 379–390. 10.1111/j.1365-313X.2010.04158.x [DOI] [PubMed] [Google Scholar]
- Ishimaru Y., Suzuki M., Tsukamoto T., Suzuki K., Nakazono M., Kobayashi T., et al. (2006). Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 45, 335–346. 10.1111/j.1365-313X.2005.02624.x [DOI] [PubMed] [Google Scholar]
- Jeong J., Connolly E. L. (2009). Iron uptake mechanisms in plants: functions of the FRO family of ferric reductases. Plant Sci. 176, 709–714. 10.1016/j.plantsci.2009.02.011 [DOI] [Google Scholar]
- Jeong J., Guerinot M. L. (2009). Homing in on iron homeostasis in plants. Trends Plant Sci. 14, 280–285. 10.1016/j.tplants.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Jeong J., Cohu C., Kerkeb L., Pilon M., Connolly E. L., Guerinot M. L. (2008). Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions. Proc. Natl. Acad. Sci. U.S.A. 105, 10619–10624. 10.1073/pnas.0708367105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., Zhou J., Chen J., Zhu L., Zhao Y., Huang Y. (2013). The genetic architecture of zinc and iron content in maize grains as revealed by QTL mapping and meta-analysis. Breed. Sci. 63, 317–324. 10.1270/jsbbs.63.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. A. T., Kyriacou B., Callahan D. L., Carruthers L., Stangoulis J., Lombi E., et al. (2011). Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 6:e24476. 10.1371/journal.pone.0024476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. L., Darah P. R., Kochian L. V. (1996). Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant Soil 180, 57–66. 10.1007/BF00015411 [DOI] [Google Scholar]
- Jukanti A. K., Gaur P. M., Gowda C. L. L., Chibbar R. N. (2012). Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. Br. J. Nutr. 108, S11–S26. 10.1017/S0007114512000797 [DOI] [PubMed] [Google Scholar]
- Kanazawa K., Higuchi K., Nishizawa N.-K., Fushiya S., Chino M., Mori S. (1994). Nicotianamine aminotransferase activities are correlated to the phytosiderophore secretions under Fe-deficient conditions in Gramineae. J. Exp. Bot. 45, 1903–1906. 10.1093/jxb/45.12.1903 [DOI] [Google Scholar]
- Kassebaum N. J. (2016). The global burden of anemia. Hematol. Oncol. Clin. North Am. 30, 247–308. 10.1016/j.hoc.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Kataki P. K. (2002). Shifts in cropping system and its effect on human nutrition: case study from India. J. Crop Product. 6, 119–144. 10.1300/J144v06n01_08 [DOI] [Google Scholar]
- Katsvairo L. (2015). Delivery of iron beans in Rwanda, in The 2nd Global Conference on Biofortification: Getting Nutritious Foods to People (Kigali: ). [Google Scholar]
- Kawai S., Kamei S., Matsuda Y., Ando R., Kondo S., Ishizawa A., et al. (2001). Concentrations of iron and phytosiderophores in xylem sap of iron-deficient barley plants. Soil Sci. Plant Nutr. 47, 265–272. 10.1080/00380768.2001.10408390 [DOI] [Google Scholar]
- Kehrer J. P. (2000). The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50. 10.1016/S0300-483X(00)00231-6 [DOI] [PubMed] [Google Scholar]
- Kennedy E. T., Bouis H. E. (1993). Linkages between Agriculture and Nutrition: Implications for Policy and Research. International Food Policy Research Institute, Washington, USA.
- Kim S. A., Guerinot M. L. (2007). Mining iron: iron uptake and transport in plants. FEBS Lett. 581, 2273–2280. 10.1016/j.febslet.2007.04.043 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nishizawa N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 63, 131–152. 10.1146/annurev-arplant-042811-105522 [DOI] [PubMed] [Google Scholar]
- Kodkany B. S., Bellad R. M., Mahantshetti N. S., Westcott J. E., Krebs N. F., Kemp J. F., et al. (2013). Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J. Nutr. 143, 1489–1493. 10.3945/jn.113.176677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Inoue H., Mizuno D., Takahashi M., Nakanishi H., Mori S., et al. (2004). OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 39, 415–424. 10.1111/j.1365-313X.2004.02146.x [DOI] [PubMed] [Google Scholar]
- Kon S., Sanchuck D. W. (1981). Phytate content and its effect on cooking quality of beans. J. Food Process. Preserv. 5, 169–178. 10.1111/j.1745-4549.1981.tb00632.x [DOI] [Google Scholar]
- Kumar S., Hash C. T., Thirunavukkarasu N., Singh G., Rajaram V., Rathore A., et al. (2016). Mapping quantitative trait loci controlling high iron and zinc content in self and open pollinated grains of pearl millet [Pennisetum glaucum (L.) R. Br.]. Front. Plant Sci. 7:1636. 10.3389/fpls.2016.01636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquar V., Lelièvre F., Bolte S., Hamès C., Alcon C., Neumann D., et al. (2005). Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 24, 4041–4051. 10.1038/sj.emboj.7600864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S. R., Rutger J. N., Young K. A., Raboy V. (2000). Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Sci. 40, 1397–1405. 10.2135/cropsci2000.4051397x [DOI] [Google Scholar]
- Larson S. R., Young K. A., Cook A., Blake T. K., Raboy V. (1998). Linkage mapping of two mutations that reduce phytic acid content of barley grain. Theor. Appl. Genet. 97, 141–146. 10.1007/s001220050878 [DOI] [Google Scholar]
- Lee S., Kim Y.-S., Jeon U., Kim Y.-K., Schjoerring J., An G. (2012). Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol. Cells 33, 269–275. 10.1007/s10059-012-2231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Persson D. P., Hansen T. H., Husted S., Schjoerring J. K., Kim Y.-S., et al. (2011). Bio-available zinc in rice seeds is increased by activation tagging of nicotianamine synthase. Plant Biotechnol. J. 9, 865–873. 10.1111/j.1467-7652.2011.00606.x [DOI] [PubMed] [Google Scholar]
- Li L., Cheng X., Ling H.-Q. (2004a). Isolation and characterization of Fe(III)-chelate reductase gene LeFRO1 in tomato. Plant Mol. Biol. 54, 125–136. 10.1023/B:PLAN.0000028774.82782.16 [DOI] [PubMed] [Google Scholar]
- Li L., Tang C., Rengel Z., Zhang F. (2003). Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 248, 297–303. 10.1023/A:1022389707051 [DOI] [Google Scholar]
- Li S. M., Li L., Zhang F. S., Tang C. (2004b). Acid phosphatase role in chickpea/maize intercropping. Ann. Bot. 94, 297–303. 10.1093/aob/mch140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhou X., Li H., Liu Y., Zhu L., Guo J., et al. (2015). Overexpression of ZmIRT1 and ZmZIP3 enhances iron and zinc accumulation in transgenic Arabidopsis. PLoS ONE 10:e0136647. 10.1371/journal.pone.0136647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-F., Liang H.-M., Yang S.-Y., Boch A., Clemens S., Chen C.-C., et al. (2009). Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 182, 392–404. 10.1111/j.1469-8137.2009.02766.x [DOI] [PubMed] [Google Scholar]
- Lolas G., Palamidis N., Markakis P. (1976). The phytic acid-total phosphorus relationship in barley, oats, soybeans, and wheat. Cereal Chem. J. 53, 867–871. [Google Scholar]
- Lönnerdal B., Bryant A., Liu X., Theil E. C. (2006). Iron absorption from soybean ferritin in nonanemic women. Am. J. Clin. Nutr. 83, 103–107. [DOI] [PubMed] [Google Scholar]
- Lucca P., Hurrell R., Potrykus I. (2001). Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor. Appl. Genet. 102, 392–397. 10.1007/s001220051659 [DOI] [Google Scholar]
- Ma J. F., Nomoto K. (1993). Two related biosynthetic pathways of mugineic acids in gramineous plants. Plant Physiol. 102, 373–378. 10.1104/pp.102.2.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi H., Labidi N., Ksouri R., Gharsalli M., Abdelly C. (2007). Differential tolerance to iron deficiency of chickpea varieties and Fe resupply effects. C. R. Biol. 330, 237–246. 10.1016/j.crvi.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Maillard A., Diquélou S., Billard V., Laîné P., Garnica M., Prudent M., et al. (2015). Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 6:317. 10.3389/fpls.2015.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Aung M., Nishizawa N. (2013a). Iron biofortification of rice using different transgenic approaches. Rice 6, 1–12. 10.1186/1939-8433-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Ishimaru Y., Aung M. S., Kobayshi T., Kakei Y., Takahashi M., et al. (2012). Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci. Rep. 2:543. 10.1038/srep00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Kobayshi T., Ishimaru Y., Takahashi M., Aung M. S., Nakanishi H., et al. (2013b). Iron-biofortification in rice by the introduction of three barley genes participated in mugineic acid biosynthesis with soybean ferritin gene. Front. Plant Sci. 4:132. 10.3389/fpls.2013.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Usuda K., Kobayashi T., Ishimaru Y., Kakei Y., Takahashi M., et al. (2009). Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2, 155–166. 10.1007/s12284-009-9031-1 [DOI] [Google Scholar]
- Matovu M. (2016). Molecular and Biochemical Characterisation of Transgenic Banana Lines Containing Iron Uptake and Storage Enhancing Genes. Ph.D. Queensland University of Technology. [Google Scholar]
- Meiners C. R., Derise N. L., Lau H. C., Crews M. G., Ritchey S. J., Murphy E. W. (1976). The content of nine mineral elements in raw and cooked mature dry legumes. J. Agric. Food Chem. 24, 1126–1130. 10.1021/jf60208a036 [DOI] [PubMed] [Google Scholar]
- Mendoza C., Viteri F. E., Lönnerdal B., Young K. A., Raboy V., Brown K. H. (1998). Effect of genetically modified, low-phytic acid maize on absorption of iron from tortillas. Am. J. Clin. Nutr. 68, 1123–1127. [DOI] [PubMed] [Google Scholar]
- Mishra P. K., Bisht S. C., Ruwari P., Joshi G. K., Singh G., Bisht J. K., et al. (2011). Bioassociative effect of cold tolerant Pseudomonas spp. and Rhizobium leguminosarum-PR1 on iron acquisition, nutrient uptake and growth of lentil (Lens culinaris L.). Eur. J. Soil Biol. 47, 35–43. 10.1016/j.ejsobi.2010.11.005 [DOI] [Google Scholar]
- Mori S., Nishizawa N. (1989). Identification of barley chromosome no. 4, possible encoder of genes of mugineic acid synthesis from 2′-deoxymugineic acid using wheat-barley addition lines. Plant Cell Physiol. 30, 1057–1061. [Google Scholar]
- Moriyama M., Oba K. (2008). Comparative study on the vitamin C contents of the food legume seeds. J. Nutr. Sci. Vitaminol. 54, 1–6. 10.3177/jnsv.54.1 [DOI] [PubMed] [Google Scholar]
- Morrissey J., Guerinot M. L. (2009). Iron uptake and transport in plants: the good, the bad, and the ionome. Chem. Rev. 109, 4553–4567. 10.1021/cr900112r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyaka A. W., Makule E. E., Oey I., Van Loey A., Hendrickx M. (2010). Thermal stability of L-ascorbic acid and ascorbic acid oxidase in broccoli (Brassica oleracea var. italica). J. Food Sci. 75, C336–C340. 10.1111/j.1750-3841.2010.01573.x [DOI] [PubMed] [Google Scholar]
- Murata Y., Ma J. F., Yamaji N., Ueno D., Nomoto K., Iwashita T. (2006). A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J. 46, 563–572. 10.1111/j.1365-313X.2006.02714.x [DOI] [PubMed] [Google Scholar]
- Namanya P. (2011). Towards the Biofortification of Banana Fruit for Enhanced Micronutrient Content. Ph.D. Queensland University of Technology. [Google Scholar]
- Narayanan N., Beyene G., Chauhan R. D., Gaitán-Solis E., Grusak M. A., Taylor N., et al. (2015). Overexpression of Arabidopsis VIT1 increases accumulation of iron in cassava roots and stems. Plant Sci. 240, 170–181. 10.1016/j.plantsci.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Nelson R. L., Yoo S. J., Tanure J. C., Andrianopoulos G., Misumi A. (1988). The effect of iron on experimental colorectal carcinogenesis. Anticancer Res. 9, 1477–1482. [PubMed] [Google Scholar]
- Nestel P., Bouis H. E., Meenakshi J. V., Pfeiffer W. (2006). Biofortification of staple food crops. J. Nutr. 136, 1064–1067. [DOI] [PubMed] [Google Scholar]
- Ning L., Sun P., Wang Q., Ma D., Hu Z., Zhang D., et al. (2015). Genetic architecture of biofortification traits in soybean (Glycine max L. Merr.) revealed through association analysis and linkage mapping. Euphytica 204, 353–369. 10.1007/s10681-014-1340-9 [DOI] [Google Scholar]
- Norton G. J., Deacon C. M., Xiong L., Huang S., Meharg A. A., Price A. H. (2010). Genetic mapping of the rice ionome in leaves and grain: identification of QTLs for 17 elements including arsenic, cadmium, iron and selenium. Plant Soil 329, 139–153. 10.1007/s11104-009-0141-8 [DOI] [Google Scholar]
- Nozoye T., Nagasaka S., Kobayashi T., Takahashi M., Sato Y., Sato Y., et al. (2011). Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem. 286, 5446–5454. 10.1074/jbc.M110.180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavan-Rad M. R., Pessarakli M. (2009). Response of wheat plants to zinc, iron, and manganese applications and uptake and concentration of zinc, iron, and manganese in wheat grains. Commun. Soil Sci. Plant Anal. 40, 1322–1332. 10.1080/00103620902761262 [DOI] [Google Scholar]
- Papanikolaou G., Pantopoulos K. (2005). Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 202, 199–211. 10.1016/j.taap.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Peng A. H., Liu X. F., He Y. R., Xu L. Z., Lei T. G., Yao L. X., et al. (2015). Characterization of transgenic Poncirus trifoliata overexpressing the ferric chelate reductase gene CjFRO2 from Citrus junos. Biol. Plant. 59, 654–660. 10.1007/s10535-015-0543-9 [DOI] [Google Scholar]
- Petry N., Egli I., Campion B., Nielsen E., Hurrell R. (2013). Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J. Nutr. 143, 1219–1224. 10.3945/jn.113.175067 [DOI] [PubMed] [Google Scholar]
- Petry N., Egli I., Gahutu J. B., Tugirimana P. L., Boy E., Hurrell R. (2012). Stable iron isotope studies in Rwandese women indicate that the common bean has limited potential as a vehicle for iron biofortification. J. Nutr. 142, 492–497. 10.3945/jn.111.149286 [DOI] [PubMed] [Google Scholar]
- Petry N., Egli I., Gahutu J. B., Tugirimana P. L., Boy E., Hurrell R. (2014). Phytic acid concentration influences iron bioavailability from biofortified beans in Rwandese women with low iron status. J. Nutr. 144, 1681–1687. 10.3945/jn.114.192989 [DOI] [PubMed] [Google Scholar]
- Petry N., Rohner F., Gahutu J. B., Campion B., Boy E., Tugirimana P. L., et al. (2016). In Rwandese women with low iron status, iron absorption from low-phytic acid beans and biofortified beans is comparable, but low-phytic acid beans cause adverse gastrointestinal symptoms. J. Nutr. 146, 970–975. 10.3945/jn.115.223693 [DOI] [PubMed] [Google Scholar]
- Phillips R. D. (1993). Starchy legumes in human nutrition, health and culture. Plant Foods Hum. Nutr. 44, 195–211. 10.1007/BF01088314 [DOI] [PubMed] [Google Scholar]
- Pianelli K., Mari S., Marquès L., Lebrun M., Czernic P. (2005). Nicotianamine over-accumulation confers resistance to nickel in Arabidopsis thaliana. Transgenic Res. 14, 739–748. 10.1007/s11248-005-7159-3 [DOI] [PubMed] [Google Scholar]
- Pich A., Scholz G., Stephan U. (1994). Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes. Nicotianamine as possible copper translocator. Plant Soil 165, 189–196. 10.1007/BF00008061 [DOI] [Google Scholar]
- Pietrangelo A. (2003). Haemochromatosis. Gut 52, ii23–ii30. 10.1136/gut.52.suppl_2.ii23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingali P. L. (2012). Green revolution: impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. U.S.A. 109, 12302–12308. 10.1073/pnas.0912953109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinstrup-Andersen P., Hazell P. B. (1985). The impact of the Green Revolution and prospects for the future. Food Rev. Int. 1, 1–25. 10.1080/87559128509540765 [DOI] [Google Scholar]
- Platel K., Srinivasan K. (2016). Bioavailability of micronutrients from plant foods: an update. Crit. Rev. Food Sci. Nutr. 56, 1608–1619. 10.1080/10408398.2013.781011 [DOI] [PubMed] [Google Scholar]
- Poltronieri F., Arêas J. A. G., Colli C. (2000). Extrusion and iron bioavailability in chickpea (Cicer arietinum L.). Food Chem. 70, 175–180. 10.1016/S0956-7135(99)00113-9 [DOI] [Google Scholar]
- Pulse Australia (2016a) Best Management Guide. Chickpea Production: Northern Region.
- Pulse Australia (2016b) What are Pulses?
- Pulse Canada (2016). Protein Quality of Cooked Pulses (PDCAAS Method).
- Rabotti G., Zocchi G. (1994). Plasma membrane-bound H+-ATPase and reductase activities in Fe-deficient cucumber roots. Physiol. Plant. 90, 779–785. 10.1111/j.1399-3054.1994.tb02537.x [DOI] [Google Scholar]
- Raboy V., Gerbasi P. F., Young K. A., Stoneberg S. D., Pickett S. G., Bauman A. T., et al. (2000). Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol. 124, 355–368. 10.1104/pp.124.1.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A., Saharan B., Nain L., Prasanna R., Shivay Y. S. (2012). Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 58, 573–582. 10.1080/00380768.2012.716750 [DOI] [Google Scholar]
- Rao M., Willey R. (1980). Evaluation of yield stability in intercropping: studies on sorghum/pigeonpea. Exp. Agric. 16, 105–116. 10.1017/S0014479700010796 [DOI] [Google Scholar]
- Rao P. P., Birthal P. S., Bhagavatula S., Bantilan M. C. S. (2010). Chickpea and Pigeonpea Economies in Asia: Facts, Trends and Outlook. Patancheru: International Crops Research Institute for the Semi-Arid Tropics. [Google Scholar]
- Rao S., Joshi S., Bhide P., Puranik B., Asawari K. (2013). Dietary diversification for prevention of anaemia among women of childbearing age from rural India. Public Health Nutr. 17, 939–947. 10.1017/S1368980013001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran V., Ravindran G., Sivalogan S. (1994). Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem. 50, 133–136. 10.1016/0308-8146(94)90109-0 [DOI] [Google Scholar]
- Ray H., Bett K., Tar'an B., Vandenberg A., Thavarajah D., Warkentin T. (2014). Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 54, 1698–1708. 10.2135/cropsci2013.08.0568 [DOI] [Google Scholar]
- Rizkalla S. W., Bellisle F., Slama G. (2002). Health benefits of low glycaemic index foods, such as pulses, in diabetic patients and healthy individuals. Br. J. Nutr. 88, 255–262. 10.1079/BJN2002715 [DOI] [PubMed] [Google Scholar]
- Robinson N. J., Procter C. M., Connolly E. L., Guerinot M. L. (1999). A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697. 10.1038/17800 [DOI] [PubMed] [Google Scholar]
- Rochfort S., Panozzo J. (2007). Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 55, 7981–7994. 10.1021/jf071704w [DOI] [PubMed] [Google Scholar]
- Rodríguez-Celma J., Lin W.-D., Fu G.-M., Abadía J., López-Millán A.-F., Schmidt W. (2013). Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula. Plant Physiol. 162, 1473–1485. 10.1104/pp.113.220426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Marschner H. (1981). Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Physiol. Plant. 53, 354–360. 10.1111/j.1399-3054.1981.tb04512.x [DOI] [Google Scholar]
- Römheld V., Marschner H. (1986). Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 80, 175–180. 10.1104/pp.80.1.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy F., Boye J. I., Simpson B. K. (2010). Bioactive proteins and peptides in pulse crops: pea, chickpea and lentil. Food Res. Int. 43, 432–442. 10.1016/j.foodres.2009.09.002 [DOI] [Google Scholar]
- Sánchez-Rodríguez A. R., del Campillo M. C., Torrent J., Jones D. L. (2014). Organic acids alleviate iron chlorosis in chickpea grown on two P-fertilized soils. J. Soil Sci. Plant Nutr. 14, 292–303. 10.4067/S0718-95162014005000024 [DOI] [Google Scholar]
- Sandberg A.-S. (2002). Bioavailability of minerals in legumes. Br. J. Nutr. 88, 281–285. 10.1079/BJN/2002718 [DOI] [PubMed] [Google Scholar]
- Sandberg A.-S., Brune M., Carlsson N.-G., Hallberg L., Skoglund E., Rossander-Hulthén L. (1999). Inositol phosphates with different numbers of phosphate groups influence iron absorption in humans. Am. J. Clin. Nutr. 70, 240–246. [DOI] [PubMed] [Google Scholar]
- Sandberg A. S., Carlsson N. G., Svanberg U. (1989). Effects of inositol tri-, tetra-, penta-, and hexaphosphates on in vitro estimation of iron availability. J. Food Sci. 54, 159–161. 10.1111/j.1365-2621.1989.tb08591.x [DOI] [Google Scholar]
- Sander J. D., Joung J. K. (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotech. 32, 347–355. 10.1038/nbt.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S., Schmidt W. (2009). Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 183, 1072–1084. 10.1111/j.1469-8137.2009.02908.x [DOI] [PubMed] [Google Scholar]
- Santi S., Cesco S., Varanini Z., Pinton R. (2005). Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol. Biochem. 43, 287–292. 10.1016/j.plaphy.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Santos C. F., Boiteux L. (2015). Genetic control and transgressive segregation of zinc, iron, potassium, phosphorus, calcium, and sodium accumulation in cowpea (Vigna unguiculata) seeds. Genetics Mol. Res. 14, 259–268. 10.4238/2015.January.16.10 [DOI] [PubMed] [Google Scholar]
- Sarmah B., Moore A., Tate W., Molvig L., Morton R., Rees D., et al. (2004). Transgenic chickpea seeds expressing high levels of a bean α-amylase inhibitor. Mol. Breed. 14, 73–82. 10.1023/B:MOLB.0000037996.01494.12 [DOI] [Google Scholar]
- Sebastiá V., Barberá R., Farré R., Lagarda M. J. (2001). Effects of legume processing on calcium, iron and zinc contents and dialysabilities. J. Sci. Food Agric. 81, 1180–1185. 10.1002/jsfa.927 [DOI] [Google Scholar]
- Sharma A., Shankhdhar D., Shankhdhar S. (2013). Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 59, 89–94. [Google Scholar]
- Sharma R. D. (1986). Phytate and the epidemiology of heart disease, renal calculi and colon cancer. Phytic Acid Chem. Appl. 161–172. [Google Scholar]
- Shi R., Weber G., Köster J., Reza-Hajirezaei M., Zou C., Zhang F., et al. (2012). Senescence-induced iron mobilization in source leaves of barley (Hordeum vulgare) plants. New Phytol. 195, 372–383. 10.1111/j.1469-8137.2012.04165.x [DOI] [PubMed] [Google Scholar]
- Siegenberg D., Baynes R. D., Bothwell T. H., Macfarlane B. J., Lamparelli R. D., Car N. G., et al. (1991). Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 53, 537–541. [DOI] [PubMed] [Google Scholar]
- Sievenpiper J. L., Kendall C. W. C., Esfahani A., Wong J. M. W., Carleton A. J., Jiang H. Y., et al. (2009). Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 52, 1479–1495. 10.1007/s00125-009-1395-7 [DOI] [PubMed] [Google Scholar]
- Sood M., Malhotra S. R. (2002). Effects of processing and cooking on ascorbic acid content of chickpea (Cicer arietinum L) varieties. J. Sci. Food Agric. 82, 65–68. 10.1002/jsfa.1001 [DOI] [Google Scholar]
- Sperotto R. A., Ricachenevsky F. K., Waldow Vde A., Fett J. P. (2012). Iron biofortification in rice: it's a long way to the top. Plant Sci. 190, 24–39. 10.1016/j.plantsci.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Stephan U. W., Scholz G. (1993). Nicotianamine: mediator of transport of iron and heavy metals in the phloem? Physiol. Plant. 88, 522–529. 10.1111/j.1399-3054.1993.tb01367.x [DOI] [Google Scholar]
- Strozycki P. M., Szczurek A., Lotocka B., Figlerowicz M., Legocki A. B. (2007). Ferritins and nodulation in Lupinus luteus: iron management in indeterminate type nodules. J. Exp. Bot. 58, 3145–3153. 10.1093/jxb/erm152 [DOI] [PubMed] [Google Scholar]
- Thavarajah D., Thavarajah P., Gupta D. S. (2014). Pulses biofortification in genomic era: multidisciplinary opportunities and challenges, in Legumes in the Omic Era, eds Gupta S., Nadarajan N., Gupta S. D. (New York, NY: Springer New York; ), 207–220. 10.1007/978-1-4614-8370-0_10 [DOI] [Google Scholar]
- Thomine S., Lanquar V. (2011). Iron transport and signaling in plants. in Transporters and Pumps in Plant Signaling. eds Geisler M. M., Venema K. (Berlin; Heidelberg: Springer Berlin Heidelberg; ), 99–131. 10.1007/978-3-642-14369-4_4 [DOI] [Google Scholar]
- Thomine S., Vert G. (2013). Iron transport in plants: better be safe than sorry. Curr. Opin. Plant Biol. 16, 322–327. 10.1016/j.pbi.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Trijatmiko K. R., Dueñas C., Tsakirpaloglou N., Torrizo L., Arines F. M., Adeva C., et al. (2016). Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 6:19792. 10.1038/srep19792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo P., Yates A. A., Schlicker S., Poos M. (2001). Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 101, 294–301. 10.1016/S0002-8223(01)00078-5 [DOI] [PubMed] [Google Scholar]
- Umaid U., Manohar S., Singh A. (1984). The anatomical structure of desi and kabuli chickpea seed coats. Int. Chickpea Newslett. 10, 26–27. [Google Scholar]
- United Nations (2015). World Population Prospects, The 2015 Revision. United Nations Department of Economic and Social Affairs.
- Upadhyaya H. D., Bajaj D., Das S., Kumar V., Gowda C. L. L., Sharma S., et al. (2016). Genetic dissection of seed-iron and zinc concentrations in chickpea. Sci. Rep. 6:24050. 10.1038/srep24050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA (2013). National Nutrient Database for Standard Reference.
- Van den Broeck I., Ludikhuyze L., Weemaes C., Van Loey A., Hendrickx M. (1998). Kinetics for isobaric–isothermal degradation of L-ascorbic acid. J. Agric. Food Chem. 46, 2001–2006. 10.1021/jf9708251 [DOI] [Google Scholar]
- Van Wuytswinkel O., Vansuyt G., Grignon N., Fourcroy P., Briat J.-F. (1999). Iron homeostasis alteration in transgenic tobacco overexpressing ferritin. Plant J. 17, 93–97. 10.1046/j.1365-313X.1999.00349.x [DOI] [PubMed] [Google Scholar]
- Varotto C., Maiwald D., Pesaresi P., Jahns P., Salamini F., Leister D. (2002). The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J. 31, 589–599. 10.1046/j.1365-313X.2002.01381.x [DOI] [PubMed] [Google Scholar]
- Varshney R. K., Song C., Saxena R. K., Azam S., Yu S., Sharpe A. G., et al. (2013). Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 31, 240–246. 10.1038/nbt.2491 [DOI] [PubMed] [Google Scholar]
- Vasconcelos M., Datta K., Oliva N., Khalekuzzaman M., Torrizo L., Krishnan S., et al. (2003). Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 164, 371–378. 10.1016/S0168-9452(02)00421-1 [DOI] [Google Scholar]
- Vasconcelos M., Eckert H., Arahana V., Graef G., Grusak M., Clemente T. (2006). Molecular and phenotypic characterization of transgenic soybean expressing the Arabidopsis ferric chelate reductase gene, FRO2. Planta 224, 1116–1128. 10.1007/s00425-006-0293-1 [DOI] [PubMed] [Google Scholar]
- Vert G. A., Briat J.-F., Curie C. (2003). Dual regulation of the Arabidopsis high-affinity root iron uptake system by local and long-distance signals. Plant Physiol. 132, 796–804. 10.1104/pp.102.016089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G., Barberon M., Zelazny E., Séguéla M., Briat J.-F., Curie C. (2009). Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells. Planta 229, 1171–1179. 10.1007/s00425-009-0904-8 [DOI] [PubMed] [Google Scholar]
- Vert G., Grotz N., Dédaldéchamp F., Gaymard F., Guerinot M. L., Briat J.-F., et al. (2002). IRT1, and Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233. 10.1105/tpc.001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wirén N., Khodr H., Hider R. C. (2000). Hydroxylated phytosiderophore species possess an enhanced chelate stability and affinity for iron(III). Plant Physiol. 124, 1149–1158. 10.1104/pp.124.3.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wirén N., Klair S., Bansal S., Briat J.-F., Khodr H., Shioiri T., et al. (1999). Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol. 119, 1107–1114. 10.1104/pp.119.3.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucenik I., Shamsuddin A. M. (2003). Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J. Nutr. 133, 3778S–3784S. [DOI] [PubMed] [Google Scholar]
- Walker E. L., Connolly E. L. (2008). Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr. Opin. Plant Biol. 11, 530–535. 10.1016/j.pbi.2008.06.013 [DOI] [PubMed] [Google Scholar]
- Walters G. O., Jacobs A., Worwood M., Trevett D., Thomson W. (1975). Iron absorption in normal subjects and patients with diopathic haemochromatosis: relationship with serum ferritin concentration. Gut 16, 188–192. 10.1136/gut.16.3.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Gruissem W., Bhullar N. (2013). Nicotianamine synthase overexpression positively modulates iron homeostasis-related genes in high iron rice. Front. Plant Sci. 4:156. 10.3389/fpls.2013.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters B. M., Troupe G. C. (2012). Natural variation in iron use efficiency and mineral remobilization in cucumber (Cucumis sativus). Plant Soil 352, 185–197. 10.1007/s11104-011-0988-3 [DOI] [Google Scholar]
- Waters B. M., Blevins D. G., Eide D. J. (2002). Characterization of FRO1, a pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol. 129, 85–94. 10.1104/pp.010829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells H. F. (2016). Vegetables and Pulses Yearbook Data. USDA, USA.
- Wheby M. S., Jones L. G., Crosby W. H. (1964). Studies on iron absorption. Intestinal regulatory mechanisms. J. Clin. Invest. 43:1433. 10.1172/JCI105019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2001). Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. Geneva: World Health Organization. [Google Scholar]
- WHO (2008). Worldwide Prevalence of Anaemia 1993-2005. World Health Organisation.
- WHO (2016). Biofortification of Staple Crops.
- WHO and FAO (2006). Guidelines on Food Fortification with Micronutrients.
- Wirth J., Poletti S., Aeschlimann B., Yakandawala N., Drosse B., Osorio S., et al. (2009). Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol. J. 7, 631–644. 10.1111/j.1467-7652.2009.00430.x [DOI] [PubMed] [Google Scholar]
- Wood J. A., Grusak M. A. (2007). Nutritional value of chickpea, in Chickpea Breeding and Management, eds Yadav S. S., Redden R. J., Chen W., Sharma B. (Trowbridge: CAB International; ), 101–142. 10.1079/9781845932138.005 [DOI] [Google Scholar]
- Wood J. A., Knights E. J., Choct M. (2011). Morphology of chickpea seeds (Cicer arietinum L.): comparison of desi and kabuli types. Int. J. Plant Sci. 172, 632–643. 10.1086/659456 [DOI] [Google Scholar]
- Xu Y., An D., Liu D., Zhang A., Xu H., Li B. (2012). Molecular mapping of QTLs for grain zinc, iron and protein concentration of wheat across two environments. Field Crops Res. 138, 57–62. 10.1016/j.fcr.2012.09.017 [DOI] [Google Scholar]
- Yadav S. S., Longnecker N., Dusunceli F., Bejiga G., Yadav M., Rizvi A. H., et al. (2007). Uses, consumption and utilisation, in Chickpea Breeding and Management, eds Yadav S., Redden R. J., Chen W., Sharma B. (Trowbridge: CAB International; ), 72–100. [Google Scholar]
- Zhang Y., Shi R., Rezaul K. M., Zhang F., Zou C. (2010). Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. J. Agric. Food Chem. 58, 12268–12274. 10.1021/jf103039k [DOI] [PubMed] [Google Scholar]
- Zheng L., Cheng Z., Ai C., Jiang X., Bei X., Zheng Y., et al. (2010). Nicotianamine, a novel enhancer of rice iron bioavailability to humans. PLoS ONE 5:e10190. 10.1371/journal.pone.0010190 [DOI] [PMC free article] [PubMed] [Google Scholar]