Anesthetics are frequently used in animal research, but their effects on physiological functions in mice have not been well defined. Here we investigated the effects of commonly used anesthetics on breathing in mice. We found that all tested anesthetics significantly reduced the hypercapnic ventilatory response (HCVR), even at subtherapeutic doses. In addition, ketamine-xylazine and urethane anesthesia altered baseline breathing. These data indicate that breathing and the HCVR in mice are highly sensitive to anesthetic modulation.
Keywords: chemoreceptor, anesthetic, ventilation, respiration, respiratory control
Abstract
Anesthetics are widely used for animal research on respiratory control in vivo, but their effect on breathing and CO2 chemoreception has not been well characterized in mice, a species now often used for these studies. We previously demonstrated that 1% isoflurane markedly reduces the hypercapnic ventilatory response (HCVR) in adult mice in vivo and masks serotonin [5-hydroxytryptamine (5-HT)] neuron chemosensitivity in vitro. Here we investigated effects of 0.5% isoflurane on breathing in adult mice and also found a large reduction in the HCVR even at this subanesthetic concentration. We then tested the effects on breathing of ketamine-xylazine and urethane, anesthetics widely used in research on breathing. We found that these agents altered baseline breathing and blunted the HCVR at doses within the range typically used experimentally. At lower doses ventilation was decreased, but mice appropriately matched their ventilation to metabolic demands due to a parallel decrease in O2 consumption. Neither ketamine nor urethane decreased chemosensitivity of 5-HT neurons. These results indicate that baseline breathing and/or CO2 chemoreception in mice are decreased by anesthetics widely viewed as not affecting respiratory control, and even at subtherapeutic doses. These effects of anesthetics on breathing may alter the interpretation of studies of respiratory physiology in vivo.
NEW & NOTEWORTHY Anesthetics are frequently used in animal research, but their effects on physiological functions in mice have not been well defined. Here we investigated the effects of commonly used anesthetics on breathing in mice. We found that all tested anesthetics significantly reduced the hypercapnic ventilatory response (HCVR), even at subtherapeutic doses. In addition, ketamine-xylazine and urethane anesthesia altered baseline breathing. These data indicate that breathing and the HCVR in mice are highly sensitive to anesthetic modulation.
breathing is normally controlled by sensory feedback from central respiratory chemoreceptors (CRCs) that monitor arterial Pco2 and peripheral respiratory chemoreceptors (PRCs) that monitor both arterial Po2 and Pco2 (Feldman et al. 2003; Richerson 2004; Richerson et al. 2005).There are many candidates for CRCs, including neurons and glia, that respond to changes in Pco2, most likely indirectly due to changes in pH (Feldman et al. 2003; Fukuda et al. 1978; Gourine et al. 2010; Mulkey et al. 2004; Nichols et al. 2008; Pineda and Aghajanian 1997; Richerson 1995, 2004; Richerson et al. 2005; Wang et al. 2001). It is well known that anesthetics affect breathing, and it has been hypothesized that some anesthetics exert their effects on CO2-sensitive CRCs (Comroe 1967). Previously, we demonstrated that 1% isoflurane, a halogenated anesthetic commonly used in research settings, abolishes spontaneous firing of 5-HT neurons and masks their pH/CO2 chemosensitivity (Massey et al. 2015). One-percent isoflurane also nearly eliminates the hypercapnic ventilatory response (HCVR) in adult mice when inspired CO2 is increased from 0 to 7% (Massey et al. 2015). In the perfused brainstem preparation, isoflurane inhibits 5-HT neuron firing in a concentration-dependent manner (Johansen et al. 2015). To accurately study breathing and its mechanisms in anesthetized animals, an anesthetic is needed that does not alter breathing and central respiratory chemoreception. Here we tested the effects on breathing and CO2 chemoreception of several anesthetics that are widely used in research, including isoflurane, ketamine-xylazine, and urethane.
Ketamine is a dissociative anesthetic used in animal research and clinical settings (Bree et al. 1967; Chen et al. 1966; Corssen and Domino 1966; Domino et al. 1965; McCarthy et al. 1965; Virtue et al. 1967). Ketamine is reported to stimulate cardiovascular function in animals, but its use is restricted due to tremors, muscle rigidity, and seizures (Bailie et al. 1979; Haskins et al. 1985; Ivankovich et al. 1974). To minimize these detrimental effects, ketamine is often combined with other drugs including benzodiazepines or xylazine (Hellyer et al. 1991; Moens and Fargetton 1990). Xylazine is an α2-adrenergic receptor agonist, and is reported to have analgesic, sedative, and muscle relaxant effects (Greene and Thurmon 1988; Hsu 1981; Kobinger 1978; Wixson et al. 1987). Ketamine-xylazine administration in male Wistar rats causes depressed heart rate and respiratory function (Sumitra et al. 2004). Sprague-Dawley rats treated with ketamine-xylazine display hypoxia, hypercapnia, and acidosis (Wixson et al. 1987). In mice, a study found that ketamine-xylazine anesthesia induces respiratory depression and decreases heart rate and arterial blood pressure (Erhardt et al. 1984).
Urethane (also known as ethyl carbamate) was first produced in 1834 and has been used for over a century as an anesthetic (Maggi and Meli 1986; Mirvish 1968; Schmiedeberg 1885). In addition to the advantage of a long-lasting level of anesthesia, urethane has also been reported to have minimal effects on cardiorespiratory function. Recordings from decerebrate rats demonstrate that there is a small drop in heart rate after urethane anesthesia is induced, but respiratory frequency (FR) and tidal volume (VT) are unchanged (Sapru and Krieger 1979). However, these same rats have a depressed increase in both frequency and VT in response to NaCN. In a second report from urethane-anesthetized rats, minute ventilation (V̇e) is reduced compared with metabolism but the hypoxic ventilatory response (HVR) and HCVR are preserved (Hughes et al. 1982). A third set of experiments from rats reported that there are no significant effects on V̇e, HVR, or HCVR with urethane anesthesia (Boon et al. 2004). Although it is generally held that urethane preserves respiratory chemoreflexes, there are data demonstrating that in some species urethane depresses the HCVR and carotid body response (Florez and Borison 1969; Sapru and Krieger 1979).
Here we tested whether there was depression of baseline breathing or CO2 chemoreception during ketamine-xylazine or urethane anesthesia. As previously shown for 1% isoflurane, we found that ketamine-xylazine or urethane markedly decreased CO2 chemoreception at doses that produced surgical anesthesia and this was not due to a decrease in O2 consumption. At subtherapeutic doses, isoflurane, ketamine-xylazine, and urethane also reduced the increase in V̇e in response to increased inspired CO2. We were unable to measure O2 consumption changes during isoflurane anesthesia, but we found that O2 consumption dropped in parallel with the decreased response to CO2 when mice were treated with a subtherapeutic dose of either ketamine-xylazine or urethane. These data suggest metabolic depression caused the decreased HCVR observed during subtherapeutic doses of these injectable agents. Additionally, isoflurane did not affect baseline ventilation, but both ketamine-xylazine and urethane altered baseline breathing. Ketamine-xylazine greatly reduced V̇e at baseline with decreases in both VT and breathing frequency. In contrast, urethane did not decrease V̇e at baseline, but there was decreased VT and increased breathing frequency. These results indicate that the effects on the respiratory network differ for the three anesthetics.
We previously demonstrated that 1% isoflurane prevents 5-HT neurons from responding to acidosis, which parallels the decreased HCVR in the whole animal. Therefore, we tested whether the effects of ketamine-xylazine and urethane on the HCVR in vivo correlated with effects on 5-HT neuron chemosensitivity in vitro. We found that unlike isoflurane, ketamine and urethane did not decrease 5-HT neuron baseline firing or inhibit chemosensitivity. These data demonstrate that ketamine-xylazine anesthesia and urethane anesthesia have larger effects on breathing and CO2 chemoreception in mice than is widely appreciated and these effects are not mediated by changes in 5-HT neuron chemosensitivity.
METHODS AND MATERIALS
Ethical Approval
All experiments were carried out with approval of The University of Iowa Institutional Animal Care and Use Committee. All animal procedures were carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, 8th ed. (National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011). The minimum number of animals was used and care was taken to reduce the possibility of discomfort.
Whole Animal Plethysmography
Isoflurane.
V̇e was measured in adult C57Bl/6 mice using standard open-flow (700 ml/min), whole body plethysmography as previously used in our laboratory (Hodges et al. 2008; Massey et al. 2015). The chamber was a commercially available model (Buxco, Wilmington, NC), but the remainder of the plethysmography equipment was custom designed and built. We performed plethysmography experiments in male and female mice for dose-dependent effects of isoflurane on the HCVR. The protocol consisted of >20 min of baseline recording in 0% CO2, 50% O2, balance N2 followed by ~7-min exposures to 3, 5, 7, and 10% CO2, 50% O2, balance N2. The same sequence of increased CO2 was then performed with 0.5% (n = 15; 10 male and five female mice) or 1% isoflurane (n = 15; 10 male and 5 female mice), which are ~0.5 and 1.0 minimum alveolar concentration (MAC), respectively. The 1.0 MAC represents the concentration at which 50% of subjects do not respond to surgical incision. Numerical values are provided in the text for data at 0, 7, and 10% CO2, whereas data at all CO2 levels are graphically represented in the figures. Mice were exposed to 0.5% isoflurane mixed with 50% O2 for at least 20 min before CO2 exposure (anesthesia was induced by 1% isoflurane in 15 min). Isoflurane levels were maintained at 0.5 or 1% using a precision vaporizer (Summit Anesthesia Solutions, Bend, OR). The plethysmograph chamber was maintained at 30°C using a heat lamp and feedback controller (TCAT-2AC; Physitemp Instruments, Clifton, NJ). Body temperature was measured with telemetry probes (IPTT-300; BMDS, Seaford, DE) inserted into the abdominal cavity at least 5 days before recordings. All data were acquired using custom-written MATLAB software.
Ketamine-xylazine and urethane.
The same setup and protocol was used to study the effects of other anesthetics on the HCVR, except that a flow rate of 200 ml/min was used. This rate was chosen because we found that at 200 ml/min our O2 and CO2 analyzers (S-3A and CD-3A; AEI Technologies; Pittsburgh, PA) were able to more accurately capture changes in O2 and CO2 than when using higher rates. O2 and CO2 levels were not measured during isoflurane experiments because engineers from AEI Technologies advised us that isoflurane would damage the analyzers. As above, five levels of CO2 were tested (mixed in 50% O2, balance N2): 0, 3, 5, 7, and 10%. Mice were exposed to this sequence of CO2 levels, and then this protocol was repeated after mice were treated with either 100 mg/kg ketamine (Ketaject; Phoenix Pharmaceutical, St. Joseph, MO) and 10 mg/kg xylazine (AnaSed; Lloyd Laboratories, Shenandoah, IA) (n = 15; 10 male and 5 female mice) or 1,500 mg/kg urethane (Sigma-Aldrich, St. Louis, MO) (n = 16; 10 male and 6 female mice). Subtherapeutic doses of both anesthetics were also tested, but since the effects were of short duration, only two levels of CO2 were tested: 0 and 7% (both in 50% O2, balance N2). Fourteen mice (10 male; 4 female) were treated with 50 mg/kg ketamine and 5 mg/kg xylazine. Fifteen mice (10 male; 5 female) were treated with 500 mg/kg urethane. Fifteen mice (10 male; 5 female) were treated with 1,000 mg/kg urethane.
Cell Culture
ePet-EYFP mice were used to prepare cultures to allow identification of 5-HT neurons before patch-clamp recordings as previously demonstrated (Massey et al. 2015; Scott et al. 2005). In these mice, the enhancer region of the Pet-1 ETS gene enables expression of enhanced yellow fluorescent protein (YFP) in 5-HT neurons. Neonatal ePet-EYFP pups (n = 20) were killed between postnatal days (P) 0–2, and a wedge of tissue from the ventromedial portion of the rostral half of the medulla (including the raphé pallidus, raphé magnus, and raphé obscurus) was removed. The tissue was digested, triturated, and plated on poly-l-ornithine- and laminin-coated coverslips. Cultures were fed and maintained as previously described (Wang et al. 1998). Recordings were performed on 5-HT neurons after P21 (19–21 days after culturing) to allow maturation of chemosensitivity (Cerpa et al. 2016; Wang and Richerson 1999). 5-HT neurons were identified by expression of EYFP using fluorescence microscopy as previously described (Massey et al. 2015; Scott et al. 2005).
Patch-Clamp Recordings
The gramicidin perforated-patch technique was used for all recordings (Ebihara et al. 1995). Electrodes (6–14 MΩ; borosilicate glass) were pulled on a micropipette puller (Model No. P-97; Sutter Instruments, Novato, CA) and filled with intracellular solution containing (in mM): 135 KOH 135 methanesulfonic acid 10 KCl, 5 HEPES, and 1 EGTA (pH 7.2; osmolarity: 275 ± 5 mosM). Coverslips were transferred to a recording chamber on the stage of an Axiovert 200 inverted microscope (Carl Zeiss, Thornwood, NY). Recordings were performed with a Multiclamp 700B microelectrode amplifier (Molecular Devices, Sunnyvale, CA), and data were collected using PClamp software and a Digidata 1440A acquisition system (Molecular Devices).
Normal artificial cerebrospinal fluid (aCSF) (pH 7.4) contained the following (in mM): 124 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2 1.3 NaH2PO4, 26 NaHCO3, and 10 dextrose. Acidic aCSF (pH 7.15) contained the following (in mM) 136 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2 1.3 NaH2PO4 13 NaHCO3, and 10 dextrose. Both solutions had an osmolarity of 305 ± 5 mosM and were maintained isocapnic by equilibration with 5% CO2-95% O2. Fast glutamatergic, glycinergic, and GABAergic ionotropic synaptic transmission was blocked by 100 μM picrotoxin (PTX), 50 μM (±)-2-amino-5-phosphonopentanoic acid (AP-5), and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Solutions for anesthesia exposure contained 120 μM ketamine (Ketaject) or 10 mM urethane. AP-5 and CNQX were obtained from Tocris (Ellisville, MI). All other drugs and chemicals were obtained from Sigma-Aldrich.
Statistical Analysis
Unless otherwise indicated, statistical significance in our plethysmography experiments was determined using a two-way repeated-measures ANOVA with an overall significance level set to P < 0.05 since our experiments manipulated two different variables: inspired CO2 levels and anesthetic treatment. We used the Holm-Sidak pairwise multiple comparison test to control for multiple comparisons between treatment conditions (GraphPad Prism V6.01). Similarly, we used a two-way repeated measures ANOVA with an overall significance level set to P < 0.05 to determine statistical significance in our patch-clamp experiments, where two variables were manipulated: bath pH and anesthetic presence. The Holm-Sidak pairwise multiple comparison test was used to control for multiple comparisons between conditions (GraphPad Prism V6.01). F-statistic values reported are for the interaction between anesthetics and pH/CO2. When data are presented as X ± Y, X is the group mean and Y is the standard deviation; all error bars represent the means ± SE. For in vivo experiments with mice, each animal was exposed to increased CO2 levels both in the absence and presence of anesthetic to act as in-subject controls.
RESULTS
Isoflurane Caused a Large Reduction in the HCVR at Subtherapeutic Doses
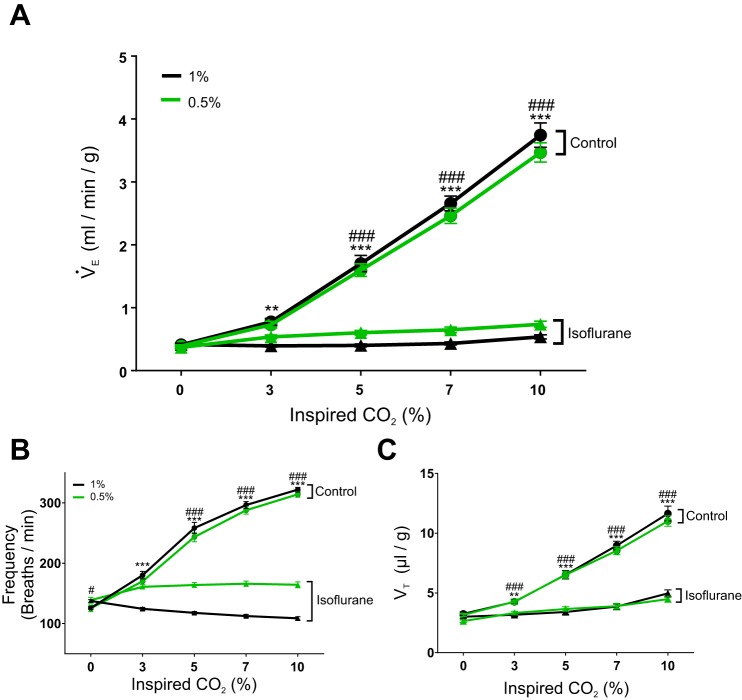
We previously reported that an anesthetic level of isoflurane (1%) causes a large decrease in the HCVR of adult C57Bl/6 mice (Massey et al. 2015). Here we examined the effect of subtherapeutic levels of isoflurane on the HCVR to determine the threshold for this effect. We tested the HCVR using a background of 50% O2 to reduce peripheral chemoreceptor activity as demonstrated previously (Lahiri and DeLaney 1975). Under control conditions in C57Bl/6 mice (n = 15), V̇e increased by 549% in response to 7% CO2 (from 0.41 ± 0.09 to 2.66 ± 0.45 ml·g−1·min−1; P < 0.001; Fig. 1A) and by 815% in response to 10% CO2 (P < 0.001; Fig. 1A). When anesthetized with 1% isoflurane, the HCVR was completely abolished, as there was no difference in V̇e at 0% CO2 compared with 7% CO2 (P = 0.9998; Fig. 1A) and 10% CO2 (P = 0.7220; Fig. 1A). 1% isoflurane reduced the change in V̇e in response to 7 and 10% CO2 to 0.4% (P < 0.001) and 4.9% (P < 0.001) of control, respectively. Baseline breathing in 0% CO2 was not reduced in 1% isoflurane in C57Bl/6 mice compared with control conditions (P > 0.9999; Fig. 1A).
Fig. 1.
Isoflurane at a subtherapeutic dose markedly reduced the hypercapnic ventilatory response (HCVR) in adult mice. A: 1% isoflurane (black lines) reduced the slope of minute ventilation (V̇e) as the percentage of CO2 increased in C57Bl/6 mice (F4,56 = 182.60, P < 0.001, n = 15). Similarly, 0.5% isoflurane (green lines) reduced the slope of V̇e as the percentage of CO2 increased in C57Bl/6 mice (F4,56 = 183.10, P < 0.001, n = 15). B: the increased respiratory frequency (FR) in response to elevated CO2 in control conditions was reversed in 1% isoflurane (black lines; F4,56 = 366.20, P < 0.001, n = 15). Isoflurane at 0.5% also reduced the increased FR in response to elevated CO2 (green lines; F4,56 = 199.3, P < 0.001, n = 15). C: anesthesia reduced the increase in tidal volume (VT) as CO2 levels rose in both 1% (black lines; F4,56 = 85.07, P < 0.001, n = 15) and 0.5% isoflurane (F4,56 = 112.20, P < 0.001, n = 15). **P < 0.05, ***P < 0.001 for 1% isoflurane (black lines); #P < 0.5, ###P < 0.001 for 0.5% isoflurane (green lines).
In a separate group of mice (n = 15), the effect of 0.5% isoflurane on the HCVR was tested. Unexpectedly, 0.5% isoflurane caused a large reduction in the HCVR despite not causing anesthesia. Under control conditions, V̇e increased by 531% in response to 7% CO2 (from 0.39 ± 0.07 to 2.46 ± 0.47 ml·g−1·min−1; P < 0.001; Fig. 1A) and by 790% in response to 10% CO2 (P < 0.001; Fig. 1A). In 0.5% isoflurane, V̇e increased by only 28% in response to 7% CO2 (from 0.37 ± 0.09 to 0.65 ± 0.17 ml·g−1·min−1; P = 0.0127; Fig. 1A) and by 100% in response to 10% CO2 (P < 0.001; Fig. 1A). Isoflurane did not affect baseline breathing in 0% CO2 (P = 0.9995; n = 15; Fig. 1A).
Isoflurane at both 1 and 0.5% substantially reduced the FR component of the HCVR (Fig. 1B). In control conditions when CO2 increased from 0 to 7%, FR increased by 135% (from 126.00 ± 9.72 breaths/min to 296.55 ± 21.50 breaths/min; P < 0.001; Fig. 1B); in response to 10% CO2, FR increased by 156% (P < 0.001; Fig. 1B). However, during 1% isoflurane anesthesia, the FR decreased by 18% in response to 7% CO2 (from 137.52 ± 8.88 to 112.49 ± 10.37 ml·g−1·min−1; P < 0.001; Fig. 1B) and by 21% in response to 10% CO2 (P < 0.001; Fig. 1B).
Isoflurane, at both 1 and 0.5%, also decreased the response of VT to a CO2 challenge. In unanesthetized mice (n = 15), VT increased by 176% in response to 7% CO2 (from 3.25 ± 0.67 to 8.96 ± 1.43 μl/g; P < 0.001; Fig. 1C) and by 258% in response to 10% CO2 (P < 0.001; Fig. 1C). In 1% isoflurane, VT increased by only 28% in response to 7% CO2 (from 3.01 ± 0.49 to 3.85 ± 0.84 μl/g; P < 0.001; Fig. 1C) and by 65% in response to 10% CO2 (P < 0.001; Fig. 1C). In the cohort of mice that received 0.5% isoflurane (n = 15), under control conditions VT increased by 170% in response to 7% CO2 (from 3.16 ± 0.50 to 8.54 ± 1.32 μl/g; P < 0.001; Fig. 1C) and by 249% in response to 10% CO2 (P < 0.001; Fig. 1C). In this same cohort in 0.5% isoflurane, VT increased by 47% in response to 7% CO2 (from 2.64 ± 0.47 to 3.89 ± 0.91 μl/g; P < 0.001; Fig. 1C) and by 70% in response to 10% CO2 (P < 0.001; Fig. 1C).
The effects on the HCVR were profound in mice treated with 0.5% isoflurane even though they were not anesthetized. These animals displayed a righting reflex and would occasionally make quick locomotor movements. These mice were mildly sedated so their movements were uncoordinated and relatively brief, but they occurred spontaneously. None of these behaviors were ever observed in 1.0% isoflurane.
Ketamine-Xylazine Also Caused a Large Reduction in the HCVR
Whole animal plethysmography was performed on adult mice before and during treatment with ketamine and xylazine, a combination that is commonly used for rodent surgery. First, in one cohort (n = 15), the normal therapeutic dose was used: 100 mg/kg ketamine plus 10 mg/kg xylazine (100/10). Then, in a second cohort (n = 14), a subtherapeutic dose was used: 50 mg/kg ketamine and 5 mg/kg xylazine (50/5). Due to a short duration of effect with the subtherapeutic dose, only 0 and 7% CO2 were tested in that cohort to ensure the HCVR was tested at the time of maximal effect. The subtherapeutic dose of ketamine-xylazine did not fully anesthetize the mice, as they still maintained their righting reflex. Instead, mice were lightly sedated and would occasionally make quick movements. Mice also exhibited whisking behavior when the side of the plethysmograph was tapped, but otherwise remained stationary. We attempted to determine the effects on breathing and the HCVR of ketamine and xylazine separately, but mice were not fully anesthetized with the same dose when the anesthetics were administered individually. Moreover, in the case of ketamine, mice were agitated and hyperactive, which complicated collection of clean breathing data while in the plethysmograph.
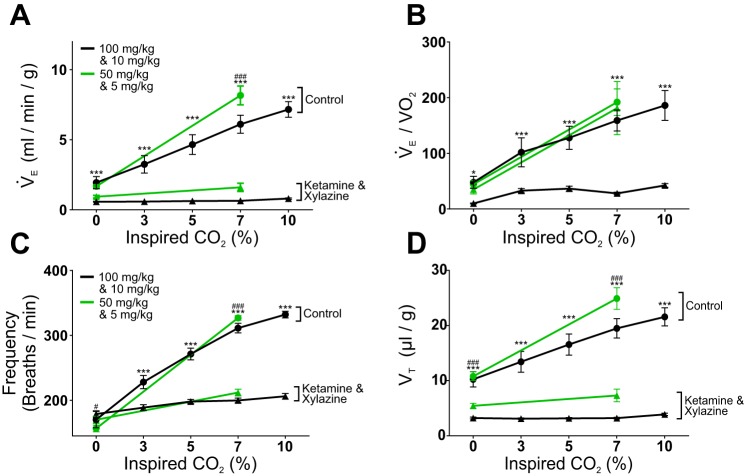
The effect on V̇e was examined first (Fig. 2A). Before being anesthetized with 100/10 ketamine-xylazine, V̇e increased in response to 10% CO2 by 269% (from 1.94 ± 1.70 to 7.16 ± 2.18 ml·g−1·min−1; P < 0.001; Fig. 2A). Baseline V̇e was substantially affected by 100/10 ketamine-xylazine. In 0% CO2, baseline V̇e was significantly reduced by 71% compared with control (from 1.94 ± 1.70 to 0.57 ± 0.11 ml·g−1·min−1; P < 0.001; Fig. 2A). During treatment with 100/10 ketamine-xylazine, the HCVR was eliminated as there was no difference between V̇e in 0% CO2 and either 7% (P > 0.9999; Fig. 2A) or 10% CO2 (P = 0.9974; Fig. 2A). Similarly, the second cohort of mice had a large increase in V̇e (386%) in response to an increase in inspired CO2 from 0 to 7% under control conditions (from 1.68 ± 0.42 to 8.16 ± 2.51 ml·g−1·min−1; P < 0.001; Fig. 2A). After this cohort received a dose of 50/5 ketamine-xylazine, there was a trend towards a reduced baseline V̇e in 0% CO2, but the difference did not reach statistical significance (P = 0.0805; Fig. 2A). The HCVR was eliminated by 50/5 ketamine-xylazine; although there was a trend to increase V̇e as CO2 increased from 0 to 7%, the difference was not significant (P < 0.1176; Fig. 2A).
Fig. 2.
Ketamine-xylazine anesthesia markedly reduced the hypercapnic ventilatory response (HCVR) in vivo. A: ketamine (100 mg/kg) and xylazine (10 mg/kg) caused a reduction in breathing at baseline (0% CO2; P < 0.001) and in the slope of V̇e as the percentage of inspired CO2 rose in C57Bl/6 mice (black lines; F4,56 = 45.60, P < 0.001, n = 15). A subtherapeutic dose of ketamine (50 mg/kg) and xylazine (5 mg/kg) also markedly decreased the slope of V̇e as CO2 increased to 7% (green lines; F1,13 = 159.3, P < 0.001, n = 14). B: ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia caused a reduction in V̇e/V̇o2 in 0% CO2 and in the slope of V̇e/V̇o2 as CO2 increased (black lines; F4,56 = 9.941, P < 0.001, n = 15). The subtherapeutic dose of ketamine (50 mg/kg) and xylazine (5 mg/kg) did not alter the V̇e/V̇o2 (green lines; F1,13 = 6.967 × 10−4, P = 0.9793, n = 14). C: the FR component of the HCVR was equally affected by both the therapeutic and subtherapeutic doses of ketamine and xylazine. Ketamine (100 mg/kg) and xylazine (10 mg/kg) showed a reduction in the increase in FR as the CO2 level was raised (black lines; F4,56 = 80.04, P < 0.001, n = 15). The subtherapeutic dose of ketamine and xylazine (50 and 5 mg/kg) also caused a marked reduction in FR in 7% CO2 (green lines; F1,13 = 359.30, P < 0.001, n = 14). D: VT did not increase in response to higher CO2 levels with ketamine and xylazine administration. Ketamine (100 mg/kg) and xylazine (10 mg/kg) reduced the VT in 0% CO2 (P < 0.001) and slope of the increase in VT in response to CO2 (black lines; F4,56 = 29.38, P < 0.001, n = 14). Similarly, the subtherapeutic dose (50 and 5 mg/kg; green lines) reduced both the baseline VT (P < 0.001) and the increase in VT in response to CO2 (F1,13 = 170.30, P < 0.001, n = 14). *P < 0.05, ***P < 0.001 for therapeutic dose (100 and 10 mg/kg; black lines); #P < 0.05, ###P < 0.001 for subtherapeutic dose (50 and 5 mg/kg; green lines).
Rodents are known to decrease their metabolic O2 consumption in hypoxic conditions, and breathing and metabolism influence each other (Frappell et al. 1992; Mortola and Maskrey 2011). Therefore, O2 consumption of mice was measured during plethysmography experiments with ketamine-xylazine and urethane to determine if a decrease in metabolism accounted for decreased ventilation and reduced HCVR. O2 consumption was then used to calculate V̇e/V̇o2 to determine if V̇e was sufficient to meet the O2 demand (Fig. 2B). In control recordings from the cohort that received 100/10 ketamine-xylazine, V̇e/V̇o2 increased by 332% as CO2 increased from 0 to 7% (from 47.82 ± 42.08 to 158.91 ± 72.98; P < 0.001; Fig. 2B) and by 389% as CO2 increased from 0 to 10% (P < 0.001; Fig. 2B). In this cohort, after treatment with 100/10 ketamine-xylazine, V̇e/V̇o2 did not change in response to an increase in CO2 from 0 to 7% (P = 0.8880; Fig. 2B) or to 10% CO2 (P = 0.2051; Fig. 2B). The slope of V̇e/V̇o2 in response to increased inspired CO2 did not change (F4,56 = 9.94, P < 0.001; Fig. 2B). In contrast, 50/5 ketamine-xylazine did not induce baseline hypoventilation as V̇e/V̇o2 did not change in 0% CO2 (P = 0.9576; Fig. 2B). This subtherapeutic dose also did not decrease the V̇e/V̇o2 response to hypercapnia, as it increased by 432% in response to an increase of CO2 from 0 to 7% (from 35.07 ± 29.42 to 181.23 ± 178.; P = 0.0022; Fig. 2B), as compared with an increase by 517% under control conditions (from 44.43 ± 23.79 to 191.89 ± 89.23; P = 0.0021; Fig. 2B). Body temperature was reduced following both doses of ketamine-xylazine, which was most likely due to decreased metabolism.
As was seen for isoflurane, the FR component of the HCVR was severely affected by ketamine-xylazine (Fig. 2C). In the first cohort under control conditions, when CO2 was increased to 7%, FR increased by 83% (from 170.11 ± 48.55 to 311.20 ± 28.60 breaths/min; P < 0.001; Fig. 2C), when CO2 was raised to 10%, FR increased by 95% (P < 0.001; Fig. 2C). After mice were treated with 100/10 ketamine-xylazine, FR increased by only 12% when CO2 was raised to 7% (from 178.80 ± 19.10 to 199.75 ± 14.33 breaths/min; P = 0.0115; Fig. 2c); when CO2 was increased to 10%, FR increased by 15% (P < 0.001; Fig. 2C). In the second cohort after treatment with 50/5 ketamine-xylazine, FR increased by only 25% in response to 7% CO2 (P < 0.001; Fig. 2C). This was only 25% of control (P < 0.001), where FR increased by 109% in response to 7% CO2 in this cohort (from 156.49 ± 18.91 to 326.83 ± 13.07 breaths/min; P < 0.001; Fig. 2C).
Ketamine-xylazine also blunted the effect of CO2 on VT (Fig. 2D). In the first cohort under control conditions, VT increased by 90% in response to 7% CO2 (from 10.24 ± 5.36 to 19.48 ± 6.79 μl/g; P < 0.001; Fig. 2D) and by 111% in response to 10% CO2 (P < 0.001; Fig. 2D). Ketamine-xylazine at 100/10 decreased baseline VT in 0% CO2 to 31% of control (P < 0.001; Fig. 2d). This therapeutic dose blocked the increase in VT in response to hypercapnia as there was no difference in VT between 0% CO2 and 7% CO2 (P > 0.9999; Fig. 2D) or 10% CO2 (P = 0.9952; Fig. 2D). In contrast, the subtherapeutic ketamine-xylazine dose affected VT to a lesser extent. In this cohort under control conditions, VT increased by 131% in response to 7% CO2 (from 10.79 ± 2.96 to 24.89 ± 7.35 μl/g; P < 0.001; Fig. 2D). During 50/5 ketamine-xylazine treatment, VT only increased by 34% (from 5.44 ± 1.67 to 7.31 ± 4.30 μl/g; P = 0.0247; Fig. 2D). This change in VT induced by 7% CO2 was 13% of control (P < 0.001; Fig. 2D).
Effect of Ketamine and Xylazine on 5-HT Neuron Chemosensitivity
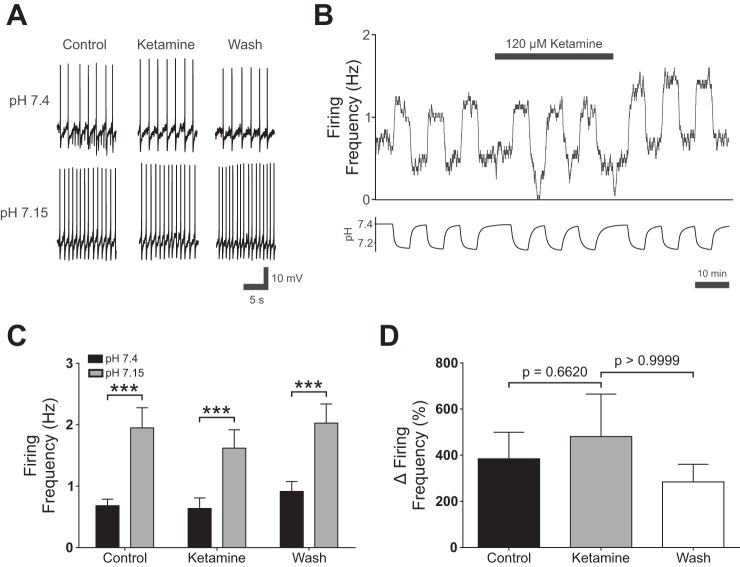
To determine if ketamine had effects on 5-HT neurons, which are putative pH/CO2 chemoreceptors (Corcoran et al. 2009; Hodges et al. 2008; Richerson 1995, 2004; Richerson et al. 2001, 2005; Richerson et al. 2001; Wang et al. 1998), patch-clamp recordings were performed from cultured ePet-EYFP 5-HT neurons (n = 12; Fig. 3, A–C). Acidosis consistently increased firing frequency in all 5-HT neurons tested (from 0.63 ± 0.37 Hz in normal aCSF to 1.95 ± 1.14 Hz in acidic aCSF; P < 0.001; Fig. 3B). Ketamine (120 µM) did not affect baseline 5-HT neuron firing in normal aCSF (0.64 ± 0.59 Hz in ketamine vs. 0.63 ± 0.37 Hz in control; P = 0.8889; Fig. 3B). In ketamine, 5-HT neurons continued to respond robustly to acidosis (from 0.64 ± 0.59 Hz to 1.62 ± 1.04 Hz; P < 0.001; Fig. 3B). Ketamine did not alter the percent increase in firing frequency in response to lowered bath pH compared with either control (P = 0.6620; Fig. 3C) or ketamine washout conditions (P > 0.9999; Fig. 3C).
Fig. 3.
Ketamine did not abolish 5-hydroxytryptamine (5-HT) neuron chemosensitivity in vitro. A: effect of ketamine (120 µM) on changes in 5-HT neuron membrane potential in response to acidic artificial cerebral spinal fluid (aCSF). B: plot of 5-HT neuron firing rate changes in response to acidosis show that chemosensitivity was not blocked by ketamine (120 µM). C: summary of current-clamp recordings with ketamine. Firing frequency of cultured 5-HT neurons increased in response to hypercapnic aCSF (control; P < 0.001, n = 12). 5-HT neurons still increased their firing frequency in response to acidosis when ketamine (120 µM) was added to the bath solution (ketamine; P < 0.001, n = 12). Cells remained chemosensitive after washout of the anesthetic (qash; P < 0.001, n = 12). A two-way repeated measures ANOVA reveled a significant effect of pH (F1,11 = 13.29, P = 0.0039, n = 12), and ketamine (F2,22 = 3.481, P = 0.0486, n = 12). There was a significant interaction between pH and ketamine (F2,22 = 4.623, P = 0.0211, n = 12). D: ketamine did not decrease the percent change in firing frequency of 5-HT neurons in response to acidosis (Friedman statistic = 2.00, P = 0.3679). P values at top were calculated using Dunn’s multiple comparisons test. ***P < 0.001.
Urethane Substantially Reduced the HCVR in Adult Mice
Urethane is widely used as an anesthetic for experiments on cardiorespiratory control, because it is thought to have relatively little effect on breathing and brainstem reflexes (Hughes et al. 1982; Sapru and Krieger 1979; Schmiedeberg 1885). Reports vary on the therapeutic dose for urethane, ranging from 1,000 to 2,000 mg/kg. Therefore, three different doses were used ranging from subtherapeutic (500 mg/kg) to the middle of the therapeutic range (1,500 mg/kg). We found that mice were not fully anesthetized at doses of either 500 or 1,000 mg/kg. Thus to ensure that HCVR measurements were collected during the plateau of the drug effect, only two CO2 levels were tested (0 and 7%) for both 500 and 1,000 mg/kg urethane, whereas mice anesthetized with 1,500 mg/kg were exposed to five CO2 levels (0, 3, 5, 7, and 10%). In the cohort of mice treated with 1,500 mg/kg urethane (n = 16), V̇e increased under control conditions in response to 7% CO2 by 303% (from 1.94 ± 1.31 to 7.81 ± 4.35 ml·g−1·min−1; P < 0.001; Fig. 4A) and by 413% in response to 10% CO2 (P < 0.001; Fig. 4A). This midtherapeutic dose of urethane (1,500 mg/kg) eliminated the HCVR, as there was no difference in V̇e in 0% CO2 compared with 7% (P = 0.9910; Fig. 4A) and 10% CO2 (P = 0.9681; Fig. 4A). In the cohort of mice treated with 1,000 mg/kg urethane (n = 15), there was a 252% increase in V̇e under control conditions in response to an increase in CO2 from 0% (1.65 ± 1.43 ml·g−1·min−1) to 7% (5.81 ± 2.96 ml·g−1·min−1; P < 0.001; Fig. 4A). The HCVR was significantly reduced by 1,000 mg/kg urethane, as there was no difference in V̇e in 0 and 7% CO2 (P = 0.1440; Fig. 4A). Despite near elimination of the HCVR, the 1,000 mg/kg dose of urethane was not sufficient to fully anesthetize animals, as mice still retained a righting reflex and would respond to sensory stimuli. The mice exhibited whisking behavior and uncoordinated movement in response to a tap against the plethysmography chamber. In the cohort of animals treated with 500 mg/kg urethane (n = 15), V̇e increased by 331% in response to an increase in CO2 from 0 to 7% under control conditions (from 1.24 ± 0.58 to 5.34 ± 0.96 ml·g−1·min−1; P < 0.001; Fig. 4A). After treatment with 500 mg/kg urethane, V̇e increased by 103% in response to a change in CO2 from 0 to 7% (from 1.56 ± 0.52 to 3.17 ± 0.89 ml·g−1·min−1; P < 0.001; Fig. 4A). However, V̇e in 7% CO2 was reduced compared with control conditions (P < 0.001; Fig. 4A) and the slope of the response was reduced to 39% of control. This lower dose of urethane was also not sufficient to anesthetize or sedate the mice; in fact, the mice were ambulatory, retained a righting reflex, and showed no behavioral effects from the drug.
Fig. 4.
Therapeutic and subtherapeutic doses of urethane reduced the HCVR in adult C57Bl/6 mice. A: urethane decreased the slope of V̇e as inspired CO2 rose in all 3 doses tested: 1,500 mg/kg (black lines; F4,60 = 40.24, P < 0.001, n = 16), 1,000 mg/kg (green lines; F1,14 = 60.57, P < 0.001, n = 15), and 500 mg/kg (blue lines; F1,14 = 170.3, P < 0.001, n = 15). B: 1,500 mg/kg urethane anesthesia reduced the slope of V̇e/V̇o2 as CO2 increased (black lines; F4,60 = 7.657, P < 0.001, n = 16). In contrast, the slope of V̇e/V̇o2 as CO2 increased was not reduced in either 1,000 mg/kg (green lines; F1,14 = 0.9686, P = 0.3417, n = 15) or 500 mg/kg (blue lines; F1,14 = 0.6293, P = 0.4409, n = 15). C: baseline breath frequency progressively increased in 500 mg/kg (blue lines; P < 0.001, n = 15), 1,000 mg/kg (green lines; P < 0.001, n = 15), and 1,500 mg/kg (black lines; P < 0.001, n = 16). The increase in FR as CO2 increases was reduced in both 1,000 mg/kg (F1,14 = 286.20, P < 0.001, n = 15) and 500 mg/kg (F1,14 = 109.40, P < 0.001, n = 15). FR decreased in 1,500 mg/kg urethane as CO2 increased (F4,60 = 112.10, P < 0.001, n = 16). D: urethane decreased the VT in 0% CO2 in both 1,500 mg/kg (F4,60 = 33.74, P < 0.001, n = 15) and 1,000 mg/kg (F1,14 = 44.27, P < 0.001, n = 15). The increase in VT in response to hypercapnia was reduced when mice were treated with 500 mg/kg (F1,14 = 59.29, P < 0.001, n = 15), 1000 mg/kg (F1,14 = 44.27, P < 0.001, n = 15), or 1500 mg/kg urethane (F4,60 = 33.74, P < 0.001, n = 16). *P < 0.05, ***P < 0.001 for the 1,500-mg/kg dose (black lines); ###P < 0.001 for the 1,000-mg/kg dose (green lines); †††P < 0.001 for the 500-mg/kg dose (blue lines).
O2 consumption was measured in these experiments, allowing calculation of V̇e/V̇o2 (Fig. 4B). In the cohort of animals treated with 1,500 mg/kg urethane (n = 16), under control conditions V̇e/V̇o2 increased by 449% as CO2 increased from 0 to 7% (from 47.90 ± 30.17 to 215.31 ± 152.95; P < 0.001; Fig. 4B) and by 488% when CO2 increased from 0 to 10% (P < 0.001; Fig. 4B). After treatment with 1,500 mg/kg urethane, V̇e/V̇o2 did not change as CO2 increased from 0 to 7% (from 42.67 ± 27.83 to 88.20 ± 74.27; P = 0.2135; Fig. 4B); however, V̇e/V̇o2 did increase by 268% when CO2 increased to 10% (from 42.67 ± 27.83 to 114.56 ± 102.78; P = 0.0053; Fig. 4B). Moreover, the 1,500 mg/kg dose of urethane reduced the slope of V̇e/V̇o2 as CO2 increased (F4,60 = 7.66; P < 0.001). As observed with 100/10 ketamine-xylazine, 1,500 mg/kg urethane caused hypoventilation relative to metabolic need. In contrast, in the group treated with 1,000 mg/kg urethane (n = 15), the drug did not cause hypoventilation. Under control conditions, V̇e/V̇o2 increased by 444% when inspired gas increased from 0 to 7% CO2 (from 30.10 ± 27.14 to 133.59 ± 98.00; P = 0.0012; Fig. 4B). After treatment with 1,000 mg/kg urethane, V̇e/V̇o2 increased by 260% when CO2 increased from 0 to 7% (from 44.11 ± 37.60 to 114.82 ± 133.10; P = 0.0189; Fig. 4B). Additionally, 1,000 mg/kg urethane did not decrease V̇e/V̇o2 at either 0% (P = 0.8078) or 7% CO2 (P = 0.6849). Similarly, 500 mg/kg urethane did not induce hypoventilation in a third cohort of mice (n = 15). There was a 450% increase in V̇e/V̇o2 under control conditions when CO2 increased from 0 to 7% (from 39.97 ± 31.55 to 179.70 ± 109.94; P = 0.0023; Fig. 4B). The slope of V̇e/V̇o2 in response to an increase in CO2 was not affected by 500 mg/kg urethane (F1,14 = 0.6293; P = 0.4409). V̇e/V̇o2 increased by 414% as CO2 increased from 0 to 7% (from 56.85 ± 26.14 to 235.10 ± 175.75; P < 0.001; Fig. 4B); there was no difference in V̇e/V̇o2 in 500 mg/kg urethane compared with control in either 0% (P = 0.8634) or 7% CO2 (P = 0.2411). In contrast to results observed in the ketamine-xylazine experiments, we did not see decreased body temperature after administering the lower doses of urethane (500 and 1,000 mg/kg), even though we saw a decrease in O2 consumption.
There was a large effect of urethane on respiratory frequency (Fig. 4C). Mice in the 1,500 mg/kg urethane treatment group (n = 16) had an 89% increase in FR in control conditions when CO2 was increased to 7% (from 162.90 ± 30.42 to 308.32 ± 23.36 breaths/min; P < 0.001; Fig. 4C); FR increased by 105% when CO2 increased from 0 to 10% (P < 0.001; Fig. 4C). After treatment with 1,500 mg/kg urethane, baseline FR (in 0% CO2) increased by 80% (from 162.90 ± 30.42 to 293.67 ± 54.13 breaths/min; P < 0.001; Fig. 4C). Surprisingly, after treatment with 1,500 mg/kg urethane anesthesia, FR decreased by 17% as CO2 increased from 0 to 7% (from 293.67 ± 54.13 to 243.74 ± 21.95 breaths/min; P < 0.001; Fig. 4C) and decreased by 21% when CO2 increased to 10% (P < 0.001; Fig. 4C). In the cohort treated with 1,000 mg/kg urethane (n = 15), FR increased in control conditions by 87% as CO2 increased to 7% (from 177.09 ± 48.56 to 330.88 ± 19.75 breaths/min; P < 0.001; Fig. 4C). In these same mice after treatment with 1,000 mg/kg urethane, baseline FR in 0% CO2 increased by 50% (from 177.09 ± 48.56 to 264.77 ± 33.24 breaths/min; P < 0.001; Fig. 4C), and FR increased by only 9% in response to 7% CO2 (from 264.77 ± 33.24 to 287.37 ± 16.51 breaths/min; P = 0.0021; Fig. 4C); the increase in FR was reduced to 15% of control (P < 0.001). Mice in the 500 mg/kg treatment group (n = 15) increased FR by 96% in control conditions in response to 7% CO2 (from 170.69 ± 32.69 to 334.26 ± 25.95 breaths/min; P < 0.001; Fig. 4C). Urethane at 500 mg/kg increased baseline FR in 0% CO2 by 35% (from 170.69 ± 32.69 to 230.19 ± 40.15 breaths/min; P = 0.002; Fig. 4C). The increase in FR in response to 7% CO2 was also reduced by 500 mg/kg urethane to 19% (from 230.19 ± 40.15 to 274.34 ± 19.75 breaths/min; P < 0.002; Fig. 4C).
Urethane anesthesia also blunted the effect of CO2 on VT (Fig. 4D). Mice in the 1,500 mg/kg urethane treatment group (n = 16) increased VT in control conditions by 113% in response to 7% CO2 (from 11.76 ± 7.67 to 25.10 ± 13.54 μl/g; P < 0.001; Fig. 4D) and by 154% in response to 10% CO2 (P < 0.001; Fig. 4D). After treatment with 1,500 mg/kg urethane, VT did not significantly increase in response to 7% CO2 (from 9.05 ± 6.60 to 11.78 ± 8.55 μl/g; P = 0.0608; Fig. 4D). Urethane at 1,500 mg/kg reduced the response of VT to 10% CO2 to 31% of control (P = 0.0011; Fig. 4D). Urethane at 1,000 mg/kg affected the VT to a lesser extent. In the 1,000 mg/kg treatment group under control conditions (n = 15), VT increased by 107% in response to 7% CO2 (from 8.41 ± 4.65 to 17.43 ± 8.31 μl/g; P < 0.001; Fig. 4D). After treatment with 1,000 mg/kg urethane, VT decreased in 0% CO2 (from 8.41 ± 4.65 to 4.95 ± 1.07 μl/g; P = 0.001; Fig. 4D). Then, VT did not significantly increase in response to 7% CO2 (from 4.94 ± 1.07 to 6.72 ± 1.83 μl/g; P = 0.0697; Fig. 4D). The lowest dose of urethane (500 mg/kg) also reduced the VT response to CO2. In control conditions, mice in the 500 mg/kg treatment group (n = 15) increased their VT in response to 7% CO2 by 128% (from 6.99 ± 2.02 to 15.93 ± 2.22 μl/g; P < 0.001; Fig. 4D). 500 mg/kg urethane reduced the increase in VT to only 54% of control in 7% CO2 (from 6.62 ± 1.47 to 11.44 ± 2.38 μl/g; P < 0.001; Fig. 4D).
Urethane Did Not Affect 5-HT Neuron Chemosensitivity
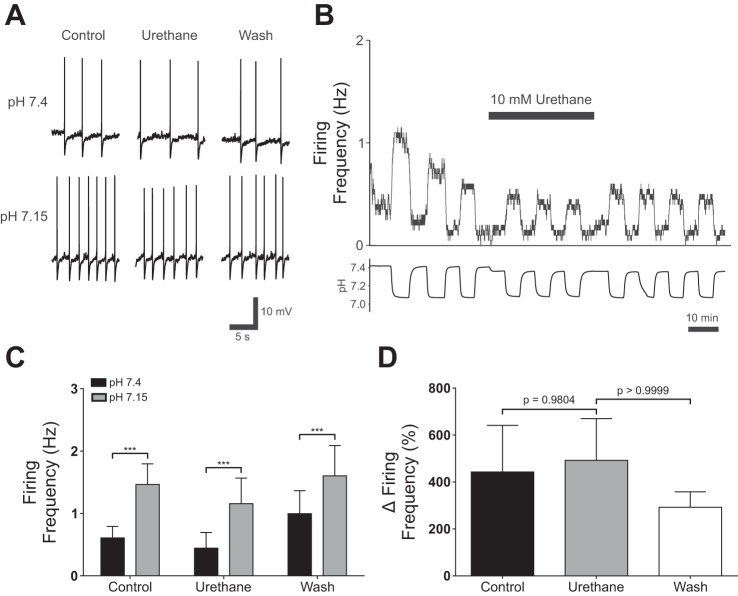
To determine whether urethane had any effect on 5-HT neurons, patch-clamp recordings were performed from cultured ePet-EYFP neurons (n = 13; Fig. 5, A–C). Acidosis increased firing frequency in all 5-HT neurons tested from 0.61 ± 0.67 Hz in normal aCSF to 1.47 ± 1.18 Hz in acidic aCSF (P < 0.001; Fig. 5B). In normal aCSF, urethane (10 mM) did not inhibit 5-HT neuron firing at baseline (0.44 ± 0.90 Hz in normal aCSF with urethane; P = 0.2860; Fig. 5b). When neurons were exposed to acidic aCSF with 10 mM urethane added, they increased their firing frequency to 1.16 ± 1.49 Hz (P < 0.001; Fig. 5B). Similarly, there was no difference in the percent change of firing frequency in response to acidic aCSF in urethane (491.90 ± 641.50%) compared with control (443.00 ± 714.40%; P = 0.9804; Fig. 5C) or washout (292.00 ± 238.00%; P > 0.9999; Fig. 5C).
Fig. 5.
Urethane did not block 5-HT chemosensitivity in vitro. A: effect of urethane (10 mM) on changes in 5-HT neuron membrane potential in response to acidic aCSF. B: plot of 5-HT neuron firing rate changes in response to acidosis show that chemosensitivity was not blocked by urethane (10 mM). C: summary of current-clamp recordings from cultured 5-HT neurons. 5-HT neurons increased their firing frequency in response to hypercapnic aCSF (pH 7.2) in control conditions (control; P < 0.001, n = 13). Urethane (10 mM) added to the bath solution did not block 5-HT neuron chemosensitivity, as neurons increased their firing frequency in response to acidosis (urethane; P < 0.001, n = 13). 5-HT neurons remained chemosensitivity after washout (wash; P < 0.001, n = 13). A two-way repeated measures ANOVA demonstrated that there was a significant effect of pH (F2,24 = 5.667, P = 0.00096, n = 13) and urethane (F2,24 = 5.667, P = 0.0798, n = 13). However, there was no significant interaction between pH and urethane (F2,24 = 1.707, P = 0.2027, n = 13). D: urethane did not alter the percent increase in the firing frequency of 5-HT neurons in response to acidosis (Friedman statistic = 2.00, P = 0.3679, n = 13). P values on the graph were calculated using Dunn’s multiple comparison test. ***P < 0.001.
DISCUSSION
Here we studied the effects of three anesthetic regimens (isoflurane, ketamine-xylazine, and urethane) on the HCVR of adult C57Bl/6 mice in vivo. We also examined the effects of ketamine and urethane on baseline firing frequency and chemosensitivity of 5-HT neurons in vitro. We found that isoflurane, ketamine-xylazine, and urethane anesthesia all markedly decreased the HCVR of adult C57Bl/6 mice at therapeutic doses. A reduction in the HCVR was observed at subtherapeutic doses of each anesthetic. However, in experiments with ketamine-xylazine and urethane, where it was possible to measure O2 consumption, mice decreased their O2 consumption with subtherapeutic doses, so that ventilation matched metabolic demand. Unlike the disruption of 5-HT neuron chemosensitivity by isoflurane that we previously reported (Massey et al. 2015), patch-clamp recordings demonstrated that ketamine and urethane did not affect 5-HT neuron chemosensitivity in vitro. Thus the effects of these two anesthetics on the HCVR are due to a different mechanism than that of isoflurane (Massey et al. 2015). The large reductions in the HCVR by ketamine-xylazine and urethane are likely due to inhibition of multiple sites involved in respiratory control, potentially including other chemoreceptor neurons or glia.
Ketamine-Xylazine and Urethane Alter Baseline Breathing
Ketamine-xylazine anesthesia is widely used in animal research; however, reports on the effects of ketamine anesthesia on breathing are variable, possibly due to differences in methodology and species studied. In newborn piglets and decerebrate adult rats, ketamine reduces baseline breathing (Bailie et al. 1979; Sapru and Krieger 1979). In contrast, baseline ventilation is increased following ketamine administration in dogs (Hirshman et al. 1975). Administration of ketamine via temporal artery catheter in goats produces increased blood pressure, heart rate, and cardiac output (Ivankovich et al. 1974). In newborn piglets treated with nitrous oxide, ketamine decreases respiratory rate, heart rate, and cardiac output compared with nitrous oxide alone (Bailie et al. 1979). Studies in humans have also been inconsistent. For example, one study reported that ketamine does not affect ventilation in spontaneously breathing subjects, whereas other reports found that ketamine either stimulates or depresses breathing (Bourke et al. 1987; Morel et al. 1986; Tokics et al. 1987).
Xylazine has different effects on cardiorespiratory function than ketamine anesthesia. Cardiac output is reduced in horses and ponies following xylazine treatment, but these values rapidly return to baseline (Clarke and Hall 1969; Garner et al. 1971b). Xylazine treatment leads to decreased heart rate and aortic blood flow after either intravenous or intramuscular administration in dogs (Klide et al. 1975). Following xylazine treatment, arterial blood pressure has a biphasic response in dogs (Antonaccio et al. 1973; Hsu et al. 1985; Klide et al. 1975; Muir and Piper 1977). Similar responses are observed in horses and ponies (Clarke and Hall 1969; Garner et al. 1971b; Kerr et al. 1972). In some studies the values for arterial pH, , and are not affected by xylazine treatment in dogs, cats, ponies, and horses (Garner et al. 1971a; Haskins et al. 1975; Klide et al. 1975; Muir and Piper 1977). However, decreases in respiratory rate are reported in xylazine-treated horses (Burns and McMullan 1972; Fessl 1970; Hoffman 1974; McCashin and Gabel 1975). The combination of ketamine and xylazine is more commonly used in research settings, and presumably the effects on cardiorespiratory function would be different than with either anesthetic alone. In studies where ketamine and xylazine are used in combination, depression of baseline breathing is observed in both mice and rats (Erhardt et al. 1984; Sumitra et al. 2004; Wixson et al. 1987).
Urethane is frequently described as preserving normal cardiorespiratory control. For example, some of the earliest reports of its use in dogs state there is no change in heart rate or breathing (Schmiedeberg 1885). Studies from the late 20th century describe minimal effects of urethane on blood gases in rats (Buelke-Sam et al. 1978; Folle and Levesque 1976). Recordings from decerebrate rats show no effect on breathing frequency or VT (Sapru and Krieger 1979). Urethane anesthesia does not change baseline ventilation in cats and rats according to two studies (Boon et al. 2004; Wang and Nims 1948). However, there is a report from urethane-anesthetized rats that demonstrates ventilation is reduced by 64–77% during the first few hours of anesthesia (Hughes et al. 1982).
Our results demonstrate clearly that ketamine-xylazine and urethane anesthesia both alter breathing in 0% CO2. Ketamine-xylazine greatly reduced baseline ventilation in 0% CO2. Although urethane anesthesia did not change baseline ventilation, it did markedly increase baseline breathing frequency while decreasing VT. Thus these anesthetics have different effects, likely due to actions on different targets in the respiratory network. In some cases the effects may be on chemoreceptors, and in other cases the effects may be on some other elements of the respiratory network. In the case of isoflurane there are strong effects on 5-HT neurons, but there are likely also inhibitory effects on multiple other cell types involved in breathing. For ketamine-xyalzine and urethane, it is not known where in the respiratory network the drugs act, but there are apparently not major effects on 5-HT neurons.
Ketamine antagonizes glutamatergic N-methyl-D-aspartate (NMDA) receptors and hyperpolarization-activated cyclic nucleotide-gated channel 1 (HCN1) chloride ion channels, which both have a role in control of breathing (Chen et al. 2009; MacDonald et al. 1987). Antagonism of NMDA receptors leads to an increase in inspiratory duration and decreased phrenic nerve discharge in adult rats (Connelly et al. 1992). HCN1 channels mediate the hyperpolarization-activated current (Ih), which is an important feature of pacemaker neurons and plays a modulatory role in the respiratory network (Thoby-Brisson et al. 2000). Xylazine activates noradrenergic α2-receptors, which inhibit respiratory neurons in the rostral ventrolateral medulla (RVLM) (Errchidi et al. 1990; Greene and Thurmon 1988; Hilaire et al. 1989; Jean-Charles and Gerard 2002). Thus the combination of ketamine and xylazine is likely to affect respiratory neurons throughout the medulla, especially those involved in generation of the respiratory rhythm.
The molecular mechanisms of urethane anesthesia remain poorly understood. Recordings from Xenopus oocytes demonstrate that urethane both enhances GABAA and glycinergic receptors and blocks glutamatergic NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (Hara and Harris 2002). However, additional recordings from neurons in rat visual cortex do not show any modulation of either GABAergic or glutamatergic receptors (Sceniak and MacIver 2006). Instead, these researchers concluded urethane was likely potentiating tandem of P-domains in a weakly inward rectifying K+ (TWIK) channels, which conduct Ba2+-sensitive K+ leak currents (Sceniak and MacIver 2006). These conflicting data make it difficult to determine the mechanism that underlies the effects of urethane on breathing, but modulation of glutamatergic, GABAergic, or TWIK channels would each alter respiratory control.
Our data demonstrate ketamine-xylazine and urethane anesthesia induce profound changes to breathing in 0% CO2 in adult C57Bl/6 mice. This is noteworthy, as many in the field use these agents as the primary anesthetic during survival surgeries or during implantation of electrodes for in vivo recordings. Although our methods do not recapitulate every protocol used with these anesthetics, and it is possible that with some protocols these anesthetics do not have such severe effects, it is important to consider adverse effects of these anesthetics. These anesthetics are widely used in experiments on breathing, and their effects are not often considered when interpreting results. Urethane in particular is widely believed to have minimal effects on breathing and chemoreception. Our data clearly demonstrate that one cannot assume that these agents have no effect on breathing. Rather, for any protocol used it is important to consider the possibility that these anesthetics affect breathing and CO2 chemoreception and demonstrate that the anesthetics do not affect the results. Without artificial ventilation, anesthetics may cause severe changes in blood gases. Furthermore, researchers often use a supplementary anesthetic during the recording phase that follows surgery in addition to ketamine-xylazine, and such a combination could further affect breathing. We did not investigate the effect of a combination of anesthetics on breathing, but caution should be taken when performing experiments on breathing with combinations of anesthetics used during surgery and maintenance phases of recordings, as interactions between anesthetics may produce different effects than each individually. Even if supplementary anesthetics are given at low doses to maintain anesthesia during recordings, our results indicate that anesthetics at subtherapeutic doses can alter breathing.
Isoflurane, Ketamine-Xylazine, and Urethane Reduce CO2 Chemoreception
Halogenated anesthetics, including isoflurane, are commonly used for research requiring surgical anesthesia (Eger 1981). We previously reported that 1% isoflurane reduces the HCVR in two different strains of mice: Lmx1bf/f and ePet-EYFP (Massey et al. 2015). We also found that these effects correlate with inhibition of 5-HT neurons in both culture and a perfused brainstem preparation (Massey et al. 2015). Results from other investigators indicate that isoflurane-induced inhibition of 5-HT neurons is dose dependent (Johansen et al. 2015). Our current data indicate that the HCVR is greatly reduced in vivo even at a subtherapeutic concentration of isoflurane (Fig. 1A). These data are important because they indicate that CO2 chemoreception is more profoundly inhibited by isoflurane than has been previously recognized. Recognition of this effect is important since lower concentrations of isoflurane (<1%) are often used as maintenance anesthesia during in vivo recordings and other postsurgical procedures, including during studies of respiratory chemoreception (Mulkey et al. 2004; Mulkey et al. 2007). Although our results do not indicate that anesthetized whole animal preparations should never be used, they do indicate that anesthetics may alter the interpretation of the results, and therefore, it is important for researchers to take these effects into account. Since isoflurane directly inhibits 5-HT neurons, the use of this anesthetic is contraindicated for any studies of these cells. In addition to the effects on CO2 chemoreception, isoflurane also reduces the HVR in both dogs and rats (Hirshman et al. 1977; Karanovic et al. 2010). Studies in humans indicate that even subtherapeutic doses of isoflurane depress the HVR (Knill et al. 1983; Pandit 2014). Thus isoflurane should not be used in studies of either CO2 or O2 chemoreception.
There have been a number of studies investigating the effects on breathing of ketamine anesthesia with or without xylazine, but few of these studies measured the response to hypercapnia. In decerebrate rats, ketamine anesthesia reduces the increased ventilation in response to NaCN administration (Sapru and Krieger 1979). In dogs, ketamine reduces both the HVR and HCVR (Hirshman et al. 1975). Studies investigating the effects of urethane anesthesia on CO2 sensitivity are also few in number, but most report minimal effects. For example, under urethane anesthesia, the HCVR is unaffected in cats and rats (Boon et al. 2004; Hughes et al. 1982; Wang and Nims 1948).
Our results demonstrate that ketamine-xylazine (100 and 10 mg/kg, respectively) and urethane (1,500 mg/kg) anesthesia both essentially eliminated the HCVR in mice. Ketamine-xylazine reduced the increase in ventilation in response to every level of CO2 tested. Breathing frequency and VT were both reduced at baseline and had minimal increases in response to CO2 during ketamine-xylazine anesthesia. Our results also disprove the widely held belief that urethane has minimal effects on baseline breathing and respiratory chemoreception and therefore provide no justification for using urethane as the agent of choice for studies of respiratory control. In fact, urethane anesthesia had profound effects on breathing frequency at baseline and in response to hypercapnia.
We chose to use hyperoxia for our whole animal plethysmography recordings to decrease the contribution of PRCs to the HCVR (Lahiri and DeLaney 1975). However, there is evidence in some recordings that hyperoxia can result in paradoxical stimulation of breathing. Although this is potentially a concern, we do not believe it would alter our conclusions for two reasons. First, previous work in our laboratory has not shown a significant effect of hyperoxia on baseline breathing or the HCVR in adult wild-type mice (Hodges et al. 2008). Second, if hyperoxia did cause hyperventilation, it would only strengthen our conclusions that anesthetics severely decrease the HCVR in mice since these drugs would inhibit both the CO2-induced stimulation of CRCs and the hyperoxia-induced hyperventilation.
Interactions Between Metabolism and Breathing
Breathing, body temperature regulation, and metabolism are strongly linked (Mortola and Maskrey 2011). During the neonatal period in many species, hypoxia induces hypometabolism and only a minimal increase in ventilation (Mortola et al. 1989). This response to hypoxia also occurs in adult rats, guinea pigs, and other small rodents (Frappell et al. 1992). At moderate levels of hypercapnia (~5%), O2 consumption changes are modest (Saiki and Mortola 1996). At higher levels of CO2, there are larger changes in O2 consumption (Morita et al. 1993; Mortola and Maskrey 2011; Schaefer et al. 1975). However, hypercapnia in these experiments is either chronic or administered during hypothermia, both of which could impact O2 consumption differently than in our experimental protocol.
To account for metabolic changes during hypercapnia and anesthesia, we measured O2 consumption during all experiments with ketamine-xylazine and urethane. Our results using therapeutic doses of ketamine-xylazine (100 and 10 mg/kg, respectively) and urethane (1,500 mg/kg) showed a reduced increase in the V̇e/V̇o2 ratio at higher levels of CO2. Thus the increase in V̇e in hypercapnic conditions was not sufficient to match O2 consumption and metabolic demand. The depressed increase in the V̇e/V̇o2 ratio in hypercapnia caused by ketamine-xylazine was more severe than urethane anesthesia.
We also observed a reduced increase in V̇e as CO2 increased during subtherapeutic doses of ketamine-xylazine (50 and 5 mg/kg, respectively) and urethane (500 and 1,000 mg/kg), indicating that the HCVR of mice is very sensitive V̇e/V̇o2 to anesthetics. However, when we accounted for decreased O2 consumption, the increase in the V̇e/V̇o2 ratio was not depressed with subtherapeutic doses of ketamine-xylazine or urethane. Thus hypoventilation did not occur because mice decreased their O2 consumption in parallel with ventilation. These data are important because they highlight that even subtherapeutic doses of anesthetics, where animals are still responsive or ambulatory, can alter breathing and metabolism. Furthermore, at therapeutic levels some anesthetics uncouple the normally tight coordination of body temperature regulation, O2 consumption, and breathing.
Role of 5-HT Neurons in Anesthesia-Induced Depression of the HCVR
Previous experiments on isoflurane indicate that reduction of the HCVR is due in part to inhibition of 5-HT neuron firing and masking chemosensitivity (Massey et al. 2015). Therefore, we wanted to determine if ketamine-xylazine and urethane anesthesia reduced the HCVR through a similar mechanism.
Neither ketamine nor urethane inhibited baseline firing frequency of 5-HT neurons in pH 7.4 aCSF. There was a statistically significant effect on 5-HT neuron chemosensitivity of ketamine. However, this effect was minimal as 5-HT neurons still had a robust increase in their firing frequency in response to acidic aCSF application. The nearly complete elimination of the HCVR could not be explained on the basis of this small effect on 5-HT neurons. There was also no effect of urethane on the response of 5-HT neurons to acidic aCSF. Therefore, the effect on the HCVR of these drugs was not due to inhibition of 5-HT neuron chemosensitivity.
These data suggest that the mechanisms of the effects on breathing of ketamine and urethane anesthesia are different from isoflurane. These results are not surprising, because both ketamine and urethane have been reported to act on fast excitatory and inhibitory neurotransmitter receptors (Hara and Harris 2002; MacDonald et al. 1987). The response of 5-HT neurons to physiologically relevant decreases in pH is an intrinsic property (Corcoran et al. 2009; Richerson 2004; Teran et al. 2014). Thus blocking fast glutamatergic and GABAergic receptors would not prevent a 5-HT neuron from increasing its firing rate in response to acidosis. Moreover, the aCSF solutions used already included antagonists of fast neurotransmitter signaling. However, it is not surprising that these anesthetics would affect CO2 chemoreception without inhibiting 5-HT neuron chemosensitivity as there are a number of other regions that have been implicated in chemoreception, including locus coeruleus, nucleus tractus solitarius, and retrotrapezoid nucleus (Feldman et al. 2003; Fukuda et al. 1978; Mulkey et al. 2004; Nichols et al. 2008; Pineda and Aghajanian 1997). It is also possible that these anesthetics could affect CO2 chemoreception via effects on neurons downstream from chemoreceptors (i.e., respiratory control nuclei).
Although hyperoxia may lead to increased production of reactive oxygen species (ROS) in in vitro preparations (Dean et al. 2004; Mulkey et al. 2003), and this could theoretically alter neuronal function, we do not believe that would change the main conclusions from our experiments because 5-HT neurons robustly increased their firing frequency in response to a decrease in bath pH and this response was not affected by anesthetics, even though ROS would be present throughout the recordings. Although production of ROS is a potential concern, it remains the standard approach to bubble bath solutions with 95% O2-5% CO2 for patch-clamp recordings.
These data highlight the fact that anesthesia alters the physiology of in vivo anesthetized preparations, even at low levels. In some cases, data obtained from brain slices, cell culture, and other reduced preparations may more closely resemble normal cell function, while also allowing better experimental control. Ultimately, the results obtained using any approach must be interpreted while keeping in mind the inherent limitations.
GRANTS
Research reported in this publication was supported by National Institute of Neurological Disorders and Stroke Grant U01-NS-090414 and National Institute of Child Health and Human Development Grants P01-HD-36379 and R01-HD-052772.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.M. and G.B.R. conceived and designed research; C.A.M. performed experiments; C.A.M. and G.B.R. analyzed data; C.A.M. and G.B.R. interpreted results of experiments; C.A.M. prepared figures; C.A.M. drafted manuscript; C.A.M. and G.B.R. edited and revised manuscript; C.A.M. and G.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Xiuqiong Zhou for mouse husbandry. We thank Lori Smith for technical contributions.
REFERENCES
- Antonaccio MJ, Robson RD, Kerwin L. Evidence for increased vagal tone and enhancement of baroreceptor reflex activity after xylazine (2-(2,6-dimethylphenylamino)-4-H-5,6-dihydro-1,3-thiazine) in anesthestized dogs. Eur J Pharmacol 23: 311–316, 1973. doi: 10.1016/0014-2999(73)90102-7. [DOI] [PubMed] [Google Scholar]
- Bailie MD, Alward CT, Sawyer DC, Hook JB. Effect of anesthesia on cardiovascular and renal function in the newborn piglet. J Pharmacol Exp Ther 208: 298–302, 1979. [PubMed] [Google Scholar]
- Boon JA, Garnett NB, Bentley JM, Milsom WK. Respiratory chemoreflexes and effects of cortical activation state in urethane anesthetized rats. Respir Physiol Neurobiol 140: 243–256, 2004. doi: 10.1016/j.resp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Bourke DL, Malit LA, Smith TC. Respiratory interactions of ketamine and morphine. Anesthesiology 66: 153–156, 1987. doi: 10.1097/00000542-198702000-00008. [DOI] [PubMed] [Google Scholar]
- Bree MM, Feller I, Corssen G. Safety and tolerance of repeated anesthesia with CI 581 (Ketamine) in monkeys. Anesth Analg 46: 596–600, 1967. [PubMed] [Google Scholar]
- Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci 28: 157–162, 1978. [PubMed] [Google Scholar]
- Burns SJ, McMullan WC. Clinical application of Bay Va 1470 in the horse. Vet Med Small Anim Clin 67: 77, 1972. [PubMed] [Google Scholar]
- Cerpa VJ, Wu Y, Bravo E, Teran FA, Flynn RS, Richerson GB. Medullary 5-HT neurons: switch from tonic respiratory drive to chemoreception during postnatal development. Neuroscience 344: 1–14, 2017. doi: 10.1016/j.neuroscience.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ensor CR, Bohner B. The neuropharmacology of 2-(omicron-chlorophenyl)-2-methylaminocyclohexanoe hydrochloride. J Pharmacol Exp Ther 152: 332–339, 1966. [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci 29: 600–609, 2009. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke KW, Hall LW. “Xylazine”–a new sedative for horses and cattle. Vet Rec 85: 512–517, 1969. doi: 10.1136/vr.85.19.512. [DOI] [PubMed] [Google Scholar]
- Comroe JH., Jr Central and reflex control of breathing. Anesth Analg 46: 367–376, 1967. doi: 10.1213/00000539-196707000-00001. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Otto-Smith MR, Feldman JL. Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res 596: 99–110, 1992. doi: 10.1016/0006-8993(92)91537-O. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg 45: 29–40, 1966. doi: 10.1213/00000539-196601000-00007. [DOI] [PubMed] [Google Scholar]
- Dean JB, Mulkey DK, Henderson RA 3rd, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol (1985) 96: 784–791, 2004. doi: 10.1152/japplphysiol.00892.2003. [DOI] [PubMed] [Google Scholar]
- Domino EF, Chodoff P, Corssen G. Pharmacologic effects of Ci-581, a new sissociative anesthetic, in man. Clin Pharmacol Ther 6: 279–291, 1965. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol 484: 77–86, 1995. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI., 2nd Isoflurane: a review. Anesthesiology 55: 559–576, 1981. doi: 10.1097/00000542-198111000-00014. [DOI] [PubMed] [Google Scholar]
- Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med (Berl) 184: 159–169, 1984. doi: 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- Errchidi S, Hilaire G, Monteau R. Permanent release of noradrenaline modulates respiratory frequency in the newborn rat: an in vitro study. J Physiol 429: 497–510, 1990. doi: 10.1113/jphysiol.1990.sp018269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessl L. [Clinical experiences with Bay Va 1470 (Rompun)]. Wien Tierarztl Monatsschr 57: 155–156, 1970. [PubMed] [Google Scholar]
- Flórez J, Borison HL. Effects of central depressant drugs on respiratory regulation in the decerebrate cat. Respir Physiol 6: 318–329, 1969. doi: 10.1016/0034-5687(69)90031-0. [DOI] [PubMed] [Google Scholar]
- Folle LE, Levesque RI. Circulatory, respiratory and acid-base balance changes produced by anesthetics in the rat. Acta Biol Med Ger 35: 605–612, 1976. [PubMed] [Google Scholar]
- Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y, Schläfke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch 376: 229–235, 1978. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- Garner HE, Amend JF, Rosborough JP. Effects of Bay VA 1470 on respiratory parameters in ponies. Vet Med Small Anim Clin 66: 921–923, 1971a. [PubMed] [Google Scholar]
- Garner HE, Amend JF, Rosborough JP. Effects on Bay Va 1470 on cardiovascular parameters in ponies. Vet Med Small Anim Clin 66: 1016–1017, 1971b. [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SA, Thurmon JC. Xylazine–a review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther 11: 295–313, 1988. doi: 10.1111/j.1365-2885.1988.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94: 313–318, 2002. [DOI] [PubMed] [Google Scholar]
- Haskins SC, Farver TB, Patz JD. Ketamine in dogs. Am J Vet Res 46: 1855–1860, 1985. [PubMed] [Google Scholar]
- Haskins SC, Peiffer RL Jr, Stowe CM. A clinical comparison of CT1341, ketamine, and xylazine in cats. Am J Vet Res 36: 1537–1543, 1975. [PubMed] [Google Scholar]
- Hellyer PW, Freeman LC, Hubbell JA. Induction of anesthesia with diazepam-ketamine and midazolam-ketamine in greyhounds. Vet Surg 20: 143–147, 1991. doi: 10.1111/j.1532-950X.1991.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res 485: 325–332, 1989. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Hypoxic ventilatory drive in dogs during thiopental, ketamine, or pentobarbital anesthesia. Anesthesiology 43: 628–634, 1975. doi: 10.1097/00000542-197512000-00004. [DOI] [PubMed] [Google Scholar]
- Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Depression of hypoxic ventilatory response by halothane, enflurane and isoflurane in dogs. Br J Anaesth 49: 957–963, 1977. doi: 10.1093/bja/49.10.957. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PE. Clinical evaluation of xylazine as a chemical restraining agent, sedative, and analgesic in horses. J Am Vet Med Assoc 164: 42–45, 1974. [PubMed] [Google Scholar]
- Hsu WH. Xylazine-induced depression and its antagonism by alpha adrenergic blocking agents. J Pharmacol Exp Ther 218: 188–192, 1981. [PubMed] [Google Scholar]
- Hsu WH, Lu ZX, Hembrough FB. Effect of xylazine on heart rate and arterial blood pressure in conscious dogs, as influenced by atropine, 4-aminopyridine, doxapram, and yohimbine. J Am Vet Med Assoc 186: 153–156, 1985. [PubMed] [Google Scholar]
- Hughes EW, Martin-Body RL, Sarelius IH, Sinclair JD. Effects of urethane-chloralose anaesthesia on respiration in the rat. Clin Exp Pharmacol Physiol 9: 119–127, 1982. doi: 10.1111/j.1440-1681.1982.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Ivankovich AD, Miletich DJ, Reimann C, Albrecht RF, Zahed B. Cardiovascular effects of centrally administered ketamine in goats. Anesth Analg 53: 924–933, 1974. doi: 10.1213/00000539-197453060-00022. [DOI] [PubMed] [Google Scholar]
- Jean-Charles V, Gérard H. Noradrenergic receptors and in vitro respiratory rhythm: possible interspecies differences between mouse and rat neonates. Neurosci Lett 324: 149–153, 2002. doi: 10.1016/S0304-3940(02)00191-X. [DOI] [PubMed] [Google Scholar]
- Johansen SL, Iceman KE, Iceman CR, Taylor BE, Harris MB. Isoflurane causes concentration-dependent inhibition of medullary raphé 5-HT neurons in situ. Auton Neurosci 193: 51–56, 2015. doi: 10.1016/j.autneu.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanovic N, Pecotic R, Valic M, Jeroncic A, Carev M, Karanovic S, Ujevic A, Dogas Z. The acute hypoxic ventilatory response under halothane, isoflurane, and sevoflurane anaesthesia in rats. Anaesthesia 65: 227–234, 2010. doi: 10.1111/j.1365-2044.2009.06194.x. [DOI] [PubMed] [Google Scholar]
- Kerr DD, Jones EW, Holbert D, Huggins K. Comparison of the effects of xylazine and acetylpromazine maleate in the horse. Am J Vet Res 33: 777–784, 1972. [PubMed] [Google Scholar]
- Klide AM, Calderwood HW, Soma LR. Cardiopulmonary effects of xylazine in dogs. Am J Vet Res 36: 931–935, 1975. [PubMed] [Google Scholar]
- Knill RL, Kieraszewicz HT, Dodgson BG, Clement JL. Chemical regulation of ventilation during isoflurane sedation and anaesthesia in humans. Can Anaesth Soc J 30: 607–614, 1983. doi: 10.1007/BF03015231. [DOI] [PubMed] [Google Scholar]
- Kobinger W. Central alpha-adrenergic systems as targets for hypotensive drugs. Rev Physiol Biochem Pharmacol 81: 39–100, 1978. doi: 10.1007/BFb0034091. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol 24: 249–266, 1975. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58: 251–266, 1987. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia 42: 109–114, 1986. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Massey CA, Iceman KE, Johansen SL, Wu Y, Harris MB, Richerson GB. Isoflurane abolishes spontaneous firing of serotonin neurons and masks their pH/CO2 chemosensitivity. J Neurophysiol 113: 2879–2888, 2015. doi: 10.1152/jn.01073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DA, Chen G, Kaump DH, Ensor C. general anesthetic and other pharmacological properties of 2-(O-chlorophenyl)-2-methylamino cyclohexanone HCl (Ci-58l). J New Drugs 5: 21–33, 1965. doi: 10.1002/j.1552-4604.1965.tb00219.x. [DOI] [PubMed] [Google Scholar]
- McCashin FB, Gabel AA. Evaluation of xylazine as a sedative and preanesthetic agent in horses. Am J Vet Res 36: 1421–1429, 1975. [PubMed] [Google Scholar]
- Mirvish SS. The carcinogenic action and metabolism of urethan and N-hydroxyurethan. Adv Cancer Res 11: 1–42, 1968. [DOI] [PubMed] [Google Scholar]
- Moens Y, Fargetton X. A comparative study of medetomidine/ketamine and xylazine/ketamine anaesthesia in dogs. Vet Rec 127: 567–571, 1990. [PubMed] [Google Scholar]
- Morel DR, Forster A, Gemperle M. Noninvasive evaluation of breathing pattern and thoraco-abdominal motion following the infusion of ketamine or droperidol in humans. Anesthesiology 65: 392–398, 1986. doi: 10.1097/00000542-198610000-00008. [DOI] [PubMed] [Google Scholar]
- Morita T, Konaka K, Kawasaki Y, Kawai F, Kanamori M, Mitsuda H. Effects of moderate hypercapnia on hypothermia induced by cold He-O2 in rats. Comp Biochem Physiol Comp Physiol 104: 215–218, 1993. doi: 10.1016/0300-9629(93)90305-N. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Maskrey M. Metabolism, temperature, and ventilation. Compr Physiol 1: 1679–1709, 2011. doi: 10.1002/cphy.c100008. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Rezzonico R, Lanthier C. Ventilation and oxygen consumption during acute hypoxia in newborn mammals: a comparative analysis. Respir Physiol 78: 31–43, 1989. doi: 10.1016/0034-5687(89)90140-0. [DOI] [PubMed] [Google Scholar]
- Muir WW, Piper FS. Effect of xylazine on indices of myocardial contractility in the dog. Am J Vet Res 38: 931–934, 1977. [PubMed] [Google Scholar]
- Mulkey DK, Henderson RA 3rd, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol (1985) 95: 910–921, 2003. doi: 10.1152/japplphysiol.00864.2002. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals (8th ed.) Washington, DC: National Academies Press, 2011. doi: 10.17226/12910. [DOI] [Google Scholar]
- Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008. doi: 10.1007/978-0-387-73693-861. [DOI] [PubMed] [Google Scholar]
- Pandit JJ. Volatile anaesthetic depression of the carotid body chemoreflex-mediated ventilatory response to hypoxia: directions for future research. Scientifica (Cairo) 2014: 394270, 2014. doi: 10.1155/2014/394270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997. doi: 10.1016/S0306-4522(96)00485-X. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266, 2005. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001. doi: 10.1016/S0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Saiki C, Mortola JP. Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol 491: 261–269, 1996. doi: 10.1113/jphysiol.1996.sp021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapru HN, Krieger AJ. Cardiovascular and respiratory effects of some anesthetics in the decerebrate rat. Eur J Pharmacol 53: 151–158, 1979. doi: 10.1016/0014-2999(79)90160-2. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, MacIver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol 95: 3865–3874, 2006. doi: 10.1152/jn.01196.2005. [DOI] [PubMed] [Google Scholar]
- Schaefer KE, Messier AA, Morgan C, Baker GT 3rd. Effect of chronic hypercapnia on body temperature regulation. J Appl Physiol 38: 900–906, 1975. [DOI] [PubMed] [Google Scholar]
- Schmiedeberg O. Ueber die pharmakologischen Wirkungen und die therapeutische Anwendung einiger Carbaminsäure-Ester. Archiv eExp Pathol Pharmakol 20: 203–216, 1885. doi: 10.1007/BF01918291. [DOI] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA 102: 16472–16477, 2005. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitra M, Manikandan P, Rao KV, Nayeem M, Manohar BM, Puvanakrishnan R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats–a comparative analysis. Life Sci 75: 1887–1896, 2004. doi: 10.1016/j.lfs.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Prog Brain Res 209: 207–233, 2014. doi: 10.1016/B978-0-444-63274-6.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Telgkamp P, Ramirez JM. The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci 20: 2994–3005, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokics L, Strandberg A, Brismar B, Lundquist H, Hedenstierna G. Computerized tomography of the chest and gas exchange measurements during ketamine anaesthesia. Acta Anaesthesiol Scand 31: 684–692, 1987. doi: 10.1111/j.1399-6576.1987.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Virtue RW, Alanis JM, Mori M, Lafargue RT, Vogel JH, Metcalf DR. An anesthetic agent: 2-orthochlorophenyl, 2-methylamino cyclohexanone HCl (CI-581). Anesthesiology 28: 823–833, 1967. doi: 10.1097/00000542-196709000-00008. [DOI] [PubMed] [Google Scholar]
- Wang SC, Nims LF. The effect of various anesthetics and decerebration on the CO2 stimulating action on respiration in cats. J Pharmacol Exp Ther 92: 187–195, 1948. [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999. doi: 10.1016/S0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001. [DOI] [PubMed] [Google Scholar]
- Wixson SK, White WJ, Hughes HC Jr, Marshall WK, Lang CM. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on noxious stimulus perception in adult male rats. Lab Anim Sci 37: 731–735, 1987. [PubMed] [Google Scholar]