Source space MEG was used to investigate sensorimotor cortical oscillations in individuals with SCI. This study provides evidence that individuals with cervical SCI exhibit decreased ERD when they attempt to grasp if they are incapable of generating muscle activity. However, there were no significant differences in ERD between paralyzed and able-bodied participants during motor imagery. These results have important implications for the design and evaluation of new therapies, such as motor imagery and neurofeedback interventions
Keywords: paralysis, spinal cord injury, sensorimotor rhythms, event-related desynchronization, magnetoencephalography
Abstract
After paralysis, the disconnection between the cortex and its peripheral targets leads to neuroplasticity throughout the nervous system. However, it is unclear how chronic paralysis specifically impacts cortical oscillations associated with attempted movement of impaired limbs. We hypothesized that μ- (8–13 Hz) and β- (15–30 Hz) event-related desynchronization (ERD) would be less modulated for individuals with hand paralysis due to cervical spinal cord injury (SCI). To test this, we compared the modulation of ERD from magnetoencephalography (MEG) during attempted and imagined grasping performed by participants with cervical SCI (n = 12) and able-bodied controls (n = 13). Seven participants with tetraplegia were able to generate some electromyography (EMG) activity during attempted grasping, whereas the other five were not. The peak and area of ERD were significantly decreased for individuals without volitional muscle activity when they attempted to grasp compared with able-bodied subjects and participants with SCI,with some residual EMG activity. However, no significant differences were found between subject groups during mentally simulated tasks (i.e., motor imagery) where no muscle activity or somatosensory consequences were expected. These findings suggest that individuals who are unable to produce muscle activity are capable of generating ERD when attempting to move, but the characteristics of this ERD are altered. However, for people who maintain volitional muscle activity after SCI, there are no significant differences in ERD characteristics compared with able-bodied controls. These results provide evidence that ERD is dependent on the level of intact muscle activity after SCI.
NEW & NOTEWORTHY Source space MEG was used to investigate sensorimotor cortical oscillations in individuals with SCI. This study provides evidence that individuals with cervical SCI exhibit decreased ERD when they attempt to grasp if they are incapable of generating muscle activity. However, there were no significant differences in ERD between paralyzed and able-bodied participants during motor imagery. These results have important implications for the design and evaluation of new therapies, such as motor imagery and neurofeedback interventions.
spinal cord injury (SCI) can lead to a debilitating loss of motor and somatosensory function that can cause plasticity in the brain. Neuroimaging evidence suggests that, after injury and impairment, cortical activation of sensorimotor networks changes in both amplitude and area (Cramer et al. 2011; Kokotilo et al. 2009). Specifically, attempted or imagined movement of impaired body parts produces reduced brain activity in motor cortex compared with able-bodied controls, whereas actual movement of intact body parts above the level of the injury, such as the hand in paraplegia, results in activation more medially in motor cortex compared with able-bodied controls (Cramer et al. 2005; Freund et al. 2013; Henderson et al. 2011; Hotz-Boendermaker et al. 2008; Raineteau and Schwab 2001; Turner et al. 2001). These more medial areas of motor cortex were previously connected to body parts below the level of the injury such as the legs or trunk. These changes in brain activity have been characterized in people with paraplegia, and to a limited degree in people with tetraplegia, using a variety of imaging modalities. However, there has been limited work investigating changes in cortical oscillations due to paralysis.
Mental rehearsal of movement, i.e., motor imagery (MI), utilizes similar cortical networks as executed movements, and has been used to study the sensorimotor system (Filimon et al. 2015; Jeannerod and Frak 1999; Park et al. 2015; Sharma and Baron 2013). Because MI does not require the ability to move but activates similar areas as motor execution, there is strong interest in developing MI as a rehabilitation intervention after paralysis to improve brain function (Cramer et al. 2007; Di Rienzo et al. 2014a, 2014c; López-Larraz et al. 2015; Mateo et al. 2015a). MI paradigms have also been used to probe aspects of the motor system after paralysis because the task is not dependent on a participant’s physical abilities (Dunlop 2008; Lotze and Halsband 2006). Behavioral and neuroimaging evidence suggests that people with paralysis exhibit differences when imagining movements as compared with attempting a movement, although no overt movement or somatosensory feedback is generated in either case (Guillot et al. 2012). MI after paralysis generates similar psychometric (e.g., electrodermal responses) and cortical activation patterns as in able-bodied controls (Alkadhi et al. 2005; Cramer et al. 2005; Di Rienzo et al. 2014a; Grangeon et al. 2012; Guillot et al. 2012; Gustin et al. 2010; Hotz-Boendermaker et al. 2008; Sabbah et al. 2002). Although some quantitative differences have been found in neuroimaging studies, the findings have been inconsistent and focused on capturing brain activity with fMRI (see Di Rienzo et al. 2014a for a review). Our goal is to increase our understanding of how SCI impacts the cortical oscillations during different movement conditions since this brain activity forms the basis for a number of rehabilitative and assistive technology-based interventions, such as MI training and brain-computer interfaces (Collinger et al. 2013; Cramer et al. 2007; Prasad et al. 2010).
Cortical oscillations, that is, rhythmic neural activity, are becoming of increased interest for understanding mechanisms of neural communication (Cheyne 2013; Donner and Siegel 2011; Engel and Fries 2010; Jones 2016; Thut et al. 2012; Tzagarakis et al. 2010; Womelsdorf et al. 2007). Several distinct frequency bands have been identified as corresponding to different neural networks and processes; μ- (8–13 Hz) and β-oscillations (15–30 Hz) make up the sensorimotor rhythms (SMR) and are modulated during movement-related tasks such as when participants are preparing and performing overt (Pfurtscheller and Lopes da Silva 1999; Tzagarakis et al. 2015) and imagined movements (Hari et al. 1997; Pfurtscheller and Neuper 1997) as well as receiving tactile stimuli (Bauer et al. 2006; Cheyne et al. 2003). During rest, activity in the μ- and β-bands is increased to inhibit the sensorimotor cortex and prevent local computations, such as inhibiting the generation of new motor commands during rest (Engel and Fries 2010; Haegens et al. 2011; Jensen and Mazaheri 2010; Takemi et al. 2013). During movement or active attention, there is a reduction in the μ- and β-band power, removing the inhibition and allowing sensory and motor computations to occur, a phenomenon referred to as event-related desynchronization (ERD) (Pfurtscheller and Lopes da Silva 1999). Evidence suggests that although the μ- and β-bands are modulated during many of the same tasks, they play different roles in sensorimotor processing (Salmelin et al. 1995). Source space MEG analysis has found that the locus of μ-activity is in the somatosensory cortex (i.e., post-central), whereas the locus of β-activity is in motor cortex (i.e., pre-central) (Salmelin et al. 1995), although there is substantial overlap in their spatial extent. This means that during preparation for and execution of overt movements the β-activity in the motor cortex is reduced from baseline, which is interpreted as a removal of inhibition (i.e., disinhibition) allowing motor plans and movement parameters to be computed (Takemi et al. 2013; Tzagarakis et al. 2015). Similarly, there is a reduction in the inhibition of the somatosensory cortex (i.e., μ-oscillation) during the expectation and attention toward somatosensory feedback (Cheyne 2013; Tzagarakis et al. 2015). Simply put, when a person is at rest, activity in the μ-band is associated with silencing task-irrelevant somatosensory area, whereas activity in the β-band is associated with preventing new movement parameters from being computed. During task preparation and execution, this activity is reduced (i.e., ERD).
Little is known about how cortical oscillations change after chronic paralysis. Previous studies have primarily quantified functional neuroplasticity using fMRI, which is agnostic to SMRs and to the inhibitory or excitatory nature of the underlying neural activity (Cramer et al. 2005; Freund et al. 2013; Henderson et al. 2011; Hotz-Boendermaker et al. 2008; Jurkiewicz et al. 2007; Turner et al. 2001). Some studies have inspected motor-related neural activity after SCI using electroencephalography (EEG) or magnetoencephalography (MEG) (Di Rienzo et al. 2014b; Green et al. 1999; Lacourse et al. 1999; Mattia et al. 2006), but no study has investigated how cortically localized SMRs differ from able-bodied controls. We tested the hypotheses that sensorimotor network modulation will decrease following chronic paralysis, which can be quantified in the μ- and β-band ERD during overt and imagined movements. To date, the only significant SMR differences found in individuals with SCI has been a decrease in event-related synchronization (ERS), where β-activity rebounds after ERD at task cessation, during attempted movement at the paralyzed ankle (Gourab and Schmit 2010). However, it is unclear whether this phenomenon could be attributed to patients’ delays in movement cessation (Cremoux et al. 2013).
We investigated how SMR activity differs in individuals with chronic tetraplegia compared with able-bodied controls during attempted or imagined hand grasping. Participants with SCI were grouped based on their ability to generate muscle activity during attempted grasping. We expected that after paralysis due to SCI there would be less modulation of the SMR during attempted movement since no overt movements or sensory consequences would occur for participants who were unable to generate muscle activity. To control for variability in motor and sensory abilities and the resultant variability in somatosensory feedback during attempted movement, SMR modulation was also investigated during imagined movements. Because of the behavioral similarities during MI between groups and the inconsistent findings in the fMRI literature, we expected to see only minimal, nonsignificant differences in SMR modulation between groups within the pre- and postcentral gyrus (Alkadhi et al. 2005; Cramer et al. 2005; Di Rienzo et al. 2014a; Gustin et al. 2010; Grangeon et al. 2012; Guillot et al. 2012; Hotz-Boendermaker et al. 2008; Sabbah et al. 2002).
METHODS
Participants.
Fourteen individuals with chronic cervical SCI were recruited into this study [mean (M) = 38, SD = 13 yr old] as part of a larger study investigating neuroplasticity after paralysis due to SCI. All participants with SCI were ≥1 yr postinjury and reported having impaired use of their hands. Demographic and impairment details are given in Table 1. An occupational therapist evaluated the participants’ injury classification using the ASIA Impairment Scale (AIS) (Kirshblum et al. 2011), which describes the extent of the spinal cord injury ranging from complete (A) loss of motor and sensory function to normal motor and sensory function (E). Fourteen able-bodied (AB) controls were recruited to match the SCI group according to sex, age, and handedness. Two participants with SCI were withdrawn from the study before neural data were collected. Data from one AB control were not included, as they were incomplete. All subjects gave written informed consent, and the study was conducted with Institutional Review Board approval from the Veterans Affairs Pittsburgh Healthcare System and the University of Pittsburgh.
Table 1.
Participant demographics and impairment
| Grip Strength, kN/m2 |
||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Injury Level | AIS | Sex | Time Since Injury, yr | Dominant Hand | Left | Right | EMG During Grasp |
| S01 | C4 | D | Male | 10 | Left | 2.3 | 20.7 | Yes |
| S02 | C4 | A | Male | 22 | Left | 27.6 | 27.6 | Yes |
| S03 | C2 | A | Male | 7 | Right | 0 | 0 | No |
| S04 | C5 | A | Male | 5 | Right | 0 | 0 | No |
| S06 | C7 | D | Male | 3 | Right | 29.9 | 6.9 | Yes |
| S07 | C6 | D | Female | 14 | Left | 29.9 | 20.7 | Yes |
| S08 | C5 | B | Female | 2 | Left | 0 | 0 | Yes |
| S10 | C5 | C | Male | 8 | Right | 0 | 0 | No |
| S11 | C2 | A | Male | 8 | Right | 124.1 | 23 | Yes |
| S12 | C5 | B | Male | 9 | Right | 0 | 0 | Yes |
| S13 | C4 | A | Male | 41 | Right | 0 | 0 | No |
| S14 | C3 | C | Female | 6 | Right | 0 | 0 | No |
AIS, ASIA Impairment Scale; EMG, electromyography. Letter categories of the AIS are as follows: A, complete; B, sensory incomplete; C, motor incomplete (less than half of the muscles below the injury level have muscle strength ≥ 3); D, motor incomplete (more than half of the muscles below the injury level have muscle strength ≥ 3).
Participants with paralysis were placed into two groups because we expected that residual motor function would impact SMR during attempted movements. Individuals with no volitional muscle activity of their hand, determined by lack of observable EMG (described below), were designated as H− (S03, S04, S10, S13, and S14), and all other participants with paralysis were grouped as H+ (S01, S02, S06, S07, S08, S11, and S12). There was no significant difference in age or sex between these three subject groups [1-way ANOVA; F(2, 22) = 0.37, P = 0.70 for age; F(2, 22) = 0.28, P = 0.76 for sex difference].
Experimental design.
Participants performed two grasping tasks during MEG recording: motor attempt (MA) and motor imagery (MI). During MA, participants with SCI were asked to try to move as best as possible, although some could not move at all. AB controls overtly performed the instructed movement. During MI, all participants were instructed to perform kinesthetic imagery (Stinear et al. 2006). Specifically, participants were shown a video of a hand performing the appropriate grasping task before the experiment and asked to imagine the sensations that would occur during actual task performance (as opposed to simple visualization of the task). It has been demonstrated that individuals with paralysis due to SCI still differentiate between MI and MA (Hotz-Boendermaker et al. 2008).
An experimental run consisted of an initial 20-s rest period, followed by 10 or 20 task blocks. Runs were randomized between the left and right hand and between MI and MA. A task block was started with a 2-s rest indicated by a red fixation cross, followed by cues to grasp (green) and relax (red) once every 2 s (i.e., 0.5-Hz pace) for nine or four grasp cycles (see Fig. 1). The number of cycles per block was decreased from nine to four partway through data collection to help encourage engagement. Four of 13 (31%) AB and five of 12 (42%) SCI participants performed nine cycles/block. To maintain a similar number of total grasp cycles, the number of blocks was increased for a total of 80 or 90 flexion/extension grasp cycles for each subject. Impairment is known to affect movement timing; therefore, this slow pace was chosen to allow participants with impairment sufficient time to complete the task (Decety and Boisson 1990). This task was designed to evaluate ERD, but not ERS. ERD was observed during the repeated movement blocks of time, and the ERS rebound was observable only at the end of the movement block but was not analyzed here. Rest blocks allowed SMR to return to baseline. Each block concluded with a 5-s rest cued by a blank screen that was used as baseline.
Fig. 1.
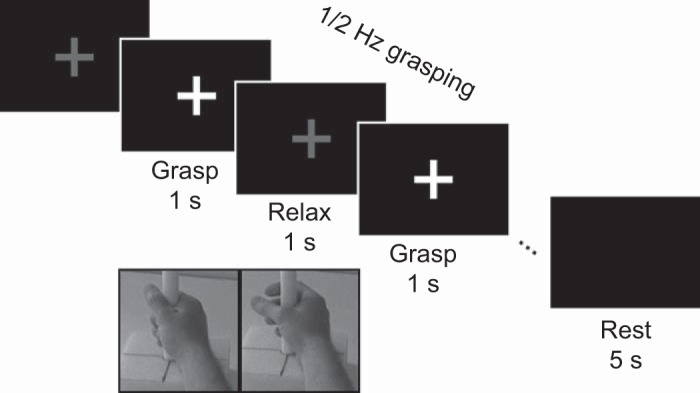
Trial design. Participants attempted or imagined grasping at a rate of 2 s/movement cycle paced. Fixation cross color indicated hand closing (i.e., flexion) or relaxing the hand to open. The expected position of the subject’s hand (if able to move overtly) is shown below the grasp and relax phases (not presented during the task). Each trial began with a 2-s preparation period (prep) and ended with a 5-s rest.
Participants sat upright in the MEG chair and watched a cue on the screen. During an experiment, the subject’s arm was comfortably supported by a table in front of them, and the subject’s hand was placed around a cylindrical object (shown in Fig. 1). Participants practiced attempting and imagining grasping for a few minutes before data collection until they were comfortable with the task. During the practice period, subjects watched a video of a person grasping the same cylindrical object at 0.5 Hz while they imagined the movements and sensations associated with grasping. Subjects were instructed to keep their eyes fixated on the middle of the screen and abstain from head movements or muscle contractions of their face and neck.
MEG recordings.
MEG data were recorded with a 306-channel whole head system (Elekta Neuromag Vectorview) with 102 sensor triplets each containing a magnetometer, longitudinal gradiometer, and latitudinal gradiometer. The locations of the participants’ nasion, left and right preauricularis, head tracking coils, and scalp (∼100 points) were digitized before the experiment. Head position was recorded at the beginning of each run using localization coils. Bipolar electromyography (EMG) was recorded from superficial flexor and extensor muscles in the forearm (flexor carpi radialis and extensor carpi radialis longus). Raw data were band-pass filtered between 0.1 and 330 Hz and then sampled at 1,000 Hz. To process the EMG, data were high-pass filtered at 30 Hz before rectifying and smoothing with a 5-Hz low-pass filter. An accelerometer was also used to track the movements of the index finger. Accelerometer data were low-pass filtered at 60 Hz, rectified, and smoothed to 5 Hz. EMG and accelerometer data were visually inspected to determine whether participants generated consistent muscle activity during attempted movement as compared with rest.
MEG sensor data were preprocessed by manually removing bad channels before temporal signal-space separation (tSSS) was performed with a 4-s buffer (Taulu and Hari 2009). To remove higher frequency noise and decrease data size, MEG data were band-pass filtered between 1 and 50 Hz and then resampled to 250 Hz. Time periods of extraneous MEG, EMG, or accelerometer activity during rest or imagined conditions were marked as erroneous and excluded. Similarly, data were also excluded if movements were not detected in the EMG or accelerometer when movements were expected from able-bodied participants.
Source space transformation.
MEG was transformed into source space using forward models and inverse solutions computed with the Brainstorm Toolbox (Tadel et al. 2011). For each experimental run, the head position within the MEG helmet was used to spatially align the MEG sensor data with an anatomic model of the brain and head from the MRI (the MRI specifics are described below) (Gross et al. 2013). Forward models were generated using the overlapping spheres method (Mosher et al. 1999). Dipole sources were modeled on the brain surface and given three orientations with a 0.4 down-weighting to orientations not normal to the cortical surface (i.e., a loose cortical orientation constraint value of 0.4) (Lin et al. 2006). This produced dipoles at 15,002 locations with three different orientations for a total of 45,006 dipoles. The inverse solution was computed using minimum norm estimate (Tadel et al. 2011), with a noise covariance matrix for each run based on broad-band resting data collected 2–4 s after rest cue onset. To coregister between subjects, the sources were projected to the MNI template brain. This resulted in 15,002 source locations with 2.26 ± 0.85 mm (mean ± SD) separation on the MNI brain surface. These source-space transformations were used to compute the source-space ERD described in Time-frequency analysis (see below).
Structural MRIs were collected to build anatomic forward models. A 3T Siemens Trio was used to collect whole brain T1-weighted images with 1-mm3 voxels. Freesurfer was used to generate three-dimensional models of the brain, CSF, skull, and scalp (Fischl 2012). Four participants with SCI were unable to undergo an MRI due to safety issues; therefore, a standardized MNI template was used instead (S08, S10, S11, and S14).
Time-frequency analysis.
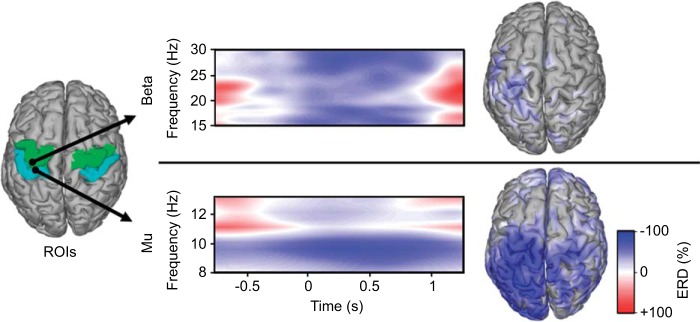
μ- and β-ERD were quantified using source-space imaging and preprocessing similar to previous literature on cortical oscillations (Cheyne et al. 2008; Jurkiewicz et al. 2006). An initial exploratory assessment was performed to identify the sources of peak ERD in the motor and somatosensory cortex before running a detailed time-frequency analysis. To do this, the spectral power was extracted from the source-space signals in the frequencies of interest (FOI), i.e., μ (8–13 Hz) and β (15–30 Hz) (Pfurtscheller and Lopes da Silva 1999), using a Hilbert transform (3rd-order Butterworth filter) (Muthukumaraswamy et al. 2004). ERD maps were created for the μ- and β-bands by computing the percent change from baseline at each source. Baseline was defined as the average resting power across a run and computed from data 3–4 s after all rest cues of each block. Multiple epochs of resting SMR were used to compute the baseline to prevent a biased estimation of rest power that could result from a signal drift that occurred over long time scales. ERD was computed from 200 ms before to 1,000 ms after movement cue. To account for the variability in reaction time likely in paralyzed populations (Cremoux et al. 2013), the time-frequency data were low-pass filtered to 2 Hz before averaging trials and determining the peak ERD. ERD of the sensory-related μ-band was limited to sources in the contralateral postcentral area and the motor-related β-band was limited to the precentral area of the Desikan-Killiany atlas (Desikan et al. 2006). ROIs were limited to the hand and arm areas defined by the hand knob and the dorsal aspects of the motor and somatosensory cortex (see Fig. 2) (Yousry et al. 1997). The source of peak ERD for the μ- and β-bands in these ROIs was analyzed in further detail using high-resolution spectral analysis.
Fig. 2.
Spectral, temporal, and spatial dynamics of event-related desynchronization (ERD) from a participant with complete paralysis (S14) during attempted right hand grasps. The high-resolution time-frequency representations from wavelet decomposition at 0.5-Hz resolution show β-ERD (top) from an M1 source [green region of interest (ROI)] and μ-ERD (bottom) from an S1 source (blue ROI). Time-frequency representations span the grasp/release cycle with the cue to grasp at t = 0. Cortical source images (right) show the spatial distribution of the ERD at the peak frequency for β (28.5 Hz) and μ (9.5 Hz).
Wavelet decomposition was used for detailed investigation of the μ- and β-waveforms at a higher frequency resolution for the sources identified above, similar to previous literature (Cheyne et al. 2008; Jurkiewicz et al. 2006). Specifically, Morlet wavelets with center frequencies at 0.5-Hz intervals and 20 cycles per time window were utilized to investigate frequencies within each FOI (Cheyne 2013; Tallon-Baudry and Bertrand 1999) using the FieldTrip toolbox (Oostenveld et al. 2011). From this wavelet analysis, the peak ERD and frequency of peak ERD were computed from the μ- and β-bands. In addition, the number of significantly modulated sources at the peak frequency with the ROIs were computed (P < 0.05 with Wilcoxon rank-sum) to investigate the spatial extent of the ERD (Cheyne 2013).
Statistical assessment.
A multivariate linear model was used to test the hypothesis that SMR modulation during attempted movement would be less for the H− group as compared with the AB and H+ groups (SPSS; IBM, Armonk, NY). The peak and spatial extent of μ- and β-activity were included as dependent variables, and subject group was included as a fixed factor. Bonferroni-corrected post hoc tests were performed between groups if the main effect of subject group was significant. A second multivariate linear model was used to test the hypothesis that SMR modulation was different between groups during imagined movements. As an exploratory analysis, we also tested for differences in the peak frequency of μ- and β-modulation between groups during attempted and imagined movements using two multivariate linear models, one for each movement condition. All participants performed tasks with their right and left hands, and so to increase statistical power, data sets for right and left hand movements were treated independently. Thus, for each subject, two data sets were included in the analysis: right and left hand movement. Statistical analysis of μ-ERD was limited to the postcentral gyrus, and β-ERD was limited to precentral gyrus based on a prior assumptions of loci of activity.
RESULTS
Figure 2 demonstrates the spectral, temporal, and spatial characteristics of the μ- and β-oscillations during attempted movements by a participant with complete hand paralysis (S14). Although the participant could not generate grasp force or detectible EMG, ERD was generated as expected.
Attempted movements.
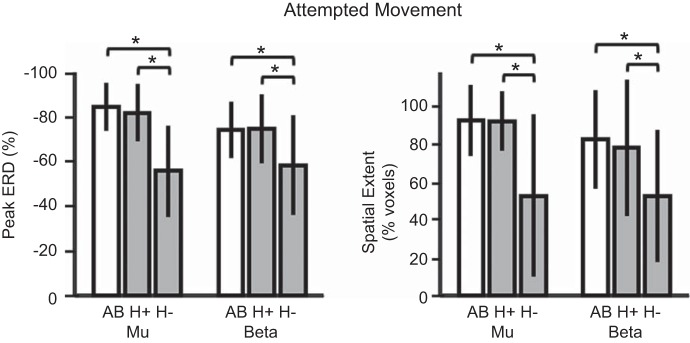
Figure 3 shows the differences in ERD modulation across subject groups during the attempted movement condition (MA). SMR modulation, measured as the peak and spatial extent of μ- and β-activity, was significantly different between groups during attempted movement [F(8, 88) = 4.01, P < 0.001]. Mean values for peak activity are reported as the percent change in activity during attempted movement as compared with rest. Spatial extent is reported as the percentage of the ROI showing significant modulation of activity as compared with rest (false discovery rate controlled with Benjamini-Hochberg procedure to account for comparisons across multiple sources in the ROI). Peak μ-activity was significantly lower for the H− group (M = 55.7, SD = 20.4) than the H+ (M = 81.8, SD = 12.8) or AB group (M = 84.5, SD = 10.8). The spatial extent of μ-activity was also less for the H− group (M = 52.9, SD = 42.9) than the H+ (M = 92.1, SD = 15.7) or AB groups (M = 92.3, SD = 18.7). Similar results were found for peak β-activity and spatial extent. Peak β-activity was significantly lower for the H− group (M = 56.1, SD = 24.1) than the H+ (M = 74.3, SD = 17.9) or AB group (M = 73.6, SD = 12.6). The spatial extent of β-activity was less for the H− group (M = 40.1, SD = 32.8) than the H+ (M = 77.9, SD = 34.3) or AB groups (M = 77.1, SD = 31.6). All P < 0.05 (Bonferroni corrected). No significant differences in SMR modulation were observed between the H+ and AB groups during attempted movement.
Fig. 3.
Peak (left) and spatial extent (right) of ERD during attempted movement tasks. Participants with spinal cord injury (SCI; gray bars) who could not generate electromyography (EMG) are labeled H− (n = 5), with all others labeled H+ (n = 7). AB, able-bodied controls (open bars; n = 12). Bars are mean values with error bars as SD. *Significant pairwise comparisons (Bonferroni-corrected post hoc t-tests, P < 0.05).
Imagined movements.
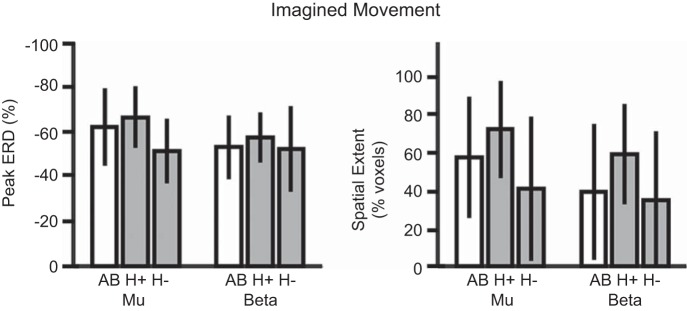
Figure 4 shows the differences in ERD modulation across subject groups during the imagined movement condition. EMG and accelerometer analysis confirmed that all data were free from overt movements to remove differences in ability across groups. During imagined movement, SMR modulation, measured as the peak and spatial extent of μ- and β-activity, was not significantly different between groups [F(8, 88) = 1.16, P = 0.331]. The effect of subject group approached significance for peak μ-activity (P = 0.075) and spatial extent of μ-activity (P = 0.066), but pairwise group comparisons were not significant. Qualitatively, the H+ group showed the largest peak ERD and spatial extent, with the H− group showing the least, but these differences were nonsignificant.
Fig. 4.
Peak (left) and spatial extent (right) of ERD during imagined movement tasks. Participants with SCI (gray bars) who could not generate EMG are labeled H− (n = 5), with all others labeled H+ (n = 7). AB, able-bodied controls (open bars; n = 12). Bars are mean values with error bars as standard deviation. No main effect of subject group was found.
Peak ERD frequency.
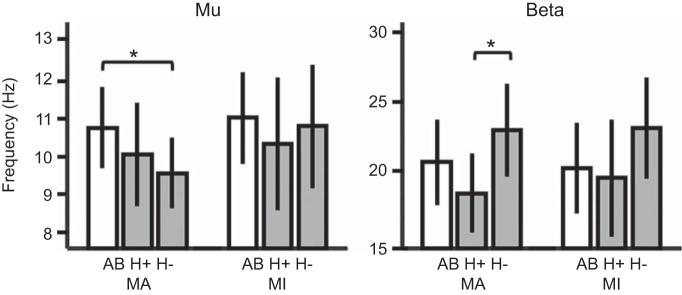
Small but significant differences were found between groups in the peak frequency of SMR during attempted movement [F(4, 92) = 5.49, P = 0.001 for both μ (P = 0.016) and β (P = 0.004); Fig. 5]. Pairwise comparisons revealed that peak μ-frequency was lower for the H− group (M = 9.5, SD = 0.36) than the AB group (M = 10.7, SD = 0.23) but not significantly different from the H+ group (M = 10.0, SD = 0.31). Peak β-frequency was highest for the H− group (M = 22.9, SD = 3.3), which was significantly different from the H+ group (M = 18.4, SD = 2.8) but not the AB group (M = 20.6, SD = 3.1). No significant differences in peak frequency of ERD were observed during imagined movements [F(4, 92) = 2.11, P = 0.085].
Fig. 5.
Frequency of peak ERD in μ- (left) and β-bands (right) during attempted [motor attempt (MA)] and imagined [motor imagery (MI)] grasping. Participants with SCI (gray bars) who could not generate EMG are labeled H− (n = 5), with all others labeled H+ (n = 7). AB, able-bodied controls (open bars; n = 12). Bars are mean values with error bars as SD. *Significant pairwise comparisons (Bonferroni-corrected post hoc t-tests, P < 0.05).
DISCUSSION
Individuals without volitional muscle activity had altered ERD compared with AB controls during attempted motor tasks. Previous work had hypothesized that SCI would lead to alterations in EEG characteristics (Lacourse et al. 1999), although it had never been confirmed before this report. People without volitional muscle activity due to SCI (H− group) showed less ERD with a smaller spatial extent within the pre- and postcentral gyrus. These participants without volitional muscle activity were unable to produce overt movement, and therefore they also did not receive task-related somatosensation from their limbs. Although ERD occurs in many situations that do not involve somatosensory feedback (e.g., during MI, motor preparation, and somatosensory anticipation and after paralysis), it is most commonly correlated to the disinhibition of the motor and somatosensory cortex during motor control. Within this framework, β-band ERD during normal movement conditions allows for movement parameters to be computed, whereas μ-band ERD allows for task-relevant somatosensory information to be interpreted. Therefore, the utility of sensorimotor ERD would be less for people with complete paralysis than for those with intact function. No differences within the pre- and postcentral gyrus were found between the groups who maintained some hand/muscle activity (AB and H+). Our findings suggest that partially intact efferent and afferent connections after incomplete SCI reduce the alterations to ERD.
SCI neuroimaging studies have shown an expansion of the cortical representations of intact movements into deafferented areas (Bruehlmeier et al. 1998; Mikulis et al. 2002). In our study, no significant difference was observed in the spatial extent of the ERD in the ROIs between able-bodied controls and participants with SCI who had some intact hand function (H+), although the function was impaired. Based on our inclusion criteria, the participants in our study with cervical SCI had limited hand movements and somatosensory feedback, which differs from previous work that evaluated fully intact movements to demonstrate expansion. Still, for individuals with SCI who did not have any volitional muscle activity (H−), the spatial extent of ERD was reduced during attempted movement compared with the other two subject groups. This indicates that less of the sensorimotor cortex is experiencing desynchronization when participants are attempting to move their fully paralyzed hand. However, because participants without volitional muscle activity (H−) generated ERD during imagined movements in a way similar to people with intact function, it suggests that a chronic disruption of somatosensation and motor consequences does not diminish the ability to generate ERD within the sensorimotor cortex. However, our analysis considered only the pre-/postcentral areas, and therefore, it cannot speak to changes in motor or whole brain networks. The use of neurofeedback training might be able to improve the modulation depth of ERD during attempted movements for people with complete, chronic paralysis to increase the utility of brain-computer interfaces (Foldes et al. 2015; Wolpaw et al. 2002) and as a potential therapeutic tool in combination with regenerative medicine therapies (Collinger et al. 2013). However, research studies are needed to determine whether normalization of cortical oscillations is a significant contributor to recovery.
Subtle but significant differences were found in the frequencies of the peak ERD between subject groups. In our study, participants without hand muscle activity (H−) had lower μ-frequencies than able-bodied controls and higher β-frequencies than the H+ group. There is limited research on the interpretation of SMR waveform properties, such as sub-band frequencies and waveform shapes. Peak SMR frequencies are not typically reported. We did not expect to find differences in peak SMR frequency a priori; however, these differences were observed and may represent an area for future investigation. Some literature has proposed functional differences between the SMRs of low and high μ (8–10 and 10–13 Hz) (Pfurtscheller et al. 2000) and low and high β (15–20 and 20–30 Hz) (Neuper and Pfurtscheller 2001). Within the μ-band, it has been speculated that higher frequencies are somatotopically specific, whereas lower μ are more generalized. The mechanisms and impact of these sub-bands are not understood. The desynchronization of somatotopic-specific μ (i.e., high μ) might be less modulated after chronic paralysis, while the generalized μ (i.e., low μ)-desynchronization may be maintained, resulting in an apparent decrease in peak μ-frequency for the H− group. The higher peak β-ERD frequency for participants without hand function could indicate a decrease in fine control networks of hand movement, although this was not tested directly. Another hypothesis is that high-frequency β is related to attention (Murthy and Fetz 1992; Sanes and Donoghue 1993). Our participants who had no somatosensory feedback during attempted movement may have needed more attention to perform the tasks than participants with some hand function. More basic science work needs to be done to understand the SMR waveform properties before we can effectively interpret pathological conditions (Fogelson et al. 2006; Moazami-Goudarzi et al. 2008).
Similar to previous work looking at motor imagery after SCI, we found that participants with paralysis did modulate their brain activity during MI (Alkadhi et al. 2005; Cramer et al. 2005; Decety and Boisson 1990; Lacourse et al. 1999. We did not find any significant differences in the peak or extent of ERD within the pre- and postcentral gyrus between subject groups during imaged grasping. This apparent conflict with some previous neuroimaging literature (Alkadhi et al. 2005; Cramer et al. 2005; Hotz-Boendermaker et al. 2008) may highlight the differences between fMRI and SMR. The BOLD signal captured with fMRI is correlated most with local field potentials that occur during local neural computations (Logothetis and Wandell 2004), such as determining kinematic parameters for motor control. On the other hand, desynchronization of the SMR (i.e., ERD) decreases the inhibition in cortical networks, thereby allowing local computations. However, the presence of ERD does not imply that local activity has increased (Donner and Siegel 2011; Ritter et al. 2009). This difference in interpretation provides a mechanism for how SMR and BOLD findings would apparently contradict, especially in a task such as MI, where inhibition plays a major role. Cortical oscillations in the broadband γ-frequency band have been linked with the BOLD response and may correspond better to the fMRI results seen in the literature (Logothetis and Wandell 2004).
Motor imagery is of particular interest as a rehabilitation intervention after paralysis (Cramer et al. 2007; Di Rienzo et al. 2014a; Driskell et al. 1994; Dunlop 2008; Lotze and Halsband 2006; Mateo et al. 2015b). A new approach to MI therapy is to supplement MI practice with real-time neurofeedback of the patient’s brain activity (Foldes et al. 2015; Ramos-Murguialday et al. 2013). Neurofeedback systems can provide patients an indication of their MI performance using μ- and β-band ERD collected noninvasively with MEG or EEG. This study suggests that individuals with paralysis generate μ- and β-ERD within the pre- and postcentral gyrus during imagined movements that is similar to activity generated in able-bodied people. However, more work is needed to separate the causal and correlative relationships of ERD and recovery of motor function. Furthermore, the ERD differences between patients without volitional muscle activity and those with it suggests that rehabilitation strategies that utilize attempted movements of paralyzed limbs, such as BCIs for controlling motor neuroprostheses, may need to be developed for specific impairment levels.
This work adds to the growing literature that highlights the richness of cortical oscillations for understanding the sensorimotor system. When SCI removes a person’s ability to generate volitional muscle activity, that person experiences a decrease in amplitude and extent of their ERD compared with able-bodied controls. However, the ERD during imagery of impaired movements was not altered compared with AB controls within the pre- and postcentral gyrus. These results suggest that ERD is dependent on the ability to generate volitional muscle activity. A better understanding of how cortical activity changes after paralysis can help in the development and evaluation of new therapies such as motor imagery and neurofeedback interventions.
GRANTS
This work was supported by the Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research & Development Service (Grant no. B7143R) and the VISN 4 Competitive Pilot Project Fund, and the Craig Neilsen Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T.F., D.J.W., and J.L.C. conceived and designed research; S.T.F. and J.L.C. performed experiments; S.T.F. analyzed data; S.T.F., D.J.W., and J.L.C. interpreted results of experiments; S.T.F. prepared figures; S.T.F. drafted manuscript; S.T.F., D.J.W., and J.L.C. edited and revised manuscript; S.T.F., D.J.W., and J.L.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We express gratitude to Emily Grattan, Betsy Harchick, Debbie Harrington, Michael Randazzo, and Dylan Royston for assistance with the experiments.
REFERENCES
- Alkadhi H, Brugger P, Boendermaker SH, Crelier G, Curt A, Hepp-Reymond MC, Kollias SS. What disconnection tells about motor imagery: evidence from paraplegic patients. Cereb Cortex 15: 131–140, 2005. doi: 10.1093/cercor/bhh116. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26: 490–501, 2006. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci 10: 3918–3922, 1998. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage 42: 332–342, 2008. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Gaetz W, Garnero L, Lachaux JP, Ducorps A, Schwartz D, Varela FJ. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Brain Res Cogn Brain Res 17: 599–611, 2003. doi: 10.1016/S0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Cheyne DO. MEG studies of sensorimotor rhythms: a review. Exp Neurol 245: 27–39, 2013. doi: 10.1016/j.expneurol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Collinger JL, Foldes S, Bruns TM, Wodlinger B, Gaunt R, Weber DJ. Neuroprosthetic technology for individuals with spinal cord injury. J Spinal Cord Med 36: 258–272, 2013. doi: 10.1179/2045772313Y.0000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain 128: 2941–2950, 2005. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Orr EL, Cohen MJ, Lacourse MG. Effects of motor imagery training after chronic, complete spinal cord injury. Exp Brain Res 177: 233–242, 2007. doi: 10.1007/s00221-006-0662-9. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D, Chen WG, Cohen LG, deCharms C, Duffy CJ, Eden GF, Fetz EE, Filart R, Freund M, Grant SJ, Haber S, Kalivas PW, Kolb B, Kramer AF, Lynch M, Mayberg HS, McQuillen PS, Nitkin R, Pascual-Leone A, Reuter-Lorenz P, Schiff N, Sharma A, Shekim L, Stryker M, Sullivan EV, Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain 134: 1591–1609, 2011. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremoux S, Tallet J, Berton E, Dal Maso F, Amarantini D. Motor-related cortical activity after cervical spinal cord injury: multifaceted EEG analysis of isometric elbow flexion contractions. Brain Res 1533: 44–51, 2013. doi: 10.1016/j.brainres.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Decety J, Boisson D. Effect of brain and spinal cord injuries on motor imagery. Eur Arch Psychiatry Clin Neurosci 240: 39–43, 1990. doi: 10.1007/BF02190091. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980, 2006. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Di Rienzo F, Collet C, Hoyek N, Guillot A. Impact of neurologic deficits on motor imagery: a systematic review of clinical evaluations. Neuropsychol Rev 24: 116–147, 2014a. doi: 10.1007/s11065-014-9257-6. [DOI] [PubMed] [Google Scholar]
- Di Rienzo F, Guillot A, Mateo S, Daligault S, Delpuech C, Rode G, Collet C. Neuroplasticity of prehensile neural networks after quadriplegia. Neuroscience 274: 82–92, 2014b. doi: 10.1016/j.neuroscience.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Di Rienzo F, Guillot A, Daligault S, Delpuech C, Rode G, Collet C. Motor inhibition during motor imagery: a MEG study with a quadriplegic patient. Neurocase 20: 524–539, 2014c. doi: 10.1080/13554794.2013.826685. [DOI] [PubMed] [Google Scholar]
- Donner TH, Siegel M. A framework for local cortical oscillation patterns. Trends Cogn Sci 15: 191–199, 2011. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Driskell JE, Copper C, Moran A. Does mental practice enhance performance? J Appl Psychol 79: 481–492, 1994. doi: 10.1037/0021-9010.79.4.481. [DOI] [Google Scholar]
- Dunlop SA. Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci 31: 410–418, 2008. doi: 10.1016/j.tins.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20: 156–165, 2010. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Filimon F, Rieth CA, Sereno MI, Cottrell GW. Observed, executed, and imagined action representations can be decoded from ventral and dorsal areas. Cereb Cortex 25: 3144–3158, 2015. doi: 10.1093/cercor/bhu110. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage 62: 774–781, 2012. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson’s disease. Cereb Cortex 16: 64–75, 2006. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- Foldes ST, Weber DJ, Collinger JL. MEG-based neurofeedback for hand rehabilitation. J Neuroeng Rehabil 12: 85, 2015. doi: 10.1186/s12984-015-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Curt A, Friston K, Thompson A. Tracking changes following spinal cord injury: insights from neuroimaging. Neuroscientist 19: 116–128, 2013. doi: 10.1177/1073858412449192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourab K, Schmit BD. Changes in movement-related β-band EEG signals in human spinal cord injury. Clin Neurophysiol 121: 2017–2023, 2010. doi: 10.1016/j.clinph.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Grangeon M, Charvier K, Guillot A, Rode G, Collet C. Using sympathetic skin responses in individuals with spinal cord injury as a quantitative evaluation of motor imagery abilities. Phys Ther 92: 831–840, 2012. doi: 10.2522/ptj.20110351. [DOI] [PubMed] [Google Scholar]
- Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical motor reorganization after paraplegia: an EEG study. Neurology 53: 736–743, 1999. doi: 10.1212/WNL.53.4.736. [DOI] [PubMed] [Google Scholar]
- Gross J, Baillet S, Barnes GR, Henson RN, Hillebrand A, Jensen O, Jerbi K, Litvak V, Maess B, Oostenveld R, Parkkonen L, Taylor JR, van Wassenhove V, Wibral M, Schoffelen JM. Good practice for conducting and reporting MEG research. Neuroimage 65: 349–363, 2013. doi: 10.1016/j.neuroimage.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot A, Di Rienzo F, Macintyre T, Moran A, Collet C. Imagining is not doing but involves specific motor commands: a review of experimental data related to motor inhibition. Front Hum Neurosci 6: 247, 2012. doi: 10.3389/fnhum.2012.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin SM, Wrigley PJ, Henderson LA, Siddall PJ. Brain circuitry underlying pain in response to imagined movement in people with spinal cord injury. Pain 148: 438–445, 2010. doi: 10.1016/j.pain.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Haegens S, Händel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci 31: 5197–5204, 2011. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M. Magnetoencephalographic cortical rhythms. Int J Psychophysiol 26: 51–62, 1997. doi: 10.1016/S0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ. Functional reorganization of the brain in humans following spinal cord injury: evidence for underlying changes in cortical anatomy. J Neurosci 31: 2630–2637, 2011. doi: 10.1523/JNEUROSCI.2717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz-Boendermaker S, Funk M, Summers P, Brugger P, Hepp-Reymond MC, Curt A, Kollias SS. Preservation of motor programs in paraplegics as demonstrated by attempted and imagined foot movements. Neuroimage 39: 383–394, 2008. doi: 10.1016/j.neuroimage.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol 9: 735–739, 1999. doi: 10.1016/S0959-4388(99)00038-0. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4: 186, 2010. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR. When brain rhythms aren’t “rhythmic”: implication for their mechanisms and meaning. Curr Opin Neurobiol 40: 72–80, 2016. doi: 10.1016/j.conb.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage 32: 1281–1289, 2006. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair 21: 527–538, 2007. doi: 10.1177/1545968307301872. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, Donovan W, Graves D, Jha A, Jones L, Mulcahey MJ, Krassioukov A. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med 34: 547–554, 2011. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma 26: 2113–2126, 2009. doi: 10.1089/neu.2008.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse MG, Cohen MJ, Lawrence KE, Romero DH. Cortical potentials during imagined movements in individuals with chronic spinal cord injuries. Behav Brain Res 104: 73–88, 1999. doi: 10.1016/S0166-4328(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Lin FH, Witzel T, Ahlfors SP, Stufflebeam SM, Belliveau JW, Hämäläinen MS. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neuroimage 31: 160–171, 2006. doi: 10.1016/j.neuroimage.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol 66: 735–769, 2004. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- López-Larraz E, Montesano L, Gil-Agudo Á, Minguez J, Oliviero A. Evolution of EEG Motor Rhythms after Spinal Cord Injury: A Longitudinal Study. PLoS One 10: e0131759, 2015. doi: 10.1371/journal.pone.0131759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Halsband U. Motor imagery. J Physiol Paris 99: 386–395, 2006. doi: 10.1016/j.jphysparis.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Mateo S, Di Rienzo F, Bergeron V, Guillot A, Collet C, Rode G. Motor imagery reinforces brain compensation of reach-to-grasp movement after cervical spinal cord injury. Front Behav Neurosci 9: 234, 2015a. doi: 10.3389/fnbeh.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo S, Di Rienzo F, Reilly KT, Revol P, Delpuech C, Daligault S, Guillot A, Jacquin-Courtois S, Luauté J, Rossetti Y, Collet C, Rode G. Improvement of grasping after motor imagery in C6–C7 tetraplegia: A kinematic and MEG pilot study. Restor Neurol Neurosci 33: 543–555, 2015b. doi: 10.3233/RNN-140466. [DOI] [PubMed] [Google Scholar]
- Mattia D, Cincotti F, Mattiocco M, Scivoletto G, Marciani MG, Babiloni F. Motor-related cortical dynamics to intact movements in tetraplegics as revealed by high-resolution EEG. Hum Brain Mapp 27: 510–519, 2006. doi: 10.1002/hbm.20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulis DJ, Jurkiewicz MT, McIlroy WE, Staines WR, Rickards L, Kalsi-Ryan S, Crawley AP, Fehlings MG, Verrier MC. Adaptation in the motor cortex following cervical spinal cord injury. Neurology 58: 794–801, 2002. doi: 10.1212/WNL.58.5.794. [DOI] [PubMed] [Google Scholar]
- Moazami-Goudarzi M, Sarnthein J, Michels L, Moukhtieva R, Jeanmonod D. Enhanced frontal low and high frequency power and synchronization in the resting EEG of parkinsonian patients. Neuroimage 41: 985–997, 2008. doi: 10.1016/j.neuroimage.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Mosher JC, Leahy RM, Lewis PS. EEG and MEG: forward solutions for inverse methods. IEEE Trans Biomed Eng 46: 245–259, 1999. doi: 10.1109/10.748978. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA 89: 5670–5674, 1992. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Brain Res Cogn Brain Res 19: 195–201, 2004. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Evidence for distinct beta resonance frequencies in human EEG related to specific sensorimotor cortical areas. Clin Neurophysiol 112: 2084–2097, 2001. doi: 10.1016/S1388-2457(01)00661-7. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang WH, Lee M, Kwon GH, Kim L, Kim ST, Kim YH. Which motor cortical region best predicts imagined movement? Neuroimage 113: 101–110, 2015. doi: 10.1016/j.neuroimage.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999. doi: 10.1016/S1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett 239: 65–68, 1997. doi: 10.1016/S0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol 111: 1873–1879, 2000. doi: 10.1016/S1388-2457(00)00428-4. [DOI] [PubMed] [Google Scholar]
- Prasad G, Herman P, Coyle D, McDonough S, Crosbie J. Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: a feasibility study. J Neuroeng Rehabil 7: 60, 2010. doi: 10.1186/1743-0003-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2: 263–273, 2001. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Läer L, Yilmaz O, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, Vyziotis A, Cho W, Agostini M, Soares E, Soekadar S, Caria A, Cohen LG, Birbaumer N. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol 74: 100–108, 2013. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp 30: 1168–1187, 2009. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah P, de Schonen C, Leveque C, Gay S, Pfefer F, Nioche C, Sarrazin JL, Barouti H, Tadie M, Cordoliani YS. Sensorimotor cortical activity in patients with complete spinal cord injury: a functional magnetic resonance imaging study. J Neurotrauma 19: 53–60, 2002. doi: 10.1089/089771502753460231. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hámáaláinen M, Kajola M, Hari R. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage 2: 237–243, 1995. doi: 10.1006/nimg.1995.1031. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA 90: 4470–4474, 1993. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Baron J-C. Does motor imagery share neural networks with executed movement: a multivariate fMRI analysis. Front Hum Neurosci 7: 564, 2013. doi: 10.3389/fnhum.2013.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Steyvers M, Levin O, Swinnen SP. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res 168: 157–164, 2006. doi: 10.1007/s00221-005-0078-y. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011: 879716, 2011. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemi M, Masakado Y, Liu M, Ushiba J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J Neurophysiol 110: 1158–1166, 2013. doi: 10.1152/jn.01092.2012. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162, 1999. doi: 10.1016/S1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Taulu S, Hari R. Removal of magnetoencephalographic artifacts with temporal signal-space separation: demonstration with single-trial auditory-evoked responses. Hum Brain Mapp 30: 1524–1534, 2009. doi: 10.1002/hbm.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J. The functional importance of rhythmic activity in the brain. Curr Biol 22: R658–R663, 2012. doi: 10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Turner JA, Lee JS, Martinez O, Medlin AL, Schandler SL, Cohen MJ. Somatotopy of the motor cortex after long-term spinal cord injury or amputation. IEEE Trans Neural Syst Rehabil Eng 9: 154–160, 2001. doi: 10.1109/7333.928575. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci 30: 11270–11277, 2010. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, West S, Pellizzer G. Brain oscillatory activity during motor preparation: effect of directional uncertainty on beta, but not alpha, frequency band. Front Neurosci 9: 246, 2015. doi: 10.3389/fnins.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol 113: 767–791, 2002. doi: 10.1016/S1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen J-M, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science 316: 1609–1612, 2007. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157, 1997. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]