Previous studies reported that high-frequency epidural stimulation (HF-ES) activates the diaphragm following acute spinal transection. This study examined HF-ES and phrenic motor output following subacute and chronic incomplete cervical spinal cord injury. Short-term potentiation of phrenic bursting following HF-ES illustrates the potential for spinal stimulation to induce respiratory neuroplasticity. Increased tonic phrenic output indicates that alternatives to the continuous stimulation paradigm used in this study will be required for respiratory muscle activation after spinal cord injury.
Keywords: spinal cord injury; epidural stimulation; phrenic, potentiation; plasticity
Abstract
C2 spinal hemilesion (C2Hx) paralyzes the ipsilateral diaphragm, but recovery is possible through activation of “crossed spinal” synaptic inputs to ipsilateral phrenic motoneurons. We tested the hypothesis that high-frequency epidural stimulation (HF-ES) would potentiate ipsilateral phrenic output after subacute and chronic C2Hx. HF-ES (300 Hz) was applied to the ventrolateral C4 or T2 spinal cord ipsilateral to C2Hx in anesthetized and mechanically ventilated adult rats. Stimulus duration was 60 s, and currents ranged from 100 to 1,000 µA. Bilateral phrenic nerve activity and ipsilateral hypoglossal (XII) nerve activity were recorded before and after HF-ES. Higher T2 stimulus currents potentiated ipsilateral phasic inspiratory activity at both 2 and 12 wk post-C2Hx, whereas higher stimulus currents delivered at C4 potentiated ipsilateral phasic phrenic activity only at 12 wk (P = 0.028). Meanwhile, tonic output in the ipsilateral phrenic nerve reached 500% of baseline values at the high currents with no difference between 2 and 12 wk. HF-ES did not trigger inspiratory burst-frequency changes. Similar responses occurred following T2 HF-ES. Increases in contralateral phrenic and XII nerve output were induced by C4 and T2 HF-ES at higher currents, but the relative magnitude of these changes was small compared with the ipsilateral phrenic response. We conclude that following incomplete cervical spinal cord injury, HF-ES of the ventrolateral midcervical or thoracic spinal cord can potentiate efferent phrenic motor output with little impact on inspiratory burst frequency. However, the substantial increases in tonic output indicate that the uninterrupted 60-s stimulation paradigm used is unlikely to be useful for respiratory muscle activation after spinal injury.
NEW & NOTEWORTHY Previous studies reported that high-frequency epidural stimulation (HF-ES) activates the diaphragm following acute spinal transection. This study examined HF-ES and phrenic motor output following subacute and chronic incomplete cervical spinal cord injury. Short-term potentiation of phrenic bursting following HF-ES illustrates the potential for spinal stimulation to induce respiratory neuroplasticity. Increased tonic phrenic output indicates that alternatives to the continuous stimulation paradigm used in this study will be required for respiratory muscle activation after spinal cord injury.
delivery of electrical currents to the dorsal, lateral, or ventral spinal dura mater (i.e., “epidural stimulation”) has been explored in animal models and humans following spinal cord injury (SCI). Much of that work has focused on enabling locomotor movement (Angeli et al. 2014; Harkema et al. 2011; Rejc et al. 2015; Sayenko et al. 2014), but epidural stimulation also has been used to activate respiratory muscles (DiMarco and Kowalski 2009, 2010, 2013b, 2015; DiMarco et al. 2002, 2014; Kowalski et al. 2013, 2016). Following a complete spinal transection at C1, high-frequency epidural stimulation (HF-ES) of the upper thoracic spinal cord can evoke bursting of diaphragm motor units during stimulation, but at rates (e.g., 10–50 Hz) well below the stimulus frequency of 300 Hz. With the use of this approach, recruitment of diaphragm motor units occurs during stimulation, and off-target effects appear to be minimal. The specific mechanisms underlying diaphragm motor unit recruitment during HF-ES of the transected spinal cord are unknown, but the phenomenon has been demonstrated in both rats (Kowalski et al. 2013) and dogs (DiMarco and Kowalski 2009).
Neuroplasticity in respiratory motoneurons and/or networks (Fuller and Mitchell 2017) is an important consideration in the context of epidural stimulation and SCI. For example, if the stimulation paradigm is intended to function as a “respiratory neuroprosthesis,” then stimulus-induced changes in synaptic efficacy could enhance (or impair) subsequent evoked motor responses. Perhaps even more important is the potential for stimulation-induced neuroplasticity to promote motor recovery (Kasten et al. 2013; McPherson et al. 2015). Electrical stimulation of the spinal cord can alter neuronal growth (Borgens et al. 1981) and synaptic plasticity (Fetz 2015; McPherson et al. 2015; Nishimura et al. 2013; Widge and Moritz 2014) and has already been used in neurorehabilitation paradigms (Harkema et al. 2011; Rejc et al. 2015, 2017; Sayenko et al. 2015).
In the current study, we investigated whether a sustained period of HF-ES would induce neuroplastic changes in phrenic motor activity similar to the well-described short-term potentiation of phrenic nerve activity that occurs following short periods of arterial hypoxia (Lee et al. 2015). The mechanisms underlying phrenic short-term potentiation induced by hypoxia are not definitively known (Lee et al. 2015), but several studies have shown that electrically stimulating synaptic inputs to spinal respiratory motoneurons can potentiate spinal respiratory output on short timescales (Hayashi et al. 2003; Johnson and Mitchell 2002; McCrimmon et al. 1997; Mercier et al. 2017). The aforementioned epidural stimulation experiments (DiMarco and Kowalski 2009, 2010, 2013b; DiMarco et al. 2002; Kowalski et al. 2013) indicate that HF-ES robustly activates synaptic inputs to phrenic motoneurons, at least following acute spinal transection. Epidural stimulation may also activate neuromodulatory neurons (e.g., raphe-spinal projections) located in proximity to phrenic motoneurons (Kinkead et al. 1998) which have the capacity to induce respiratory neuroplasticity (Fuller and Mitchell 2017). Accordingly, in this study we hypothesized that a brief period of HF-ES in rats with incomplete cervical SCI would induce short-term potentiation in phrenic motor output. Experiments were conducted in adult rats following hemilesion of the spinal cord at C2 (C2Hx; Goshgarian 2003; Sandhu et al. 2009). The C2Hx lesion transiently eliminates ipsilateral inspiratory phrenic activity, but this is followed by a gradual and spontaneous return of a small degree of inspiratory activity (Nantwi et al. 1999; Pitts 1940) coupled with increases in tonic discharge (Fuller et al. 2008). Epidural stimulation was applied to the ventrolateral wall of the midcervical (C4) or high thoracic (T2) spinal cord, ipsilateral to C2Hx, and both phrenic and supraspinal (hypoglossal, XII) respiratory output were recorded. Experiments were conducted after both subacute (2 wk) and chronic (12 wk) injury because the spontaneous neuroplastic processes that drive phrenic motor recovery after C2Hx (Golder and Mitchell 2005) may also impact the response to HF-ES. The results indicate that HF-ES can potentiate motor output in the phrenic nerve with impaired output (i.e., ipsilateral to C2Hx), with some evidence for improved efficacy at 12 vs. 2 wk post-C2Hx. However, large increases in tonic phrenic motor output following stimulation masked the increases in phasic (inspiratory) phrenic bursting.
MATERIALS AND METHODS
Experiments were conducted on adult, female Sprague-Dawley rats (Harlan, Indianapolis, IN) with C2Hx injury. All rats were housed in pairs in a controlled environment (12:12-h light-dark cycles) with food and water ad libitum. The C4 or T2 spinal cord was stimulated in separate rats at either 2 or 12 wk post-C2Hx: 2-wk C4, n = 8; 12-wk C4, n = 8; 2-wk T2, n = 7; and 12-wk T2, n = 7. A summary of the experimental approach including the SCI model and location of stimulation is shown in Fig. 1. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Fig. 1.
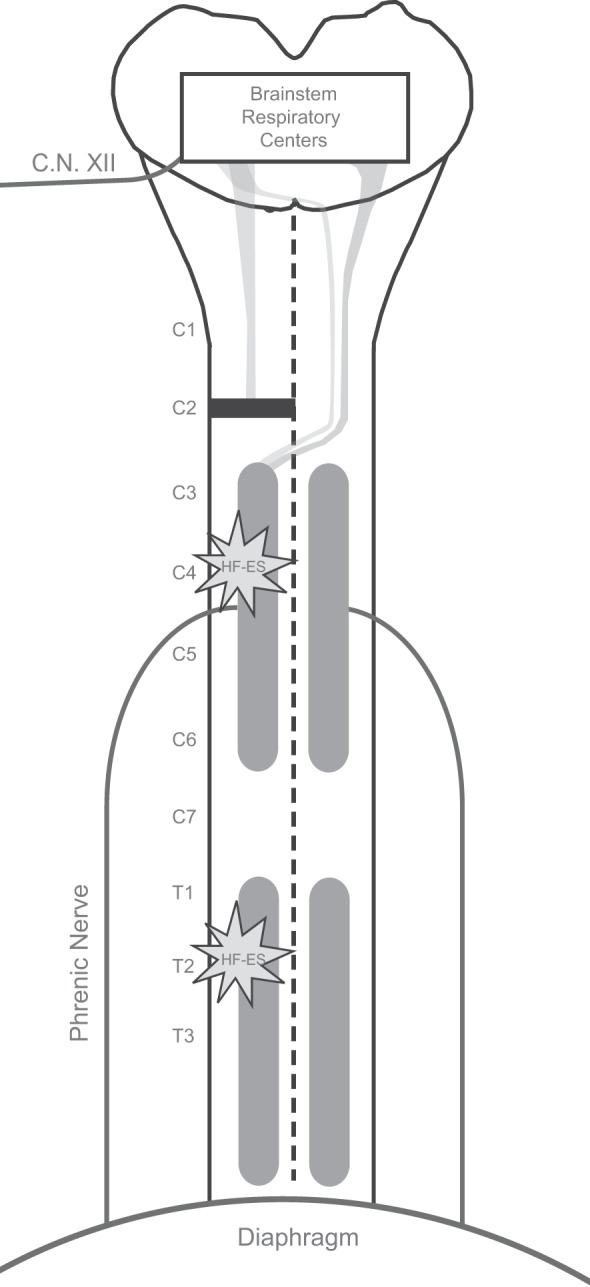
Schematic diagram illustrating the experimental model. Lateral C2 spinal cord hemisection injury disrupts the descending bulbospinal projections innervating the phrenic motor pool on one side, rendering the ipsilateral hemidiaphragm paralyzed. Modest but incomplete spontaneous recovery of ipsilateral diaphragm function occurs in the weeks following injury. At either 2 or 12 wk following injury, HF-ES was delivered to the ventrolateral surface of the spinal cord at either the midcervical (C4) or upper thoracic (T2) level ipsilateral to the side of injury. C.N. XII, cranial nerve XII.
SCI surgery.
Anesthesia and C2Hx methods have been previously described (Doperalski et al. 2008; Dougherty et al. 2012; Fuller et al. 2008). Rats were anesthetized by 3% isoflurane in a closed chamber and then maintained at a surgical plane by using a nose cone to inspire 1–2% isoflurane. A 1-in. dorsal midline incision was made from the base of the skull to approximately the fourth cervical segment (C4). After C2 laminectomy, a small incision was made in the dura, followed by lateral hemisection of the left spinal cord, using a microscalpel and gentle aspiration. After completeness of the lesion was verified visually, the dura was closed with interrupted 9-0 sutures. The overlying muscle was sutured in layers, and the skin was closed with surgical wound clips. Rats were given injections of buprenorphine (0.03 mg/kg sc; Hospira, Lake Forest, IL) for analgesia and sterile lactated Ringer’s solution (5 ml sc) to prevent dehydration. Buprenorphine (0.03 mg/kg sc) was given at 12-h intervals for the initial 48 h. Lactated Ringer’s solution (5 ml/day sc) and oral Nutri-Cal supplements (1–3 ml; Webster Veterinary Supply, Devens, MA) were given until adequate volitional drinking and eating resumed.
Phrenic and XII nerve recordings.
Isoflurane anesthesia (3–4%) was induced in a closed chamber followed by intravenous infusion of urethane (1.7–1.8 g/kg; Sigma, St. Louis, MO) via a tail vein catheter. The trachea was cannulated with polyethylene (PE-240) tubing, and rats were mechanically ventilated for the remainder of the experiment with 50% O2. Tracheal pressure was continuously monitored with a pressure transducer (DTXPlus pressure transducer, Argon Critical Care Systems, Singapore; model TA-100 strain gauge amplifier, CWE, Ardmore, PA) connected to the tracheal cannula. The vagus nerves were sectioned in the midcervical region to prevent entrainment of phrenic motor output with the ventilator and rats were paralyzed with pancuronium bromide (2.5 mg/kg iv; Hospira) to eliminate spontaneous breathing efforts. After paralysis, arterial blood pressure and phrenic nerve response to toe pinch were monitored to ensure the depth of anesthesia, and supplemental urethane was given if indicated (0.3 g/kg iv). A femoral arterial catheter (PE-50) was inserted to measure blood pressure (DTXPlus, Argon Critical Care Systems; model TA-100 strain gauge amplifier, CWE) and end-tidal carbon dioxide partial pressure () to periodically withdraw blood samples. Heart rate was extracted from the arterial pressure trace and calculated in real time using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Arterial Po2 () and Pco2 () and pH were determined from 0.2-ml arterial blood samples using an i-Stat blood gas analyzer (Heska, Fort Collins, CO). Rectal temperature was maintained at 37 ± 1°C using a rectal thermistor connected to a servo-controlled heating pad (model TC-1000; CWE). The was measured using a rapidly responding mainstream CO2 analyzer positioned a few centimeters from the tracheostomy tube (Capnogard; Novametrix Medical Systems, Wallingford, CT).
With the use of a dorsal surgical approach, the phrenic and hypoglossal nerves were isolated, cut distally, and partially desheathed (Mahamed et al. 2011). Electrical activity in the nerves was recorded using custom-made bipolar silver wire suction electrodes filled with 0.9% saline. Signals were amplified (×10,000), filtered (bandpass = 3–30 kHz), digitized (16-bit, 25-kHz sampling frequency/channel; CED Power 1401 data acquisition interface), and recorded using Spike2 software. The amplified signals were full-wave rectified in real time using Spike2 software.
Stimulation of the spinal cord.
After isolation of the phrenic and XII nerves, a laminectomy was performed at either C3–C5 or T1–T3 (Fig. 1). Two Teflon-coated silver wires (diameter: 0.010 in. bare, 0.013 in. coated; A-M Systems) with 1 mm of insulation removed at the tip were used to stimulate the spinal cord. Electrode tips were separated by 1.5 mm and were slid between the dorsal roots ipsilateral to the side of injury to wrap around to the ventrolateral surface of the spinal cord. A Grass S-88 stimulator was used to pass electrical current through the wires.
Experimental protocol.
Baseline conditions were established by adjusting the ventilator rate to maintain between 44 and 48 mmHg; this ensured that a robust and rhythmic inspiratory burst could be recorded in the phrenic and XII nerves. All baseline recordings were made for a minimum of 10 min, during which the burst amplitude remained consistent. The stimulation protocol consisted of three 60-s trains of high-frequency (300 Hz) stimulation delivered at 100, 500, and 1,000 μA (pulse duration = 0.2 ms). A 3-min recovery period followed each bout of stimulation. Throughout the protocol, measurements were used as a guide to help maintain stability; arterial blood gases were measured at baseline and after the final bout of stimulation.
Data analysis.
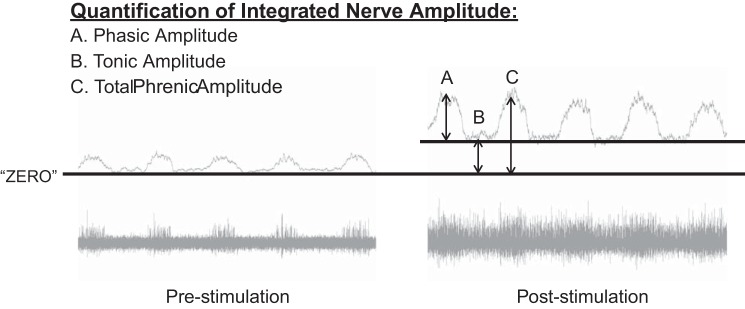
The amplitude and frequency (burst/min) of the integrated phrenic and hypoglossal signals were quantified at baseline and after each bout of stimulation. Figure 2 provides an overview of how the phasic inspiratory-related discharge and the tonic discharge were evaluated. The integrated signals were compared with a “zero” point, which was determined at the end of the experimental protocol, when each nerve was bathed in lidocaine to silence all neural activity. Tonic activity was measured as the integrated amplitude during the expiratory phase of the respiratory cycle relative to the calculated zero. Phasic (inspiratory) activity was quantified as the peak of the integrated inspiratory burst amplitude relative to the amplitude of the expiratory phase preceding it. The total activity during the inspiratory phase was quantified as the peak of the integrated inspiratory burst amplitude relative to zero. Integrated burst amplitudes were quantified as 1) absolute voltages (i.e., arbitrary units, a.u.), 2) relative to the amplitude recorded during the baseline condition (% baseline), and 3) as a “signal-to-noise” ratio, calculated to express the relationship of phasic to tonic nerve activity in a given nerve at each time point. Baseline nerve activity was calculated over a stable 3-min period just before initiation of stimulations. Poststimulation nerve activity was assessed as 1) an average over the 3 min following termination of the stimulation, 2) an average of the first 10 inspiratory cycles following termination of the stimulation, and 3) the peak amplitude following termination of stimulation. Mean arterial pressure and heart rate were assessed throughout the entire protocol. Statistical analyses were performed using SigmaStat software. Comparisons of baseline blood gases, cardiovascular parameters (blood pressure, heart rate), and nerve data (frequency and amplitude) were made across groups using one-way analysis of variance (ANOVA). Changes in arterial blood gases, cardiovascular parameters, and neural output following stimulation were compared across groups using two-way repeated-measures analyses of variance (RM ANOVA) and Holm-Sidak post hoc tests. All data are means ± SE. A P value <0.05 was considered statistically significant.
Fig. 2.
Quantification of electrical activity recorded in the phrenic and XII nerves. Integrated nerve signals were compared with a “zero” point, which was determined at the end of the experimental protocol, when each nerve was bathed in lidocaine to silence all neural activity. “Phasic” amplitude (A) was quantified as the integrated amplitude of the inspiratory burst relative to the amplitude of the expiratory phase preceding it. “Tonic” amplitude (B) was quantified as the integrated amplitude during the expiratory phase relative to “zero.” “Total” amplitude (C) was quantified as the integrated amplitude of the inspiratory burst relative to “zero.”
RESULTS
Baseline respiratory and cardiovascular parameters.
Robust inspiratory signals were recorded from the left XII nerve (ipsilateral to C2Hx) and the right (contralateral) phrenic nerve in all experiments (Fig. 3). Baseline inspiratory-related bursting in the left (ipsilateral) phrenic nerve was also present in all animals, and amplitude was attenuated by 41 ± 10% acompared with the contralateral side (P < 0.001; Fig. 3, A and B, left). For each of the three motor outputs, the absolute value (mV) of the baseline burst amplitude was similar across the four experimental groups (ipsilateral phrenic: P = 0.661; contralateral phrenic: P = 0.485; XII: P = 0.564). The normalized ipsilateral phrenic burst showed a progressive increase in amplitude between 2 wk (19 ± 7% of the contralateral burst amplitude) and 12 wk postinjury (57 ± 14%), but the difference between these two time points was just above the threshold for statistical significance (P = 0.061). Inspiratory frequency (bursts/min) at baseline was similar across the four groups (P = 0.244; 2-wk cervical: 53 ± 2; 12-wk cervical: 48 ± 2; 2-wk thoracic: 50 ± 2; 12-wk thoracic: 51 ± 2). Baseline cardiovascular parameters including heart rate (HR), mean arterial blood pressure (MAP; Table 1), and arterial blood gases (Table 2) were also similar between the four experimental groups (P > 0.3 for all).
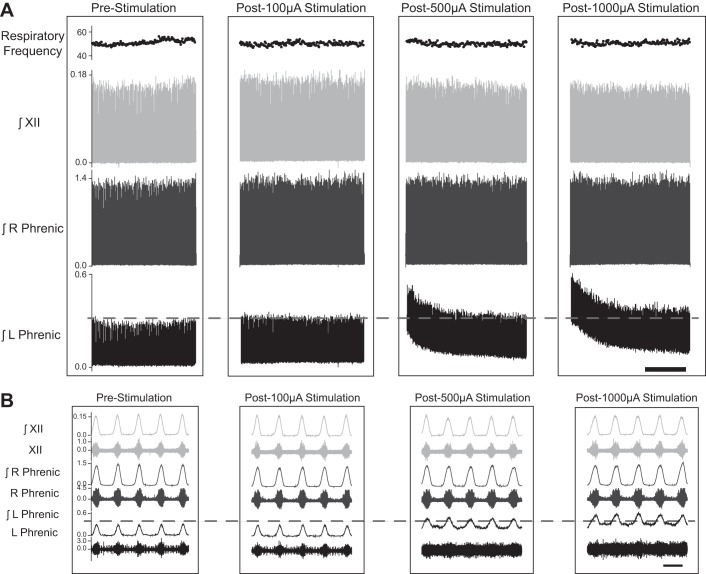
Fig. 3.
Representative examples illustrating the impact of cervical HF-ES on phrenic and XII nerve activity. Following a left-sided C2 spinal cord hemisection injury, HF-ES was delivered to the ventrolateral epidural surface of the C4 spinal cord, ipsilateral to the side of injury. A: representative compressed traces depict integrated (∫)phrenic and hypoglossal (XII) nerve activity before and after HF-ES at 100, 500, and 1,000 µA from a rat that is 12 wk post-C2Hx. B: expanded traces depict both raw and integrated phrenic and hypoglossal nerve activity at baseline and following each bout of stimulation. Scale bars represent 1 min (A) and 1 s (B).
Table 1.
Cardiovascular parameters before, during, and after HF-ES
| Mean Arterial Pressure, mmHg |
Heart Rate, beats/min |
|||||||
|---|---|---|---|---|---|---|---|---|
| Stimulation | 2-wk C4 | 2-wk T2 | 12-wk C4 | 12-wk T2 | 2-wk C4 | 2-wk T2 | 12-wk C4 | 12-wk T2 |
| Baseline | 100 ± 9 | 101 ± 5 | 118 ± 13 | 96 ± 10 | 409 ± 5 | 416 ± 9 | 402 ± 10 | 401 ± 7 |
| 100 µA | 101 ± 9 | 101 ± 5 | 117 ± 13 | 97 ± 10 | 407 ± 4 | 416 ± 9 | 401 ± 9 | 400 ± 8 |
| Post 100 µA | 101 ± 9 | 101 ± 5 | 116 ± 13 | 97 ± 10 | 406 ± 4 | 417 ± 9 | 400 ± 9 | 400 ± 8 |
| 500 µA | 103 ± 9* | 104 ± 4 | 120 ± 14* | 104 ± 11 | 405 ± 4 | 417 ± 8 | 401 ± 9 | 402 ± 8 |
| Post 500 µA | 102 ± 10 | 103 ± 5 | 117 ± 13 | 97 ± 9 | 405 ± 4 | 417 ± 8 | 400 ± 9 | 400 ± 8 |
| 1,000 µA | 106 ± 10* | 108 ± 4* | 123 ± 13* | 106 ± 11* | 406 ± 4 | 418 ± 8 | 403 ± 9 | 402 ± 9 |
| Post 1,000 µA | 102 ± 10 | 102 ± 6 | 118 ± 14 | 93 ± 10 | 406 ± 4 | 420 ± 7 | 400 ± 9 | 401 ± 9 |
Mean arterial blood pressure was significantly increased during 500- and 1,000-µA stimulation in rats receiving stimulation at C4 and during 1,000-µA stimulation in rats receiving stimulation at T2. Heart rate was significantly increased during 1,000-µA stimulation in rats receiving HF-ES to the C4 spinal cord at 12 wk postinjury. No other statistically significant changes in cardiovascular parameters were observed during or after stimulation.
P < 0.05, significantly different from baseline, 100 µA, post 100 µA, post 500 µA, and post 1,000 µA.
P < 0.05, significantly different from T2 stimulation (2-way repeated-measures ANOVA with Holm-Sidak post hoc tests for individual comparisons).
Table 2.
Arterial blood gas parameters before and after HF-ES
| , mmHg | , mmHg | pH | |
|---|---|---|---|
| 2-wk C4 | |||
| Baseline | 50.0 ± 2.6 | 171.4 ± 15.1 | 7.3 ± 0.0 |
| Poststimulation | 46.4 ± 2.6 | 162.3 ± 13.9* | 7.3 ± 0.0 |
| 2-wk T2 | |||
| Baseline | 51.7 ± 2.6 | 161.9 ± 17.8 | 7.3 ± 0.0 |
| Poststimulation | 51.9 ± 1.6 | 156.6 ± 20.2* | 7.3 ± 0.0 |
| 12-wk C4 | |||
| Baseline | 50.3 ± 1.0 | 178.6 ± 15.1 | 7.3 ± 0.1 |
| Poststimulation | 48.6 ± 2.8 | 174.6 ± 15.2* | 7.3 ± 0.0 |
| 12-wk T2 | |||
| Baseline | 50.5 ± 1.9 | 195.0 ± 19.1 | 7.3 ± 0.0 |
| Poststimulation | 52.4 ± 2.5 | 187.8 ± 20.6* | 7.3 ± 0.0 |
Arterial partial pressure of CO2 () and O2 () and pH are shown by group, before and after HF-ES. No changes in or pH were observed poststimulation. Although was slightly reduced poststimulation, all animals remained well oxygenated.
P < 0.05, significantly different from baseline (2-way repeated-measures ANOVA with Holm-Sidak post hoc tests for individual comparisons).
Phrenic and XII neural output following cervical HF-ES.
Representative traces of phrenic and XII nerve output following brief periods of C4 epidural stimulation are provided in Fig. 3. A few features can be appreciated from these example records. First, the recording configuration precluded assessment of respiratory nerve activity during the period of stimulation due to a large stimulus artifact that obviated the underlying signal. Second, robust short-term potentiation of the ipsilateral phrenic signal is evident following HF-ES. The lowest current (100 µA) typically evoked minimal, if any, potentiation of respiratory nerve output, but stimulation at both 500 and 1,000 µA resulted in a substantial increase in the overall output of the ipsilateral phrenic nerve. In the example shown in Fig. 3 there was no appreciable impact on XII or contralateral phrenic output, even at the higher stimulus currents. Figure 4 provides an “overlay” of ipsilateral phrenic waveform averages, obtained immediately following HF-ES. The overlay plots further illustrate the potentiation of both phasic (inspiratory) and tonic phrenic output that occurred following the higher stimulus currents.
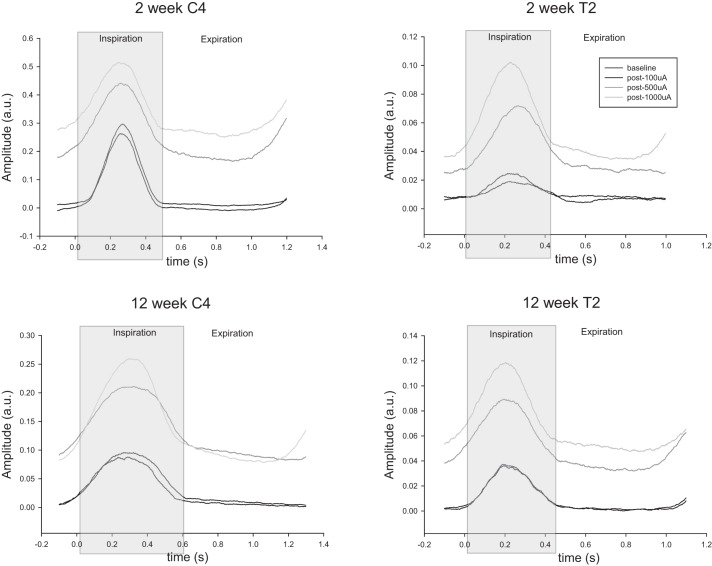
Fig. 4.
Impact of HF-ES on the phrenic activity recorded ipsilateral to C2Hx. Representative traces of integrated ipsilateral phrenic waveform averages are shown from rats that were either 2 or 12 wk post-C2Hx injury and received HF-ES to either the cervical or thoracic spinal cord. Waveform averages were calculated from the 10 respiratory cycles immediately preceding the initiation of stimulation (baseline) and from the 10 cycles immediately following each bout of stimulation. For each plot, time 0 represents the start of the inspiratory phase of the respiratory cycle, which is shaded in gray. Note the increase in tonic phrenic output in both the inspiratory and expiratory phases of the respiratory cycle following 500- and 1,000-µA stimulation (as evidenced by the upward shift in the waveform baseline).
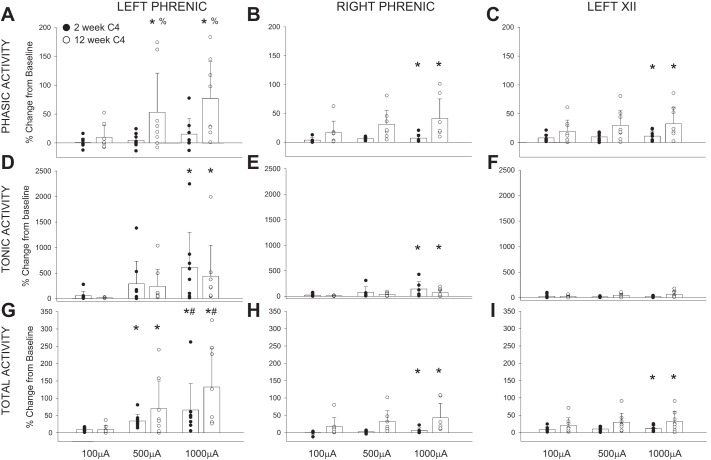
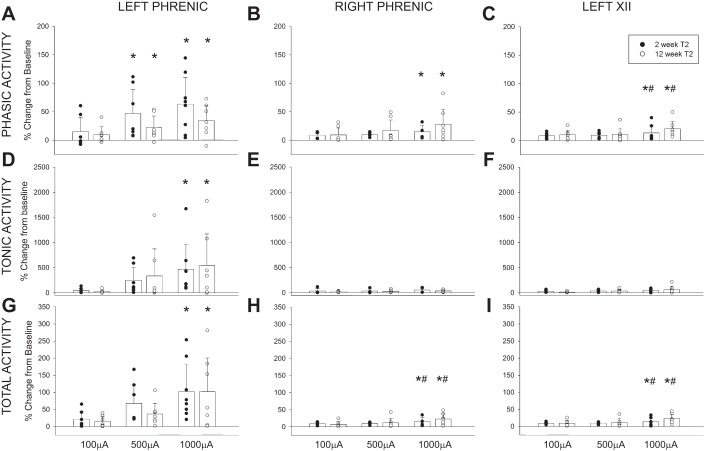
The average changes in phasic, tonic, and total activity in the phrenic and XII nerve recordings following C4 HF-ES are presented in Fig. 5. In addition to the mean and SE, each individual data point is provided in keeping with recent recommendations for data reporting in preclinical research (Landis et al. 2012). Evaluation of phasic inspiratory activity in the left (ipsilateral) phrenic nerve revealed a statistical interaction (P = 0.028) between the stimulus current (i.e., 100, 500, and 1,000 µA) and the time postinjury (i.e., 2 vs. 12 wk post-C2Hx). Thus higher C4 stimulus currents evoked increases in phasic ipsilateral phrenic activity at 12 but not 2 wk following C2Hx (Fig. 5A). A similar response occurred in the phrenic nerve contralateral to the lesion (P < 0.05; Fig. 5B); however, neither the time postinjury (P = 0.051) nor the time-stimulus current interaction (P = 0.079) reached the threshold for statistical significance. Although there was no significant impact of time postinjury, contralateral phrenic output did increase with stimulus current (P = 0.014).
Fig. 5.
Average changes in phrenic and hypoglossal nerve activity following C4 HF-ES. Depicted are changes in phasic (A–C), tonic (D–F), and total (G–I) phrenic and hypoglossal nerve activity following HF-ES of the C4 spinal cord, delivered at 2 and 12 wk postinjury. Data were calculated over the 3 min following each bout of stimulation and are represented as %change from baseline activity. HF-ES delivered to the C4 spinal cord at 12 wk post-C2Hx resulted in an increase in phasic phrenic activity. No other group differences were observed, although dose-dependent increases in amplitude (%change above baseline) were observed following HF-ES across both groups, with the greatest amplitude changes occurring following the highest stimulation intensities (e.g., 500 and 1,000 µA). Note that profound increases in tonic activity comprised much of the increase in total activity and that the ipsilateral phrenic nerve demonstrated the most dramatic changes in nerve amplitude relative to baseline. *P < 0.05, different from 100 µA. #P < 0.05, different from 500 µA. %P < 0.05, different from 2 wk at same stimulus current. Data were evaluated using 2-way repeated-measures ANOVA with Holm-Sidak post hoc tests for individual comparisons (groups: 2-wk C4, n = 8; 12-wk C4, n = 8).
The C4 stimulation paradigm also influenced the medulla, as confirmed by small increases in XII motor output following HF-ES. The relative changes were small compared with the ipsilateral phrenic signal, but the XII inspiratory burst (Fig. 5C) increased with the stimulus current (P = 0.027), and potentiation of XII bursting tended to be greater at 12 vs. 2 wk post-C2Hx (P = 0.070).
The average changes in tonic neural activity following C4 stimulation are provided in Fig. 5, D–F. Note that the relative magnitude (%baseline) of the increased tonic discharge was considerably greater for tonic compared with phasic output. Both the left (Fig. 5D; P = 0.001) and right phrenic nerves (Fig. 5E; P = 0.002) showed increases in tonic activity as the stimulus current increased. The most robust potentiation occurred in the left phrenic nerve, with increases reaching ~500% of the baseline output at the highest stimulation intensity. There was no evidence for a time-dependent effect in either the left or right phrenic neurogram (i.e., tonic output was similar at 2 and 12 wk post-C2Hx). Tonic XII discharge tended to be greater following C4 stimulation at 12 vs. 2 wk, but this was not statistically significant (Fig. 5F; P = 0.083). Changes in total phrenic and XII activity (i.e., including both the phasic and tonic components) are presented in Fig. 5, G–I. All three recordings showed a progressive increase in total activity as the stimulus current was increased.
Although the XII data suggest an impact of HF-ES on medullary neurons (Fig. 5), no changes in inspiratory burst frequency were observed following stimulation of the cervical spinal cord (Table 3; P = 0.402).
Table 3.
Respiratory frequency before and after HF-ES
| Stimulation |
||||
|---|---|---|---|---|
| Baseline | 100 µA | 500 µA | 1,000 µA | |
| 2-wk C4 | 52.5 ± 1.5 | 50.0 ± 1.4 | 51.0 ± 1.2 | 52.0 ± 0.7 |
| 2-wk T2 | 50.3 ± 1.5 | 47.7 ± 2.1 | 49.7 ± 1.8 | 50.9 ± 2.4 |
| 12-wk C4 | 47.5 ± 1.6 | 47.5 ± 1.6 | 47.0 ± 1.5 | 48.0 ± 1.2 |
| 12-wk T2 | 51.4 ± 1.9 | 50.9 ± 1.2 | 49.7 ± 1.3 | 48.6 ± 1.4 |
Average respiratory frequency (breaths per minute) are shown by group, prior to and following HF-ES. No changes in respiratory frequency were observed following stimulation (2-way repeated-measures ANOVA).
Phrenic and XII neural output following thoracic HF-ES.
The impact of thoracic (T2) stimulation on phasic, tonic, and total activity in the left and right phrenic nerves and the left XII nerve is summarized in Fig. 6. At the 100-µA stimulus current, the majority of rats showed a small increase in phasic discharge in all three motor outputs. The only exception was in the left phrenic recording at the 2-wk time point, where only 3 of 6 rats showed an increase in phasic discharge (Fig. 6A). The magnitude of potentiation increased with stimulus current in the left phrenic (Fig. 6A; P = 0.004), right phrenic (Fig. 6B; P = 0.011), and left XII recordings (Fig. 6C; P = 0.021). However, time-dependent effects (e.g., 2 vs. 12 wk) on phasic output were not detected for any of the recordings (all P > 0.265).
Fig. 6.
Average changes in phrenic and hypoglossal nerve activity following T2 HF-ES. Shown are changes in phasic (A–C), tonic (D–F), and total (G–I) phrenic and hypoglossal nerve activity following HF-ES of the T2 spinal cord, delivered at 2 and 12 wk postinjury. Data were calculated over the 3 min following each bout of stimulation and are represented as %change from baseline activity. A dose-dependent increase in amplitude (%change above baseline) was observed in all nerves (*P < 0.05, different from 100µA; #P < 0.05, different from 500 µA) across all outcomes (phasic, tonic, and total nerve activity), with the greatest amplitude changes occurring following the highest stimulation intensities. Note that profound increases in tonic activity comprised much of the increase in total activity and that the ipsilateral phrenic nerve demonstrated the most dramatic changes in nerve amplitude relative to baseline. No significant group differences were observed in any nerve in any of the outcomes assessed. Data were evaluated using 2-way repeated-measures ANOVA with Holm-Sidak post hoc tests for individual comparisons (groups: 2-wk T2, n = 7; 12-wk T2, n = 7).
Tonic output was potentiated in all three nerves following T2 stimulation in nearly all experiments at both the 2- and 12-wk time points (Fig. 6, D–F). In the left phrenic signal, tonic discharge increased with stimulus intensity (P = 0.004) and reached ~500% of baseline at the highest stimulus current. Increases in tonic discharge in the right phrenic and left XII recordings were typically in the range of 50–100% (Fig. 6, E and F). Time-dependent effects on the tonic response to T2 stimulation were not statistically significant for any of the neural outcomes assessed (all P > 0.557). The magnitude by which total activity was potentiated increased with stimulus intensity in all recordings (all P < 0.007; Fig. 6, G–I), and there were no significant differences in the total activity between the two time points (all P > 0.075).
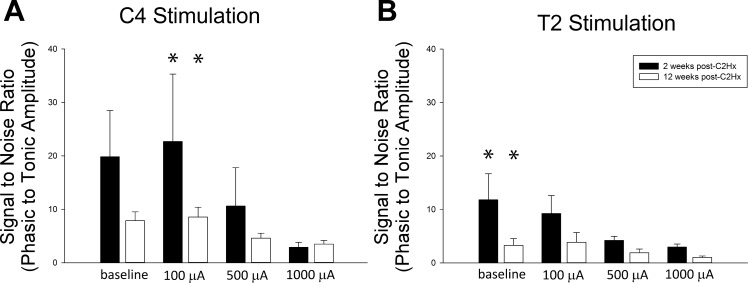
The ratio of phasic to tonic activity within each nerve is presented in Fig. 7. For both C4 and T2 stimulation, higher HF-ES currents were associated with the most dramatic reductions in this ratio, consistent with the observed increase in tonic output relative to phasic output. Although not statistically significant, there appeared to be a greater impact of stimulation on the ratio of phasic to tonic activity at the more acute time points.
Fig. 7.
Ratio of phasic to tonic ipsilateral phrenic nerve activity following C4 and T2 HF-ES. Shown are the average “signal-to-noise ratios” of phasic to tonic ipsilateral phrenic nerve activity at baseline and following each bout of HF-ES delivered to either the C4 (A) or T2 (B) spinal cord at 2 and 12 wk postinjury. A dose-dependent reduction in this ratio was observed following both C4 and T2 stimulation (*P < 0.05, different from 1,000 µA) across all outcomes (phasic, tonic, and total nerve activity), with the greatest reductions in this ratio occurring following the highest stimulation intensities. No significant time-dependent group differences were observed, although this ratio was consistently lower in the chronic postinjury time point, a finding that is consistent with increased baseline tonic activity in chronically injured rats. Data were evaluated using 2-way repeated-measures ANOVA with Holm-Sidak post hoc tests for individual comparisons (groups: 2-wk C4, n = 8; 12-wk C4, n = 8; 2-wk T2, n = 7; 12-wk T2, n = 7).
Similar to the cervical HF-ES data, changes in inspiratory burst frequency were not observed following stimulation of the thoracic spinal cord (Table 3; P = 0.281).
Cardiovascular parameters during and after HF-ES.
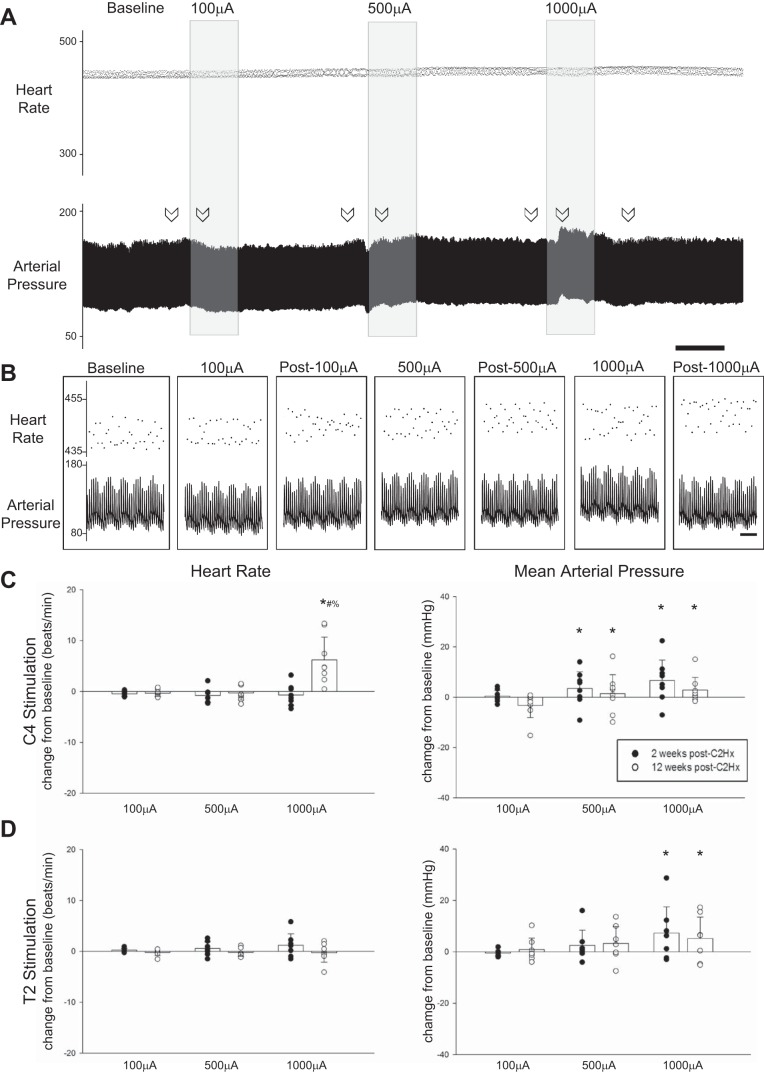
Representative traces depicting the impact of C4 stimulation on HR and MAP are shown in Fig. 8, A and B; average responses along with individual data points are provided in Fig. 8, C and D. HR remained stable in all groups during and after each bout of stimulation. MAP increased during C4 stimulation at 500 (P = 0.046) and 1,000 µA (P < 0.001), but with no evidence of a time-dependent change between 2 and 12 wk (P = 0.445; Fig. 8C). MAP also increased during T2 stimulation at 1,000 µA (P = 0.039), but no time-dependent changes were detected (P = 0.963). At both 2 and 12 wk, MAP returned ack to baseline values following cessation of stimulation, regardless of the level of stimulation (all P > 0.627). The mean HR and MAP data are provided in Table 1.
Fig. 8.
Impact of HF-ES on cardiovascular parameters. A: representative traces depict instantaneous heart rate (HR; beats/min) and mean arterial pressure (MAP; mmHg) from a rat that received stimulation to the C4 spinal cord 2 wk postinjury. B: expanded traces depict instantaneous HR and MAP at baseline as well as during and after each bout of stimulation. Arrows in A indicate locations from which the expanded traces shown in B were obtained. Scale bars represent 1 min (A) and 1 s (B). C and D: stimulation-induced changes in HR and MAP for rats receiving HF-ES at either the C4 (C) or the T2 (D) spinal level. A significant increase in HR was observed following C4 stimulation in rats that were 12 wk postinjury. MAP was significantly increased relative to baseline during stimulation at both 500 (#P < 0.05) and 1,000 µA (*P < 0.05). Following T2 stimulation, MAP was only increased with stimulation at 1,000 µA (*P < 0.05). In all groups, HR and MAP returned to baseline following cessation of stimulation. *P < 0.05, different from 100 µA. #P < 0.05, different from 500 µA. %P < 0.05, different from 2 wk. Data were evaluated using 2-way repeated-measures ANOVA with Holm-Sidak post hoc tests for individual comparisons (groups: 2-wk C4, n = 8; 12-wk C4, n = 8; 2-wk T2, n = 7; 12-wk T2, n = 7).
Arterial blood was assessed for , , and pH values before and after the stimulation protocol. As shown in Table 2, no group differences in or pH were detected at either time point (all P > 0.38). Despite a slight reduction in following stimulation (P = 0.012), all rats remained well oxygenated throughout the experimental protocol, with values >98 mmHg in all cases.
DISCUSSION
In regard to respiratory muscle activation, the utility of HF-ES was first demonstrated in terminal studies in dogs and rats following complete transection at the C1 level (DiMarco and Kowalski 2009, 2011, 2013b; Kowalski et al. 2013). In the current study we tested HF-ES following an incomplete high cervical SCI that spared bulbospinal synaptic inputs to contralateral phrenic motoneurons while preserving a limited number of bulbospinal inputs to ipsilateral motoneurons via collaterals from contralateral fibers (Fuller et al. 2006; Goshgarian 2003, 2009; Goshgarian et al. 1991; Lane et al. 2008; Moreno et al. 1992; Nantwi et al. 1999). The results show that prolonged HF-ES of the ventrolateral C4 or T2 spinal cord can cause an increase in ipsilateral phrenic discharge that persists after the stimulation. This short-term potentiation of phrenic bursting illustrates the potential for spinal cord stimulation to trigger respiratory plasticity (Mercier et al. 2017), a concept that could be useful in designing neurorehabilitation paradigms (Kasten et al. 2013; McPherson et al. 2015). However, the large increases in tonic phrenic output triggered by HF-ES indicate that this particular paradigm is unlikely to have application as a “respiratory neuroprosthesis” for activation of the respiratory muscles after incomplete SCI.
HF-ES after SCI.
Application of HF-ES to the ventral spinal cord following C1 spinal cord transection can produce diaphragm and intercostal activation motor unit activation that is asynchronous relative to the stimulus (DiMarco and Kowalski 2009, 2010, 2011, 2013a, 2013b, 2015; Hormigo et al. 2017; Kowalski et al. 2013). In these prior reports, respiratory motor unit discharge during HF-ES occurred at rates well below the stimulation frequency, and respiratory motor unit activation resembled discharge patterns seen during spontaneous breathing. Therefore, it appears that HF-ES can activate a spinal network capable of providing “physiologically appropriate” synaptic input to respiratory motoneurons, resulting in discharge patterns that resemble what is observed during spontaneous breathing. In contrast, direct nerve or muscle electrical stimulation techniques (e.g., functional electrical stimulation, or FES) typically induce synchronous activation of axons within the electrical field and do not recruit motor units in an orderly fashion, which could contribute to motor unit fatigue (Levy et al. 1990). This has led to the suggestion that spinal stimulation methods may be advantageous compared with FES approaches (Bamford and Mushahwar 2011; DiMarco and Kowalski 2013a; Mondello et al. 2014; Tator et al. 2012).
The current study is the first to explore the impact of HF-ES on phrenic and XII motor output after subacute and chronic incomplete cervical SCI. We utilized a C2Hx injury, which axotomizes bulbospinal neurons projecting to the ipsilateral phrenic motor nucleus (Goshgarian 2003). The resulting paralysis of the ipsilateral diaphragm is transient, and a small but functionally relevant (Dougherty et al. 2012) return of ipsilateral diaphragm inspiratory bursting occurs over weeks to months post-C2Hx (Nantwi et al. 1999; Pitts 1940). Spontaneous activation of the ipsilateral diaphragm after C2Hx results at least in part due to activation of latent crossed bulbospinal synaptic projections to the ipsilateral phrenic motor nucleus (Goshgarian 2003, 2009). There also may be a contribution of midcervical interneurons to the “crossed phrenic pathway” (Lane 2011; Sandhu et al. 2009). Regardless of the specific neuroanatomical substrate, it is well established that crossed phrenic pathways are capable of considerable plasticity and that the relative extent of ipsilateral phrenic motor recovery after C2Hx can be enhanced (Fuller and Mitchell 2017). Treatments that can increase phrenic recovery include spinal serotonin receptor activation (Ling et al. 1994; Zhou and Goshgarian 1999, 2000), increased brain-derived neurotrophic factor availability (Gransee et al. 2013; Mantilla et al. 2013), preconditioning nerve lesions (Fuller et al. 2002; Johnson et al. 2000), and intermittent hypoxia exposure (Fuller et al. 2003; Golder and Mitchell 2005; Lovett-Barr et al. 2012). Here we investigated whether applying HF-ES with a continuous paradigm, similarly to stimulation studies targeting increased limb muscle activation (Angeli et al. 2014; Harkema et al. 2011), can induce phrenic motor plasticity on short timescales in rats with subacute (2 wk) or chronic (12 wk) C2Hx. Both thoracic and cervical HF-ES were able to potentiate phrenic bursting, but some differences were noted. With thoracic stimulation, increases in phasic (i.e., inspiratory related) activity occurred after both subacute and chronic injury. In contrast, cervical HF-ES did not increase phasic phrenic bursting at the subacute time point, but did so after chronic injury. Inflammatory processes and other aspects of “spinal shock” (Hagg and Oudega 2006) occurring in the cervical cord will be more prominent at the subacute time point, and these may limit the ability for cervical HF-ES to activate synaptic pathways to phrenic motoneurons to increase inspiratory bursting. It should be emphasized, however, that HF-ES evoked robust increases in tonic phrenic output in subacutely injured rats, similar to the tonic response after chronic injury. This differential tonic vs. phasic response at the subacute time point suggests that HF-ES is regulating these outputs via distinct mechanisms.
It is worth noting that that tonic discharge observed in our studies is similar to that in a report from Alilain et al. (2008), who used light-based activation of cervical neurons. Stimulation of the midcervical spinal cord using a channel rhodopsin method also evoked increases in phrenic bursting across the entire respiratory cycle. In that report, light-induced neuronal activation was not restricted to phrenic motoneurons, and the result was tonic phrenic motoneuron activation, similar to the current data. Regardless of the underlying mechanisms, the robust increase in tonic phrenic discharge raises concerns about the impact of this particular HF-ES paradigm on respiratory biomechanics. More specifically, the tonic activity and sustained diaphragm contraction are unlikely to facilitate lung inflation and alveolar ventilation. Accordingly, future studies of spinal epidural stimulation after incomplete SCI need to focus on the pattern of diaphragm motor unit activation both during (e.g., DiMarco and Kowalski 2009, 2011, 2013b; Kowalski et al. 2013) and after stimulation (e.g., as in the current report). Thus it appears that diffuse stimulation of the midcervical spinal cord has a high probability of evoking tonic diaphragm activation, at least after incomplete cervical SCI.
Mechanisms by which HF-ES may potentiate phasic and tonic respiratory motor output.
Modeling studies indicate that epidural stimulation is not effective at direct motoneuron activation and that activation of transynaptic pathways is likely to underlie many of the observed motor responses (Capogrosso et al. 2013). In addition, the HF-ES approach used in the current study likely caused diffuse neuronal activation in the vicinity of the electrode. Accordingly, there are multiple mechanisms that may contribute to HF-ES induced short-term potentiation of ipsilateral phrenic discharge. A predominantly local (spinal) mechanism (e.g., at or near the ipsilateral phrenic motor pool) seems most likely to underlie the impact of HF-ES on ipsilateral phrenic motor output, because contralateral phrenic and XII responses were much lower. However, plasticity in respiratory premotor inputs cannot be excluded as a contributing factor, because HF-ES also induced small changes in both phasic and tonic supraspinal (XII) motor output. On the other hand, there was no sustained impact of HF-ES on brain stem processes driving the respiratory rhythm, because the frequency of breathing did not show any appreciable changes.
Activation of neuromodulatory inputs to phrenic motoneurons could have contributed to persistently increased activity following HF-ES. The caudal raphe nuclei project a dense serotonergic input to the spinal cord (Alvarez et al. 1998; Bowker et al. 1981a, 1981b, 1981c; Steinbusch 1981), including phrenic motoneurons (Satriotomo et al. 2012) as well as interneurons that are synaptically coupled to the phrenic motor pool (Gonzalez-Rothi EJ and Fuller DD, unpublished observations). Following C2Hx injury, there is a gradual return of serotonergic innervation to ipsilateral phrenic motoneurons (Tai et al. 1997), and the amount of serotonin immunostaining in this region correlates with the extent of phrenic recovery (Golder and Mitchell 2005). As noted above, C4 HF-ES did not increase phasic inspiratory output in the phrenic nerve ipsilateral to C2Hx at 2 wk postinjury, a time point when serotonergic innervation of ipsilateral phrenic nucleus is quite low (Golder and Mitchell 2005). On the other hand, the tonic phrenic response was very strong when HF-ES was given at the 2-wk time point. A similarly robust increase in tonic phrenic activity can be evoked by systemic delivery of the serotonin precursor molecule 5-hydroxytryptophan after both acute and chronic C2Hx injury (Ling et al. 1994; Zhou and Goshgarian 2000). Application of serotonin in an in vitro rat preparation trigger also increases tonic activity to such an extent that it can obscure rhythmic inspiratory bursting in the 4th cervical ventral root (Lindsay and Feldman 1993). The similarity between the impact of pharmacologically driven serotonin receptor activation (Ling et al. 1994; Zhou and Goshgarian 2000) and the response to HF-ES may indicate similar mechanisms are driving the increases in tonic activity.
Another possibility is that short-term potentiation of phrenic output reflected a direct impact of HF-ES on phrenic motoneuron membrane biophysical properties. For example, persistent inward currents (PICs) are possible in phrenic motoneurons, albeit extremely rare (Enríquez Denton et al. 2012). Enriquez Denton and colleagues reported that phrenic motoneurons are immunopositive for voltage-dependent calcium channels (Cav1.3), and through neurophysiological recordings they also identified a phrenic motoneuron with PIC activation. The caveat was that the PIC was only identified following interruption of bulbospinal inputs to the phrenic motoneuron pool (i.e., as occurs for ipsilateral phrenic motoneurons following C2Hx lesion). Most prominently, phrenic motoneurons showed a voltage-dependent amplification of output through NMDA receptor activation (Enríquez Denton et al. 2012). The HF-ES may have directly (or indirectly, e.g., via triggering serotonin release; Capogrosso et al. 2013) triggered motoneuron depolarization and modulation of voltage-dependent synaptic currents, or possibly even PIC activation in these spinally injured animals.
A final consideration is that high-frequency stimulation may have altered inhibitory synaptic inputs to phrenic motoneurons. Studies of deep brain stimulation have reported that stimulation frequencies greater than 50 Hz can suppress neuronal discharge (Lafreniere-Roula et al. 2010), and this inhibition can influence target/downstream neural circuits. In regard to the phrenic motor system, inhibitory inputs to phrenic motoneurons are active across the respiratory cycle (Berger 1979) and play a role in shaping the pattern of respiratory output (Marchenko et al. 2015). Thus it is plausible that HF-ES may have triggered disinhibition of phrenic motoneurons, resulting in an increase in tonic activity throughout the respiratory cycle.
C4 vs. T2 stimulation.
Qualitatively similar potentiation of phrenic motor output occurred regardless of whether HF-ES was applied to the cervical or thoracic spinal cord, particularly after chronic injury. The most likely explanation is that cervical and thoracic stimulation activated the same propriospinal neurons innervating the ipsilateral phrenic motoneuron pool. Decima and colleagues (Decima and von Euler 1969a, 1969b; Decima et al. 1967, 1969) provided evidence that long propriospinal tracts connect thoracic intercostal motoneurons with cervical phrenic motoneurons, and these tracts may be activated by the HF-ES paradigm. Long propriospinal tracts also originate in the lumbar spinal cord, and traverse the thoracic spinal cord to terminate in the cervical region (Dutton et al. 2006). Indeed, in their earlier work, Dimarco and Kowalski (2013b) concluded that long propriospinal tracts located in the lateral funiculus were the likely candidate neuronal pathways mediating respiratory related activation of diaphragm motor units via HF-ES of the thoracic spinal cord.
Conclusion.
We conclude that HF-ES to the ventrolateral spinal cord can potentiate ipsilateral phrenic motor output following incomplete high cervical SCI and that this appears to reflect primarily a local spinal mechanism. Similar phrenic potentiation occurred with stimulation of the midcervical or high thoracic spinal cord, indicating that a highly selective electrode placement is not needed to evoke this response. Short-term potentiation of phrenic bursting illustrates that stimulation of the injured spinal cord can trigger respiratory plasticity, and this potentially could be harnessed in the context of neurorehabilitation (Kasten et al. 2013; McPherson et al. 2015; Mercier et al. 2017). However, the substantial increases in tonic phrenic output evoked by HF-ES in our study indicate that the continuous stimulation paradigm used is unlikely to be useful for respiratory muscle activation after SCI. The increased tonic phrenic discharge highlights the need for future studies of epidural stimulation to carefully evaluate the pattern of diaphragm activation evoked by stimulation. In this regard, lower stimulation frequencies (Gad et al. 2013; Lu et al. 2016; Shah et al. 2016) may be more appropriate for activation of respiratory motor units after incomplete SCI. A recent publication explored the impact of midcervical (C3) epidural stimulation in anesthetized mice by using a relatively high current (1.5 mA) delivered at a rate of 20 Hz (Huang et al. 2016). This low-frequency paradigm did not impact tidal volume but did produce an increase in respiratory rate and also increased the occurrence of spontaneous sighs. However, we are not aware of any published reports regarding the impact of low-frequency epidural stimulation on respiratory outcomes after SCI, in either the acute or chronic setting.
GRANTS
Funding was provided by National Institutes of Health (NIH) Grants 1R01NS080180-01A1 (to D. D. Fuller), 1R01NS054025-06 (to P. J. Reier), Department of Defense Award SC120209 (to P. J. Reier), NIH Training Grant K12HDO55929 (to E. J. Gonzalex-Rothi), NIH Research Supplement to Promote Diversity NS80180 (to E. J. Gonzalez-Rothi), and the State of Florida Brain and Spinal Cord Injury Research Trust Fund (to D. D. Fuller and P. J. Reier), awarded through the McKnight Brain Institute at the University of Florida.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.J.G.-R., D.M.B., and D.D.F. conceived and designed research; E.J.G.-R., K.A.S., and D.M.B. performed experiments; E.J.G.-R., K.A.S., M.H.H., and A.C.S. analyzed data; E.J.G.-R., K.A.S., P.J.R., and D.D.F. interpreted results of experiments; E.J.G.-R. prepared figures; E.J.G.-R. drafted manuscript; E.J.G.-R., K.A.S., P.J.R., D.M.B., and D.D.F. edited and revised manuscript; E.J.G.-R., K.A.S., M.H.H., A.C.S., P.J.R., D.M.B., and D.D.F. approved final version of manuscript.
REFERENCES
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci 28: 11862–11870, 2008. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on α-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393: 69–83, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137: 1394–1409, 2014. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res 194: 227–239, 2011. doi: 10.1016/B978-0-444-53815-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90, 1979. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Roederer E, Cohen MJ. Enhanced spinal cord regeneration in lamprey by applied electric fields. Science 213: 611–617, 1981. doi: 10.1126/science.7256258. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Steinbusch HW, Coulter JD. Serotonergic and peptidergic projections to the spinal cord demonstrated by a combined retrograde HRP histochemical and immunocytochemical staining method. Brain Res 211: 412–417, 1981a. doi: 10.1016/0006-8993(81)90965-3. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res 226: 187–199, 1981b. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Serotonergic projections to the spinal cord from the midbrain in the rat: an immunocytochemical and retrograde transport study. Neurosci Lett 24: 221–226, 1981c. doi: 10.1016/0304-3940(81)90160-9. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, Courtine G, Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33: 19326–19340, 2013. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Excitability of phrenic motoneurones to afferent input from lower intercostal nerves in the spinal cat. Acta Physiol Scand 75: 580–591, 1969a. doi: 10.1111/j.1748-1716.1969.tb04413.x. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Intercostal and cerebellar influences on efferent phrenic activity in the decerebrate cat. Acta Physiol Scand 76: 148–158, 1969b. doi: 10.1111/j.1748-1716.1969.tb04459.x. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature 214: 312–313, 1967. doi: 10.1038/214312a0. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand 75: 568–579, 1969. doi: 10.1111/j.1748-1716.1969.tb04412.x. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol (1985) 107: 662–669, 2009. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir Physiol Neurobiol 171: 218–224, 2010. doi: 10.1016/j.resp.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Distribution of electrical activation to the external intercostal muscles during high frequency spinal cord stimulation in dogs. J Physiol 589: 1383–1395, 2011. doi: 10.1113/jphysiol.2010.199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Activation of inspiratory muscles via spinal cord stimulation. Respir Physiol Neurobiol 189: 438–449, 2013a. doi: 10.1016/j.resp.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Spinal pathways mediating phrenic activation during high frequency spinal cord stimulation. Respir Physiol Neurobiol 186: 1–6, 2013b. doi: 10.1016/j.resp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Electrical activation to the parasternal intercostal muscles during high-frequency spinal cord stimulation in dogs. J Appl Physiol (1985) 118: 148–155, 2015. doi: 10.1152/japplphysiol.01321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT. Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med 37: 380–388, 2014. doi: 10.1179/2045772313Y.0000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Supinski G, Romaniuk JR. Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol (1985) 92: 2341–2346, 2002. doi: 10.1152/japplphysiol.01231.2001. [DOI] [PubMed] [Google Scholar]
- Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir Physiol Neurobiol 162: 160–167, 2008. doi: 10.1016/j.resp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol (1985) 112: 96–105, 2012. doi: 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton RC, Carstens MI, Antognini JF, Carstens E. Long ascending propriospinal projections from lumbosacral to upper cervical spinal cord in the rat. Brain Res 1119: 76–85, 2006. doi: 10.1016/j.brainres.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Enríquez Denton M, Wienecke J, Zhang M, Hultborn H, Kirkwood PA. Voltage-dependent amplification of synaptic inputs in respiratory motoneurones. J Physiol 590: 3067–3090, 2012. doi: 10.1113/jphysiol.2011.225789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE. Restoring motor function with bidirectional neural interfaces. Prog Brain Res 218: 241–252, 2015. doi: 10.1016/bs.pbr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211: 97–106, 2008. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol (1985) 100: 800–806, 2006. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett 323: 25–28, 2002. doi: 10.1016/S0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Mitchell GS. Respiratory neuroplasticity - Overview, significance and future directions. Exp Neurol 287: 144–152, 2017. doi: 10.1016/j.expneurol.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Gad P, Choe J, Shah P, Garcia-Alias G, Rath M, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J Neuroeng Rehabil 10: 108, 2013. doi: 10.1186/1743-0003-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25: 2925–2932, 2005. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol (1985) 94: 795–810, 2003. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol 169: 85–93, 2009. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol 111: 135–139, 1991. doi: 10.1016/0014-4886(91)90061-G. [DOI] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8: e64755, 2013. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma 23: 263–280, 2006. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol (1985) 94: 1421–1430, 2003. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Hormigo KM, Zholudeva LV, Spruance VM, Marchenko V, Cote MP, Vinit S, Giszter S, Bezdudnaya T, Lane MA. Enhancing neural activity to drive respiratory plasticity following cervical spinal cord injury. Exp Neurol 287: 276–287, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Baca SM, Worrell JW, Liu X, Seo Y, Leiter JC, Lu DC. Modulation of respiratory output by cervical epidural stimulation in the anesthetized mouse. J Appl Physiol (1985) 121: 1272–1281, 2016. doi: 10.1152/japplphysiol.00473.2016. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Okragly AJ, Haak-Frendscho M, Mitchell GS. Cervical dorsal rhizotomy increases brain-derived neurotrophic factor and neurotrophin-3 expression in the ventral spinal cord. J Neurosci 20: RC77, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. Activity-dependent plasticity in descending synaptic inputs to respiratory spinal motoneurons. Respir Physiol Neurobiol 131: 79–90, 2002. doi: 10.1016/S1569-9048(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Kasten MR, Sunshine MD, Secrist ES. Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury. J Neural Eng 10: 044001, 2013. doi: 10.1088/1741-2560/10/4/044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci 18: 8436–8443, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski KE, Hsieh YH, Dick TE, DiMarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 247: 689–693, 2013. doi: 10.1016/j.expneurol.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski KE, Romaniuk JR, Brose SW, Richmond MA, Kowalski T, DiMarco AF. High frequency spinal cord stimulation-New method to restore cough. Respir Physiol Neurobiol 232: 54–56, 2016. doi: 10.1016/j.resp.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M, Kim E, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. High-frequency microstimulation in human globus pallidus and substantia nigra. Exp Brain Res 205: 251–261, 2010. doi: 10.1007/s00221-010-2362-8. [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT III, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490: 187–191, 2012. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol 179: 3–13, 2011. doi: 10.1016/j.resp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Hypoxia triggers short term potentiation of phrenic motoneuron discharge after chronic cervical spinal cord injury. Exp Neurol 263: 314–324, 2015. doi: 10.1016/j.expneurol.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Mizrahi J, Susak Z. Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng 12: 150–156, 1990. doi: 10.1016/0141-5425(90)90136-B. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol 461: 213–233, 1993. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res 101: 35–43, 1994. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, Sarino ME, Sarrafzadeh M, Nuwer MR, Roy RR, Gerasimenko Y. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabil Neural Repair 30: 951–962, 2016. doi: 10.1177/1545968316644344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Strey KA, Mitchell GS, Baker-Herman TL. Reduced respiratory neural activity elicits phrenic motor facilitation. Respir Physiol Neurobiol 175: 303–309, 2011. doi: 10.1016/j.resp.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol 247: 101–109, 2013. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko V, Ghali MG, Rogers RF. The role of spinal GABAergic circuits in the control of phrenic nerve motor output. Am J Physiol Regul Integr Comp Physiol 308: R916–R926, 2015. doi: 10.1152/ajpregu.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol 110: 161–176, 1997. doi: 10.1016/S0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- McPherson JG, Miller RR, Perlmutter SI. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc Natl Acad Sci USA 112: 12193–12198, 2015. doi: 10.1073/pnas.1505383112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier LM, Gonzalez-Rothi EJ, Streeter KA, Posgai SS, Poirier AS, Fuller DD, Reier PJ, Baekey DM. Intraspinal microstimulation and diaphragm activation following cervical spinal cord injury. J Neurophysiol 117: 767–776, 2017. doi: 10.1152/jn.00721.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello SE, Kasten MR, Horner PJ, Moritz CT. Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Front Neurosci 8: 21, 2014. doi: 10.3389/fnins.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol 116: 219–228, 1992. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Nantwi K, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair 13: 225–234, 1999. doi: 10.1177/154596839901300404. [DOI] [Google Scholar]
- Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron 80: 1301–1309, 2013. doi: 10.1016/j.neuron.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RF. The respiratory center and its descending pathways. J Comp Neurol 72: 605–625, 1940. doi: 10.1002/cne.900720309. [DOI] [Google Scholar]
- Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One 10: e0133998, 2015. doi: 10.1371/journal.pone.0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejc E, Angeli CA, Bryant N, Harkema S. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma 34: 1787–1802, 2017. doi: 10.1089/neu.2016.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol 169: 94–101, 2009. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237: 103–115, 2012. doi: 10.1016/j.expneurol.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayenko DG, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol 111: 1088–1099, 2014. doi: 10.1152/jn.00489.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayenko DG, Atkinson DA, Floyd TC, Gorodnichev RM, Moshonkina TR, Harkema SJ, Edgerton VR, Gerasimenko YP. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci Lett 609: 229–234, 2015. doi: 10.1016/j.neulet.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Sureddi S, Alam M, Zhong H, Roy RR, Edgerton VR, Gerasimenko Y. Unique spatiotemporal neuromodulation of the lumbosacral circuitry shapes locomotor success after spinal cord injury. J Neurotrauma 33: 1709–1723, 2016. doi: 10.1089/neu.2015.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Tai Q, Palazzolo KL, Goshgarian HG. Synaptic plasticity of 5-hydroxytryptamine-immunoreactive terminals in the phrenic nucleus following spinal cord injury: a quantitative electron microscopic analysis. J Comp Neurol 386: 613–624, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Tator CH, Minassian K, Mushahwar VK. Spinal cord stimulation: therapeutic benefits and movement generation after spinal cord injury. Handb Clin Neurol 109: 283–296, 2012. doi: 10.1016/B978-0-444-52137-8.00018-8. [DOI] [PubMed] [Google Scholar]
- Widge AS, Moritz CT. Pre-frontal control of closed-loop limbic neurostimulation by rodents using a brain-computer interface. J Neural Eng 11: 024001, 2014. doi: 10.1088/1741-2560/11/2/024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol 160: 446–453, 1999. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol (1985) 89: 1528–1536, 2000. [DOI] [PubMed] [Google Scholar]