Arginine Vasopressin, Copeptin, and Renal Function in Diabetes (Responsible Authors: Ronan Roussel, Christopher Taveau, Nadine Bouby)
Arginine Vasopressin and Diabetes
The worldwide prevalence of diabetes mellitus is expected to rise to over 550 million by 2030; this continuous increase being driven by aging and obesity [1]. Diabetes has direct human and monetary costs, but the main burden for patients and health care systems comes from vascular complications of diabetes. If diabetes is well recognized as a risk factor for myocardial infarction, stroke, and peripheral artery disease, it has also to be seen as a major provider of end-stage renal disease (ESRD) [2]. High plasma vasopressin concentrations have been consistently reported in experimental models of diabetes and patients with diabetes, either of type 1 or type 2 [3,4,5]. Interestingly, this was described in patients with uncontrolled diabetes, but also in those with a good glycemic control. However, the study of large populations was problematic due to the low stability of vasopressin, a short peptide, and the limited availability of high-quality assays. A few years ago, an assay for copeptin, the stable C-terminal portion of the precursor of vasopressin and as such a good surrogate for the secretion of vasopressin, was made commercially available [6]. Since then, nearly 300 papers based on copeptin measurements were published. Several works not only confirmed the elevation of copeptin in diabetic patients, but also extended this observation. Actually, plasma copeptin was already elevated in patients with metabolic abnormalities such as those preceding type 2 diabetes (metabolic syndrome) [7]. It has been validated as a prognostic marker of diabetes, independent of other classical risk factors like BMI or age.
Water intake is a major determinant of vasopressin secretion. In the French D.E.S.I.R. study, a cohort recruited in the population, our group found that the risk of new-onset hyperglycemia during a 9-year follow-up was inversely and independently associated with daily water intake [8]. After adjustment for classical confounders and intake of alcoholic beverages and sweet drinks, participants who reported drinking more than 1 l water per day were 21% less likely to develop hyperglycemia than those with a water intake below 0.5 l/day (p = 0.016).
Several lines of evidence support a causal role of vasopressin in these associations. First, in the liver, vasopressin stimulates glycogenolysis and gluconeogenesis through V1a receptor in vitro [9,10,11]. V1b, another G-protein-coupled vasopressin receptor, is expressed in pancreatic Langerhans islets, and perfused pancreas experiments revealed a role of vasopressin in glucagon and insulin secretion [12]. In healthy humans, acute infusion of vasopressin increases plasma glucose, due to an increased glucose production, which is consistent with the expected vasopressin action on the liver [13]. Finally, genetic association studies found a link between vasopressin receptor polymorphisms and metabolic disorders [14].
Diabetic Nephropathy
Diabetic nephropathy accounts for up to 45% of incident cases of ESRD in the USA [2]. Prevention and treatment include blood glucose and blood pressure control, and the use of renin-angiotensin system (RAS) blockers is mandatory when diabetic nephropathy is diagnosed. Despite the best recommended care, a residual risk of development and progression of diabetic nephropathy remains. Once the decline of renal function is established, even with optimal treatment, up to 20% of patients with diabetes and proteinuria develop ESRD within only a 3-year follow-up [15]. Many approaches have been tested in response to the huge unmet need of persistent proteinuria and loss of renal function in diabetic patients despite recommended care, but they failed to demonstrate any favorable risk-to-benefit balance, for example dual blockade of RAS, anti-inflammatory and anti-oxidant therapies, just to name a few recent reports of negative results [16,17]. To explore new therapeutic avenues, physicians need to better understand the pathogenesis of the disease; many decades after the studies of Brenner and co-workers, this is still a relevant area of research.
The pathogenesis of diabetic nephropathy is multifactorial. Hemodynamic and metabolic factors are involved. The role of hyperglycemia is central, of course, but some factors associated with the progress of the disease are shared with other causes of chronic kidney disease (CKD) involving primarily the glomerulus; such factors include hypertension or dyslipidemia. The natural history of diabetic nephropathy has been recently challenged. Classically, it is the sequence of hyperfiltration, albuminuria of increasing amplitude, and then progressive loss of nephrons and glomerular filtration rate (GFR) down to ESRD. The observation of a non-negligible proportion of patients with CKD despite persistent normal or only slightly elevated levels of albuminuria illustrates the actual heterogeneity of the course of nephropathy associated with diabetes.
Hyperfiltration is likely linked to several mechanisms leading to intraglomerular hypertension [18]. Endothelial dysfunction, secondary to hyperglycemia, causes an imbalance between afferent and efferent arterioles, with a net effect of relatively increased efferent arteriole resistance and an increase in glomerular hydrostatic pressure, the main driving force of filtration. Many regulatory systems are involved in renal hemodynamic perturbations related to diabetes, but activation of the intrarenal renin-angiotensin system is considered to be the key event. Moreover, direct toxic effects of glucose on arteriolar endothelium are likely not to be the only determinants of hyperfiltration. The increased amount of glucose filtered, due to the high level of plasma glucose, elevates the reabsorption of glucose in the proximal tubule. This is done through sodium-glucose co-transporters, which are up-regulated and activated in uncontrolled diabetes. Increased proximal reabsorption reduces salt delivery to the macula densa. Via tubuloglomerular feedback, it leads to reduced resistance to blood flow in the afferent arteriole, contributing to increased glomerular pressure.
In addition to these vascular and tubular effects, high plasma glucose induces alterations of the cellular homeostasis, relevant for every cell types in the kidney: oxidative stress, activation of the formation of advanced end products and the expression of their receptors, increased flux through the polyol and hexosamine pathways, activation of protein kinase C isoforms, production of pro-inflammatory cytokines, profibrotic growth factors and vascular growth factors, finally leading to an inflammatory response and functional and structural renal injury with increased leak of albumin, an early landmark of diabetic nephropathy.
Arginine Vasopressin and Albuminuria
Experimental studies in the late 1990s supported a role for vasopressin in diabetic nephropathy. Brattleboro rats are spontaneously deficient in vasopressin, due to a mutation in the vasopressin gene. When treated with streptozotocin, a chemical that is toxic to the insulin-producing beta cells of the pancreas, they develop diabetes, but do not show similar changes in kidney function as controls; they do not hyperfiltrate as diabetic Long-Evans rats do, and they have a much lower urinary albumin excretion rate during the follow-up [19]. In streptozotocin-treated diabetic Wistar rats, albuminuria increase is blocked by a selective vasopressin V2 receptor antagonist [20]. Albuminuria and other markers of renal damages were correlated to the urine-concentrating activity of the kidney. In humans also (a cohort of type 1 patients with diabetes), intra-individual plasma vasopressin changes were shown to be independently associated with variations in GFR [21]. The effect on kidney function of an acute administration of a V2 agonist was tested by Bardoux et al. [19] in healthy humans. The increased V2 receptor-mediated antidiuretic action lead to a reduced urinary flow rate and increased urinary electrolytes concentrations, but also tripled the urinary albumin excretion rate, an effect not observed in patients who were genetically unable to respond to vasopressin receptor stimulation (diabetes insipidus due to aquaporin-2 gene mutations). These results strongly support a direct V2 receptor-mediated role of vasopressin in the development of renal damage. They are extended by several epidemiological studies, including in the specific context of diabetes. In the large cross-sectional population-based PREVEND study [22], the 24-hour urinary albumin excretion was measured, and prevalence of microalbuminuria (increased albuminuria not reaching the proteinuria stage) was positively associated with plasma copeptin concentrations. In a 15-year follow-up study which was also based on the general population [23], baseline copeptin concentration was independently associated with later onset of microalbuminuria. In elderly patients with diabetes, high baseline plasma copeptin was also associated with higher baseline urinary albumin [24]. Moreover, the association of baseline plasma copeptin with progression of albuminuria and decline of estimated GFR was also observed during the 6.5-year follow-up of this study.
Arginine Vasopressin, Glomerular Filtration Decline, and End-Stage Renal Disease in Patients with Diabetes and Proteinuria
Despite this consistent evidence, data were lacking regarding the association of plasma copeptin with the rate of clinically relevant renal events, namely severe decline of GFR or ESRD, and its independency of classical risk factors such as baseline albuminuria and baseline GFR. We examined this association in 3,101 persons with type 2 diabetes and microalbuminuria or macroalbuminuria from the prospective DIABHYCAR study [25], with a 6-year follow-up. We found that plasma copeptin was strongly associated with the rate of these renal events, or with the annual loss of estimated GFR in the whole population, but the association was even stronger in the subset of 729 patients with proteinuria at baseline. The association was independent of relevant covariates such as sex, age, duration of diabetes, blood pressure, and baseline levels of HbA1c, albuminuria, and GFR. There was no interaction with the treatment by renin-angiotensin system blockers. Death was not a competing risk in the association of copeptin levels with renal events. Despite a limited number of occurrences of ESRD (19 patients) during the follow-up, we were able to show that the hazard ratio (adjusted on other prognostic factors) associated with the third tertile (higher values) of baseline copeptin compared to the first tertile was as dramatically elevated as 15.92 (95% CI 3.17-289.23, p = 0.0001). In the multivariate analysis, plasma copeptin and the rate of albuminuria, a well-established renal risk factor, had prognostic values of similar amplitudes.
Conclusion: Blockade of Vasopressin Action as a Therapeutic Perspective
Taken together, these observations raise exciting hypotheses of new therapeutic options in diabetic nephropathy. Plasma copeptin can help target patients with the highest risk of CKD progression, as shown above, but also with a likely high response rate to blockade of vasopressin action. Increasing water intake (or even simply correcting insufficient water intake) may lower vasopressin secretion; such ‘medicinal use of water’ has been discussed for kidney diseases other than diabetic nephropathy elsewhere in this issue. Also, vaptans (vasopressin receptor antagonists) could selectively target the V2 receptor-mediated deleterious effects of vasopressin. Pros and cons of both options are debatable [26]. If the increase of water intake or V2 receptor antagonists were shown to carry a benefit, then this could allow individualization of renoprotective therapy and cover the specific unmet need of patients with diabetes and persistent albuminuria despite renin-angiotensin system blockers. Indeed, these interventions could be used specifically in subjects with high copeptin levels who are likely to benefit the most from the reduction of vasopressin effects. Further clinical studies are needed to test these hypotheses.
Water as Therapy in Kidney Diseases (Responsible Author: Connie J. Wang)
Introduction
Water, long recognized as the element essential for life, has important therapeutic benefits on many organ systems [27], including the kidney. Water's benefit in the prevention of kidney stones has been appreciated for several decades, while in more recent years large observational studies have suggested a therapeutic role in CKD prevention. Additionally, a potential benefit of water in autosomal dominant polycystic kidney disease (ADPKD) has been proposed based on data from in vitro and in vivo studies. This review presents the current state of knowledge regarding water's potential roles in nephrolithiasis, CKD and ADPKD, and addresses the safety concerns associated with increasing water intake.
Relationship between Solute Excretion and Urinary Volume
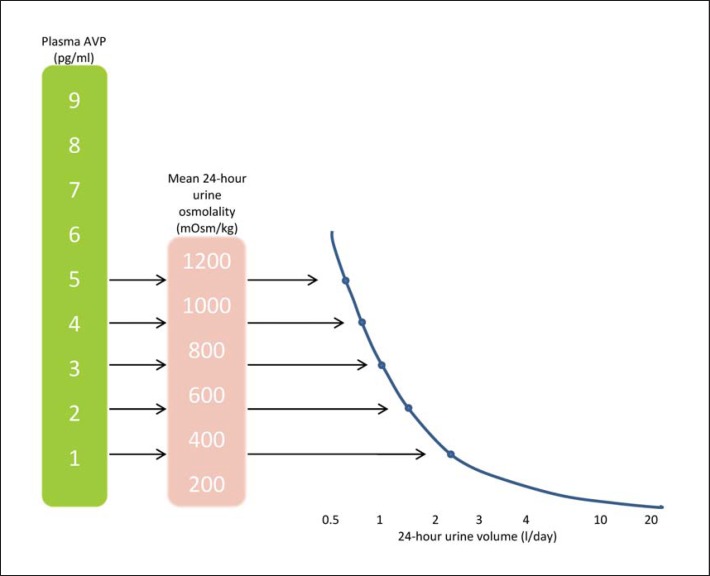
Maintenance of solute and water homeostasis is an essential function of the kidney. The relationship between solute and water excretion can be expressed as:
24-hour osmolar excretion / 24-hour urine volume = mean 24-hour urine osmolality (see also fig. 1).
Fig. 1.
Relationship among plasma AVP, 24-hour urine osmolality and 24-hour urine volume (based on daily osmolar intake at 800 mOsm).
Responding to daily osmolar and water intake, the normal kidney can generate a wide range of urine osmolalities, from 50 mosm/kg at the dilute end to 1,200 mosm/kg at the concentrated end. Urine volume can therefore vary considerably as a result. However, when maximal urine concentrating ability is impaired, as in CKD, the minimal, or obligatory, urine volume must increase in order to avoid solute accumulation.
Therapeutic Roles of Water in Kidney Diseases
Nephrolithiasis
The relationship between water and kidney stones was first surmised in the 1960s, when the incidence of stone formation was observed to be higher among soldiers stationed in areas with less access to water, compared to those working in water-rich areas [28]. Increasing water intake after the first symptomatic stone soon became the standard of care. Four retrospective studies subsequently attempted to systematically investigate the putative benefit of ‘prescribing’ increased water intake in the prevention of stone recurrence (that is, secondary prevention) [29,30,31,32]. Individuals without a recurrence had, on average, a greater increase in urine volume than those with (approximately 0.5 l vs 0.25 l, respectively), suggesting that adherence to increased water intake was beneficial. In a subsequent randomized clinical trial (RCT), Borghi et al. [33] found that urine volume > 2 l/day reduced stone recurrence to 12%, which is significantly lower than the 27% in the control group the mean urine volume of which was 1 l/day (p < 0.0001). Similarly, an increase of urine volume to 2.5 l was found to be associated with a much lower stone recurrence rate compared to no intervention (8.7% vs. 55.0%; p < 0.05) [34].
The benefit of water in primary (as opposed to secondary) stone prevention has been reported in four studies utilizing two large population-based cohorts. The National Health Professionals cohort included > 90,000 men aged 44-69 years who were followed up for up to 12 years [35,36], while the National Nurse Association cohort included > 20,000 women aged 33-60 years followed up for up to 14 years [37,38]. All four studies found that the incidence of stone occurrence was inversely related to urine volume. For instance, the relative risk of stone formation in women with daily urine volume > 2.5 l was reduced to 0.58 compared to those with daily urine volume < 1.3 l [38]; similar findings were demonstrated in men.
Collectively, the evidence suggests that increased water intake is associated with reduced incidence of both initial and subsequent kidney stones.
Chronic Kidney Disease
Whether increased water intake prevents or slows CKD progression remains a topic of great interest. Water intake suppresses plasma levels of arginine vasopressin (AVP), high levels of which have been suggested to play deleterious roles in animal models of kidney disease. Administration of AVP resulted in worsening renal histology and albuminuria in both normal and hypertensive rats [39,40], while pharmacological inhibition of V2 vasopressin receptors yielded clinical and histological improvement [39,41]. Several potential mechanisms have been implicated in mediating the effects of AVP on the kidney, including induction of glomerular hyperfiltration, stimulation of renin secretion, and promotion of mesangial cell proliferation [42].
Several recent observational studies have examined the role of water intake in CKD progression. Strippoli et al. [43] observed a statistically significant trend of lower CKD prevalence in individuals who had higher fluid intake in two Australian cross-sectional cohorts. Fluid intake of ≥3.2 l/day (which defined the highest quartile) was associated with a 50% reduction of CKD prevalence compared to 1.8 l/day (the lowest quartile). Clark et al. [44] found in a prospective Canadian cohort followed up for 6 years that the rate of estimated glomerular filtration rate (eGFR) decline was inversely related to increased 24-hour urine volume. The adjusted odds ratio of developing rapid renal decline, defined as eGFR loss > 5%/year, was 0.46 for individuals with urine volume > 3 l/day compared with the reference group (urine volume 1-1.9 l/day). Most recently, Sontrop et al. [45] conducted a cross-sectional analysis of the 2005-2006 U.S. National Health and Nutrition Examination Survey, and found higher CKD prevalence among those with fluid intake of <2 l/day versus >4.3 l/day (adjusted odds ratio 2.52). Interestingly, it was intake in the form of plain water, rather than from other beverages, that was associated with lower CKD prevalence. Taken together, these three large population-based studies demonstrated the association between high fluid intake and preservation of kidney function, at least among those with generally well-preserved renal function (98.6% with eGFR > 60 ml/min/1.73 m2[44], mean eGFR 87 ml/min/1.73 m2[43], and mean eGFR 95 ml/min/1.73 m2[45]).
These findings are not universal; a smaller (n = 581) retrospective study by Hebert et al. [46] reported a more rapid eGFR decline associated with higher urine volume. However, the statistical significance of the association was lost after adjustment for diuretic intake, suggesting that factors other than water intake may have been responsible for their findings. An additional important caveat of this study is that participants had a low GFR (25-55 ml/min/1.73 m2) at baseline. As renal function declines, concentrating ability of the kidney is impaired, requiring more urine output for a given level of solute excretion. Thus, the high urine volume in this setting may well be a result, rather than a cause, of more rapid CKD progression.
The association between greater water intake and preservation of renal function is bolstered by studies of insufficient water intake. Peraza et al. [47] studied 638 healthy participants in Central America and found that the development of CKD was higher in individuals who worked in hotter environments or who engaged in more strenuous physical activities. The odds ratio of having an elevated serum creatinine concentration was 3.1 for men and 2.3 for women who worked for ≥10 years at a coastal sugarcane or cotton production facility, compared to those who never worked in these strenuous settings. However, as with all observational studies, caution should be exercised regarding these conclusions, as an RCT is required to prove causality.
Autosomal Dominant Polycystic Kidney Disease
ADPKD, the most common genetic renal disease, is characterized by progressive enlargement of cysts that eventually disrupt renal parenchyma. Breakthroughs in molecular and cellular biology have led to a greater understanding of the pathogenesis of cyst formation as well as to the discovery of potential therapeutic approaches to retard these processes. AVP-mediated intracellular cAMP generation has been implicated in cyst growth by both in vivo and in vitro studies [48]. Inhibition of AVP's effect on the kidney, by either pharmacological blockade of V2 receptors or by water intake, has been shown to slow cyst growth and preserve renal function [49,50].
The effects of tolvaptan, a selective V2 receptor antagonist, were first investigated in a single-arm study of ADPKD patients [51]. 21 patients, treated with tolvaptan for 3 years, were compared to 42 historical controls. Tolvaptan use was associated with a slower increase in kidney volume (1.7%/year in tolvaptan vs. 5.8%/year in controls, p < 0.001) and less eGFR decline (−0.71 vs. −2.1 ml/min/1.73 m2 per year; p = 0.01). More definitive evidence comes from the subsequent RCT [24], which enrolled 1,445 ADPKD patients with preserved kidney function (mean eGFR 81 ml/min/1.73 m2) who were followed up for 3 years. Tolvaptan again demonstrated significant benefit in slowing the growth of kidney volume (2.8 vs. 5.5%/year; p < 0.001) and the decline of kidney function (1/serum creatinine annualized rate of −2.61 vs. −3.81 (mg/ml)-1/year; p < 0.001).
In addition to pharmacological blockade of the V2 receptor, inhibition of circulating AVP via increased water intake has the potential to provide a cost-saving alternative, if proven effective. Nagao et al. [49] has shown that polycystic kidney disease rats with high water intake had lower renal weight, smaller renal cyst area, and a lower blood urea nitrogen levels than controls. Data from comparable human studies have not been undertaken, but a pilot study [52] determined the amount of water intake in ADPKD patients that could achieve isosthenuria (thereby strongly inhibiting AVP). An individually prescribed daily fluid intake regimen (based on daily solute excretion and designed to achieve isosthenuria) was well-tolerated, with target urine osmolality achieved in 62.5% of the participants.
Two studies are currently underway to test the efficacy of high water intake in reducing renal cyst growth in ADPKD patients (NCT01348035 and NCT00784030).
Safety and Tolerability of High Water Intake
Plasma AVP can be suppressed to <2 pg/ml, a nominal level [53], when mean 24-hour urine osmolality is diluted to that of plasma (e.g., 285mosm/kg) via water intake (fig. 1). The amount of water sufficient to achieve isosthenuria can then be computed based on daily osmolar intake (estimated as 800 mosm for women and 1,100 mosm for men). Thus, a urine volume of 2.8 l for women and 3.7 l for men – which can be translated into a water intake of 3 l for women and 4 l for men (taking into account insensible losses) – can provide adequate suppression of plasma AVP. In general, this degree of water is likely to be well-tolerated, even by those with advanced age [54] or GFR < 20 ml/min/1.73 m2[55]. Potential dangers do exist, however. Excessive or extremely rapid water intake can result in hyponatremia, hypertension, or volume retention. Patients at particular risk for adverse events are those who have conditions that may impair renal diluting capacity, such as thiazide diuretic ingestion and ultra-low salt restriction.
Conclusions
The role of water in kidney diseases has been increasingly recognized. In nephrolithiasis, dilution likely reduces the saturation of the stone-forming elements, while in CKD and ADPKD, water's inhibitory effect on AVP is the presumptive mechanism. However, lack of direct and definitive evidence for water's therapeutic benefit in humans with CKD or ADPKD calls for future RCTs.
Hydration in Chronic Kidney Disease: Observational Studies Lead to a Randomized Control Trial (Responsible Author: William F. Clark)
Water, Arginine Vasopressin and Chronic Kidney Disease
It is well known that AVP is a potent antidiuretic hormone that regulates thirst and water conservation in man [56,57,58]. Its essential role in water regulation is best known; however, there is a growing body of evidence that chronic increased levels of AVP may have negative effects on kidney function [59,60,61,62,63]. Infusion of this hormone has been shown to increase proteinuria, renal plasma flow, and hyperfiltration (the hallmark of progressive renal injury in all forms of kidney disease) while administration of AVP antagonists reduces proteinuria and lowers blood pressure [59,61,64,65].
Animal studies have shown that the use of an AVP antagonist or increased hydration, which results in suppression of AVP, is associated with a slowing in the progression of renal disease in models of accelerated kidney injury [57,66,67]. Do we have similar evidence in man? Earlier observations in humans have noted that urea clearance increased as urine flow doubled from 1 to 2 ml/min and blood urea nitrogen is lower in patients who on average drink more fluid [68]. Glomerular filtration rate as measured by inulin clearance declines in humans experiencing dehydration [69]. There are recent reports that show a positive association with micro-albuminuria and AVP levels in the general population and evidence in animals and man that AVP infusion causes albuminuria [64,70]. Meijer et al. [71] noted that copeptin, a surrogate marker of AVP, is associated with rapid renal decline in renal transplant recipients. Recently chronic dehydration from heat stress, which is associated with very high levels of vasopressin, was thought to have been the causal factor in a perplexing epidemic of CKD in Central America [72,73]. This highly cited observation has received widespread notice and increased interest in the potential of vasopressin to accelerate kidney injury as well as its potential inhibition by increased hydration with water.
Observational Studies: Water Intake and Chronic Kidney Disease (Retrospective Studies)
There is conflicting evidence in observational studies in man about the potential protective role of increased hydration with water in patients with CKD. Hebert et al. [74] initially noted that increased urine output was associated with a faster renal decline in patients with CKD. This retrospective study reported on 581 patients with CKD of which 440 had polycystic kidney disease. The baseline GFR for the group was 25-55 ml/min. The key outcome measure in this study was the annualized GFR slope in relation to mean 24-hour urine volume. In this population higher urine volume was associated with a faster GFR decline. However, this association was negated when subjects were controlled for diuretic and antihypertensive use. The association may be explained by the need for greater diuretic use resulting in a greater urine output in those with greater kidney dysfunction since this is an observational study and temporality is bi-directional. This study clearly demonstrates the power of selection bias and the need in observational studies to adjust for baseline imbalances.
Prospective Observational Studies
Since these original retrospective observations there have been three prospective larger observational studies that have looked at the effect of increased hydration on kidney function [75,76,77]. The first was a cross-sectional study carried out by Strippoli et al. [75] in Australia and composed two analyses involving adults older than 50 years of age (some individuals in the initial cohort were included in the later cohort) and living in a well-defined suburban area. In this door-to-door census, fluid intake was assessed by a validated nutrition and food frequency questionnaire. The primary outcome was CKD (eGFR < 60 ml/min/1.73 m2). Fluid (total content of fluid and drinks) was stratified in quintiles and association with CKD analyzed by logistic regression expressed as unadjusted and adjusted odds ratios, with testing for linear trends. The proportion of participants who completed the questionnaire and had GFR measured was 2,744/3,654 (75%) for the first and 2,476/3,508 for the second survey. Participants who had the highest quintile (3.2 l/day) had a significantly lower risk of CKD (odds ratio 0.5, 95% CI 0.32-0.77, p for trend = 0.003). These findings were consistent across both study periods, and the authors concluded that a higher fluid intake appeared to protect against CKD. They also suggested that CKD may be preventable at a population level with low cost by increased fluid intake. However the association noted in their cross-sectional observational study, even though adjusted for recognized bias, does not exclude the possibility that it is a result of unknown bias. In other words, due to the cross-sectional nature of their study they could have concluded that people with CKD drink less fluid or healthier people who are less likely to have kidney failure drink more fluid, and all we are noting is selection bias rather than a causal relationship. In recognition of this weakness they did emphasize the need for causal verification from longitudinal observational studies as well as well constructed RCTs to establish the benefits of increased hydration.
We were involved in a prospective 7-year longitudinal community-based cohort study of 2,141 participants who were free of CKD at baseline, provided a 24-hour urinary sample (verified by gender adjusted urinary creatinine measurement), and had their kidney function measured annually over the following 6 years [76]. The decline in kidney function was significantly lower in participants with higher than in those with lower urine volume, and with 0.6 ml/min/1.73 m2 per year the age- and sex-related average decline in the eGFR was slower for those with urine volume greater than 3 l/day compared to those with smaller urine volumes. For each increasing category of 24-hour urine volume (less than 1 l/day, 1-1.9 l/day, 2-2.9 l/day, greater than 3 l/day) percentage annual eGFR decline was progressively slower (1.3%, 1%, 0.8%, 0.5%; p = 0.02). As well, after adjusting for age, gender and baseline cardiovascular disease (CVD), those with the largest urine volume (>3 l/day) were the least likely to demonstrate mild to moderate renal decline, (odds ratio 0.66, 95% CI 0.46-0.94) or rapid renal decline (odds ratio 0.46, 95% CI 0.23-0.92). In this longitudinal multivariant-adjusted study, the demonstration of a significant inverse relationship between urine volume and annual loss of kidney function does suggest causality. However, this association – even after multivariant adjustment – may be the result of unknown selection bias. Thus to confirm a causal relationship between increased hydration and decreased loss of kidney function, a properly constructed RCT is required.
Further evidence of the potential role of increased fluid intake in slowing the progression of renal disease is reported in the recent analysis of NHANES data by Sontrop et al. [77]. This study examined the associations between water intake, CKD and self-reported CVD. The inclusion of CVD was due to the Adventist Health Study that noted that fatal coronary heart disease was significantly lower among participants who drank 5 or more cups of water daily compared with those drinking less than 2 cups daily; however, no association was noted for beverages other than water [78]. Sontrop et al. [77] also examined if these relationships differed for intake of plain water versus other beverages. They carried out a cross-sectional analysis of the 2005-2006 National Health and Examination Survey and included non-pregnant adults with an estimated GFR of greater than 30 ml/min/1.73 m2. The total water intake from food and beverages was categorized as low (less than 2 l/day), moderate (2-4.3 l/day) and high (greater than 4.3 l/day). Participants were excluded if they had kidney cancer, taking lithium or diuretics, or were on dialysis. There were 3,427 adults with a mean age of 46 (range 20-84) years with a mean eGFR of 95 (range 30-161) ml/min/1.73 m2; 13% had CKD, and 18% had CVD. Total water intake was defined as total water from all foods/beverages consumed in the last 24 h. Plain water included water from a tap, drinking fountain, or water cooler, non-carbonated bottled water, or spring water. All analyses were weighted using the NHANES dietary examination sample weights, and logistic regression was used to estimate the odds of CKD or CVD in those with low versus high fluid intake. All analyses, controlled for age and sex models, were tested for potential confounders: ethnicity, education, BMI, smoking, dietary sodium, physical activity, hypertension, diabetes. CKD was defined as an eGFR < 60 ml/min/1.73 m2, and CVD was self-reported physician-diagnosed coronary heart disease, heart attack, stroke, congestive heart failure, or angina pectoris. CKD was higher among those with the lowest (less than 2 l/day) versus the highest total water intake (greater than 4.3 l/day). The adjusted odds ratio was 2.52 with 95% CI of 0.91-6.96. When stratifying the intake of plain water and other beverages, CKD was associated with low intake of plain water(adjusted odds ratio was 2.36 with a 95% CI of 1.10-5.06), but not with other beverages. The authors concluded that even if increased hydration does protect the kidney, any beneficial effect may be offset if total fluid consumption comes from sugar-sweetened beverages. No association was observed between water intake and CVD. The strengths of this study are the large representative community-based sample of >3,000 participants and the objective measure (eGFR not self-reported) of kidney function. This study does suffer all the limitations previously noted for a cross-sectional analysis plus a 24-hour dietary recall that may not capture long-term intake. However, this study provides additional evidence of a potential protective effect of high water intake, particularly plain water, on kidney function.
Pilot Randomized Controlled Trial of Increased Water Intake in Chronic Kidney Disease
It seems plausible, certainly from the basic studies in animals and more recently from observational studies in man, that increased hydration may slow the decline in kidney function in patients with CKD and that marked dehydration may hasten the loss of kidney function. The evidence to date suggests the need for a RCT to examine whether hyperhydration does slow the decline in kidney function in patients with established CKD. Prior to initiating a large RCT, we examined the safety and feasibility of asking adults with CKD to increase their water intake in a pilot study [79]. This pilot study included 29 adults with CKD (eGFR of less than 30-60 ml/min/1.73 m2) and albuminuria (albumin:creatinine ratio of >2.8 mg/mmol for females and >2 mg/mmol for males or a spot urine sample with trace of protein measured by Albustix). Exclusion criteria were as follows: required fluid restriction for kidney disease, heart failure or liver disease, self-reported fluid intake of >10 cups/day or a 24-hour urine volume of >3 l, pregnancy or breastfeeding, kidney transplant recipient, history of kidney stones in the past 5 years, life expectancy < 2 years, serum sodium < 130 mmol/l, serum calcium > 2.6 mmol/l, current taking of lithium or high daily doses of diuretics (hydrochlorothiazide > 25 mg, indapamide > 1.25 mg, furosemide > 40 mg or metolazone >2.5 mg). The 29 eligible adults were randomized in a 2:1 ratio to a hydration (increased hydration, 18 participants) or a control group (normal hydration, 11 participants). The hydration group was asked to increase their water intake by 1-1.5 l/day relative to their weight, gender and 24-hour urine osmolality, in addition to their usually consumed beverages, for a 6-week time period. To encourage adherence to their fluid goals, research personnel maintained regular contact with participants and inquired about regimen tolerance and adherence. Participants collected 24-hour urine samples at baseline as well as 2 weeks and 6 weeks after randomization. Our primary outcome was a between-group difference and a changed 24-hour urine volume from baseline to 6 weeks. 63% of our participants were male; 91% were Caucasian, and the baseline eGFR was 40 ml/min/1.73 m2, with a standard deviation of 11 ml/min/1.73 m2; the median albumin:creatinine ratio was 19 mg/mmol with an interquartile range of 6-74 mg/mmol. Between baseline and 6-week follow-up the hydration group's 24-hour urine volume increased by 0.7 l/day; from 2.3 to 3 l/day, whereas the control group's 24-hour urine volume decreased by 0.3 l/day (2.0 to 1.7 l/day). The between-group difference in change was 0.9 l/day (p = 0.002). We found no significant change in the urine serum osmolality and electrolyte concentration or eGFR, and no serious adverse events were reported or observed. From this pilot study it can be concluded that patients with stage III CKD can successfully and safely increase their water intake by 1-1.5 l/day in addition to their usual fluid intake without any obvious harm. This study has really prepared the grounds for a larger RCT including more than 700 participants with CKD and albuminuria that we have registered at Clinical Trials.gov NCT01766687 and was initiated in June 2013.
Conclusion
There appears to be ample evidence from both animal and more laterally human observational studies to support a RCT to assess the potential of hyperhydration in slowing the progression of CKD. A recent pilot study [79] suggests that it is feasible to carry out such an RCT. In this study the effects of increased hydration (plus 1-1.5 l/day relative to their gender and body weight) will be tested against normal hydration in patients with CKD and albuminuria to clarify if there are any beneficial effects in these patients.
Disclosure Statement
NB and CT did not provide a disclosure statement.
WFC: Honoraria: Canadian Society of Nephrology, American Society of Nephrology, Alexion and Danone Research. Grants: Canadian Institute of Health Research, Kidney Foundation of Canada and Danone Research. Consultant Danone Research.
RR: Research grants, travel or congress grants, and board/lecture honoraria from Astra-Zeneca, Boehringer Ingelheim, sanofi-aventis, MSD Chibret, Servier, Roche, Eli Lilly, Novartis, Danone Research, Novo Nordisk, and Johnson & Johnson.
CJW has nothing to disclose.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Williams ME. Diabetic CKD/ESRD 2010: a progress report? Semin Dial. 2010;23:129–133. doi: 10.1111/j.1525-139X.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 3.Zerbe RL, Vinicor F, Robertson GL. Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes. 1979;28:503–508. doi: 10.2337/diab.28.5.503. [DOI] [PubMed] [Google Scholar]
- 4.Zerbe RL, Vinicor F, Robertson GL. Regulation of plasma vasopressin in insulin-dependent diabetes mellitus. Am J Physiol. 1985;249:E317–E325. doi: 10.1152/ajpendo.1985.249.3.E317. [DOI] [PubMed] [Google Scholar]
- 5.Bankir L, Bardoux P, Ahloulay M. Vasopressin and diabetes mellitus. Nephron. 2001;87:8–18. doi: 10.1159/000045879. [DOI] [PubMed] [Google Scholar]
- 6.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 7.Enhörning S, Struck J, Wirfält E, Hedblad B, Morgenthaler NG, Melander O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1065–E1072. doi: 10.1210/jc.2010-2981. [DOI] [PubMed] [Google Scholar]
- 8.Roussel R, Fezeu L, Bouby N, Balkau B, Lantieri O, Alhenc-Gelas F, Marre M, Bankir L, D.E.S.I.R. Study Group Low water intake and risk for new-onset hyperglycemia. Diabetes Care. 2011;34:2551–2554. doi: 10.2337/dc11-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hems DA, Whitton PD. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973;136:705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin G, Baverel G. Vasopressin promotes the metabolism of near-physiological concentration of glutamine in isolated rat liver cells. Biosci Rep. 1984;4:171–176. doi: 10.1007/BF01120314. [DOI] [PubMed] [Google Scholar]
- 11.Whitton PD, Rodrigues LM, Hems DA. Stimulation by vasopressin, angiotensin and oxytocin of gluconeogenesis in hepatocyte suspensions. Biochem J. 1978;176:893–898. doi: 10.1042/bj1760893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Basha EA, Yibchok-Anun S, Hsu WH. Glucose dependency of arginine vasopressin-induced insulin and glucagon release from the perfused rat pancreas. Metabolism. 2002;51:1184–1190. doi: 10.1053/meta.2002.34052. [DOI] [PubMed] [Google Scholar]
- 13.Spruce BA, McCulloch AJ, Burd J, Orskov H, Heaton A, Baylis PH, Alberti KG. The effect of vasopressin infusion on glucose metabolism in man. Clin Endocrinol (Oxf) 1985;22:463–468. doi: 10.1111/j.1365-2265.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 14.Enhörning S, Leosdottir M, Wallström P, Gullberg B, Berglund G, Wirfält E, Melander O. Relation between human vasopressin 1a gene variance, fat intake, and diabetes. Am J Clin Nutr. 2009;89:400–406. doi: 10.3945/ajcn.2008.26382. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 16.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA NEPHRON-D Investigators Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 17.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, BEACON Trial Investigators Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–1972. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- 19.Bardoux P, Martin H, Ahloulay M, Schmitt F, Bouby N, Trinh-Trang-Tan MM, Bankir L. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressindeficient Brattleboro rats. Proc Natl Acad Sci U S A. 1999;96:10397–10402. doi: 10.1073/pnas.96.18.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the reninangiotensin system. Nephrol Dial Transplant. 2003;18:497–506. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen MM, Christiansen JS, Pedersen EB, Mogensen CE. Determinants of intra-individual variation in kidney function in normoalbuminuric insulin-dependent diabetic patients: importance of atrial natriuretic peptide and glycaemic control. Clin Sci (Lond) 1992;83:445–451. doi: 10.1042/cs0830445. [DOI] [PubMed] [Google Scholar]
- 22.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010;77:29–36. doi: 10.1038/ki.2009.397. [DOI] [PubMed] [Google Scholar]
- 23.Enhörning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, Morgenthaler NG, Nilsson PM, Melander O. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: the prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes (Lond) 2013;37:598–603. doi: 10.1038/ijo.2012.88. [DOI] [PubMed] [Google Scholar]
- 24.Boertien WE, Riphagen IJ, Drion I, Alkhalaf A, Bakker SJ, Groenier KH, Struck J, de Jong PE, Bilo HJ, Kleefstra N, Gansevoort RT. Copeptin, a surrogate marker for arginine vasopressin, is associated with declining glomerular filtration in patients with diabetes mellitus (ZODIAC-33) Diabetologia. 2013;56:1680–1688. doi: 10.1007/s00125-013-2922-0. [DOI] [PubMed] [Google Scholar]
- 25.Velho G, Bouby N, Hadjadj S, Matallah N, Mohammedi K, Fumeron F, Potier L, Bellili-Munoz N, Taveau C, Alhenc-Gelas F, Bankir L, Marre M, Roussel R. Plasma copeptin and renal outcomes in patients with type 2 diabetes and albuminuria. Diabetes Care. 2013;36:3639–3645. doi: 10.2337/dc13-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9:223–239. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 27.Kleiner SM. Water: an essential but overlooked nutrient. J Am Diet Assoc. 1999;99:200–206. doi: 10.1016/S0002-8223(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 28.Frank M, De Vries A, Atsmon A, Lazebnik J, Kochwa S. Epidemiological investigation of urolithiasis in Israel. J Urol. 1959;81:497–505. doi: 10.1016/S0022-5347(17)66053-1. [DOI] [PubMed] [Google Scholar]
- 29.Strauss AL, Coe FL, Deutsch L, Parks JH. Factors that predict relapse of calcium nephrolithiasis during treatment: a prospective study. Am J Med. 1982;72:17–24. doi: 10.1016/0002-9343(82)90566-6. [DOI] [PubMed] [Google Scholar]
- 30.Hosking DH, Erickson SB, Van den Berg CJ, Wilson DM, Smith LH. The stone clinic effect in patients with idiopathic calcium urolithiasis. J Urol. 1983;130:1115–1118. doi: 10.1016/s0022-5347(17)51711-5. [DOI] [PubMed] [Google Scholar]
- 31.Embon OM, Rose GA, Rosenbaum T. Chronic dehydration stone disease. Br J Urol. 1990;66:357–362. doi: 10.1111/j.1464-410x.1990.tb14954.x. [DOI] [PubMed] [Google Scholar]
- 32.Daudon M, Hennequin C, Boujelben G, Lacour B, Jungers P. Serial crystalluria determination and the risk of recurrence in calcium stone formers. Kidney Int. 2005;67:1934–1943. doi: 10.1111/j.1523-1755.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 33.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–843. [PubMed] [Google Scholar]
- 34.Sarica K, Inal Y, Erturhan S, Yagci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res. 2006;34:184–189. doi: 10.1007/s00240-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 35.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 37.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Arch Intern Med. 2004;164:885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 38.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568–1573. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 39.Naito A, Hasegawa H, Kurasawa T, Ohtake Y, Matsukawa H, Ezure Y, Koike K, Shigenobu K. Histopathological study of kidney abnormalities in an experimental SIADH rat model and its application to the evaluation of the pharmacologic profile of VP-343, a selective vasopressin V2 receptor antagonist. Biol Pharm Bull. 2001;24:897–901. doi: 10.1248/bpb.24.897. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes S, Bruneval P, Hagege A, Heudes D, Ghostine S, Bouby N. Chronic V2 vasopressin receptor stimulation increases basal blood pressure and exacerbates deoxycorticosterone acetate-salt hypertension. Endocrinology. 2002;143:2759–2766. doi: 10.1210/endo.143.7.8918. [DOI] [PubMed] [Google Scholar]
- 41.Perico N, Zoja C, Corna D, Rottoli D, Gaspari F, Haskell L, Remuzzi G. V1/V2 vasopressin receptor antagonism potentiates the renoprotection of renin-angiotensin system inhibition in rats with renal mass reduction. Kidney Int. 2009;76:960–967. doi: 10.1038/ki.2009.267. [DOI] [PubMed] [Google Scholar]
- 42.Bolignano D, Zoccali C. Vasopressin beyond water: implications for renal diseases. Curr Opin Nephrol Hypertens. 2010;19:499–504. doi: 10.1097/MNH.0b013e32833d35cf. [DOI] [PubMed] [Google Scholar]
- 43.Strippoli GF, Craig JC, Rochtchina E, Flood VM, Wang JJ, Mitchell P. Fluid and nutrient intake and risk of chronic kidney disease. Nephrology (Carlton) 2011;16:326–334. doi: 10.1111/j.1440-1797.2010.01415.x. [DOI] [PubMed] [Google Scholar]
- 44.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX. Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol. 2011;6:2634–2641. doi: 10.2215/CJN.01990211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sontrop JM, Dixon SN, Garg AX, Buendia-Jimenez I, Dohein O, Huang SH, Clark WF. Association between water intake, chronic kidney disease, and cardiovascular disease: a cross-sectional analysis of NHANES data. Am J Nephrol. 2013;37:434–442. doi: 10.1159/000350377. [DOI] [PubMed] [Google Scholar]
- 46.Hebert LA, Greene T, Levey A, Falkenhain ME, Klahr S. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 47.Peraza S, Wesseling C, Aragon A, Leiva R, Garcia-Trabanino RA, Torres C, Jakobsson K, Elinder CG, Hogstedt C. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Torres VE. Vasopressin in chronic kidney disease: AN elephant in the room? Kidney Int. 2009;76:925–928. doi: 10.1038/ki.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17:2220–2227. doi: 10.1681/ASN.2006030251. [DOI] [PubMed] [Google Scholar]
- 50.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, Nutahara K, Ouyang J, Krasa HB, Czerwiec FS. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. Clin J Am Soc Nephrol. 2011;6:2499–2507. doi: 10.2215/CJN.03530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6:192–197. doi: 10.2215/CJN.03950510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson AG. Disorders of antidiuretic hormone secretion. Clin Endocrinol Metab. 1985;14:55–88. doi: 10.1016/s0300-595x(85)80065-7. [DOI] [PubMed] [Google Scholar]
- 54.Spigt MG, Knottnerus JA, Westerterp KR, Olde Rikkert MG, Schayck CP. The effects of 6 months of increased water intake on blood sodium, glomerular filtration rate, blood pressure, and quality of life in elderly (aged 55-75) men. J Am Geriatr Soc. 2006;54:438–443. doi: 10.1111/j.1532-5415.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 55.Passfall J, Pai J, Spies KP, Haller H, Luft FC. Effect of water and bicarbonate loading in patients with chronic renal failure. Clin Nephrol. 1997;47:92–98. [PubMed] [Google Scholar]
- 56.Shore AC, Markandu ND, Sagnella GA, Singer DR, Forsling ML, Buckley MG, Sugden AL, MacGregor GA. Endocrine and renal response to water loading and water restriction in normal man. Clin Sci (Lond) 1988;75:171–177. doi: 10.1042/cs0750171. [DOI] [PubMed] [Google Scholar]
- 57.Bouby N, Bachmann S, Bichet D, Bankir L. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol. 1990;258:F973. doi: 10.1152/ajprenal.1990.258.4.F973. [DOI] [PubMed] [Google Scholar]
- 58.May M, Jordan J. The osmopressor response to water drinking. Am J Physiol Regul Integr Comp Physiol. 2011;300:R40–R46. doi: 10.1152/ajpregu.00544.2010. [DOI] [PubMed] [Google Scholar]
- 59.Torres VE. Vasopressin in chronic kidney disease: an elephant in the room? Kidney Int. 2009;76:925–928. doi: 10.1038/ki.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards RM, Trizna W, Kinter LB. Renal microvascular effects of vasopressin and vasopressin antagonists. Am J Physiol. 1989;256:F274–F278. doi: 10.1152/ajprenal.1989.256.2.F274. [DOI] [PubMed] [Google Scholar]
- 61.Perico N, Zoja C, Corna D, Rottoli D, Gaspari F, Haskell L, Remuzzi G. V1/V2 vasopressin receptor antagonism potentiates the renoprotection of renin-angiotensin system inhibition in rats with renal mass reduction. Kidney Int. 2009;76:960–967. doi: 10.1038/ki.2009.267. [DOI] [PubMed] [Google Scholar]
- 62.Luft FC. Vasopressin, urine concentration, and hypertension: a new perspective on an old story. Clin J Am Soc Nephrol. 2007;2:196–197. doi: 10.2215/CJN.04161206. [DOI] [PubMed] [Google Scholar]
- 63.Cirillo M. Determinants of kidney dysfunction: is vasopressin a new player in the arena? Kidney Int. 2010;77:5–6. doi: 10.1038/ki.2009.408. [DOI] [PubMed] [Google Scholar]
- 64.Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the reninangiotensin system. Nephrol Dial Transplant. 2003;18:497–506. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 65.Bolignano D, Zoccali C. Vasopressin beyond water: implications for renal diseases. Curr Opin Nephrol Hypertens. 2010;19:499–504. doi: 10.1097/MNH.0b013e32833d35cf. [DOI] [PubMed] [Google Scholar]
- 66.Bouby N, Hassler C, Bankir L. Contribution of vasopressin to progression of chronic renal failure: study in Brattleboro rats. Life Sci. 1999;65:991–1004. doi: 10.1016/s0024-3205(99)00330-6. [DOI] [PubMed] [Google Scholar]
- 67.Okada H, Suzuki H, Kanno Y, Yamamura Y, Saruta T. Effects of vasopressin V1 and V2 receptor antagonists on progressive renal failure in rats. Clin Sci. 1994;86:399–404. doi: 10.1042/cs0860399. [DOI] [PubMed] [Google Scholar]
- 68.Anastasio P, Cirillo M, Spitali L, Frangiosa A, Pollastro RM, De Santo NG. Level of hydration and renal function in healthy humans. Kidney Int. 2001;60:748–756. doi: 10.1046/j.1523-1755.2001.060002748.x. [DOI] [PubMed] [Google Scholar]
- 69.McCance RA, Young WF, Black DA. The secretion of urine during dehydration and rehydration. J Physiol. 1944;102:415–428. doi: 10.1113/jphysiol.1944.sp004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meijer E, Bakker SJL, Halbesma N, deJong PE, Struck J, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010;77:29–36. doi: 10.1038/ki.2009.397. [DOI] [PubMed] [Google Scholar]
- 71.Meijer E, Bakker SJ, deJong PE, Homan van der Heide JJ, von Son WJ, Struck J, Lems SP, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with accelerated renal function decline in renal transplant recipients. Transplantation. 2009;88:561–567. doi: 10.1097/TP.0b013e3181b11ae4. [DOI] [PubMed] [Google Scholar]
- 72.Brooks DR, Ramirez-Rubino O, Amador JJ. CKD in Central America: a hot issue. Am J Kidney Dis. 2012;59:481–484. doi: 10.1053/j.ajkd.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Peraza S, Wesseling C, Aragon A, Leiva R, Garcia-Trabanino RA, Torres C, Jakobsson K, Elinder CG, Hogstedt C. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 74.Hebert LA, Greene T, Levey A, Falkenstein ME, Klahr S. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 75.Strippoli GF, Craig JC, Rochchina E, Flood VM, Wang JJ, Mitchell P. Fluid and nutrient intake and risk of chronic kidney disease. Nephrology. 2011;16:326–334. doi: 10.1111/j.1440-1797.2010.01415.x. [DOI] [PubMed] [Google Scholar]
- 76.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX. Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol. 2011;6:2634–2641. doi: 10.2215/CJN.01990211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sontrop JM, Dixon SN, Garg AX, Buendia-Jimenez I, Dohein O, Huang SH, Clark WF. Association between water intake, chronic kidney disease, and cardiovascular disease: a cross-sectional analysis of NHANES data. Am J Nephrol. 2013;37:434–442. doi: 10.1159/000350377. [DOI] [PubMed] [Google Scholar]
- 78.Chan J, Knutsen SF, Blix GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease. Am J Epidemiol. 2002;155:827–833. doi: 10.1093/aje/155.9.827. [DOI] [PubMed] [Google Scholar]
- 79.Clark WF, Sontrop JM, Huang S-H, Gallo K, Moist L, House AA, Weir MA, Garg AX. The chronic kidney disease Water Intake Trial (WIT): results from the pilot randomized controlled trial. BMJ Open. 2013;3:e003666. doi: 10.1136/bmjopen-2013-003666. [DOI] [PMC free article] [PubMed] [Google Scholar]