Abstract
In a 10-year retrospective study we assessed the epidemiology of candidemia and the association between the presence and removal of indwelling central venous catheters, antifungal use and clinical outcomes among hospitalised children. Demographic and clinical information were retrieved from the electronic medical records. One hundred six episodes of candidemia were identified in 83 unique patients. Candida parapsilosis was the most prevalent (52%) species, followed by C. albicans (25%). Non-oncologic children receiving fluconazole within 30 days of developing candidemia were most likely to develop C. parapsilosis infection (40%, P = 0.006), independent of total parenteral nutrition (odds ratio (OR) 2.5, 95% confidence interval (CI): 0.6–11, P = 0.3). Crude mortality rate was 12% and significantly higher for children less than 2 years (OR: 6.7, 95% CI: 1.9–23, P = 0.003), and those infected with C. lusitaniae (OR: 9, 95% CI: 1.6–51, P = 0.02). The aggregate use of antifungal agents decreased overtime (χ2: 16.7, P < 0.0001). Fluconazole remained the most common antifungal agent used during the study.
Keywords: Candida spp, candidemia, paediatrics
Background
Over the past two decades, the incidence of bloodstream candidiasis among hospitalised patients has continued to increase world-wide.1 In the United States, candidemia is the fourth most common nosocomial bloodstream infection.1,2 Among adult patients, Candida albicans remains the most common species, despite the increased use of fluconazole. Among hospitalised children, some reports have noted a proportional increased in candidemia caused by non-Candida albicans species, especially C. parapsilosis, and independent of the underlying comorbidity.3 Nevertheless, rates of candidemia remained stable, possible due to an increased used in fluconazole, either as prophylaxis or empiric therapy.3
The aim of this study was to describe the epidemiology of candidemia among hospitalised paediatric patients over a 10-year period and the association between with the use of fluconazole within 30 days of developing infection and the aggregate use of antifungal agents at a single paediatric teaching institution.
Methods
Settings and study design
In a retrospective epidemiologic study, we evaluated children less than 21 years of age with candidemia admitted to Alfred I. duPont Hospital for Children (AIDHC) in Wilmington, DE, from 1 January 2000 through 31 December 2009. AIDHC is a 180-bed tertiary care paediatric teaching hospital affiliated with Thomas Jefferson University in Philadelphia, PA.4 The Nemours Institutional Review Board approved the study.
Definitions and data collection
An episode of candidemia was defined as the recovery of any Candida species from a blood culture. We identified episodes of candidemia using the computerised clinical microbiology laboratory database (Misys, Misys Healthcare System, Inc., Raleigh, NC, USA). Patients from whom the same Candida species was recovered on multiple consecutive days were counted as a single event for study purposes. Subjects with candidemia due to the same species that elapsed for four or more weeks (with negative blood culture between episodes) were counted as unique episodes. Electronic medical records of all candidemia episodes were reviewed by research team members. Data were collected on patient demographic characteristics, underlying diagnosis, days of candidemia, presence and removal of central venous catheter (CVC), antifungal therapy, use of antifungal agents before and after infection and 30-day crude mortality data. Duration of candidemia was determined based on days of positive blood cultures. Subjects were categorised into two subgroups based on their comorbid conditions. Oncologic patients included subjects with diagnosis of haematologic malignancies, solid organ malignancies and/or stem cell transplantation, whereas non-oncologic patients included all others (medically complex patients including those with gastrointestinal disorders). Rates of candidemia were calculated as the number of episodes per 1000 hospital admissions. Crude mortality was defined as death regardless of cause that occurred within 30 days of candidemia episode.
An antimicrobial stewardship programme (ASP) was implemented at AIDHC in 2004 as previously described.4 Targeted antifungal agents subject to prospective audit included fluconazole, voriconazole and liposomal formulations of amphotericin B.4 Briefly, clinical and laboratory data of patients receiving targeted therapies were reviewed by the infectious disease pharmacist with the ASP medical director. Feedback, including alternative antifungal therapy and dosing recommendations, was performed through direct one-on-one communication with the prescribing physician when indicated.4 The use of antifungal agents within 30 days preceding the first positive culture was recorded for every individual patient. Antifungal treatment was defined as antifungal agents given to the patient within 14 days of the positive blood cultures. Aggregate use of antifungal agents was calculated based on number of doses-administered normalised by 1000 patients-days as previously described.4–7
Quantitative variables are summarised by mean (standard deviation (SD) ) and ranges. Categorical variables are summarised using frequencies and percentages. Univariable logistic regression was performed to detect the association between outcome variables with each of the predictors. Multivariable logistic regression was performed to detect the association between mortality and duration of candidemia after adjustment for the presence and removal of CVC. Odds ratio, along with P-value or 95% CI, is presented. A χ2 trend test for proportion was used to test the trend in antifungal use and rates of candidemia overtime. All tests were two-tailed at 5% level of significance. Analyses were performed using IBM SPSS software (Version 22, IBM Corp., Chicago, IL, USA) and R 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
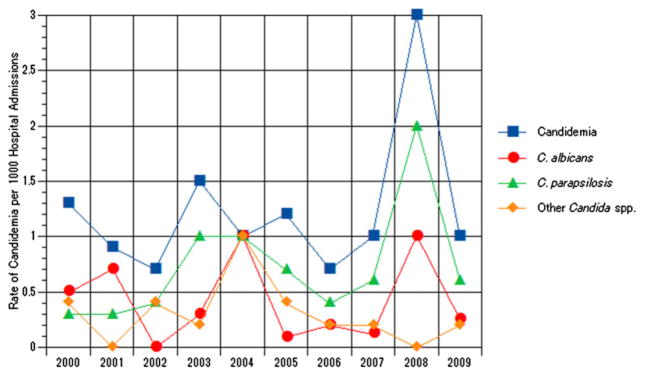
Candidemia was identified in 83 unique patients who developed 106 episodes of candidemia between 1 January 2000 and 31 December 2009. As shown in Figure 1, the overall rate of candidemia (χ2: 0.35; P = 0.6), C. albicans (χ2: 0.03; P = 0.8), C. parapsilosis (χ2: 0.84; P = 0.3) and other Candida spp. (χ2: 0.19; P = 0.7) bloodstream infections per 1000 hospitalisations did not change significantly overtime. Table 1 depicts the demographic characteristics of children with candidemia. The majority of the non-oncologic patients were total parenteral nutrition (TPN) dependent (59%). Additional comorbid conditions in this patient population included congenital heart disease (13%), neurological (13%) and genetic (4%) disorders, prematurity (3%), respiratory distress (2%), and other, for example trauma, biliary atresia and complicated appendicitis (6%). The median time of duration of candidemia for both cohorts was 2.5 days (mean 3.2, range: 1–19) (Table 2). The duration of candidemia did not correlate with underlying diagnosis (r2 = 0.24; P = 0.79).
Fig. 1.
Rates of candidemia per 1000 hospitalisations, Alfred I. duPont Hospital for Children, 2000–2009.
 , Candidemia;
, Candidemia;
 , C. albicans;
, C. albicans;
 , C. parapsilosis;
, C. parapsilosis;
 , other Candida spp.
, other Candida spp.
Table 1.
Demographic characteristics of children with Candidemia, Alfred I. duPont Hospital for Children, 2000–2009
| Cohort n = 106 |
Oncologic n = 14 (13.2%) |
Non-oncologic n = 92 (86.8%) |
|
|---|---|---|---|
| Age: | |||
| Mean (±SD) | 6.3 (±6.1) years | 4.7 (±3.7) years | 6.5 (±6.3) years |
| Range | 7 days–20 years | 1–13 years | 7 days–20 years |
| Age groups | |||
| Neonates | 3 (3%) | – | 3 (3.3%) |
| >1 month–2 years | 31 (29%) | 4 (29%) | 27 (29.4%) |
| >2–15 years | 57 (54%) | 10 (71%) | 47 (51%) |
| >15 years | 15 (14%) | – | 15 (16.3%) |
| Gender (male) | 66 (62.3%) | 7 (50%) | 59 (64.2%) |
| Ethnicity | |||
| White | 60 (57%) | 7 (50%) | 53 (57.6%) |
| African American | 9 (8%) | 2 (14%) | 7 (7.6%) |
| Hispanic | 16 (15%) | 3 (22%) | 12 (14.1%) |
| Unknown | 21 (20%) | 2 (14%) | 19 (20.7%) |
Table 2.
Clinical characteristics and Candida spp. crude mortality rate
| Cohort n = 106 |
Oncologic n = 14 (13.2%) |
Non-oncologic n = 92 (86.8%) |
|
|---|---|---|---|
| Central venous catheter | 102 (96.2%) | 14 (100%) | 88 (96%) |
| Days of candidemia mean (range) | 3.2 (1–19) | 2.6 (1–11) | 3.3 (1–19) |
| Crude mortality rate | 13 (12.3%) | 0 | 13 (14.1%) |
| C. albicans | 5 (18.5%) | 5 (18.5%) | |
| C. parapsilosis | 3 (5.4%) | 3 (5.4%) | |
| C. lusitaniae | 3 (50%) | 3 (50%) | |
| C. krusei | 1 (33%) | 1 (33%) | |
| C. tropicalis | 1 (1.5) | 1 (1.5) | |
| C. glabrata | 0 | 0 |
CVC were present in 102 (96.2 %) episodes at the time of positive blood culture (Table 2). Removal of the CVC was documented in 93% (95 of 102) of these episodes. No significant differences were noted in rates of catheter removal between oncology (86%; 12 of 14) and non-oncologic patients (94%; 83 of 88) (χ2: 1.3, P = 0.27). Catheter removal did not reduce the days to mycologic eradication to <5 days (odds ratio (OR) = 1.280, 95% confidence interval (CI) 0.133–12.121, P = 0.830), or mortality (OR = 0.3, 95% CI 0.033–3.5, P = 0.41).
Throughout the study period, C. parapsilosis (52%) was the predominant species, followed by C. albicans (25%). Other species causing candidemia included C. tropicales (7.5%), C. glabrata (6.6%), C. lusitaniae (5.6%) and C. krusei (2.8%). After controlling for CVC removal, patients infected with C. parapsilosis were more prone to develop prolonged candidemia (≥5 days) when compared with patients infected with C. albicans (OR: 4.22, 95% CI: 0.491–36.309, P = 0.19), though this was not statistically significant. For all other Candida species, days of candidemia remained similar (P = 0.3).
Thirty-eight patients (36%) received fluconazole within 30 days of developing candidemia. C. parapsilosis was recovered in 79% (30 of 38) (OR: 1.5; 95% CI 0.6–3.7; P = 0.5) of these patients. C. parapsilosis candidemia was more common among non-oncology patients receiving fluconazole (40%; P = 0.006) but was independent of TPN (OR: 2.5; 95% CI: 0.6–11; P = 0.3). Mortality risk was not statistically different for patients receiving fluconazole within 30 days of developing candidemia (OR: 0.5; 95% CI 0.1–2; P = 0.4).
Antifungal therapy was prescribed in 97% (103) of patients who experienced candidemia (Table 3). Non-oncologic children receiving voriconazole had the highest mortality rate (OR: 14.8; 95% CI 2.1–101; P = 0.01). Three patients did not receive antifungal therapy; two oncology patients (solid-organ tumours) infected with C. parapsilosis, which resolved after CVL removal and a teenage girl (with Sturge-Weber) with a postmortem diagnosis of C. albicans candidemia. Although not statistically significant, the crude mortality risk was higher in patients who did not receive antifungal therapy (OR: 3.8; 95% CI 0.3–45; P = 0.3).
Table 3.
Antifungal therapy administered and 30-day crude mortality rate in paediatric patients with candidemia, Alfred I. duPont Hospital for Children, 2000–2009
| Cohort n = 106 |
Oncologic n = 14 (13.2%) |
Non-oncologic n = 92 (86.8%) |
Crude mortality n (%) |
|
|---|---|---|---|---|
| Any antifungal agent | 103 (97%) | 12 (86%) | 91 (99%) | 12 (12%) |
| Fluconazole | 47 (46%) | 5 (35.7%) | 42 (46%) | 3 (6%) |
| Amphotericin B | 16 (15%) | 2 (14%) | 14 (15%) | 2 (13%) |
| Voriconazole | 5 (5%) | 1 | 4 | 3 (60%) |
|
| ||||
| Combination therapy | ||||
|
| ||||
| Fluconazole – echinocandins | 4 (4%) | – | 4 | – |
| Fluconazole – Amphotericin B | 31 (29%) | 4 (29%) | 27 (29%) | 4 (13%) |
From 2001 to 2005, the aggregate use of antifungal agents steadily increased from 65 doses administered/1000 patients-days/year to 202 doses administered/1000 patients-days/year. By the end of the study and after the implementation of prospective audit and feedback to providers in 2005, antifungal use decreased to 120 doses administered/1000 patients-days/year (χ2: 16.7; P < 0.0001).
Fluconazole remained the most commonly used antifungal agent throughout the 10 years of the study. Fluconazole use peaked in 2004–2005 at 202 doses administered/1000 patients-days/year and declined to 73 doses administered/1000 patients-days/year in 2009–2010 (χ2: 45; P < 0.0001). Voriconazole was added to the formulary in 2004, and its use increased overtime to 20 doses administered/1000 patients-days/year (χ2: 5.4; P = 0.02). Posaconazole use was insignificant through the study period. Liposomal amphotericin B formulations use decreased over time from 14 doses administered/1000 patients-days/year in 2003 to 2.4 doses administered/1000 patients-days/year in 2009–2010 (χ2: 5.98, P = 0.01). Echinocandins were introduced in 2008, and its use did not change significantly in the subsequent two years (χ2: 1.2, P = 0.2).
Among all study subjects, the 30-day crude mortality rate was 12.3% (Table 2). Independent of their underlying condition, children younger than 2 years of age had a higher mortality risk than older children (OR = 6.7, 95% CI 1.9–23, P = 0.003). Table 2 depicts the crude mortality rate for all Candida spp. based on patient’s comorbidities. Children with C. lusitaniae candidemia experienced the higher mortality rates, 50% (3 of 6) when compared with other Candida spp. (OR = 9, 95% CI 1.6–51, P = 0.02). In our cohort, the crude mortality rate associated with C. krusei was 33% (1 of 3) but not significantly higher when compared with other Candida spp. (OR = 3.7, 95% CI 0.3–45, P = 0.3). The mortality risk for patients infected with C. albicans was higher than for those infected with C. parapsilosis, though this finding was not statistically significant (OR = 3.9, 95% CI, 0.9–18, P = 0.1). Mortality was low among infections with C. tropicalis (1.5%, 1 of 7) and C. glabrata (0 of 7). Although not statistically significant, we found that the mortality risk decreased after CVC removal (OR = 0.3; 95% CI, 0.033–3.5, P = 0.4).
Discussion
The rates of candidemia among hospitalised children did not change significantly over the 10-year study. Nonetheless, these rates were significantly lower than previously described in paediatric patients with similar age distribution.3 We did not find a significant change on the distribution of Candida species throughout the study period. Similar to other studies, C. parapsilosis was the predominant species among hospitalised paediatric patients.3,8 Nevertheless, in the 5-year study by Neu et al., C. parapsilosis was the predominant species regardless of the patient’s comorbidity.3 In our patient population, the crude mortality rate was significantly lower than previously reported but significantly higher for young children and those infected with C. lusitaniae.9
Despite the lack of statistical significance among all patients in our cohort, the use of fluconazole within 30 days of developing candidemia did not prevent infection with C. parapsilosis in non-oncologic patients, independent of TPN as previously published.
Our study is consistent with previously published data showing that early CVC removal did not improve survival or time to mycogical eradication.10,11 However, in our cohort, these findings did not pertain to children with C. lusitaniae infection.
Several potential limitations can be recognised in this study. Despite the 10-year analysis, the number of children with candidemia is relatively small. At AIDHC, peripheral blood cultures are not routinely obtained in children with CVC, limiting our ability to identify the role of CVC removal in clearing the infection. In addition, antifungal susceptibilities were not assessed to determine if antifungal therapy correlated with mortality. Lastly, the metric used in our study (doses administered per 1000 patient days) to measure aggregate antifungal use has not been standardised; however, we previously reported a strong correlation between days of therapy and doses administered, particularly for antimicrobials requiring daily or twice daily dosing.5
In conclusion, candidemia remains an important threat for hospitalised children, particularly children under 2 years of age infected with non-albicans species, despite the introduction of new antifungal agents. Risk factors for mortality in this patient population remain poorly understood and warrant larger epidemiologic studies to further design intervention strategies.
Key points.
Candidemia is the fourth most common nosocomial bloodstream infection.
Over the past decade, C. parapsilosis has emerged as the predominant species, particularly among patients receiving fluconazole prophylaxis.
C. lusitaniae bloodstream infection was associated with the highest mortality rate when compared with other Candida species.
Footnotes
Conflict of interest: All authors report no conflicts of interest relevant to this article.
References
- 1.Morgan J. Global trends in candidemia: review of reports from 1995–2005. Curr Infect Dis Rep. 2005;7:429–39. doi: 10.1007/s11908-005-0044-7. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neu N, Malik M, Lunding A, et al. Epidemiology of candidemia at a Children’s hospital, 2002 to 2006. Pediatr Infect Dis J. 2009;28:806–9. doi: 10.1097/INF.0b013e3181a0d78d. [DOI] [PubMed] [Google Scholar]
- 4.Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children’s hospital. Pediatrics. 2011;128:1062–70. doi: 10.1542/peds.2010-3589. [DOI] [PubMed] [Google Scholar]
- 5.Rose L, Coulter MM, Chan S, Hossain J, Di Pentima MC. The quest for the best metric of antibiotic use and its correlation with the emergence of fluoroquinolone resistance in children. Pediatr Infect Dis J. 2014;33:e158–61. doi: 10.1097/INF.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Pentima M, Chan S, Briody C, Power M, Hossain J. Driving forces of vancomycin-resistant E. faecium and E. faecalis blood-stream infections in children. Antimicrob Resist Infect Control. 2014;3:1–5. doi: 10.1186/2047-2994-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Pentima MC, Chan S. Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29:707–11. doi: 10.1097/INF.0b013e3181d683f8. [DOI] [PubMed] [Google Scholar]
- 8.Sobel JD. The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Curr Infect Dis Rep. 2006;8:427–33. doi: 10.1007/s11908-006-0016-6. [DOI] [PubMed] [Google Scholar]
- 9.Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J. 2004;23:635–41. doi: 10.1097/01.inf.0000128781.77600.6f. [DOI] [PubMed] [Google Scholar]
- 10.Nucci M, Anaissie E, Betts RF, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis. 2010;51:295–303. doi: 10.1086/653935. [DOI] [PubMed] [Google Scholar]
- 11.Zaoutis TE, Coffin SE, Chu JH, et al. Risk factors for mortality in children with candidemia. Pediatr Infect Dis J. 2005;24:736–9. doi: 10.1097/01.inf.0000172938.76561.8e. [DOI] [PubMed] [Google Scholar]