Abstract
A wide range of health benefits have been ascribed to soya intake including a lowered risk of osteoporosis, heart disease, breast cancer, and menopausal symptoms. Because it is a hormonally active diet, however, soya can also be endocrine disrupting, suggesting that intake has the potential to cause adverse health effects in certain circumstances, particularly when exposure occurs during development. Consequently, the question of whether or not soya phyto-oestrogens are beneficial or harmful to human health is neither straightforward nor universally applicable to all groups. Possible benefits and risks depend on age, health status, and even the presence or absence of specific gut microflora. As global consumption increases, greater awareness and consideration of the endocrine-disrupting properties of soya by nutrition specialists and other health practitioners is needed. Consumption by infants and small children is of particular concern because their hormone-sensitive organs, including the brain and reproductive system, are still undergoing sexual differentiation and maturation. Thus, their susceptibility to the endocrine-disrupting activities of soya phyto-oestrogens may be especially high. As oestrogen receptor partial agonists with molecular and cellular properties similar to anthropogenic endocrine disruptors such as bisphenol A, the soya phyto-oestrogens provide an interesting model for how attitudes about what is ‘synthetic’ v. what is ‘natural,’ shapes understanding and perception of what it means for a compound to be endocrine disrupting and/or potentially harmful. This review describes the endocrine-disrupting properties of soya phyto-oestrogens with a focus on neuroendocrine development and behaviour.
Keywords: Soya, Isoflavones, Genistein, Equol, Endocrine disruption, Oestrogen, ERα, ERβ, Brain, Hypothalamus
A plant-based diet has many undeniable ecological and health benefits. As a food or food additive, soya is appealing because it is a complete protein that is cholesterol-free, lactose-free, high in fibre and rich in complex carbohydrates, antioxidants and unsaturated fats. Soya is also replete with phyto-oestrogens, which makes it a hormonally active food. For many, the consequences of this activity will be minimal, oreven potentially beneficial, but for others the endocrine-disrupting properties of soya should not be discounted and health practitioners should be more broadly aware of this phenomenon and potential outcomes. The pros and cons of a phyto-oestrogen-rich diet on many aspects of human health, including breast and prostate cancer, reproductive maturation and function, cardiovascular health, bone health and menopausal symptoms have been reviewed previously by myself and others(1–6). The present review specifically focuses on the endocrine-disrupting properties of soya isoflavones, particularly within the neuroendocrine system, and highlights our most recent findings along those lines.
Phyto-oestrogens are naturally occurring plant compounds that are structurally and/or functionally similar to mammalian oestrogens and their active metabolites(7). There are several phyto-oestrogen classes, but the most hormonally active are the phenolic compounds of which the isoflavones and coumestans are the most widely studied groups. Isoflavones are most abundant in soyabeans and other legumes but also found in berries, wine, grains, nuts and soya-fortified foods(8). Although present as inactive glycoside conjugates (containing glucose or carbohydrate moieties) and unconjugated (aglycone) forms in food, only the latter are bioactive. Fermented soya, such as tem-peh or miso, typically contains higher aglycone levels than other soya-based foods. Once consumed, isoflavones are rapidly metabolised and absorbed, entering systemic circulation predominantly as conjugates with limited bioavailability and bioactivity, leaving only a tiny fraction of the ‘free’ bioactive form in systemic circulation. Typically, metabolites are less bioactive than the parent compounds but equol, a metabolite of daidzein, is a notable exception(9). At best, only 30–50 % of individuals are capable of bioconverting daidzein to its more oestrogenic metabolite equol with vegetarians and individuals of Asian origin being most likely(10,11). Age and health status, particularly the use of antibiotics, can significantly impact the production and absorption of bioactive isoflavones, including equol.
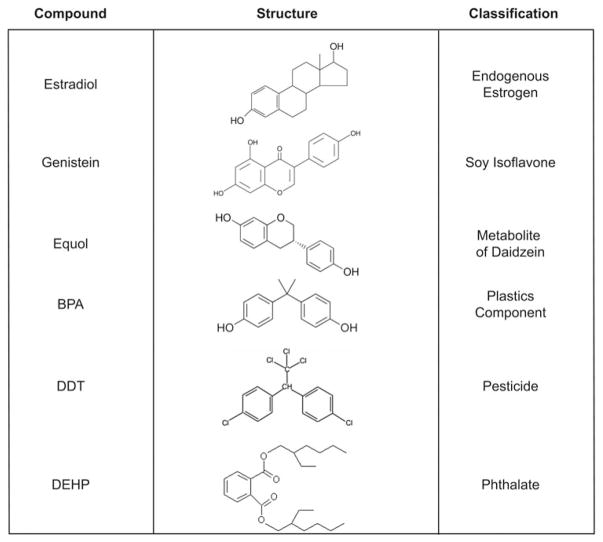
Although they are structurally similar to anthropogenic endocrine-disrupting compounds (EDC) and behave similarly on numerous molecular and cellular targets, intake of soya phyto-oestrogens is broadly encouraged and regarded as healthy, while their synthetic counterparts are increasingly viewed with caution and met with repeated calls to ban or restrict their use (Fig. 1). This attitudinal discordance is almost entirely based on the source of the compounds (soya is ‘natural’, while synthetic chemicals are not) rather than the scientific evidence regarding their hormone-disrupting activities. While it is clear that for many people soya diets are a healthful option, particularly when meat and saturated fat intake is concomitantly reduced, a growing chorus of scepticism is cautioning that the health benefits popularly ascribed to soya may be overstated and minimally supported by robustly conducted, statistically sound scientific studies(12–14). It has also been recognised for nearly a century that phyto-oestrogens have endocrine-disrupting properties in vertebrates, including human subjects, and that exposure to these compounds may pose a risk to some groups, particularly infants and the unborn(15–19).
Fig. 1.
Structures of some well-known anthropogenic and naturally occurring endocrine-disrupting compounds. BPA, bisphenol A; DDT, dichlorodiphenyltrichloroethane; DEPH, di(2-ethylhexyl)phthalate.
Endocrine-disrupting activities of phyto-oestrogens in vertebrates and human subjects
An EDC is defined by the Endocrine Society as a compound that interferes with any aspect of hormone action(20). The word ‘interferes’ is important because many things can have hormone action as part of maintaining homeostasis and interacting with the environment, such as the simple act of eating or standing in sunlight. An EDC is a compound that interferes with the way in which the pancreas responds to a meal, or disrupts the vitamin-D-producing capacity of sunbathed skin. In the case of isoflavones, the target of this ‘interference’ is primarily thyroid hormone and oestrogen. Although a formal definition has not yet been established, the term neuroendocrine disruption has been used to broadly describe chemical impacts on endocrine-related brain development and function(21,22). In the case of phyto-oestrogens, the vast majority of research effort has concentrated on the reproductive neuroendocrine system, which includes the hypothalamic–pituitary–gonadal (HPG) axis. Importantly, neuroendocrine disruption isdistinct from, and should not be conflated with, neurotoxicity, which characterises processes contributing to neuronal cell death and related downstream consequences (e.g. dopaminergic cell death and Parkinson’s like symptoms) and peripheral neuropathies. Isoflavones are not neurotoxic.
That phyto-oestrogens are endocrine disrupting has been known since at least the 1940s when ewes grazing on clover rich pastures in Australia were observed to have abnormally high rates of infertility, abortion and reproductive abnormalities in their offspring(23–25). Consequently, management of phyto-oestrogen levels has been the subject of grazing/feeding practices within the agricultural community for decades, including, most recently, in aquaculture(26,27). Phyto-oestrogens have proven to be potently endocrine disrupting for a wide range of vertebrates, including rodents(1,28), birds(29), cheetahs(30), multiple species of fish(31,32), and grazing mammals such as cattle, sheep and even the southern white rhinoceros(23,25,33).
Evidence of endocrine disruption by soya in human subjects also dates back decades. Soya has been known to be goitrogenic for nearly a century(34,35) necessitating the addition of iodine to soya infant formula and other soya-rich foods. Both genistein and daidzein potently block thyroxine synthesis by serving as alternate substrates and blocking thyroid peroxidase catalysed tyrosine iodination. Soya also decreases absorption of synthetic thyroid hormone(36) potentially necessitating higher doses in hypothyroid patients. Thus, for these and other patients at risk for clinical or subclinical hypothyroid, compensatory iodine intake is advisable if soya is part of the regular diet. Additionally, although research regarding the relationship between soya intake and thyroid levels during pregnancy is extremely limited(37), because thyroid hormone is essential for normal brain development, pregnant women regularly consuming soya should be particularly mindful of this endocrine-disrupting property of soya.
Soya can also impact reproductive function in women. Suppression of circulating steroid hormone levels and attenuation of the preovulatory gonadotropin surge have been repeatedly observed and a 2009 meta-analysis concluded that isoflavone intake moderately increases cycle length and suppresses luteinising hormone and follicle-stimulating hormone levels(38). A 2008 clinical case report described three women (aged 35–56 years) experiencing a suite of symptoms related to excessive soya intake (estimated to exceed 40 g/d), including abnormal uterine bleeding, endometrial pathology and dysmenorrhea, all of which resolved when soya intake was discontinued or reduced(39). Importantly, as for all EDC, timing of exposure is important when considering the potential for long-term effects. The youngest of the three patients had been on a soya-rich diet since age 14 years and was experiencing secondary infertility, a condition that resolved and resulted in a pregnancy once she cut back on her soya consumption. Of even greater concern is what might happen in infant girls who consume high levels of soya, while their reproductive systems are still developing. Exposure earlier in life may have more lasting effects because disruption of the organisational actions of hormones may produce permanent structural and/or functional changes(40).
The earliest evidence for developmental reproductive health effects came from two studies, conducted in the mid-1980s, which associated neonatal phyto-oestrogen exposure with thelarche before age 2 years in a population of Puerto Rican girls. A number of confounding factors, however, including the consumption of meat that had been fattened with potent oestrogens, including the notorious endocrine disruptor diethylstilbestrol, make the data problematic and difficult to interpret(41,42). A highly cited retrospective cohort study of 952 women found that young women reared on soya-based infant formula (248 women) as part of a controlled, University of Iowa feeding study, reported longer menstrual bleeding and menstrual discomfort than those who were fed a non-soya based formula (563 women)(43). At the time the study was conducted, the women were too young to comprehensively examine pregnancy or fertility outcomes, but, now that nearly a decade has past, this area is ripe for reevaluation. Soya formula consumption has also been linked to a greater risk of developing uterine fibroids(44). A prospective study reported oestrogenised vaginal epithelium in female soya formula-fed infants, an important finding confirming soya infant formula is oestrogenic in human subjects(45). Other studies, however, have found no link between soya infant formula and developmental reproductive parameters, including breast, ovarian or testes volume(45,46), and impacts of soya formula intake and on age at menarche are mixed(47,48). That soya is hormonally active is irrefutable. Whether or not soya intake, particularly during infancy, can have long-term health effects remains the subject of debate, but parents should be made aware of possible oestrogenic effects if they choose to feed their infants a soya-based formula.
Mechanisms of endocrine disruption by isoflavones
EDC can act via a myriad of mechanisms but the most fundamental include: (1) mimicking the effects of natural hormones by acting as a ligand at their binding sites; (2) antagonising the effect of these hormones by blocking their interaction with their physiological binding sites; (3) reacting directly and indirectly with the hormone in question; (4) altering the natural pattern of synthesis/degredation of hormones; or (5) altering cellular hormone receptor levels(40,49,50). Isoflavones have been shown to interfere with oestrogen action via all of these. They also have other biological activities, which is not atypical as one of the hallmarks of EDC is that they simultaneously affect multiple hormonal systems, and act by multiple mechanisms. Genistein is thought to slow tumourigenesis, for example, via inhibition of protein tyrosine kinases and inhibition of DNA topoisomerases I and II, along with other chemoprotective mechanisms(1,6,51). Phyto-oestrogens are also good anti-oxidants and anti-inflammatory agents.
The primary mode of isoflavone endocrine disruption is interference with oestrogen. At almost the same instant that a second subform of the nuclear oestrogen receptor (ER) was discovered (termed ERβ) it was recognised that isoflavones bind and activate transcription via both forms (ERα and ERβ), but generally have a higher relative binding affinity for ERβ(52–55). Potency estimates vary by assay, but most isoflavones bind nuclear ER far more readily than their synthetic endocrine-disrupting counterparts including bisphenol a(52). Exposure is also consistently higher, often orders of magnitude higher, making them one of the most significant EDC in the human landscape(1,56). Once bound, isoflavones act as partial agonists, with activity varying across tissue types and local levels of endogenous oestrogen. ER subtype distribution varies across tissues and cell types, particularly in the brain, changes over the lifespan, and is sexually dimorphic(57–59). Because ERα and ERβ are differentially distributed throughout the body and the brain, including neuroendocrine pathways, which coordinate reproductive function, that isoflavones are more bio-active via ERβ is functionally significant(60–64). ERα and ERβ regulate different aspects of reproduction, behaviour and neuroendocrine function across the lifespan, although their relative roles are more clearly elucidated in animal models than in human subjects, in some tissues than others and, in some cases, one sex than another(65–68). For example, ERβ in the paraventricular nucleus of the hypothalamus (PVN), a region important for the coordination of reproductive, social and stress-related behaviours, suppresses anxiety-related behaviours and enhances production of the neuropeptide oxytocin (OT)(69–71). ERβ is also expressed at higher levels than ERα in the basal forebrain, hippocampus, dorsal raphe and cerebral cortex in the adult(60,72,73), all brain regions critical to neuroendocrine function and mood-related behaviours. ERβ is particularly abundant in the prenatal brain and plays a key role in brain morphogenesis by affecting cortical layering and interneuron migration(73).
Once bound to ER, phyto-oestrogens can initiate transcription classically through interactions with the oestrogen response element or by binding early immediate genes, such as Jun and Fos(74). Steroid hormones, particularly oestrogens, can also initiate rapid, non-genomic actions at the cell surface via a range of mechanisms, including the binding of specialised steroid membrane receptors or ion channel subunits(75–78). The vast majority of rapid actions are thought to originate at oestrogen-binding sites at the extracellular surface of the cell membrane, meaning that a potential EDC does not have to enter the cell to be active. Binding then activates second messenger pathways leading to cellular responses such as increased intracellular calcium or cAMP levels, or promoting nitric oxide release resulting in the stimulation of signal transduction pathways important for neuronal signalling, differentiation and other cellular processes(79). The best-known transmembrane ER, the G-protein-coupled oestrogen receptor, was cloned as the orphan receptor GPR30 two decades ago and is now known to be capable of binding a wide range of EDC, including genistein(80). The functional significance of this pathway, or its disruption, has yet to be fully described but G-protein-coupled oestrogen receptor plays an important role in rapid vascular oestrogen signalling along with ERα and ERβ(81). Emerging data reveals that phyto-oestrogens have epigenetic activity and can alter activities of DNA and histone methyltransferases, NAD-dependent histone deacetylases and other modifiers of chromatin structure(82–84).
Phyto-oestrogens have also been shown to interfere with the enzymes needed for steroid biosynthesis and/or degradation. Coumestrol, for example, attenuates the conversion of [3H]-estrone to [3H]-estradiol in vitro by inhibiting the enzyme 17β-hydroxysteroid oxidoreductase Type 1 in a dose-dependent fashion(85). Genistein, though weaker, has a similar dose-dependent inhibitory effect. In rats, genistein can alter folliculogenesis, an outcome postulated to result, at least in part, from dysregulation of steroidogenic enzymes(86). In porcine granulosa cells, genistein decreases the activity of cholesterol side-chain cleavage enzyme (P450scc) and 3β-hydroxysteroid dehydrogenase(87). Genistein has also been characterised as a non-competitive inhibitor of 11β-hydroxysteroid dehydrogenase type 1, which produces bioactive glucocorticoids, such as cortisol, from inactive precursors(88). Disruption of aromatase and 5α-reductase by a number of phyto-oestrogens has also been demonstrated in vitro but this potential activity in mammalian tissues remains controversial(2). Disruption of biosynthetic/degradative enzymes could significantly alter local endogenous hormone levels but not manifest as a change in circulating hormone levels. This may be particularly important for brain and hormone-sensitive subregions such as the hypothalamus as growing evidence strongly suggests that neural cells have the capacity to synthesise steroid hormones de novo(89–91).
Another mechanism by which phyto-oestrogens can perturb steroid bioavailability and transport is by altering sex hormone-binding globulin synthesis and availability. Isoflavones have long been known to appreciably stimulate sex hormone-binding globulin production, particularly in individuals who have levels on the low range of normal(92). Heightened sex hormone-binding globulin levels are thought to be one mechanism by which soya may lower breast cancer risk because bioavailable levels of circulating oestrogens are concomitantly reduced(93). Similarly, suppression of circulating androgens, particularly dihydrotestosterone by equol, is hypothesised to be one way in which soya might protect against prostate cancer(51,94). Notably, a subset of studies have found no impact of isoflavones on circulating sex hormone-binding globulin or steroid hormone levels in human subjects (e.g.(95)). One found suppressed luteal oestrogen levels following increased soya intake, but only in women of Asian descent(96), indicating ethnicity and/or the capacity to produce equol could be an underappreciated factor-mediating interindividual variability in responsiveness(97,98).
Endocrine-disrupting effects of soya isoflavones on the adult neuroendocrine system
Impacts on the mature reproductive axis in human subjects and other vertebrates have already been summarised and include altered serum hormone levels and suppression of ovulation. Elevated urine levels of genistein and daidzein have been associated with idopathic infertility and lower semen quality in Chinese men(99), and a slightly lower percentage of normal sperm in US men whose partners were attempting pregnancy(100), but supporting evidence in other populations or species for effects on spermatogenesis is limited. In contrast, the animal literature has explored a wider age range and a more diverse array of endocrine-disrupting effects.
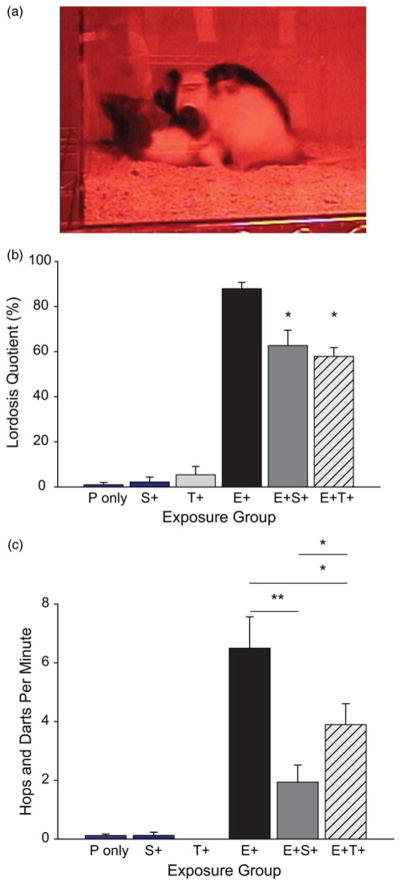
Work in our laboratory focuses on neuroendocrine pathways underlying sexually dimorphic behaviours and, using a variety of animal models, we and others have shown that isoflavone intake interferes with oestrogen-mediated behaviours, including female sexual motivation. For example, consumption of a commercially prepared isoflavone supplement to adult female rats, at a dose that results in serum levels between those seen in Western and Asian (human) adults, attenuated lordosis (a reflexive posture indicating sexual receptivity) to the same degree as tamoxifen(101,102). The supplement also suppressed proceptive behaviours even more profoundly than tamoxifen suggesting that soya isoflavones can suppress female sexual motivation and solicitation (Fig. 2). Administration of genistein alone did not recapitulate these effects(103). Whether or not libido is altered in human subjects appears to be completely unknown. Remarkably, a Pubmed search with the keywords ‘soya’ and ‘libido’ produced only nine published papers, not all of which were relevant. One was a case report describing a case of bilateral gynecomastia, erectile dysfunction and loss of libido in a 60-year-old man, which resolved when he discontinued drinking three quarts of soya milk daily(104). Another reported a beneficial effect of soya protein dietary supplements on libido in post-menopausal women but there was an equally beneficial placebo effect suggesting that the soya effect was spurious(105). Further inquiry revealed no studies, which have tackled this question in younger populations, or with a large-enough sample size to achieve reasonably robust statistical power. Given that soya appears to have a consistently suppressive effect on circulating steroid hormone levels it is not implausible that libido may also be suppressed but this appears to be, by and large, an unanswered question.
Fig. 2.
(Colour online) In ovariectomised, hormone replaced female rats, sexual behaviour is suppressed by a soya isoflavone supplement. (a) Lordosis is a hallmark receptive posture in the rat and the frequency of lordosis in response to male mounting, which can be induced in ovariectomised females with progesterone (P) and estradiol benzoate (E), but not P alone. (b) In the presence of E and P tamoxifen (E + T+) or a soya supplement (E + S+) significantly decrease lordosis in female rats. (c) Similarly, proceptive behaviour, including hopping and darting, is also suppressed in hormonally replaced female rats on tamoxifen (E + T+) and, to an even greater degree, the soya isoflavone supplement (E + S+). *P ≤ 0·05; **P ≤ 0·01; means ± SEM. Figure adapted from Patisaul et al.(101)
Mood and anxiety-related behaviours may also be impacted by adult soya intake(106). In human subjects, nearly all studies along these lines have focused on post-menopausal women and evidence for improvement of mood is minimal and sporadic(107). Results across animal studies are mixed and sex dependent with females generally showing decreased anxiety and males showing heightened(108,109). This pattern tends to abrogate or reverse expected sex differences in assessments of anxiety-related behaviours(110) and appears to involve the neuropeptides OT and vasopressin (AVP). For example, male cynomolgus monkeys fed soya protein isolate containing 1·88 mg isoflavones/g protein over 18 months demonstrated higher frequencies of intense aggressive (67 % higher) and submissive (203 % higher) behaviours as well as a decreased proportion of time (68 % reduction) spent in physical contact with other monkeys(111). Male rats maintained on a diet containing 150 μg/g genistein and daidzein displayed increased anxiety and elevated stress-induced plasma AVP and corticosterone levels(112). Increased hypothalamic AVP has also been reported in Sprague–Dawley rats fed a diet containing 1250 ppm genistein across the lifespan in a study run at a US Food and Drug Association research center(113). We found that the same isoflavone supplement found to suppress reproductive behavior and motivation in female rats (described earlier) abrogated the oestrogen-dependent up-regulation of OT receptors in the ventromedial nucleus of the hypothalamus and heightened ERβ expression levels in the PVN, an effect opposite to that of estradiol(102).
Involvement of the PVN is consistent with both the oestrogen and thyroid-disrupting properties of soya, and the high potency of isoflavones at ERβ. The PVN, which contains little to no ERα but high levels of ERβ(114), is a primary site of OT and AVP production, peptide hormones important for social behaviour and the facilitation of sexual behaviour(115), as well as thyroid hormone releasing hormone and corticotropin-releasing hormone. Oestrogen-dependent stimulation of PVN OT and AVP production requires ERβ(116,117). OT then binds to its receptor throughout the brain, including the ventromedial nucleus, a nucleus critical for mediating the lordosis response in females(118). Isoflavone-related effects on these and other oestrogen dependent systems in the adult rodent brain have previously been reviewed(109,119–121) but a concerted focus on OT/AVP systems in human subjects remains lacking.
Evidence for developmental neuroendocrine disruption in animals and human subjects
Neuroendocrine disruption by soya isoflavones in mature neuroendocrine systems is by and large reversible with dietary modification and thus, with the exception of some hypersensitive groups such as hypothyroid and oncology patients, soya likely poses no long term health risk and may even confer modest benefits. Of greater concern is that phyto-oestrogens may interfere with the organisational role of oestrogen in the developing brain and reproductive system. Data from a diversity of animal models have repeatedly shown that manipulation of oestrogen during specific critical windows of development throughout gestation and early infancy leads to a myriad of adverse outcomes in the HPG axis including malformations in the ovary, uterus, mammary gland and prostate, early puberty, reduced fertility, disrupted brain organisation, and reproductive tract cancers(66,122–126). The disruptor diethylstilbestrol story also starkly illustrates the broad spectrum of sex-specific consequences on neuroendocrine systems following fetal oestrogen exposure(127,128). Although isoflavones and other EDC are far less potent than disruptor diethylstilbestrol, human exposure is ubiquitous and there is growing acceptance that EDC are contributing to adverse reproductive health trends in Western nations including median age at menarche, first breast development, and sexual precocity(40,129–131). Advanced pubertal onset in girls adopted from developing countries by Western parents supports a role for environmental factors(129). Emerging but controversial data suggest that EDC may also be shifting age at puberal onset in boys(132). Among men, sperm counts in the USA and Europe appear to have declined by approximately half over the past 50 years(133,134) with upwards of 30 % in the subfertile range in places like Denmark where exposure to persistent environmental pollutants is particularly high(135). A provocative but limited study associated increased incidence of hypospadias (malformation of the male external genitalia) with maternal vegetarianism(136) but this effect has not been replicated. Synthetic EDC which interfere with androgen biosynthesis or activity are also associated with disorders of male genital development(137,138) thus it is not implausible that equol may be endocrine disrupting in this regard. Increased prevalence of reproductive health disorders is likely not attributable to a single factor, not even a single environmental factor, but EDC are causal to some degree and isoflavones are hypothesised to play a contributing role(5,40,139–141).
Disruption of reproductive tract development
The vast majority of studies exploring the impact of early life isoflavone exposure on HPG differentiation and function have used rodent models, with the compounds administered either prenatally to the pregnant dam or postnatally to the pups. This aspect of the literature has been extensively reviewed and will thus not be recapitulated in detail here but adverse outcomes in female rodents include disrupted timing of vaginal opening (pubertal onset), altered ovarian development, impaired oestrous cyclicity and ovulation, and disrupted HPG steroid feedback(1,2,5,142). We have recently shown, for example, that female rats reared on a soya-rich diet across the lifespan (gestation through adulthood) have earlier pubertal onset (defined as the day of vaginal opening in the rat), and a greater number of corpera lutea post-puberty but took longer to establish regular oestrus cycles than their conspecifics on soya-free diet. Cycle regularity then degraded with time and soya-reared animals had a greater number of cystic follicles in early adulthood(143). Notably, not all pathology is readily obvious. Emerging evidence suggests that the oviductal and uterine environments in mice developmentally exposed to human-relevant gensitein levels are not suitable to maintain pregnancy, which manifests as the incapacity of the uterus to support implantation and embryonic development(144). Moreover, embryo transfer experiments have shown that the uterus of genistein-treated mice is not capable of sustaining pregnancy even if the blastocysts arise from control mice(145). These data are consistent with effects seen in sheep and other species suggesting that developmental isoflavone exposure induces permanent changes in the function of the female reproductive tract that may be subtle but can result in complete infertility, particularly as the animal ages.
There is a surprising paucity of data on the impact of developmental isoflavone exposure on male neuroendocrine physiology (reviewed in(146)). There is some sporadic evidence in animal models that developmental isoflavone exposure affects testicular function, but many studies find no effects, which makes it challenging to draw definitive conclusions(4). A transformative pair of high-impact studies, which greatly contributed to health advisories in Europe, was conducted in marmosets. Twins were fed either soya or milk formula. Males on the soya diet had lower serum testosterone concentrations and higher numbers of Leydig cells then their milk-fed twins. As adults, the soya fed marmosets had larger testes and lower serum testosterone levels, demonstrating that the impacts were persistent(147,148), but fertility was not compromised. Two rat studies conducted using classical toxicological testing parameters and long-term multi-generational oral exposure protocols spanning gestation through adulthood linked genistein with abnormalities in spermatogenesis(149,150). One also found genistein-related alterations in sperm motility and a reduction in litter size accompanied by evidence of post-implantation embryo loss when the adult rats underwent fertility testing(149). Chronically exposed males have also been shown to develop mammary gland hypertrophy at doses at or above 11 mg/kg, and mammary gland hyperplasia at doses at or above 29 mg/kg (ductal/alveolar hyperplasia was observed in females as well)(151). This effect was confirmed in a subsequent study by a different research group even though exposure was restricted to the peri-natal period, suggesting that the sensitive period of exposure is pre-pubertal(152). The male mammary gland may be one of the most sensitive targets for endocrine disruption but is rarely examined in EDC studies, leading some to advocate for its inclusion in chemical test guideline studies and risk assessment(153,154).
Disruption of brain sexual differentiation and neuroendocrine organisation
Work in our laboratory focuses on sexually dimorphic, oestrogen-sensitive hypothalamic systems and we have repeatedly shown that the sex-specific ontogeny of these systems is vulnerable to synthetic and naturally occurring EDC including soya isoflavones(1,121,155). In rodents, hormone mediated morphological and functional organisation within the neuroendocrine system occur during a series of well-defined critical periods spanning gestation through puberty(66,124,156). Although most sex differences are established during prenatal and neonatal development, in the rat new cells (neurons and glia) are added to sexually dimorphic nuclei during adolescence in response to steroid hormone treatments(157,158), demonstrating the long-term sensitivity of sexually dimorphic brain regions to steroid hormone-mediated signalling. Interference with the hormone-sensitive organisation of neuroendocrine pathways could result in irreversible developmental defects and disruption of sex-typical behaviours, emphasising that development is likely the most susceptible periods for EDC exposure over the lifespan. Although it does not readily transfer lactationally, genistein efficiently crosses the rat placenta and the bioactive aglycone form of genistein is present in the fetal brain at levels comparable to circulating levels in the dam(159,160). Moreover, the transfer of genistein to the brain from systemic circulation appears to be more efficient in prenatal animals than adults(161) demonstrating that it and other isoflavone phyto-oestrogens are capable of directly interfering with the organisation of neuroendocrine signalling pathways in the developing brain.
The sexually dimorphic brain region most frequently used as a biomarker of endocrine disruption in rats is the sexually dimorphic nucleus of the preoptic area (SDN-POA). The volume of the SDN-POA is enhanced by estradiol aromatised from perinatal, testicular androgen(124), is five to six times larger in males than females(162), and is thought to play a role in male reproductive behaviours and mate choice. Although both ERα and ERβ are expressed in the SDN-POA across the lifespan, ERα appears to play a dominant role in masculinising SDN-POA morphometrics(61,163,164), a process which has now been elucidated in detail and is largely complete by the second week of life(67,165). In rats, numerous studies have queried the extent to which soya isoflavones alter SDN-POA volume in both sexes and, while not always in complete accordance, the data are generally consistent with oestrogenic effects. For example, when administered prenatally through adulthood, genistein increases SDN-POA volume in males but not females(166). No enhancement, however, was observed in males exposed from birth through weaning(167) or in males exposed on only the first few days of life(168) suggesting that exposure must be ongoing to maintain the enlargement. Masculinising effects on female SDN-POA volume have only been observed following high-dose exposure(169) and some studies have not found genistein to be endocrine disrupting in the female rat SDN-POA, even at doses high enough to be uterotrophic(167,170).
An additional area of focus for our studies is the anterior ventral periventricular nucleus (AVPV), which, like the SDN-POA, is sexually differentiated by endogenous gonadal hormones during a series of pre- and peri-natal critical periods but is larger in females than males(171,172). The presence and density of the two ER subtypes varies across species but both are present in the rat(58). The AVPV is essential for coordinating the preovulatory gonadatropin surge and plays a central role in female sexual behaviour(173–175). In human subjects, the neural machinery controlling gonadotropin pulsatility is functional by the end of the first trimester(176), while in rodents this system does not fully sexually differentiate until the first few days of the neonatal period(171). In male rodents, testicular androgen is aromatised to oestrogen in the brain, and it is this locally derived oestrogen, working primarily through ERα-dependent pathways, that is primarily responsible for defeminising/masculinising the AVPV(67,177,178). At birth exogenous oestrogen administration can defeminise the female AVPV and surrounding structures thereby eliminating lordosis and the capacity for steroid-positive feedback. By extension, if endogenous oestrogen is blocked in males, either by castration, by aromatase inhibition, or antagonism of hypothalamic ER, the AVPV and surrounding structures fails to defeminise and the capacity to elicit lordosis and a gonadal surge remains. Therefore, interference with oestrogen at birth, in either sex, can result in the improper differentiation and function of the HPG axis across the lifespan.
We have shown that subcutaneous administration of 10 mg/kg genistein, a dose that is approximately equivalent to the total amount of isoflavones ingested by infants fed soya formula, over the first 4 d of life, advances vaginal opening and compromises the ability to maintain a regular oestrous cycle in female rats(179). This outcome was accompanied by an impaired ability to stimulate gonadotropin releasing hormone activity (as measured by the co-immunoreactivity of gonadotropin releasing hormone and Fos) following ovariectomy and hormone priming. We have further shown that neonatal exposure to 10 mg/kg genistein significantly decreases the density of kisspeptin immunoreactive fibres in the AVPV of female rats(180,181). Exciting work over the past decade has identified kisspeptin neurons as the primary gatekeepers of gonadotropin releasing hormone release in many species, including human subjects(182,183). Therefore, our findings suggest that disrupted organisation of kisspeptin signalling pathways may be a novel mechanism by which isoflavones and other EDC may induce a suite of HPG-related abnormalities, including advanced pubertal onset, irregular oestrous cycles and premature anovulation(184).
How much is too much: human isoflavone intake, metabolism and excretion
Ultimately risk of harm comes down to two primary factors: dose and timing of exposure. Undoubtedly, development is the most sensitive period for the endocrine-disrupting consequences of soya isoflavone exposure, thus it is not surprising that concerns have been expressed regarding the safety of soya-based infant formula. Initially developed as an alternative to bovine milk formulas for babies with milk allergy, use of soya infant formula in the USA is a popular choice and constitutes an estimated 25 % of the formula market(185–187). The safety of soya formula has been rigorously discussed from several perspectives, and a litany of review articles and position papers have been published on the subject(188–195). Societies including the American Academy of Pediatrics and the European Society for Pediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition have issued guidelines recommending against the exclusive use of soya formula except in the rare cases of true milk allergy or lactose intolerance. The US National Toxicology Programme completed its most recent safety assessment of soya infant formula in 2010 (monograph available at http://ntp.niehs.nih.gov/pubhealth/hat/noms/formula/index.html) and concluded there is ‘minimal concern for adverse developmental effects.’ For comparison, this is the same level of concern initially expressed for bisphenol a until the Food and Drug Association elevated that advisory to ‘some concern’ in January, 2010 based on new data (and then subsequently lowered it again). Notably, the National Toxicology Programme could not issue a conclusive recommendation regarding potential long-term reproductive effects of soya infant formula largely because of limited and poor-quality human data. An apparent lack of adverse effects is one reason why so many consumers, clinicians and public health agencies consider regular use of soya formula to be safe, even beneficial. However, the absence of evidence is not evidence of safety so this problematic data gap regarding the long-term impacts of soya formula use remains in serious need of attention.
When considering the potential safety of soya formula, it is frequently argued that Asian populations have been consuming soya for centuries, with no obvious consequences. This argument fails to recognise, however, that exposure patterns differ in key ways between Asians consuming a traditional soya-rich diet and Caucasians eating a typical Western diet(185). This timing of exposure is critical. In a traditional Asian diet, soya consumption is moderate across the entire lifespan, but because isoflavones do not effectively transfer via lactation, exposure in breastfeeding infants is extremely low. By contrast, Western babies on soya infant formula have their highest exposures in the first year of life then exposure rapidly plummets. In that regard the two populations are not comparable because their exposure patterns during a critical window of development are so dramatically different. Other diet and lifestyle differences may also be confounding when evaluating the potential health benefits and risks of soya. For example, Asian populations on traditional diets eat less processed foods, considerably higher levels of seafood and lower levels of animal fat than Western populations.
So how much is too much? There is no ‘typical’ level of isoflavone intake as consumption patterns vary widely across populations, and geographic regions. For Asians, vegetarians and other groups in which soya is foundational to the diet, isoflavone consumption can be as high as 100 mg/d (intake range is about 0·3–1·5 mg/kg body weight)(6,192,196–198). Western diet intake estimates range from 1 to 3 mg/d(198–201). For their weight, infants exclusively fed soya-based formula have the highest mean daily consumption of total isoflavones, ranging from 6 to 9 mg/kg body weight per d in 4-month-old infants, an amount that is up to seven times higher than Asians consuming a traditional soya-based diet.
The isoflavone content of an array of foods and food products is now available via online databases (reviewed in:(202)) including one maintained by the USDA(203). Food isoflavone content varies widely, even in the same foods, because of local and/or seasonal differences in growing conditions so its difficulty to accurately estimate intake(198). Additionally, soya is found in upwards of 60 % of processed foods and ground meats(204). Textured soya protein (50–70 % soya protein) is used as a meat substitute or filler for hotdogs, hamburgers, sausages and other meat products(205,206), while soya protein isolate (90 % soya protein) is frequently used to enrich energy bars and sports drinks (particularly those advertising high protein levels), cereals, granola bars, infant formula, imitation dairy products, ice cream and cheese. Soya isoflavones and other phyto-oestrogens are also widely available as dietary supplements(207,208), typically containing concentrations far higher than those found in food(209).
Not surprisingly, blood isoflavone levels also vary widely, and can be orders of magnitude different between individuals based on dietary preferences and individual differences in phyto-oestrogen absorption and metabolism(210–212). Blood genistein levels are generally in the range of 25 ng/ml for Asian women, slightly less for vegetarian women, and under 2 μg/ml for US women(213). Isoflavones can pass from mother to fetus through the placenta, and have been found in human umbilical cord blood and amniotic fluid at levels comparable with concentrations seen in maternal plasma, demonstrating that fetal exposure can be significant(214). Infants on soya formula can have plasma levels exceeding 1000 ng/ml(209) which is 13 000–22 000 times higher than their own endogenous oestrogen levels, 50–100 times higher than oestradiol levels in pregnant women, and 3000 times higher than oestradiol levels at ovulation(185,215,216). In contrast, infants fed cow’s milk formula or human breast milk have plasma isoflavone levels of 9·4 and 4·7 ng/ml, respectively(192,196,216). Notably, levels in infants and vegetarians easily far surpass, sometimes by several orders of magnitude, internal levels other endocrine disruptors of concern, including bisphenol a and phthalates(126).
Conclusions and recommendations
Soya isoflavones are clearly endocrine disrupting, but although they are similar to their synthetic brethren in terms of their cellular and molecular mechanisms of action on neuroendocrine structure and function, and the scope of adverse outcomes they can inflict, society embraces these compounds at the same time it rejects, often with vigour, exposure to their synthetic brethren. Thus, phyto-oestrogens both challenge our attitudes regarding EDC and highlight how profoundly the direction and interpretation of research and available data can be influenced by source. While some beneficial effects might be conferred by including moderate levels of dietary soya, particularly in adults eating a diet high in saturated fat and animal protein, the potentially adverse effects of these compounds for some groups are likely underappreciated. An abundance of animal data unequivocally demonstrates that soya isoflavone exposure, at doses and plasma concentrations attainable in human subjects, including soya-reared infants, can permanently alter the structure and function of neuroendocrine pathways in both sexes. Infants fed soya formula have the highest exposure to any non-pharmacological source of oestrogen-like compounds, and yet greater anxiety surrounds compounds like bisphenol a and the phthalates which have far lower potency on neuroendocrine targets and to which exposure is far lower. Although relatively few adverse effects have been reported, that is somewhat a consequence of lack of data rather than lack of measurable effects. Although unsatisfying, a parsimonious approach to soya intake is to follow the classic adage and consume in moderation. Development of dietary guidelines should consider the endocrine-disrupting properties of soya and other hormonally active foods, particularly for vulnerable groups such as pregnant women and hypothyroid individuals.
Acknowledgments
Financial Support
This effort was supported by NIEHS R21ES021233 to H. P. B. and pilot funds from the NCSU Center for Human Health and the Environment.
Abbreviations
- AVP
vasopressin
- AVPV
anterior ventral periventricular nucleus
- EDC
endocrine-disrupting compounds
- ER
oestrogen receptor
- HPG
hypothalamic–pituitary–gonadal
- OT
oxytocin
- PVN
paraventricular nucleus
- SDN-POA
sexually dimorphic nucleus of the preoptic area
Footnotes
Conflicts of Interest
None.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.
References
- 1.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–260. doi: 10.1530/REP-11-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–226. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- 4.Cederroth CR, Auger J, Zimmermann C, et al. Soy, phyto-oestrogens and male reproductive function: a review. Int J Androl. 2010;33:304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 5.Cederroth CR, Zimmermann C, Nef S. Soy, phytoestrogens and their impact on reproductive health. Mol Cell Endocrinol. 2012;355:192–200. doi: 10.1016/j.mce.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140:1350S–1354S. doi: 10.3945/jn.109.118315. [DOI] [PubMed] [Google Scholar]
- 7.Whitten PL, Kudo S, Okubo KK. Isoflavonoids. In: D’Mello JPF, editor. Handbook of Plant and Fungal Toxicants. Boca Raton: CRC Press; 1997. pp. 117–137. [Google Scholar]
- 8.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 9.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 10.Setchell KD, Brown NM, Desai PB, et al. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 11.Lampe J, Karr S, Hutchins A, et al. Urinary equol excretion with a soy challenge: influence of habitual diet. PSEBM. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 12.Balk E, Chung M, Chew P, et al. Effects of soy on health outcomes. Evid Rep Technol Assess (Summ) 2005;126:1–8. doi: 10.1037/e439502005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs A, Wegewitz U, Sommerfeld C, et al. Efficacy of isoflavones in relieving vasomotor menopausal symptoms – a systematic review. Mol Nutr Food Res. 2009;53:1084–1097. doi: 10.1002/mnfr.200800552. [DOI] [PubMed] [Google Scholar]
- 14.Sacks FM, Lichtenstein A, Van Horn L, et al. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 15.Rozman KK, Bhatia J, Calafat AM, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol. 2006;77:485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Toxicology Program. NTP multigenerational reproductive study of Genistein (CAS no. 446-72-0) in Sprague–Dawley rats (Feed Study) Natl Toxicol Program Tech Rep Ser. 2008:1–266. [PubMed] [Google Scholar]
- 17.Whitten PL, Patisaul HB. Cross-species and inter-assay comparisons of phytoestrogen action. Environ Health Perspect. 2001;109:5–23. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patisaul HB. Dietary phytoestrogens. In: Naz RK, editor. Endocrine Disruptors: Effects on Male and Female Reproductive Systems. 2. Boca Raton, FL: CRC Press; 2004. pp. 135–173. [Google Scholar]
- 19.Barrett J. Phytoestrogens. Friends or foes? Environ Health Perspect. 1996;104:478–482. doi: 10.1289/ehp.96104478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waye A, Trudeau VL. Neuroendocrine disruption: more than hormones are upset. J Toxicol Environ Health B Crit Rev. 2011;14:270–291. doi: 10.1080/10937404.2011.578273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gore AC, Patisaul HB. Neuroendocrine disruption: historical roots, current progress, questions for the future. Front Neuroendocrinol. 2010;31:395–399. doi: 10.1016/j.yfrne.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J. 1946;22:2. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 24.Braden A, Hart N, Lamberton J. Oestrogenic activity and metabolism of certain isoflavones in sheep. Aust J Agric Res. 1967;18:348–355. [Google Scholar]
- 25.Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- 26.Green CC, Kelly AM. Effects of the estrogen mimic genistein as a dietary component on sex differentiation and ethoxyresorufin-O-deethylase (EROD) activity in channel catfish (Ictalurus punctatus) Fish Physiol Biochem. 2009;35:377–384. doi: 10.1007/s10695-008-9260-z. [DOI] [PubMed] [Google Scholar]
- 27.Gontier-Latonnelle K, Cravedi JP, Laurentie M, et al. Disposition of genistein in rainbow trout (Oncorhynchus mykiss) and siberian sturgeon (Acipenser baeri) Gen Comp Endocrinol. 2007;150:298–308. doi: 10.1016/j.ygcen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Whitten PL, Naftolin F. Dietary plant estrogens: a biologically active background for estrogen action. In: Hochberg RB, NF, editors. The New Biology of Steroid Hormones. Vol. 74. New York: Raven Press; 1991. pp. 155–167. [Google Scholar]
- 29.Leopold A, Erwin M, Oh J, et al. Phytoestrogens: adverse effects on reproduction in California quail. Science. 1976;191:98–100. doi: 10.1126/science.1246602. [DOI] [PubMed] [Google Scholar]
- 30.Setchell K, Gosselin S, Welsh M, et al. Dietary estrogens – a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93:225–233. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- 31.Sassi-Messai S, Gibert Y, Bernard L, et al. The phytoestrogen genistein affects zebrafish development through two different pathways. PLoS ONE. 2009;4:e4935. doi: 10.1371/journal.pone.0004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clotfelter ED, Rodriguez AC. Behavioral changes in fish exposed to phytoestrogens. Environ Pollut. 2006;144:833–839. doi: 10.1016/j.envpol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Tubbs C, Hartig P, Cardon M, et al. Activation of Southern White Rhinoceros (Ceratotherium simum simum) estrogen receptors by phytoestrogens: potential role in the reproductive failure of captive-born females? Endocrinology. 2012;153:1444–1452. doi: 10.1210/en.2011-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarrison R. The goitrogenic action of soya-bean and ground-nut. Indian J Med Res. 1933;21:179–181. [Google Scholar]
- 35.Divi RL, Chang HC, Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54:1087–1096. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 36.Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–258. doi: 10.1089/thy.2006.16.249. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Teng X, Wang W, et al. Effects of dietary soy intake on maternal thyroid functions and serum anti-thyroperoxidase antibody level during early pregnancy. J Med Food. 2011;14:543–550. doi: 10.1089/jmf.2010.1078. [DOI] [PubMed] [Google Scholar]
- 38.Hooper L, Ryder JJ, Kurzer MS, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandrareddy A, Muneyyirci-Delale O, McFarlane SI, et al. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complem Ther Clin Pract. 2008;14:132–135. doi: 10.1016/j.ctcp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freni-Titulaer LLW, Cordero JJF, Haddock LL, et al. Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child. 1986;140:1263–1267. doi: 10.1001/archpedi.1986.02140260065028. [DOI] [PubMed] [Google Scholar]
- 42.Schoental R. Precocious sexual development in Puerto Rico and oestrogenic mycotoxins (zearalenone) Lancet. 1983;1:537. doi: 10.1016/s0140-6736(83)92229-8. [DOI] [PubMed] [Google Scholar]
- 43.Strom BL, Schinnar R, Ziegler EE, et al. Exposureto soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;286:807–814. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 44.D’Aloisio AA, Baird DD, DeRoo LA, et al. Association of intrauterine and early life exposures with diagnosis of uterine leiomyomata by age 35 in the sister study. Environ Health Perspect. 2009;118:375–381. doi: 10.1289/ehp.0901423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernbaum JC, Umbach DM, Ragan NB, et al. Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect. 2008;116:416–420. doi: 10.1289/ehp.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andres A, Moore MB, Linam LE, et al. Compared with feeding infants breast milk or cow-milk formula, soy formula feeding does not affect subsequent reproductive organ size at 5 years of age. J Nutr. 2015;145:871–875. doi: 10.3945/jn.114.206201. [DOI] [PubMed] [Google Scholar]
- 47.D’Aloisio AA, DeRoo LA, Baird DD, et al. Prenatal and infant exposures and age at menarche. Epidemiology. 2013;24:277–284. doi: 10.1097/EDE.0b013e31828062b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adgent MA, Daniels JL, Rogan WJ, et al. Early-life soy exposure and age at menarche. Paediatr Perinat Epidemiol. 2012;26:163–175. doi: 10.1111/j.1365-3016.2011.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frye CA, Bo E, Calamandrei G, et al. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol. 2012;24:144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuiper GGJM, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 53.Pfitscher A, Reiter E, Jungbauer A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J Steroid Biochem Mol Biol. 2008;112:87–94. doi: 10.1016/j.jsbmb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Casanova M, You L, Gaido K, et al. Developmental effects of dietary phytoestrogens in Sprague–Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci. 1999;51:236–244. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 55.Kuiper GGJM, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 56.Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science. 2010;330:460–461. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao J, Patisaul HB. Sex specific expression of estrogen receptors alpha and beta and kiss1 in the postnatal rat amygdala. J Comp Neurol. 2013;521:465–478. doi: 10.1002/cne.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shughrue P, Lane M, Scrimo P, et al. Comparative distribution of estrogen receptor-α (ERα) and β (ERβ) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 61.Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 62.Koehler KF, Helguero LA, Haldosen LA, et al. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 63.Drummond A, Fuller P. The importance of ER {beta} signalling in ovarian function. J Endocrinol. 2009;205:15–23. doi: 10.1677/JOE-09-0379. [DOI] [PubMed] [Google Scholar]
- 64.Handa RJ, Ogawa S, Wang JM, et al. Roles for oestrogen receptor beta in adult brain function. J Neuroendocrinol. 2012;24:160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 67.Wright CL, Schwarz JS, Dean SL, et al. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trend Endocrinol Metab. 2010;21:553–561. doi: 10.1016/j.tem.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29:557–568. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Lund TD, Rovis T, Chung WC, et al. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 70.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Acevedo-Rodriguez A, Mani SK, Handa RJ. Oxytocin and estrogen receptor beta in the brain: an overview. Front Endocrinol (Lausanne) 2015;6:160. doi: 10.3389/fendo.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang JQ, Cai WQ, de Zhou S, et al. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 73.Fan X, Xu H, Warner M, et al. ERbeta in CNS: new roles in development and function. Prog Brain Res. 2010;181:233–250. doi: 10.1016/S0079-6123(08)81013-8. [DOI] [PubMed] [Google Scholar]
- 74.Kushner PJ, Agard DA, Greene GL, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 75.Levin ER. Membrane oestrogen receptor alpha signalling to cell functions. J Physiol. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Kow LM, Pfaff DW. Rapid estrogen actions on ion channels: a survey in search for mechanisms. Steroids. 2016;111:46–53. doi: 10.1016/j.steroids.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belcher SM, Zsarnovszky A. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J Pharmacol Exp Ther. 2001;299:408–414. [PubMed] [Google Scholar]
- 80.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 81.Barton M. Not lost in translation: emerging clinical importance of the G protein-coupled estrogen receptor GPER. Steroids. 2016;111:37–45. doi: 10.1016/j.steroids.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 82.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Tollefsbol TO. Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem. 2010;17:2141–2151. doi: 10.2174/092986710791299966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Labinskyy N, Csiszar A, Veress G, et al. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13:989–996. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franke AA, Custer LJ. High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J Chromatogr B Biomed Appl. 1994;662:47–60. doi: 10.1016/0378-4347(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 86.Patel S, Zhou C, Rattan S, et al. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. 2015;93:20. doi: 10.1095/biolreprod.115.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piasecka-Srader J, Kolomycka A, Nynca A, et al. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and phytoestrogen genistein on the activity and the presence of steroi-dogenic enzyme proteins in cultured granulosa cells of pigs. Anim Reprod Sci. 2014;148:171–181. doi: 10.1016/j.anireprosci.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 88.Tagawa N, Kubota S, Kobayashi Y, et al. Genistein inhibits glucocorticoid amplification in adipose tissue by suppression of 11beta-hydroxysteroid dehydrogenase type 1. Steroids. 2015;93:77–86. doi: 10.1016/j.steroids.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Taves MD, Plumb AW, Sandkam BA, et al. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology. 2015;156:511–522. doi: 10.1210/en.2013-1606. [DOI] [PubMed] [Google Scholar]
- 90.Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2010;39:72–81. doi: 10.1016/j.jchemneu.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fokidis HB, Adomat HH, Kharmate G, et al. Regulation of local steroidogenesis in the brain and in prostate cancer: lessons learned from interdisciplinary collaboration. Front Neuroendocrinol. 2015;36:108–129. doi: 10.1016/j.yfrne.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Pino AM, Valladares LE, Palma MA, et al. Dietary isoflavones affect sex hormone-binding globulin levels in postmenopausal women. J Clin Endocrinol Metab. 2000;85:2797–2800. doi: 10.1210/jcem.85.8.6750. [DOI] [PubMed] [Google Scholar]
- 93.Low YL, Dunning AM, Dowsett M, et al. Implications of gene-environment interaction in studies of gene variants in breast cancer: an example of dietary isoflavones and the D356N polymorphism in the sex hormone-binding globulin gene. Cancer Res. 2006;66:8980–8983. doi: 10.1158/0008-5472.CAN-06-2432. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka M, Fujimoto K, Chihara Y, et al. Isoflavone supplements stimulated the production of serum equol and decreased the serum dihydrotestosterone levels in healthy male volunteers. Prostate Cancer Prostatic Dis. 2009;12:247–252. doi: 10.1038/pcan.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maskarinec G, Williams AE, Inouye JS, et al. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:195–201. [PubMed] [Google Scholar]
- 96.Wu AH, Stanczyk FZ, Hendrich S, et al. Effects of soy foods on ovarian function in premenopausal women. Br J Cancer. 2000;82:1879–1886. doi: 10.1054/bjoc.1999.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Atkinson C, Newton KM, Stanczyk FZ, et al. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control. 2008;19:1085–1093. doi: 10.1007/s10552-008-9172-3. [DOI] [PubMed] [Google Scholar]
- 98.Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr Rev. 2011;69:432–448. doi: 10.1111/j.1753-4887.2011.00400.x. [DOI] [PubMed] [Google Scholar]
- 99.Xia Y, Chen M, Zhu P, et al. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ Int. 2013;59:161–167. doi: 10.1016/j.envint.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 100.Mumford SL, Kim S, Chen Z, et al. Urinary phytoestrogens are associated with subtle indicators of semen quality among male partners of couples desiring pregnancy. J Nutr. 2015;145:2535–2541. doi: 10.3945/jn.115.214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm Behav. 2004;45:270–277. doi: 10.1016/j.yhbeh.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Patisaul HB, Dindo M, Whitten PL, et al. Soy isoflavone supplements antagonize reproductive behavior and ERα- and ERβ-dependent gene expression in the brain. Endocrinology. 2001;142:2946–2952. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- 103.Patisaul HB, Melby M, Whitten PL, et al. Genistein affects ERβ-but not ERα-dependent gene expression in the hypothalamus. Endocrinology. 2002;143:2189–2197. doi: 10.1210/endo.143.6.8843. [DOI] [PubMed] [Google Scholar]
- 104.Martinez J, Lewi JE. An unusual case of gynecomastia associated with soy product consumption. Endocr Pract. 2008;14:415–418. doi: 10.4158/EP.14.4.415. [DOI] [PubMed] [Google Scholar]
- 105.Kotsopoulos D, Dalais FS, Liang YL, et al. The effects of soy protein containing phytoestrogens on menopausal symptoms in postmenopausal women. Climacteric. 2000;3:161–167. doi: 10.1080/13697130008500108. [DOI] [PubMed] [Google Scholar]
- 106.Lephart ED, Setchell KD, Handa RJ, et al. Behavioral effects of endocrine-disrupting substances: phytoestrogens. Ilar J. 2004;45:443–454. doi: 10.1093/ilar.45.4.443. [DOI] [PubMed] [Google Scholar]
- 107.Thomas AJ, Ismail R, Taylor-Swanson L, et al. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: a systematic review. Maturitas. 2014;78:263–276. doi: 10.1016/j.maturitas.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patisaul HB, Blum A, Luskin JR, et al. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav Neurosci. 2005;119:587–594. doi: 10.1037/0735-7044.119.2.587. [DOI] [PubMed] [Google Scholar]
- 109.Patisaul HB. Phytoestrogen action in the adult and developing brain. J Neuroendocrinol. 2005;17:57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- 110.Patisaul HB, Sullivan AW, Radford ME, et al. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS ONE. 2012;7:e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simon NG, Kaplan JR, Hu S, et al. Increased aggressive behavior and decreased affiliative behavior in adult male monkeys after long-term consumption of diets rich in soy protein and isoflavones. Horm Behav. 2004;45:278–284. doi: 10.1016/j.yhbeh.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 112.Hartley DE, Edwards JE, Spiller CE, et al. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- 113.Scallet AC, Wofford M, Meredith JC, et al. Dietary exposure to genistein increases vasopressin but does not alter beta-endorphin in the rat hypothalamus. Toxicol Sci. 2003;72:296–300. doi: 10.1093/toxsci/kfg029. [DOI] [PubMed] [Google Scholar]
- 114.Hrabovszky E, Kallo I, Hajszan T, et al. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 115.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patisaul HB, Scordalakes EM, Young LJ, et al. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 117.Nomura M, McKenna E, Korach K, et al. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Mol Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 118.Pfaff D. Drive. Cambridge, MASS: MIT Press; 1999. [Google Scholar]
- 119.Lephart ED, West T, Weber KS, et al. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 120.Lephart ED, Setchell KD, Lund TD. Phytoestrogens: hormonal action and brain plasticity. Brain Res Bull. 2005;65:193–198. doi: 10.1016/j.brainresbull.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 121.Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Res Rev. 2008;57:352–362. doi: 10.1016/j.brainresrev.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 122.Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertil Steril. 2008;89:e55–e56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 123.Gorski RA. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am J Physiol. 1963;205:842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- 124.Gorski RA. Sexual dimorphisms of the brain. J Anim Sci 61, Suppl. 1985;3:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- 125.Lindzey J, Korach KS. Developmental and physiological effects of estrogen receptor gene disruption in mice. Trends Endocrinol Metab. 1997;8:137–145. doi: 10.1016/s1043-2760(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 126.Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today. 2013;99:134–146. doi: 10.1002/bdrc.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 129.Aksglaede L, Sorensen K, Petersen JH, et al. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123:e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 130.Parent AS, Teilmann G, Juul A, et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 131.Mouritsen A, Aksglaede L, Sorensen K, et al. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int J Androl. 2010;33:346–359. doi: 10.1111/j.1365-2605.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 132.Zawatski W, Lee MM. Male pubertal development: are endocrine-disrupting compounds shifting the norms? J Endocrinol. 2013;218:R1–R12. doi: 10.1530/JOE-12-0449. [DOI] [PubMed] [Google Scholar]
- 133.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hauser R. The environment and male fertility: recent research on emerging chemicals and semen quality. Semin Reprod Med. 2006;24:156–167. doi: 10.1055/s-2006-944422. [DOI] [PubMed] [Google Scholar]
- 135.Joensen UN, Jorgensen N, Rajpert-De Meyts E, et al. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol. 2008;102:155–161. doi: 10.1111/j.1742-7843.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 136.North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. BJU Int. 2000;85:107–113. doi: 10.1046/j.1464-410x.2000.00436.x. [DOI] [PubMed] [Google Scholar]
- 137.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 138.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 139.Massart F, Parrino R, Seppia P, et al. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006;58:247–254. [PubMed] [Google Scholar]
- 140.Fowler PA, Bellingham M, Sinclair KD, et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 141.West MC, Anderson L, McClure N, et al. Dietary oestrogens and male fertility potential. Hum Fertil (Camb) 2005;8:197–207. doi: 10.1080/14647270500030266. [DOI] [PubMed] [Google Scholar]
- 142.Jefferson WN, Newbold RR. Potential endocrine-modulating effects of various phytoestrogens in the diet. Nutrition. 2000;16:658–662. doi: 10.1016/s0899-9007(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 143.Patisaul HB, Mabrey N, Adewale HB, et al. Soy but not bisphenol A (BPA) induces hallmarks of polycystic ovary syndrome (PCOS) and related metabolic co-morbidities in rats. Reprod Toxicol. 2014;49C:209–218. doi: 10.1016/j.reprotox.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 145.Jefferson WN, Padilla-Banks E, Goulding EH, et al. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod. 2009;80:425–431. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cederroth CR, Auger J, Zimmermann C, et al. Soy, phyto-oestrogens and male reproductive function: a review. Int J Androl. 2009;33:304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 147.Tan KA, Walker M, Morris K, et al. Infant feeding with soy formula milk: effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Hum Reprod. 2006;21:896–904. doi: 10.1093/humrep/dei421. [DOI] [PubMed] [Google Scholar]
- 148.Sharpe RM, Martin B, Morris K, et al. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 2002;17:1692–1703. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- 149.Eustache F, Mondon F, Canivenc-Lavier MC, et al. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117:1272–1279. doi: 10.1289/ehp.0800158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Delclos KB, Bucci TJ, Lomax LG, et al. Effects of dietary genistein exposure during development on male and female CD (Sprague–Dawley) rats. Reprod Toxicol. 2001;15:647–663. doi: 10.1016/s0890-6238(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 151.Delclos KB, Newbold R. NTP Toxicity Report of Reproductive Dose Range-Finding Study of Genistein (CAS No. 446-72-0) Administered in Feed to Sprague–Dawley Rats. Toxicity Report Series. 2007 1-C2. [PubMed] [Google Scholar]
- 152.Boberg J, Mandrup KR, Jacobsen PR, et al. Endocrine disrupting effects in rats perinatally exposed to a dietary relevant mixture of phytoestrogens. Reprod Toxicol. 2013;40:41–51. doi: 10.1016/j.reprotox.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Rudel RA, Fenton SE, Ackerman JM, et al. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119:1053–1061. doi: 10.1289/ehp.1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 155.Rebuli ME, Patisaul HB. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J Steroid Biochem Mol Biol. 2015;160:148–159. doi: 10.1016/j.jsbmb.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cooke B, Hegstrom C, Villeneuve L, et al. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 157.Ahmed EI, Zehr JL, Schulz KM, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sisk CL. Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Curr Opin Neurobiol. 2016;38:63–68. doi: 10.1016/j.conb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doerge DR, Churchwell MI, Chang HC, et al. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod Toxicol. 2001;15:105–110. doi: 10.1016/s0890-6238(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 160.Doerge DR, Twaddle NC, Churchwell MI, et al. Lactational transfer of the soy isoflavone, genistein, in Sprague–Dawley rats consuming dietary genistein. Reprod Toxicol. 2006;21:307–312. doi: 10.1016/j.reprotox.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 161.Chang HC, Churchwell MI, Delclos KB, et al. Mass spectrometric determination of genistein tissue distribution in diet-exposed Sprague–Dawley rats. J Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 162.Gorski RA, Gordon JH, Shryne JE, et al. Evidence for a morphological sex difference within the medial pre-optic area of the rat brain. Brain Res. 1978;143:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 163.Shughrue PL, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor α and β mRNA intherat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 164.Patchev AV, Gotz F, Rohde W. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB J. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- 165.Sakuma Y. Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol. 2009;21:410–414. [Google Scholar]
- 166.Scallet AC, Divine RL, Newbold RR, et al. Increased volume of the calbindin D28k-labeled sexually dimorphic hypothalamus in genistein and nonylphenol-treated male rats1. Toxicol Sci. 2004;82:570–576. doi: 10.1093/toxsci/kfh297. [DOI] [PubMed] [Google Scholar]
- 167.Lewis RW, Brooks N, Milburn GM, et al. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- 168.Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 169.Faber KA, Hughes CL., Jr Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotropin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod Toxicol. 1993;7:35–39. doi: 10.1016/0890-6238(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 170.Masutomi N, Shibutani M, Takagi H, et al. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–170. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 171.Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- 172.Cooke B, Hegstrom CD, Villeneuve LS, et al. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 173.Gorski RA, Mennin SP, Kubo K. The neural and hormonal bases of the reproductive cycle of the rat. Adv Exp Med Biol. 1975;54:115–153. doi: 10.1007/978-1-4684-8715-2_6. [DOI] [PubMed] [Google Scholar]
- 174.Elkind-Hirsch K, King JC, Gerall AA, et al. The luteinizing hormone-releasing hormone (LHRH) system in normal and estrogenized neonatal rats. Brain Res Bull. 1981;7:645–654. doi: 10.1016/0361-9230(81)90112-x. [DOI] [PubMed] [Google Scholar]
- 175.Semaan SJ, Kauffman AS. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol. 2010;20:424–431. doi: 10.1016/j.conb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res 57, Suppl. 2002;2:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 177.McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J Steroid Biochem Mol Biol. 2008;109:300–306. doi: 10.1016/j.jsbmb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–997. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Patisaul HB, Todd KL, Mickens JA, et al. Impact of neonatal exposure to the ERα agonist PPT, bisphenol-a or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;3:10. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–997. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Piet R, de Croft S, Liu X, et al. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol. 2015;36:15–27. doi: 10.1016/j.yfrne.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 184.Patisaul HB. Effects of environmental endocrine disruptors and phytoestrogens on the kisspeptin system. Adv Exp Med Biol. 2013;784:455–479. doi: 10.1007/978-1-4614-6199-9_21. [DOI] [PubMed] [Google Scholar]
- 185.Badger TM, Ronis MJ, Hakkak R, et al. The health consequences of early soy consumption. J Nutr. 2002;132:559S–565S. doi: 10.1093/jn/132.3.559S. [DOI] [PubMed] [Google Scholar]
- 186.Forsyth BW, McCarthy PL, Leventhal JM. Problems of early infancy, formula changes, and mothers’ beliefs about their infants. J Pediatr. 1985;106:1012–1017. doi: 10.1016/s0022-3476(85)80260-2. [DOI] [PubMed] [Google Scholar]
- 187.Barrett JR. The science of soy: what do we really know? Environ Health Perspect. 2006;114:A352–A358. doi: 10.1289/ehp.114-a352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121:1062–1068. doi: 10.1542/peds.2008-0564. [DOI] [PubMed] [Google Scholar]
- 189.Badger TM, Gilchrist JM, Pivik RT, et al. The health implications of soy infant formula. Am J Clin Nutr. 2009;89:1668S–1672S. doi: 10.3945/ajcn.2009.26736U. [DOI] [PubMed] [Google Scholar]
- 190.Barrett JR. Soy and children’s health: a formula for trouble. Environ Health Perspect. 2002;110:A294–A296. doi: 10.1289/ehp.110-a294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Goldman LR, Newbold R, Swan SH. Exposure to soy-based formula in infancy. JAMA. 2001;286:2402–2403. doi: 10.1001/jama.286.19.2402. [DOI] [PubMed] [Google Scholar]
- 192.Setchell KDR, Zimmer-Nechemias L, Cai J, et al. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 193.Tuohy PG. Soy infant formula and phytoestrogens. J Paediatr Child Health. 2003;39:401–405. doi: 10.1046/j.1440-1754.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 194.Zoppi G, Guandalini S. The story of soy formula feeding in infants: a road paved with good intentions. J Pediatr Gastroenterol Nutr. 1999;28:541–543. doi: 10.1097/00005176-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 195.Chen A, Rogan WJ. Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu Rev Nutr. 2004;24:33–54. doi: 10.1146/annurev.nutr.24.101603.064950. [DOI] [PubMed] [Google Scholar]
- 196.Franke AA, Custer LJ, Tanaka Y. Isoflavones in human breast milk and other biological fluids. Am J Clin Nutr. 1998;68:1466S–1473S. doi: 10.1093/ajcn/68.6.1466S. [DOI] [PubMed] [Google Scholar]
- 197.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 198.Mortensen A, Kulling SE, Schwartz H, et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res 53, Suppl. 2009;2:S266–S309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 199.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 200.Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc. 2009;109:245–254. doi: 10.1016/j.jada.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 201.Horn-Ross PL, Barnes S, Lee VS, et al. Reliability and validity of an assessment of usual phytoestrogen consumption (United States) Cancer Causes Control. 2006;17:85–93. doi: 10.1007/s10552-005-0391-6. [DOI] [PubMed] [Google Scholar]
- 202.Schwarz H, Sontag G, Plumb J. Inventory of phytoestrogen databases. Food Chem. 2009;113:736–747. [Google Scholar]
- 203.Service USDoAAR. Database for the Isoflavone Content of Selected Foods, Release 2.0. 2008 htpp://www.ars.usda.gov/nutrientdata/isoflav.
- 204.UK-Committee-on-Toxicity. Phytoestrogens and Health. Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. London: Food Standards Agency; 2003. [Google Scholar]
- 205.Thomas JM, Lutz SF. Soy protein lowers fat and saturated fat in school lunch beef and pork entrees. J Am Diet Assoc. 2001;101:461–463. doi: 10.1016/S0002-8223(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 206.Senti F. Soy protein foods in U.S. assistance programs. J Am Oil Chem Soc. 1974;51:138A–140A. [Google Scholar]
- 207.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 208.Piotrowska E, Jakobkiewicz-Banecka J, Wegrzyn G. Different amounts of isoflavones in various commercially available soy extracts in the light of gene expression-targeted isoflavone therapy. Phytother Res 24, Suppl. 2009;1:S109–113. doi: 10.1002/ptr.2944. [DOI] [PubMed] [Google Scholar]
- 209.Cao Y, Calafat AM, Doerge DR, et al. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol. 2009;19:223–234. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hutchins AM, Slavin JL, Lampe JW. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. J Am Diet Assoc. 1995;95:545–551. doi: 10.1016/S0002-8223(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 211.Markiewicz L, Garey J, Adlercreutz H, et al. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45:399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 212.Rolwand I, Wiseman H, Sanders T, et al. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 213.Verkasalo PK, Appleby PN, Allen NE, et al. Soya intake and plasma concentrations of daidzein and genistein: validity of dietary assessment among eighty British women (Oxford arm of the European Prospective Investigation into Cancer and Nutrition) Br J Nutr. 2001;86:415–421. doi: 10.1079/bjn2001424. [DOI] [PubMed] [Google Scholar]
- 214.Adlercreutz H, Yamada T, Wahala K, et al. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 215.Winter JSD, Hughes IA, Reyes FI, et al. Pituitary–gonadal relations in infancy: patterns of serum gonadal steroid concentrations in man from birth to two years of age. J Clin Endocrinol Metab. 1976;42:679–686. doi: 10.1210/jcem-42-4-679. [DOI] [PubMed] [Google Scholar]
- 216.Setchell KD, Zimmer-Nechemias L, Cai J, et al. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]