Abstract
Background
Generalized Anxiety Disorder (GAD) is one of the most common anxiety disorders treated in primary care, yet current therapies have limited efficacy and substantial side effects.
Purpose
To evaluate long-term chamomile (Matricaria chamomilla L.) use for prevention of GAD symptom relapse.
Methods
Outpatients from primary care practices and local communities with a primary diagnosis of moderate-to-severe GAD were enrolled for this two-phase study at a large US academic medical center. During Phase 1, eligible participants received 12 weeks of open-label therapy with chamomile pharmaceutical grade extract 1500 mg (500-mg capsule 3 times daily). During Phase 2, treatment responders were randomized to either 26 weeks of continuation chamomile therapy or placebo in a double-blinded, placebo-substitution design. The primary outcome was time to relapse during continuation therapy, analysed using Cox proportional hazards. Secondary outcomes included the proportion who relapsed, treatment-emergent adverse events, and vital sign changes. This study is registered at ClinicalTrials.gov, identifier NCT01072344.
Results
Between March 1, 2010, and June 30, 2015, we enrolled 179 participants. Of those, 93 (51.9%) were responders and agreed to continue in the double-blind randomized controlled trial. A numerically greater number of placebo-switched (n = 12/47; 25.5%) versus chamomile-continuation (n = 7/46; 15.2%) participants relapsed during follow-up. Mean time to relapse was 11.4 ± 8.4 weeks for chamomile and 6.3 ± 3.9 weeks for placebo. Hazard of relapse was non-significantly lower for chamomile (hazard ratio, 0.52; 95% CI, 0.20–1.33; P = 0.16). During follow-up, chamomile participants maintained significantly lower GAD symptoms than placebo (P = 0.0032), with significant reductions in body weight (P = 0.046) and mean arterial blood pressure (P = 0.0063). Both treatments had similar low adverse event rates.
Conclusions
Long-term chamomile was safe and significantly reduced moderate-to-severe GAD symptoms, but did not significantly reduce rate of relapse. Our limited sample size and lower than expected rate of placebo group relapse likely contributed to the non-significant primary outcome finding. Possible chamomile superiority over placebo requires further examination in large-scale studies.
Keywords: Chamomile, Herbal Medicine, Clinical Trials, Generalized Anxiety Disorder
Graphical abstract
Introduction
Generalized Anxiety Disorder (GAD) is characterized by excessive worry about daily matters and the presence of restlessness, fatigue, difficulty concentrating, irritability, muscle tension, and sleep problems (Andrews et al., 2010). GAD has a lifetime prevalence of approximately 5%, with nearly 9 million affected adults in the United States (Kessler et al., 2005). As the second most frequently treated psychiatric disorder in the primary care setting, GAD results in substantial distress and disability comparable only to that of the most frequently treated disorder, major depression (Kessler et al., 1999).
Current psychopharmacological treatments for GAD include benzodiazepines and selective serotonin-reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors (SSRI/SNRIs) (Reinhold and Rickels, 2015), but some patients do not respond to these therapies, while others cannot tolerate their side effects (Mitte et al., 2005). As a result, individuals suffering from anxiety commonly seek out complementary and integrative medicine, including herbal medicine products to treat this disorder (Bystritsky et al., 2012).
Chamomile (Matricaria chamomilla L.) is one of the most widely used herbal remedies in the world. It is included in the pharmacopoeia of 26 countries (Salamon, 1992). Limited basic science research suggests that chamomile and several of its flavonoid components may have anxiolytic and antidepressant activity (Nakazawa et al., 2003; Reis et al., 2006). Although chamomile is used extensively throughout the world as a calming agent, research in humans is very limited. In the only existing randomized controlled trial, our group found a significantly greater reduction in mean general anxiety symptom scores for chamomile as compared with placebo after 8 weeks of therapy (Amsterdam et al., 2009).
Recognizing that GAD is a recurrent disorder that often requires long-term therapy (Yonkers et al., 2003), we sought to extend our preliminary results by conducting a randomized, double-blind, placebo-substitution, long-term safety and efficacy study of chamomile therapy in GAD. Our primary aim was to examine if long-term chamomile therapy prolonged the time to relapse of anxiety symptoms following recovery from GAD, relative to placebo. Our secondary aim was to evaluate the relative safety and tolerability of long-term chamomile therapy as compared with placebo in participants who had recovered from GAD.
Materials and methods
Study Design
We conducted a randomized controlled trial from March 2010 through June 2015 at the Hospital of the University of Pennsylvania, an academic medical center in Philadelphia. After 12 weeks of open-label therapy with chamomile for moderate to severe GAD (Keefe et al Open-Label citation TBD), treatment responders were randomized to receive 26 weeks of either continuation chamomile or placebo in a double-blinded, placebo-substitution design. This is a well-established design strategy used in prior long-term efficacy trials of anxiety disorders (Allgulander et al., 2006; Amsterdam et al., 2004; Davidson et al., 2004; Gelenberg et al., 2000; Keller, 2002; Montgomery et al., 2004; Stocchi et al., 2003; Walker et al., 2000).The Institutional Review Board of the University of Pennsylvania approved the study protocol. We previously published the details of the protocol (Mao et al., 2014). This study is registered at ClinicalTrials.gov, number NCT01072344.
Participants
We recruited participants from primary care practices and local communities. Eligible patients were 18 years or older with a DSM-IV Axis-I diagnosis of GAD as their primary disorder as per the Structured Clinical Interview for Diagnosis of DSM-IV disorders (SCID-I) (First et al., 2001). Patients were also required to obtain a score of at least 10 on the GAD-7, a validated self-report measure of GAD symptomatology (Spitzer et al., 2006), and were rated by an assessor as moderately symptomatic or higher per the Clinical Global Impression (CGI) scale (Guy, 1976). Patients could not be currently taking an anti-anxiety medication (defined as benzodiazepines, buspirone, SSRIs, and SNRIs), antidepressant, mood stabilizer, or a tranquilizer for a prior DSM-IV Axis-I condition in remission, and/or alternative therapies (e.g., hypericum, valerian root, ginseng, chamomile tea). In addition, a list of concomitant medications was obtained from each patient during baseline and each study visit to evaluate the potential for herb-drug interactions. Patients with a psychotic-spectrum or bipolar-spectrum illness or who were active substance abusers were excluded. Women who were pregnant or nursing were also excluded. All enrolled patients provided written informed consent at their intake evaluation.
Randomization and masking
The total duration of the trial was 38 weeks. Participants were treated with 1500 mg (3 capsules daily) of open-label chamomile extract for up to 12 weeks. Individuals who had successfully attained a clinical response to treatment (defined as a reduction in GAD-7 score from baseline of at least 50% and a score of 3 or below on the CGI–Severity [CGI-S] scale) over 12 weeks were then randomized to either continuation chamomile or placebo for an additional 26 weeks. Randomization was stratified by gender with varying block sizes. Additional details of randomization and blinding procedures are in our published protocol (Mao et al., 2014), and briefly described below. The blinding methods used were determined to be successful in our prior chamomile study (Amsterdam et al., 2009). Participants meeting criteria for relapse or a new onset disorder were discontinued from the trial and treated as clinically warranted. No further data was collected upon their discontinuation.
Procedures
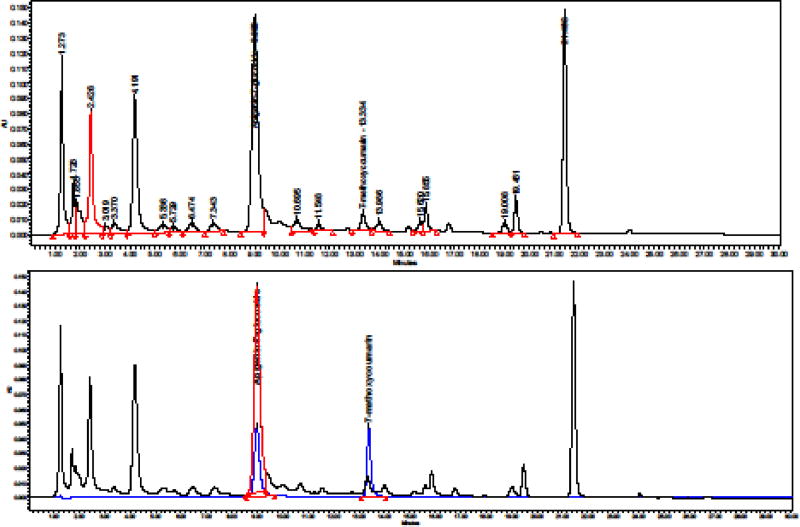
Each capsule contains 500 mg of extract (as dry extract) from Matricaria chamomilla L. flowers (equivalent to 2.0 g of German chamomile flowers), corresponding to 6 mg of total apigenin-7-glycosides (Api-7Glc). Extraction solvents were ethanol 70%, V/V and water (second extraction). Pharmaceutical grade German chamomile dry extract SHC-1 (DER 4:1, batch Nr 50053, Swedish Herbal Institute AB, Vallberga, Sweden) was standardized to a content of 1.2% Api-7Glc and 0.2–0,6% tetracoumaroyl spermine (TCS). The content of Api-7Glc in herbal substance and herbal preparation was analyzed according to European Pharmacopoeia 8.8 monograph 04/2016:0404 and according to the United States Pharmacopeia-30 which are quite similar. Api-7Glc is present in the herb both in free and esterified form. For measurement of total apigenin-7-glucosides, the herb and extracts were separately subjected to alkaline hydrolysis, in which various acetylated derivatives of Api-7Glc are converted to Api-7Glc. The hydrolysates were subjected to high performance liquid chromatography (HPLC) using the Waters Empower system. An HPLC fingerprint of the extract is shown in Fig. 1.
Fig. 1.
Upper panel: HPLC fingerprint of the extract after alkaline hydrolysis. Lower panel: Overlay of the chromatograms of the hydrolyzed extract with the chromatogram of the reference standards, USP Apigenin-7-glucoside RS and of 7-methoxycoumarin.
The content of Matricariae flower genuine extract (DERnative – 6.2 :1) in SHC-1 (containing 35% of maltodextrin as a carrier) comprises 65%. The samples of herbal substance and herbal preparations are retained at the manufacturer quality control laboratory. A current certificate of analysis of purity and suitability for human use was provided, and the product was approved for use in GAD in a “Safe to Proceed” letter by the Food and Drug Administration on December 17, 2009 (IND 107,206). Chamomile capsules were prepared and packaged by the University of Pennsylvania Investigational Drug Service under Good Manufacturing Practice Guidelines, in a HEPA-filtered ISO-8 production facility. A dose of 500 mg of dry extract SHC-1 was filled into a gelatin capsule shell without any additional filler. All participants in the open-label phase, and those subsequently randomized to receive the same active therapy versus placebo during the continuation phase, took 1500 mg of extract (500 mg per capsule). Pharmaceutical-grade lactose monohydrate NF (Spectrum® Quality Products, New Brunswick, NJ) was used as the placebo filling and packed in an identical capsule form to the chamomile capsule.
Participants in both chamomile and placebo groups were asked to take 3 capsules daily. Assessments of all outcome measures took place at study weeks 14, 16, 20, 28, and 38, corresponding to 2, 4, 8, 16, and 26 weeks post-randomization (Table 3 of published protocol) (Mao et al., 2014).
Outcome measures
Primary Outcome (Time to Relapse)
The CGI-S scale and the SCID-I were used to determine whether a patient had experienced GAD relapse. The CGI-S is an assessor-rated global measure of severity that correlates with other symptom severity outcome ratings, and has been validated in several trials across psychiatric conditions (Guy, 1976). Relapse was defined in the trial protocol as requiring 2 criteria: 1) an increase in the CGI-State score to a 4 (moderately symptomatic) or greater on 2 consecutively scheduled or unscheduled study visits at least 2 weeks apart; and 2) re-meeting DSM-IV criteria for GAD as per the SCID-I.
Secondary Outcomes (Symptoms)
GAD-specific symptomatology was assessed over the course of treatment using the GAD-7, described above. Overall patient quality of life and well-being was assessed using the Psychological General Well-Being Index (PGWBI), a validated patient-reported outcome (PRO) instrument assessing 6 quality of life domains that sum to an interpretable total score (Wiklund et al., 1992). In addition, general anxiety symptoms were assessed using the Hamilton Anxiety Rating Scale (HAM-A), a commonly used observer-rated outcome measure (Hamilton, 1959), and the Beck Anxiety Inventory (BAI), a well-validated PRO instrument (Beck et al., 1988).
Adverse events and side effects experienced by patients during the study were evaluated using the Treatment Emergent Symptom Scale, an assessor-rated measure (National Institute of Mental Health, 1985). Adverse event information was obtained via spontaneous subject report at assessments, assessor or doctor query, and changes in physical and laboratory findings. Any event determined to be at least possibly or probably related to treatment was considered an adverse event. Serum labs, urine tests, and ECGs were performed at Baseline, Week 8, and end-of-study participation to evaluate potential biological adverse events. Vital signs such as weight, blood pressure, and pulse were monitored at all study visits.
Statistical analyses
Analyses were conducted using STATA (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.) and SAS/STAT (Version 9.3, SAS Institute Inc.). The primary outcome of time to relapse was analyzed using Cox proportional hazards modeling to estimate the relative risk of relapse for treatment conditions. Secondary symptomatic outcomes and vital signs (weight, blood pressure) were analyzed using linear mixed-effect models (Raudenbush and Bryk, 2002). The fixed effects in the linear mixed-effects model for each secondary outcome were treatment, time, the treatment by time interaction, and baseline outcome. Subject-specific random intercepts were used to account for the correlation between repeated measures of each secondary outcome.
Prospective power analyses were conducted using the nQuery Advisor sample size software. Based on these analyses, we planned to enroll 180 participants in the trial. We estimated a 10% screen failure rate and a 40% non-response rate in the open-label phase, leading to an estimate of 90 participants entering the blinded randomization phase. With 45 participants per group, we estimated that our study would have 80% power to detect a difference between conditions at the .05 level using a 2-sided log-rank test, assuming the proportion of subjects who experienced a relapse by Week 38 was 55% for those taking placebo versus 26% for those taking chamomile. An external Data and Safety Monitoring Board oversaw the progress of the study and conducted study reviews at 6-month intervals.
Results
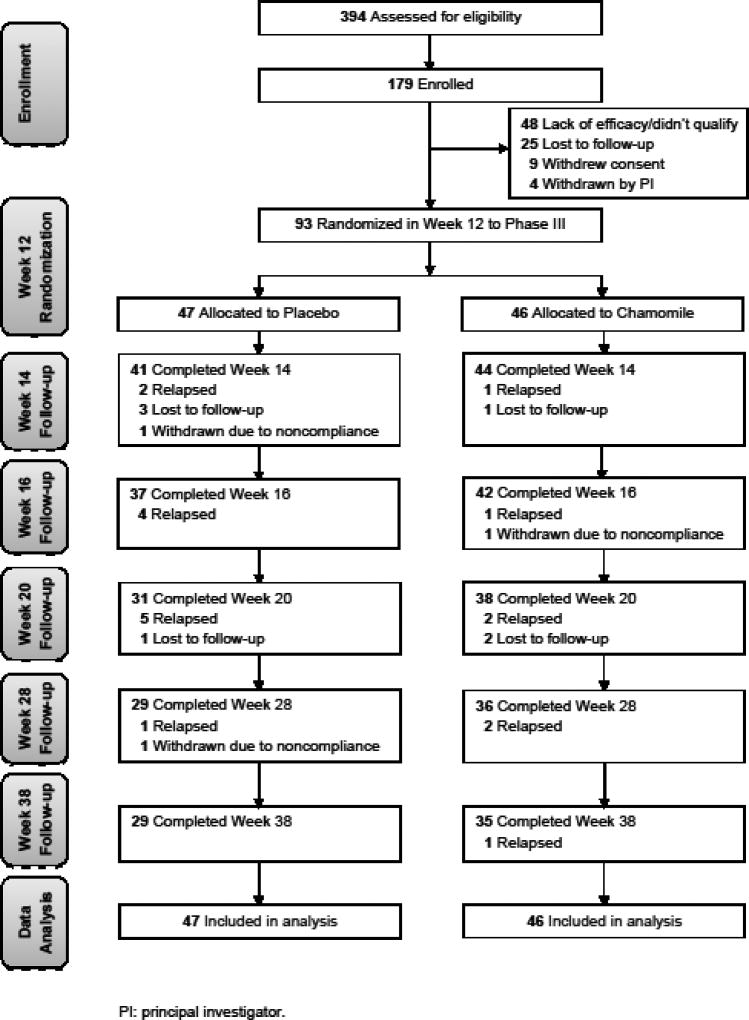
Between March 1, 2010, and June 30, 2015, we evaluated 394 individuals and enrolled a total of 179 participants. Of these, 93 (51.9%) met criteria for clinical response and were then randomized to either placebo (n = 47) or chamomile continuation (n = 46). Among participants who were randomized, 7 (7.5%) were lost to follow-up; 3 (3.2%) were withdrawn by the PI due to non-compliance; 19 (20.4%) met criteria for relapse; and 64 (68.8%) were no longer clinically ill by the Week 38 follow-up (Fig. 2, CONSORT diagram). Overall, participants were 95.7% compliant in the chamomile group and 97.8% compliant in the placebo group.
Fig. 2.
Screening, Randomization, and Completion of 38-Week Evaluations
Table 1 shows the demographics and clinical characteristics for open-label responders who entered the randomization phase. The mean age of the participants was 47.3 years (range, 19.7 to 73.9); 65 (69.9%) were women; 73 (78.5%) were white. At the time of enrollment, 86.0% had moderate GAD symptoms and 14.0% had moderately severe to severe GAD symptoms. The mean onset age of GAD was 22.0 years (range, 4 to 73) and the mean duration of the current GAD episode was 9.3 ± 14.2 years. At the time of randomization, all participant characteristics were well balanced.
Table 1.
Baseline characteristics of study participants.
| Variables | Placebo (n = 47) |
Chamomile (n = 46) |
|---|---|---|
|
| ||
| Baseline Enrollment | ||
| Sex, n (%) | ||
| Men | 16 (34) | 12 (26) |
| Women | 31 (66) | 34 (74) |
| Race, n (%)a | ||
| White | 37 (79) | 36 (78) |
| Non-white | 10 (21) | 10 (22) |
| Age, y | 45.4±16.1 | 49.2±14.3 |
| Age at 1st GAD episode | 20.9±14.4 | 23.2±17.1 |
| Duration of current GAD episode, y | 9.0±15.9 | 9.6±12.7 |
| GAD-7 scoreb | 13.1±2.4 | 13.9±3.0 |
| HAM-A scorec | 14.6±3.1 | 14.7±3.7 |
| CGI-S Anxiety, n (%)d | ||
| Moderate | 41 (87) | 39 (85) |
| Moderately severe | 6 (13) | 6 (13) |
| Severe | 0 | 1 (2) |
| BAI scoree | 15.3±9.2 | 17.4±9.4 |
| PGWBIf | ||
| Anxiety | 9.5±3.6 | 9.0±4.0 |
| PGWBI total | 55.6±12.7 | 53.7±14.9 |
|
| ||
| Randomization | ||
|
| ||
| GAD-7 score | 2.9±1.8 | 2.8±1.6 |
| HAM-A score | 2.6±2.0 | 2.6±1.9 |
| CGI-S Anxiety, No. (%) | ||
| Not ill | 34 (72) | 34 (74) |
| Borderline | 9 (19) | 10 (22) |
| Mild | 4 (9) | 2 (4) |
| BAI score | 4.0±3.3 | 4.1±3.8 |
| PGWBI | ||
| Anxiety | 17.6±3.7 | 17.8±3.1 |
| PGWBI total | 80.4±14.8 | 82.5±11.0 |
BAI: Beck Anxiety Inventory; CGI-S: Clinical Global Impression-Severity; GAD: Generalized Anxiety Disorder; HAM-A: Hamilton Anxiety Rating Scale; PGWBI: Psychological General Well-Being Index.
Majority of non-white participants are African American.
GAD-7 is a 7-item, patient-rated measure of GAD that is linked to DSM IV-TR criteria and validated via the MINI-SCID. Scores range from 0–21, with higher scores indicating more anxious mood.
HAM-A is a psychological questionnaire used by clinicians to rate the severity of a patient's anxiety. It consists of 14 items, and the total score ranges from 0–56, with higher scores indicating more severe anxiety symptoms.
CGI-S is a 7-point scale that requires the clinician to rate the severity of the patient's illness at the time of assessment. Scores range from 1–7, with higher scores indicating more severe symptoms.
BAI is a 21-question multiple choice self-report inventory that is used for measuring severity of anxiety. Total scores range from 0–63, with higher scores indicating more severe anxiety.
PGWBI is a 22-item self-report instrument used to assess health and quality of life of the general population and people with chronic disease. The higher the score, the better the quality of life and general well-being.
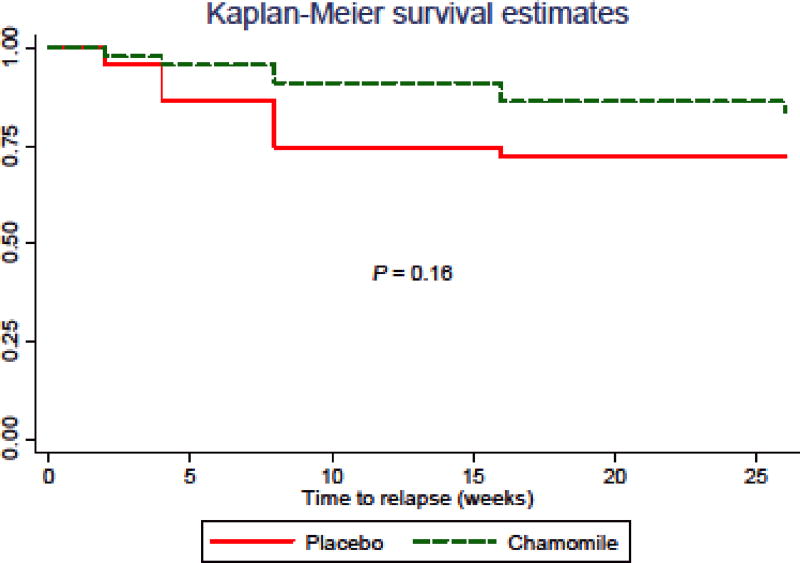
During the 26-week follow-up period after randomization, 7 (15.2%) in the chamomile group compared with 12 (25.5%) in the placebo group relapsed (P = 0.15). The mean time to relapse was 11.4 ± 8.4 weeks in the chamomile group versus 6.3 ± 3.9 weeks for placebo. Median survival time was not available for either group because only a small percentage of subjects actually experienced a relapse. Compared with placebo, chamomile was associated with a non-significant reduction in relapse of GAD symptoms (hazard ratio, 0.52; 95% CI, 0.20–1.33; P = 0.16; Fig. 3).
Fig. 3.
Time to Relapse of GAD Symptoms Between Chamomile and Placebo
Relative to participants randomized to placebo, those who continued on chamomile experienced a lesser increase in GAD-7 symptoms (P = 0.0032) and had overall better psychological well-being (P = 0.013), specifically in anxiety impacts on well-being (P = 0.0094). Anxiety symptoms measured by the HAM-A and BAI showed the same trends, but were not significant (Table 2).
Table 2.
Changes in psychological outcomes.
| Variables | Mean change from baseline (95% CI) | P valuea | |
|---|---|---|---|
| Placebo (n = 47) | Chamomile (n = 46) | ||
| GAD-7b | 0.0032 | ||
| Week 2 | 2.3 (1.1 to 3.5) | 0.2 (−0.4 to 0.7) | 0.0047 |
| Week 4 | 2.0 (0.8 to 3.2) | 0.4 (−0.4 to 1.2) | 0.016 |
| Week 8 | 1.1 (−0.2 to 2.4) | 1.2 (−0.06 to 2.5) | 0.81 |
| Week 16 | 0.6 (−0.7 to 1.9) | 0.8 (−0.2 to 1.9) | 0.84 |
| Week 26 | −0.4 (−1.2 to 0.4) | 0.5 (−0.4 to 1.5) | 0.55 |
|
| |||
| HAM-Ab | 0.27 | ||
| Week 2 | 2.0 (0.9 to 3.1) | 0.8 (0.1 to 1.4) | 0.15 |
| Week 4 | 2.8 (1.5 to 4.2) | 1.3 (0.3 to 2.4) | 0.06 |
| Week 8 | 1.5 (0.1 to 2.9) | 1.0 (−0.3 to 2.4) | 0.42 |
| Week 16 | 0.5 (−0.9 to 2.0) | 0.4 (−0.7 to 1.6) | 0.63 |
| Week 26 | −0.4 (−1.3 to 0.4) | 0.1 (−0.9 to 1.1) | 0.74 |
|
| |||
| BAIb | 0.089 | ||
| Week 2 | 1.5 (−0.03 to 3.0) | 0.1 (−0.8 to 1.0) | 0.17 |
| Week 4 | 1.4 (−0.4 to 3.1) | 0.2 (−1.0 to 1.3) | 0.19 |
| Week 8 | 0.08 (−1.4 to 1.6) | 0.6 (−1.2 to 2.4) | 0.67 |
| Week 16 | −0.8 (−2.0 to 0.4) | 0.7 (−0.9 to 2.4) | 0.23 |
| Week 26 | −0.8 (−1.7 to 0.08) | −0.2 (−1.1 to 0.7) | 0.65 |
|
| |||
| PGWBIc | |||
| Anxiety | 0.0094 | ||
| Week 2 | −2.2 (−3.7 to −0.6) | −0.7 (−1.8 to 0.4) | 0.23 |
| Week 4 | −3.2 (−4.9 to −1.6) | −0.5 (−1.7 to 0.6) | 0.0088 |
| Week 8 | −1.2 (−2.8 to 0.4) | −1.6 (−3.1 to −0.1) | 0.94 |
| Week 26 | 0.2 (−1.5 to 2.0) | −1.0 (−2.5 to 0.5) | 0.39 |
|
| |||
| PGWBI Global | 0.013 | ||
| Week 2 | −5.4 (−9.8 to −0.9) | −1.6 (−4.8 to 1.6) | 0.31 |
| Week 4 | −6.1 (−10.8 to −1.3) | −2.2 (−6.0 to 1.6) | 0.21 |
| Week 8 | −1.6 (−6.6 to 3.3) | −6.9 (−12.1 to −1.6) | 0.15 |
| Week 26 | 2.7 (−2.9 to 8.4) | −3.2 (−7.9 to 1.5) | 0.12 |
BAI: Beck Anxiety Inventory; GAD: Generalized Anxiety Disorder; HAM-A: Hamilton Anxiety Rating Scale; PGWBI: Psychological General Well-Being Index.
P values were calculated using the mixed-effects model.
GAD-7, HAM-A, BAI: Increasing scores indicates worsening anxiety.
An increase in all PGWBI domains represents improvement in symptoms.
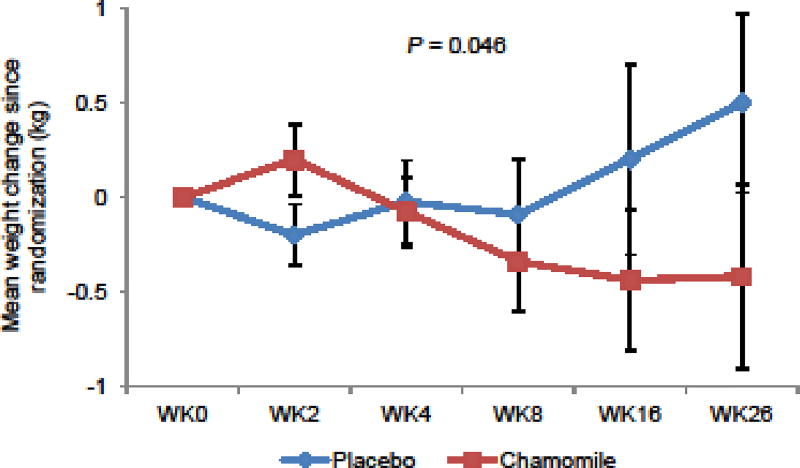
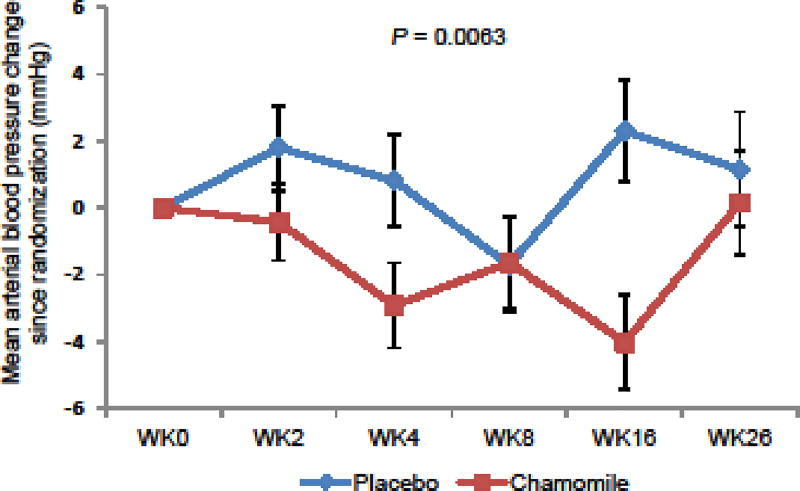
Table 3 shows adverse events experienced by participants. In the randomization phase, 8 (17.4%) in the chamomile group versus 9 (19.1%) in the placebo group experienced possibly or probably related adverse events (P = 0.22). All adverse events were graded mild and did not require any medical treatment. No participants were withdrawn due to adverse events. During the 26-week continuation phase, the chamomile group had a relatively lower weight than the placebo group (P = 0.046; Fig. 4). The chamomile group also had significantly lower mean arterial blood pressure (P = 0.0063; Fig. 5) and systolic pressure (P = 0.0012), and had a non-significant trend toward lower diastolic pressure (P = 0.057). No difference in pulse change was observed between groups. No ECG or laboratory abnormalities were observed.
Table 3.
AE profiles between treatment groups.
| Placebo (n = 47) |
Chamomile (n = 46) |
P valuea | |
|---|---|---|---|
| Subjects experiencing an AE, n (%) | 9 (19) | 8 (17) | 0.22 |
| AEs | |||
| Bruising | 2 | 0 | |
| Congestion | 1 | 1 | |
| Decreased platelet count | 2 | 0 | |
| Diarrhea | 0 | 1 | |
| Dizziness | 0 | 1 | |
| Drowsiness | 0 | 1 | |
| Dry mouth | 0 | 2 | |
| Fatigue | 0 | 1 | |
| Flushing | 0 | 1 | |
| Herbal taste | 0 | 1 | |
| Nausea | 0 | 3 | |
| Rash/Itchy skin | 1 | 0 | |
| Ringing in ears | 1 | 0 | |
| Sleep paralysis | 1 | 0 | |
| Taste perversion | 2 | 1 | |
| Urinary frequency | 0 | 1 | |
| Vivid dream | 1 | 0 | |
| Wobbly leg | 0 | 1 |
AE: adverse event. Some patients experienced >1 AE.
P value was calculated using a chi-square test.
Fig. 4.
Mean Weight Change by Treatment Groups
Fig. 5.
Mean Arterial Blood Pressure Change by Treatment Groups
Discussion
GAD is a common chronic psychological disorder seen in primary and mental health care settings. Current psychopharmacological treatments have both limited efficacy and substantial side effects (Mitte et al., 2005; Reinhold and Rickels, 2015), and many patients self-medicate with herbal supplements to alleviate chronic anxiety (McIntyre et al., 2015). To our knowledge, very little research has been conducted to date that evaluates the long-term safety and efficacy of herbal medicines for anxiety. In this 38-week randomized placebo-controlled trial, we found that among responders to chamomile therapy, the magnitude of difference (approximately 50%) in time to relapse between chamomile and placebo, our primary endpoint, was clinically important even though it did not reach statistical significance. Secondary outcomes of the GAD-7 and PGWBI showed supportive findings as continuation-chamomile participants experienced lower GAD-specific symptoms and better psychological well-being relative to those switched to placebo. Further, long-term intake of 1500 mg of chamomile extract appeared to be safe, with potentially desirable weight and blood pressure profiles compared with placebo. In addition, rates of acute response to chamomile in the open-label phase were in the range of pharmaceutical response reported in the literature (Mitte et al., 2005). Taken together, these results suggest that further study is warranted in a future adequately-powered trial to clarify whether long-term chamomile extract may be developed into a safe and effective therapeutic agent for treatment of GAD.
Our trial contributes to a relatively small number of studies that have examined whether long-term use of medications can prevent the relapse of GAD symptoms. Most prior pharmaceutical trials of GAD responders who either received long-term placebo or switched from active to placebo treatment have reported much higher rates of relapse over a similar time-course (40–70%) (Allgulander et al., 2006; Davidson et al., 2008; Gelenberg et al., 2000; Rickels et al., 2010; Stocchi et al., 2003), with some exceptions (Montgomery et al., 2002; Stein et al., 2012). Rates of relapse in both groups observed in our trial (15.2% chamomile; 25.5% placebo) were overall lower than rates commonly reported in the literature and assumed in power analysis calculation for this trial. Our limited sample size and lower than expected rate of placebo group relapse may have contributed to our inability to find a statistically significant difference between the placebo and chamomile group.
To our knowledge, there has been no research done to evaluate the long-term safety of high-dose chamomile oral extract. Our data suggest oral intake of pharmaceutical grade chamomile is safe, with few mild side effects that are statistically and clinically indistinguishable from placebo. There were no signs of sexual side effects or weight gain, both of which are common in SSRI/SNRIs and can lead to treatment discontinuation (Fava, 2000; Montejo et al., 2001). On the contrary, we observed modest weight loss and decreased blood pressure in the chamomile group relative to placebo, a finding that needs to be confirmed in future studies. Chamomile’s side effect profile may be particularly attractive to patients who cannot tolerate SSRI/SNRIs, or to those who prefer natural products or refuse conventional pharmaceutics due to stigma and other socio-cultural reasons (Kessler et al., 2001).
Several study limitations need to be acknowledged. First, as mentioned above, our relatively low rate of placebo group relapse compared with that previously reported in the literature contributed to our inability to identify a statistically significant difference between chamomile and placebo. Second, participants were removed from the trial as they met criteria for relapse; this resulted in a disproportionate distribution of missing data in the 2 groups for the long-term follow-up, which may have introduced bias in estimating the effects of our continuous secondary outcomes (eg, GAD-7, PGWBI, weight, blood pressure). Third, this was a single-institution study, which limits the generalizability of our findings. Lastly, given the safety signals, we don’t know whether an even higher dose of chamomile would produce an even more robust clinical response.
Conclusions
Our study is the first long-term placebo-controlled trial of chamomile conducted in humans. Chamomile appeared to be safe, with few mild side effects that were indistinguishable from placebo. Continued use of chamomile was associated with a non-significant reduction in GAD relapse among responders to initial therapy, in addition to significantly better GAD symptoms and improved psychological well-being. Furthermore, long-term chamomile use may be associated with improved blood pressure and weight profiles. These promising long-term results need to be confirmed in a well-powered multicenter clinical trial to establish chamomile oral extract as a safe and effective therapy for patients with GAD.
Acknowledgments
We thank all of the clinical coordinators and research assistants for their dedication to clinical trial coordination, data collection, and management. We also thank Kenneth Rockwell Jr, PharmD, MS, Director of Research Pharmacy, University of Pennsylvania School of Medicine and the staff at the Penn Investigational Drug Service for their contributions in study drug preparation and dispensing. We thank the Swedish Herbal Institute for processing, producing, and providing the standardized oral chamomile extract product. We also thank our Data Safety and Monitoring Board members, Drs Andy Nierenberg and Sara Ratcliffe, for volunteering their time and expertise. We thank Christina Seluzicki and Ingrid Haviland for their editorial assistance in the preparation of this manuscript, which was funded by the aforementioned grants for this study. Sincere thanks go to all the patients who participated in this study. The funding agencies had no role in the design, conduct, or analysis of the study, or the decision to submit the manuscript for publication.
Funding: This study is supported by grants from the National Institutes of Health (NIH) / National Center for Complementary and Integrative Health [Grant Number R01 AT005074], and NIH / National Cancer Institute [Cancer Center Support Grant Number P30 CA008748].
Abbreviations
- BAI
Beck Anxiety Inventory
- CGI-S
Clinical Global Impression–Severity
- GAD
Generalized Anxiety Disorder
- HAM-A
Hamilton Anxiety Rating Scale
- PGWBI
Psychological General Well-Being Index
- PRO
Patient-reported outcome
- SCID-I
Structured Clinical Interview for Diagnosis of DSM-IV Axis I Disorders
- SNRI
Serotonin-norepinephrine reuptake inhibitors
- SSRI
Serotonin-reuptake inhibitors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration Number: NCT01072344 at ClinicalTrials.gov
FDA: IND 107,206
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- Allgulander C, Florea I, Huusom AK. Prevention of relapse in generalized anxiety disorder by escitalopram treatment. Int. J. Neuropsychopharmacol. 2006;9:495–505. doi: 10.1017/S1461145705005973. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Brunswick DJ, Gibertini M. Sustained efficacy of gepirone-IR in major depressive disorder: a double-blind placebo substitution trial. J. Psychiatr. Res. 2004;38:259–265. doi: 10.1016/j.jpsychires.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J. Clin. Psychopharmacol. 2009;29:378–382. doi: 10.1097/JCP.0b013e3181ac935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Hobbs MJ, Borkovec TD, Beesdo K, Craske MG, Heimberg RG, Rapee RM, Ruscio AM, Stanley MA. Generalized worry disorder: a review of DSM-IV generalized anxiety disorder and options for DSM-V. Depress. Anxiety. 2010;27:134–147. doi: 10.1002/da.20658. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Hovav S, Sherbourne C, Stein MB, Rose RD, Campbell-Sills L, Golinelli D, Sullivan G, Craske MG, Roy-Byrne PP. Use of complementary and alternative medicine in a large sample of anxiety patients. Psychosomatics. 2012;53:266–272. doi: 10.1016/j.psym.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress. Anxiety. 2004;19:234–240. doi: 10.1002/da.10146. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Wittchen HU, Llorca PM, Erickson J, Detke M, Ball SG, Russell JM. Duloxetine treatment for relapse prevention in adults with generalized anxiety disorder: a double-blind placebo-controlled trial. Eur. Neuropsychopharmacol. 2008;18:673–681. doi: 10.1016/j.euroneuro.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Fava M. Weight gain and antidepressants. J. Clin. Psychiatry. 2000;61(Suppl 11):37–41. [PubMed] [Google Scholar]
- First MS, Spitzer L, Gibbon M, Williams J. Biometrics Research. New York State Psychiatric Institute; New York, NY: 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/P W/PSY Screen) [Google Scholar]
- Gelenberg AJ, Lydiard RB, Rudolph RL, Aguiar L, Haskins JT, Salinas E. Efficacy of venlafaxine extended-release capsules in nondepressed outpatients with generalized anxiety disorder: a 6-month randomized controlled trial. JAMA. 2000;283:3082–3088. doi: 10.1001/jama.283.23.3082. [DOI] [PubMed] [Google Scholar]
- Guy W. Assessment manual for psychopharmacology. DHEW publication ADM. 1976:76–338. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Keller MB. The long-term clinical course of generalized anxiety disorder. J. Clin. Psychiatry. 2002;63(Suppl 8):11–16. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, DuPont RL, Berglund P, Wittchen HU. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am. J. Psychiatry. 1999;156:1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, Van Rompay MI, Eisenberg DM. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am. J. Psychiatry. 2001;158:289–294. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Li QS, Soeller I, Rockwell K, Xie SX, Amsterdam JD. Long-term chamomile therapy of generalized anxiety disorder: a study protocol for a randomized, double-blind, placebo-controlled trial. J Clin Trials. 2014;4:188. doi: 10.4172/2167-0870.1000188. doi:110.4172/2167-0870.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre E, Saliba AJ, Moran CC. Herbal medicine use in adults who experience anxiety: A qualitative exploration. International journal of qualitative studies on health and well-being. 2015;10:29275. doi: 10.3402/qhw.v10.29275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitte K, Noack P, Steil R, Hautzinger M. A meta-analytic review of the efficacy of drug treatment in generalized anxiety disorder. J. Clin. Psychopharmacol. 2005;25:141–150. doi: 10.1097/01.jcp.0000155821.74832.f9. [DOI] [PubMed] [Google Scholar]
- Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J. Clin. Psychiatry. 2001;62(Suppl 3):10–21. [PubMed] [Google Scholar]
- Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. Int. Clin. Psychopharmacol. 2004;19:271–280. doi: 10.1097/01.yic.0000137184.64610.c8. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Sheehan DV, Meoni P, Haudiquet V, Hackett D. Characterization of the longitudinal course of improvement in generalized anxiety disorder during long-term treatment with venlafaxine XR. J. Psychiatr. Res. 2002;36:209–217. doi: 10.1016/s0022-3956(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Yasuda T, Ueda J, Ohsawa K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003;26:474–480. doi: 10.1248/bpb.26.474. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. Treatment emergent symptoms scale. Psychopharmacological Bulletin. 1985;21:1069–1073. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2. Sage Publications; Thousand Oaks, California: 2002. [Google Scholar]
- Reinhold JA, Rickels K. Pharmacological treatment for generalized anxiety disorder in adults: an update. Expert Opin. Pharmacother. 2015;16:1669–1681. doi: 10.1517/14656566.2015.1059424. [DOI] [PubMed] [Google Scholar]
- Reis LS, Pardo PE, Oba E, Kronka Sdo N, Frazatti-Gallina NM. Matricaria chamomilla CH12 decreases handling stress in Nelore calves. J. Vet. Sci. 2006;7:189–192. doi: 10.4142/jvs.2006.7.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels K, Etemad B, Khalid-Khan S, Lohoff FW, Rynn MA, Gallop RJ. Time to relapse after 6 and 12 months' treatment of generalized anxiety disorder with venlafaxine extended release. Arch. Gen. Psychiatry. 2010;67:1274–1281. doi: 10.1001/archgenpsychiatry.2010.170. [DOI] [PubMed] [Google Scholar]
- Salamon I. Chamomile, a medicinal plant. Herb Spice Med Plant Digest. 1992;10:1–4. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Ahokas A, Albarran C, Olivier V, Allgulander C. Agomelatine prevents relapse in generalized anxiety disorder: a 6-month randomized, double-blind, placebo-controlled discontinuation study. J. Clin. Psychiatry. 2012;73:1002–1008. doi: 10.4088/JCP.11m07493. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Nordera G, Jokinen RH, Lepola UM, Hewett K, Bryson H, Iyengar MK. Efficacy and tolerability of paroxetine for the long-term treatment of generalized anxiety disorder. J. Clin. Psychiatry. 2003;64:250–258. doi: 10.4088/jcp.v64n0305. [DOI] [PubMed] [Google Scholar]
- Walker JR, Van Ameringen MA, Swinson R, Bowen RC, Chokka PR, Goldner E, Johnston DC, Lavallie YJ, Nandy S, Pecknold JC, Hadrava V, Lane RM. Prevention of relapse in generalized social phobia: results of a 24-week study in responders to 20 weeks of sertraline treatment. J. Clin. Psychopharmacol. 2000;20:636–644. doi: 10.1097/00004714-200012000-00009. [DOI] [PubMed] [Google Scholar]
- Wiklund I, Holst J, Karlberg J, Mattsson LA, Samsioe G, Sandin K, Uvebrant M, von Schoultz B. A new methodological approach to the evaluation of quality of life in postmenopausal women. Maturitas. 1992;14:211–224. doi: 10.1016/0378-5122(92)90116-l. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Bruce SE, Dyck IR, Keller MB. Chronicity, relapse, and illness--course of panic disorder, social phobia, and generalized anxiety disorder: findings in men and women from 8 years of follow-up. Depress. Anxiety. 2003;17:173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]