ABSTRACT
Mycobacterium lepraemurium is the causative agent of murine leprosy, a chronic, granulomatous disease similar to human leprosy. Due to the similar clinical manifestations of human and murine leprosy and the difficulty of growing both bacilli axenically, Mycobacterium leprae and M. lepraemurium were once thought to be closely related, although it was later suggested that M. lepraemurium might be related to Mycobacterium avium. In this study, the complete genome of M. lepraemurium was sequenced using a combination of PacBio and Illumina sequencing. Phylogenomic analyses confirmed that M. lepraemurium is a distinct species within the M. avium complex (MAC). The M. lepraemurium genome is 4.05 Mb in length, which is considerably smaller than other MAC genomes, and it comprises 2,682 functional genes and 1,139 pseudogenes, which indicates that M. lepraemurium has undergone genome reduction. An error-prone repair homologue of the DNA polymerase III α-subunit was found to be nonfunctional in M. lepraemurium, which might contribute to pseudogene formation due to the accumulation of mutations in nonessential genes. M. lepraemurium has retained the functionality of several genes thought to influence virulence among members of the MAC.
KEYWORDS: Mycobacterium lepraemurium, comparative genomics, genome sequencing, murine leprosy
IMPORTANCE
Mycobacterium lepraemurium seems to be evolving toward a minimal set of genes required for an obligatory intracellular lifestyle within its host, a niche seldom adopted by most mycobacteria, as they are free-living. M. lepraemurium could be used as a model to elucidate functions of genes shared with other members of the MAC. Its reduced gene set can be exploited for studying the essentiality of genes in related pathogenic species, which might lead to discovery of common virulence factors or clarify host-pathogen interactions. M. lepraemurium can be cultivated in vitro only under specific conditions and even then with difficulty. Elucidating the metabolic (in)capabilities of M. lepraemurium will help develop suitable axenic media and facilitate genetic studies.
OBSERVATION
Murine leprosy is a chronic, granulomatous disease caused by Mycobacterium lepraemurium. Murine leprosy mainly affects the skin, mucosa of the upper respiratory tract, and eyes; however, unlike human leprosy, the viscera are commonly affected, whereas the peripheral nerves are not (1). Murine leprosy was first reported in the early 20th century in rats in Ukraine (2), after which similar cases were reported from other countries (3, 4). M. lepraemurium is one of the causative agents of leprosy in cats and causes granulomatous skin lesions that often involve ulceration (5).
In humans, leprosy is primarily caused by Mycobacterium leprae and Mycobacterium lepromatosis. Numerous similarities exist between human and murine leprosy, including disease transmission through abrasions in the skin and the mucosal respiratory surfaces, a similar spectrum of disease progression, suppression of cell-mediated immunity, and strong humoral immunity (1). This led to the hypothesis that these species were closely related, and hence, it was thought that murine leprosy might serve as a model for human leprosy (6–8). DNA hybridization studies and analysis of the 16S rRNA gene sequences suggested that M. lepraemurium might actually be more related to the Mycobacterium avium complex (MAC) (9, 10). However, to date, no phylogenomic study of M. lepraemurium has been conducted, and the lack of a genome sequence has restricted our understanding of its biology and evolution.
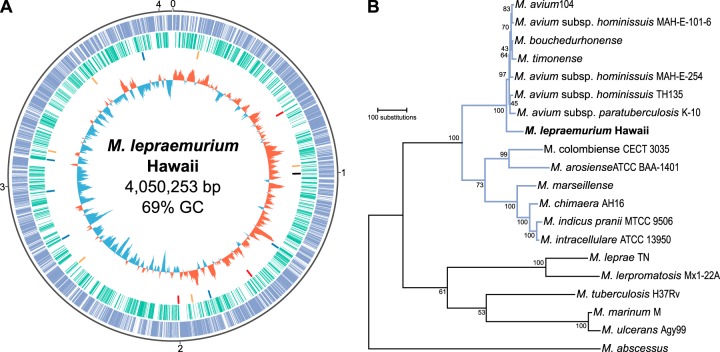
Here, we describe the complete genome of M. lepraemurium, which was sequenced using single-molecule real-time (SMRT; Pacific Biosciences) and Illumina technologies. The M. lepraemurium genome was found to be circular, with a total GC content of 68.99%, and 4,050,523 bp in length. No plasmids were found. The genome comprises 3,821 “protein-coding” genes, of which 2,682 are functional genes and 1,139 are pseudogenes (Fig. 1A). M. lepraemurium belongs to the MAC and is more closely related to the M. avium clade than to the M. intracellulare clade (Fig. 1B and see Fig. S1 and S2 in the supplemental material).
FIG 1 .
The genome of Mycobacterium lepraemurium strain Hawaii. (A) Graphical representation of the genome and its features. The origin of replication is at 12 o’clock, and the genome sequence runs clockwise. Ticks around the outermost circle mark million bases. Looking inwards, the outermost track or the first track (blue) shows functional genes. The second track (green) shows pseudogenes. The third track shows insertion sequences (all dysfunctional) colored to distinguish families (orange, red, black, and blue) and exaggerated in size for visibility. The fourth track shows the GC skew, calculated for a 20-kb window sliding every 1 kb, represented as a histogram with positive values pointing outward (red bars) and negative values pointing inward (blue bars). (B) Maximum parsimony tree of M. lepraemurium and selected mycobacterial species. The tree was created using MEGA7 from concatenated amino acid sequences (3,936 positions) of 11 proteins (DnaN, RplI, GrpE, MetG, RplY, PheT, FtsQ, HolA, MiaA, FtsY, and FtsX). Branches corresponding to the Mycobacterium avium complex are in blue. Bootstrap support, estimated from 500 replicates, is given below each branch. An expanded version of this tree, including additional genomes, is in Fig. S1 in the supplemental material.
Maximum parsimony tree of M. lepraemurium and other mycobacterial species. The tree was created using MEGA7 from concatenated amino acid sequences (3,948 positions) of 11 proteins (DnaN, RplI, GrpE, MetG, RplY, PheT, FtsQ, HolA, MiaA, FtsY, and FtsX). Bootstrap support, estimated from 500 replicates, is given below each branch. Download FIG S1, EPS file, 0.2 MB (202KB, eps) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogeny of M. lepraemurium and selected mycobacterial species based on whole-genome sequence alignments. Species belonging to the M. avium complex are highlighted in blue and M. lepraemurium is denoted in red. M. abscessus was used as the outgroup. (A) Maximum likelihood tree. The tree was created using RAxML based on 460,625 variable nucleotide sites and a general time reversible (GTR) model with gamma distribution. Bootstrap support estimated from 100 replicates is given below each branch. (B) Neighbor-joining tree. The tree was created using MEGA7 based on 460,625 variable nucleotide sites and the p-distance method. Bootstrap support estimated from 500 replicates is given below each branch. (C) Maximum parsimony tree. The tree was created using MEGA7 based on 460,625 variable nucleotide sites and the SPR algorithm. Bootstrap support estimated from 500 replicates is given below each branch. Download FIG S2, EPS file, 0.2 MB (167.5KB, eps) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genome downsizing and pseudogene formation.
At 4.05 Mb, the M. lepraemurium genome is the smallest within the MAC and one of the smallest among mycobacteria (9th out of the 350 sequenced mycobacterial species). More strikingly, within the mycobacteria, only the M. leprae and M. lepromatosis genomes contain fewer functional protein-coding genes than the M. lepraemurium genome. The presence of 1,139 pseudogenes indicates that M. lepraemurium underwent reductive evolution, which is characteristic for strictly host-associated organisms. Analyses of pseudogene families within a diverse set of prokaryotes have shown that pseudogenes are most likely to occur in ABC transporter, short-chain dehydrogenase/reductase, sugar transporter, cytochrome P450, and proline-glutamate (PE)/proline-proline-glutamate (PPE) gene families (11). The M. lepraemurium genome was found to contain pseudogenes in all these families.
M. lepraemurium is the third mycobacterial species known to have undergone reductive evolution. The other species include the common ancestor of M. leprae and the closely related M. lepromatosis, which underwent genome reduction before the two species diverged, as well as Mycobacterium ulcerans, which is in a state of intermediate reductive genome evolution (12–14). Genome size among M. avium subspecies varies considerably (around 4.8 to 5.5 Mb), but no extensive pseudogenization was observed in these organisms. Curiously, M. lepraemurium seems to be evolving convergently toward a minimal gene set such as the one retained by M. leprae (Fig. 2). This may be due to both species having adapted to a similar niche, consequently resulting in similar pathological manifestations.
FIG 2 .
Heatmap of the gene orthology between M. avium subsp. paratuberculosis K-10, M. lepraemurium, M. leprae, and M. ulcerans. Genes are shown in black, and the absence of genes is shown in white. The raw height corresponds to the number of genes.
Loss of the DnaQ-mediated proofreading mechanism of DNA polymerase III α-subunit has been hypothesized as the cause of pseudogene formation in M. leprae (12), and a similar pattern has been observed in other obligate pathogens and symbionts (15, 16). In M. lepraemurium, a homologue of the error-prone repair DNA polymerase III α-subunit (MLM_3495) is nonfunctional, which might contribute to a higher error rate leading to pseudogene formation in this species.
The AT content of the pathogen/symbiont genome seems to correlate with the age of the strict association with the host (16). As expected, the genome of M. leprae has the highest AT content of all mycobacteria (42.2%), especially in pseudogenes (43.5%). On the other hand, the genome of M. lepraemurium is as AT-poor as that of its closest relative, M. avium (31%), and shows only a small difference in the AT content of functional genes and pseudogenes (30.46% and 31.05%, respectively). This suggests that M. lepraemurium adopted the “strictly intracellular” niche relatively recently, as evidenced by its phylogenetic relatedness to the free-living M. avium (Fig. S1). Genomic data for other M. lepraemurium strains would allow us to determine whether the pseudogene content varies between strains, which would indicate ongoing reductive evolution.
Repetitive sequences and mobile elements.
Repeats and mobile elements are the major vehicles leading to genomic rearrangements and large deletions, and hence, they are thought to play an important role in genome downsizing. Yet, there seems to be a negative correlation between the stage of reductive evolution and repeat content (17). This is likely because deletions and pseudogenization impact fitness as more genes become nonfunctional, and the remaining genes become indispensable. Therefore, it has been suggested that repeats might be involved in the early phase of reductive evolution and that a different mechanism is responsible for the gradual deletion of pseudogenes (17). M. lepraemurium has only a few repeats (Table S1), which mostly derive from three families of dysfunctional insertion sequences (IS), each consisting of up to six copies.
M. lepraemurium repetitive genomic regions. Download TABLE S1, DOCX file, 0.01 MB (15.4KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Curiously, one IS family consisting of six copies (MLM_1414, MLM_1873, MLM_2691, MLM_2913, MLM_3078, and MLM_3876) shows 84% nucleotide identity with the ISMsm2 mobile element from Mycobacterium smegmatis (NCBI accession number WP_003887303). A BLAST search showed that this IS family resides in only a few other unrelated mycobacteria and not in the MAC, with the exception of a more diverged copy (with 78% identity) present in the plasmid of M. avium subsp. hominissuis strain 88Br (GenBank accession number KR997898.1) and a truncated copy (with 81% identity) in the genome of M. avium subsp. hominissuis strain H87 (GenBank accession number CP018363.1). Strikingly, when we analyzed the genomic synteny between M. lepraemurium, M. intracellulare, and M. avium subsp. hominissuis, all the synteny breaks in M. lepraemurium occurred at the locations of this particular IS (Fig. S3A and B). The synteny plot between M. lepraemurium and M. avium subsp. paratuberculosis looked different from the aforementioned genomes, which is probably due to genomic rearrangements in M. avium subsp. paratuberculosis (Fig. S3C).
Synteny plots between M. lepraemurium and selected members of the Mycobacterium avium complex. Genome sequences are represented as colored ideograms. Numbered ticks mark million bases. Transposable elements are shown as ticks in the second track, with the ISMsm2-like family in magenta. Inner links are LAST hits (see Text S1 in the supplemental material for details). Download FIG S3, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The presence of identical copies of dysfunctional transposable elements indicates that M. lepraemurium lost functional transposases relatively recently. As mentioned above, at least one IS family was responsible for large genomic rearrangements in M. lepraemurium, and a deeper analysis of the genome sequence might shed light on the driving force that caused major deletions in this genome.
M. lepraemurium-specific sequences.
Comparison of M. lepraemurium with other members of the MAC revealed a total of 32.2 kb of genomic sequence that was not found in any publicly available genome sequence. These regions contain 35 genes, of which only 12 are functional (Table S2). One of these genes, MLM_3300, codes for a Fic family protein. Fic proteins have been associated with pathogenicity in bacteria, often acting as toxins that interfere with the host cell in different ways (18).
M. lepraemurium-specific genomic regions and genes. Download TABLE S2, DOCX file, 0.01 MB (16KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Interaction with macrophages.
After entering the macrophage, members of the MAC reside within phagosomes (19). There they inhibit phagosome maturation by preventing acidification to a pH below 6.4, and thus, prevent fusion with the extremely acidic lysosome. In M. avium, mutations in the PPE gene MAV_2928 and the PE gene MAV_1346 inhibit maturation and acidification of phagosomes, resulting in decreased virulence (20, 21). In M. lepraemurium, gene MLM_2357 (homologous to MAV_2928) and MLM_1265 (homologous to MAV_1346) are functional, suggesting that they may help its survival in macrophages. Additionally, the gene MLM_2012 encodes the lipoprotein, LppM, which is an important virulence factor in M. tuberculosis and interferes with phagosomal maturation in macrophages (22).
Reactive oxygen species (ROS) are produced by the macrophage as a bactericidal mechanism in response to infection. The ability of pathogens to produce enzymes such as catalase-peroxidase, epoxide hydrolase, and superoxide dismutase (SOD), which remove ROS, enable their survival within macrophages. While it has been suggested that M. lepraemurium abolishes the production of ROS upon entering the macrophage (29), probably as a survival mechanism, it remains unclear whether small quantities of ROS are produced upon infection and handled by the bacterium. M. lepraemurium has retained four functional epoxide hydrolases (MLM_0642, MLM_0684, MLM_1194, and MLM_1485) and one catalase-peroxidase (MLM_2092), which explains its observed catalase-peroxidase activity (23). Moreover, M. lepraemurium is able to produce two superoxide dismutases, SodA and SodC (MLM_0123 and MLM_2650).
Glycopeptidolipid synthesis.
Glycopeptidolipids (GPLs) are synthesized by several nontuberculosis mycobacteria, including members of the MAC. GPLs are present on the outermost layer of the cell wall, and therefore, they play an important role in sliding motility, biofilm formation, and pathogenesis (24). This is even more relevant within the MAC, whose members can produce a number of serovar-specific variations of GPLs. The M. lepraemurium cell wall contains glycolipids and amino acids (25, 26); however, production of GPLs in M. lepraemurium has not been studied. Most of the genes known to be involved in the biosynthesis of non-serovar-specific GPLs are intact in M. lepraemurium, albeit with a few exceptions (Table S3). Although it is difficult at this moment to predict the effects of these mutations, it appears that M. lepraemurium should be able to produce GPLs, as all of the core genes are present.
Genes implicated in the biosynthesis of GPLs in the Mycobacterium avium complex. Download TABLE S3, DOCX file, 0.02 MB (17.9KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Virulence.
While the ESX-1 system is the main determinant of virulence in M. tuberculosis and M. leprae (27), it is absent in the MAC. However, ESX-5, which is also associated with virulence in pathogenic mycobacteria, is mostly intact in M. lepraemurium, except for the cytochrome P450 hydroxylase (MLM_2361), which is also nonfunctional in M. leprae. The duplicated four-gene region, ESX-5a, encoding EsxI, EsxJ, a PPE, and a PE protein may serve as an accessory system for transport of a subset of ESX-5 proteins (28) and is still functional in M. lepraemurium. In M. lepraemurium, the PPE and PE genes are merged into a single open reading frame, as in M. avium subsp. paratuberculosis but not in other members of the MAC. An interesting observation is that in MAC, esxI and esxJ are 100% identical to esxN and esxM from the main ESX-5 locus (unlike in M. tuberculosis), which indicates a novel and recent duplication event and suggests that a crucial function might lie behind redundancy of the ESX-5 components.
The pks12 gene is involved in the biosynthesis of mannosyl-β-1-phosphomycoketides (MPM) and is found only in the slow-growing mycobacteria. In different pathogenic mycobacteria, including M. avium (20), pks12 was shown to be necessary for the virulence, and this gene is functional in M. lepraemurium (MLM_2156).
Experimental procedures.
M. lepraemurium strain Hawaii was grown in BALB/c mice. The DNA was sequenced using Illumina and PacBio technologies, followed by sequence assembly and annotation. More details are given in Text S1 in the supplemental material.
Supplemental Materials and Methods. Download TEXT S1, DOCX file, 0.03 MB (33.6KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Accession number(s).
The annotated genome was submitted to GenBank under GenBank accession number CP021238.
ACKNOWLEDGMENTS
We thank the Genomic Technologies Facility at the University of Lausanne, Switzerland, for Illumina and PacBio sequencing. We thank Philippe Busso (École Polytechnique Fédérale de Lausanne [EPFL], Lausanne, Switzerland) for helping with the DNA extraction.
This work was supported by the Fondation Raoul Follereau and the Swiss National Science Foundation grant IZRJZ3_164174.
Footnotes
Citation Benjak A, Honap TP, Avanzi C, Becerril-Villanueva E, Estrada-García I, Rojas-Espinosa O, Stone AC, Cole ST. 2017. Insights from the genome sequence of Mycobacterium lepraemurium: massive gene decay and reductive evolution. mBio 8:e01283-17. https://doi.org/10.1128/mBio.01283-17.
Contributor Information
Roland Brosch, Institut Pasteur.
Christina L. Stallings, Washington University in St. Louis School of Medicine.
REFERENCES
- 1.Rojas-Espinosa O. 2009. Murine leprosy revisited, p 97–140. In Tomioka H (ed), Current topics on the profiles of host immunological response to mycobacterial infections, 1st ed. Research Signpost, Trivandrum, Kerala, India. [Google Scholar]
- 2.Stefansky WK. 1902. Zabolevanija u krys, vyzvannyja kislotoupornoj palotsjkoj. Rus Vratsj 47:1726–1727. [Google Scholar]
- 3.Marchoux E, Sorel F. 1912. Recherches sur la lèpre: la lèpre des rats (Lepra murium). Ann Inst Pasteur 56:778–801. [Google Scholar]
- 4.Dean G. 1903. A disease of the rat caused by an acid-fast bacillus. Cent F Bakteriol 34:222–224. [Google Scholar]
- 5.O’Brien CR, Malik R, Globan M, Reppas G, McCowan C, Fyfe JA. 2017. Feline leprosy due to Mycobacterium lepraemurium. J Feline Med Surg 19:737–746. doi: 10.1177/1098612X17706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker EL, Sweeney MA. 1929. The identity of human leprosy and rat leprosy. J Prev Med 3:325–333. [Google Scholar]
- 7.Schmitt LS. 1911. On the relation between rat and human leprosy. Pathologe 2:29–37. [Google Scholar]
- 8.Dean G. 1905. Further observations on a leprosy-like disease of the rat. J Hyg 5:99–112. doi: 10.1017/S0022172400002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athwal RS, Deo SS, Imaeda T. 1984. Deoxyribonucleic acid relatedness among Mycobacterium leprae, Mycobacterium lepraemurium, and selected bacteria by dot blot and spectrophotometric deoxyribonucleic acid hybridization assays. Int J Syst Bacteriol 34:371–375. doi: 10.1099/00207713-34-4-371. [DOI] [Google Scholar]
- 10.Hughes MS, Ball NW, Beck LA, De Lisle GW, Skuce RA, Neill SD. 1997. Determination of the etiology of presumptive feline leprosy by 16S rRNA gene analysis. J Clin Microbiol 35:2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Harrison PM, Kunin V, Gerstein M. 2004. Comprehensive analysis of pseudogenes in prokaryotes: widespread gene decay and failure of putative horizontally transferred genes. Genome Biol 5:R64. doi: 10.1186/gb-2004-5-9-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream M-A, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 13.Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, Nieselt K, Krause J, Vera-Cabrera L, Cole ST. 2015. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc Natl Acad Sci U S A 112:4459–4464. doi: 10.1073/pnas.1421504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, Tichit M, Porter JL, Ryan J, Johnson PDR, Davies JK, Jenkin GA, Small PLC, Jones LM, Tekaia F, Laval F, Daffé M, Parkhill J, Cole ST. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale C, Wang B, Moran N, Ochman H. 2003. Loss of DNA recombinational repair enzymes in the initial stages of genome degeneration. Mol Biol Evol 20:1188–1194. doi: 10.1093/molbev/msg138. [DOI] [PubMed] [Google Scholar]
- 16.Moya A, Peretó J, Gil R, Latorre A. 2008. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet 9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 17.Frank AC, Amiri H, Andersson SGE. 2002. Genome deterioration: loss of repeated sequences and accumulation of junk DNA. Genetica 115:1–12. doi: 10.1023/A:1016064511533. [DOI] [PubMed] [Google Scholar]
- 18.Roy CR, Cherfils J. 2015. Structure and function of Fic proteins. Nat Rev Microbiol 13:631–640. doi: 10.1038/nrmicro3520. [DOI] [PubMed] [Google Scholar]
- 19.Ignatov D, Kondratieva E, Azhikina T, Apt A. 2012. Mycobacterium avium-triggered diseases: pathogenomics. Cell Microbiol 14:808–818. doi: 10.1111/j.1462-5822.2012.01776.x. [DOI] [PubMed] [Google Scholar]
- 20.Li Y-J, Danelishvili L, Wagner D, Petrofsky M, Bermudez LE. 2010. Identification of virulence determinants of Mycobacterium avium that impact on the ability to resist host killing mechanisms. J Med Microbiol 59:8–16. doi: 10.1099/jmm.0.012864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Miltner E, Wu M, Petrofsky M, Bermudez LE. 2005. A Mycobacterium avium PPE gene is associated with the ability of the bacterium to grow in macrophages and virulence in mice. Cell Microbiol 7:539–548. doi: 10.1111/j.1462-5822.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 22.Deboosère N, Iantomasi R, Queval CJ, Song OR, Deloison G, Jouny S, Debrie AS, Chamaillard M, Nigou J, Cohen-Gonsaud M, Locht C, Brodin P, Veyron-Churlet R. 2017. LppM impact on the colonization of macrophages by Mycobacterium tuberculosis. Cell Microbiol 19:e12619. doi: 10.1111/cmi.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lygren ST, Closs O, Bercouvier H, Wayne LG. 1986. Catalases, peroxidases, and superoxide dismutases in Mycobacterium leprae and other mycobacteria studied by crossed immunoelectrophoresis and polyacrylamide gel electrophoresis. Infect Immun 54:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang L, Tian X, Pan W, Xie J. 2013. Structure and function of mycobacterium glycopeptidolipids from comparative genomics perspective. J Cell Biochem 114:1705–1713. doi: 10.1002/jcb.24515. [DOI] [PubMed] [Google Scholar]
- 25.Draper P. 1971. The walls of Mycobacterium lepraemurium: chemistry and ultrastructure. J Gen Microbiol 80:313–324. [DOI] [PubMed] [Google Scholar]
- 26.Luna-Herrera J, Rojas-Espinosa O, Estrada-Parra S. 1996. Recognition of lipid antigens by sera of mice infected with Mycobacterium lepraemurium. Int J Lepr Other Mycobact Dis 64:299–305. [PubMed] [Google Scholar]
- 27.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. 2016. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol 14:677–691. doi: 10.1038/nrmicro.2016.131. [DOI] [PubMed] [Google Scholar]
- 28.Shah S, Briken V. 2016. Modular organization of the ESX-5 secretion system in Mycobacterium tuberculosis. Front Cell Infect Microbiol 6:49. doi: 10.3389/fcimb.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas-Espinosa O, Camarena-Servin V, Estrada-Garcia I, Arce-Paredes P, Wek-Rodriguez K. 1998. Mycobacterium lepraemurium, a well-adapted parasite of macrophages: I. Oxygen metabolites. Int J Lepr Other Mycobact Dis 66:365–373. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum parsimony tree of M. lepraemurium and other mycobacterial species. The tree was created using MEGA7 from concatenated amino acid sequences (3,948 positions) of 11 proteins (DnaN, RplI, GrpE, MetG, RplY, PheT, FtsQ, HolA, MiaA, FtsY, and FtsX). Bootstrap support, estimated from 500 replicates, is given below each branch. Download FIG S1, EPS file, 0.2 MB (202KB, eps) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phylogeny of M. lepraemurium and selected mycobacterial species based on whole-genome sequence alignments. Species belonging to the M. avium complex are highlighted in blue and M. lepraemurium is denoted in red. M. abscessus was used as the outgroup. (A) Maximum likelihood tree. The tree was created using RAxML based on 460,625 variable nucleotide sites and a general time reversible (GTR) model with gamma distribution. Bootstrap support estimated from 100 replicates is given below each branch. (B) Neighbor-joining tree. The tree was created using MEGA7 based on 460,625 variable nucleotide sites and the p-distance method. Bootstrap support estimated from 500 replicates is given below each branch. (C) Maximum parsimony tree. The tree was created using MEGA7 based on 460,625 variable nucleotide sites and the SPR algorithm. Bootstrap support estimated from 500 replicates is given below each branch. Download FIG S2, EPS file, 0.2 MB (167.5KB, eps) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
M. lepraemurium repetitive genomic regions. Download TABLE S1, DOCX file, 0.01 MB (15.4KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Synteny plots between M. lepraemurium and selected members of the Mycobacterium avium complex. Genome sequences are represented as colored ideograms. Numbered ticks mark million bases. Transposable elements are shown as ticks in the second track, with the ISMsm2-like family in magenta. Inner links are LAST hits (see Text S1 in the supplemental material for details). Download FIG S3, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
M. lepraemurium-specific genomic regions and genes. Download TABLE S2, DOCX file, 0.01 MB (16KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genes implicated in the biosynthesis of GPLs in the Mycobacterium avium complex. Download TABLE S3, DOCX file, 0.02 MB (17.9KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental Materials and Methods. Download TEXT S1, DOCX file, 0.03 MB (33.6KB, docx) .
Copyright © 2017 Benjak et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.