Abstract
Introduction
Cannabis users have been reported to have decreased regional cerebral glucose metabolism after short periods of abstinence. The purpose of this study was to measure striatal dopamine receptor (D2/D3) availability and cerebral glucose metabolism with positron emission tomography (PET) in young adults who had a prolonged exposure to cannabis and who had been abstinent for a period of at least 12 weeks.
Materials and methods
Six 18–21-year-old male subjects with cannabis dependence in early full remission and six age- and sex-matched healthy subjects underwent PET scans for D2/D3 receptor availability measured with [C11]-raclopride and glucose metabolism measured with [18F]-FDG. All subjects were sober for at least 12 weeks before PET scan procedures. PET data were analyzed with statistical parametric mapping software (SPM99; uncorrected p<0.001, corrected p<0.05 at the cluster level). Toxicology screening was performed prior to the PET scan to confirm the lack of drugs of abuse.
Observation and results
Striatal D2/D3 receptor availability did not differ significantly between groups. Compared to controls, subjects with cannabis dependence had lower normalized glucose metabolism in the right orbitofrontal cortex, putamen bilaterally, and precuneus. There were no significant correlations between striatal D2/D3 receptor availability and normalized glucose metabolism in any region of the frontal cortex or striatum.
Conclusion
These findings may reflect both cannabis exposure and adaptive changes that occur after a prolonged period of abstinence. Subsequent studies should address whether metabolic and dopamine receptor effects are associated with either active use or longer-term withdrawal in these relatively young subjects.
Keywords: Cannabis, Dependence, Glucose metabolism, D2/D3 receptor, ([18F]-FDG, [11C]-raclopride
Introduction
Cannabis is the most widely used illicit drug among young adults (SAMHSA 2006). The mechanisms underlying the addictive effects of cannabis are not well understood. Converging evidence from animal and human studies suggests that dysfunction of the dopaminergic brain reward circuit is involved in the various aspects of drug addiction, including reinforcing responses to drugs during intoxication, activation during craving, and deactivation during withdrawal (Kalivas 2002). In the dopaminergic reward circuit, the ascending mesocorticolimbic projections of the ventral tegmental area (VTA) dopaminergic neurons appear to be a common substrate for the rewarding properties of many drugs of abuse (including tetrahydrocannabinol (THC) and cannabinomimetic drugs). These drugs increase cell firing in VTA dopaminergic neurons and dopamine release in terminal areas such as the nucleus accumbens and prefrontal cortex (for reviews, see for example Gardner 2005; Koob et al. 1998; Wise and Bozarth 1985). It has been suggested that the dopamine activation in the striatum involves direct interactions between the dopamine D2 receptors and type 1 cannabinoid (CB1) receptors. The highest densities of CB1 receptors are in the substantia nigra, cerebellum, hippocampus, striatum, and frontal cortex, and there is a high co-localization of the dopamine and CB1 receptors in neurons of the basal ganglia and of the limbic cortex (Glass et al. 1997; Herkenham et al. 1990; Mailleux and Vanderhaeghen 1992). The endogenous cannabinoid, anandamide, is released in the striatum during neural activity and appears to be a primary component of a network of neurally active substances that regulate striatal function. Functional interactions between the endogenous cannabinoid anandamide and dopamine suggest a possible participation of the endogenous cannabinoid system in disorders that involve dysregulated dopamine neurotransmission (Giuffrida et al. 1999). The mesocortical dopamine circuit, which includes the prefrontal, anterior cingulate cortices, and orbitofrontal cortices, is likely to be involved in the conscious experience of drug intoxication, drug incentive salience, drug expectation or craving, and compulsive drug administration (Volkow and Fowler 2000). The mesolimbic system including the nucleus accumbens, amygdala, and hippocampus is thought to be involved in drug reward and conditioned responses (Koob and Bloom 1988). Thus, cannabinoid receptors are located within the target regions of the reward circuit, particularly in regions innervated by dopamine, and may have a modulatory role on dopamine function. These preclinical data suggest that the evaluation of the functional circuitry affected by cannabis dependence and alterations in D2/D3 receptor availability is a logical step for the present study.
It has been suggested that decreases in dopamine (D2) receptors and dopamine release cause a decreased sensitivity of reward circuits to stimulation by natural rewards (Noble et al. 1991; Volkow et al. 2002). This hypothesis is consistent with functional imaging studies integrating measures of cerebral glucose metabolism and striatal dopamine (D2) receptor availability in adult subjects dependent on cocaine (Volkow et al. 1993) and methamphetamine (Volkow et al. 2001). These methods have enabled investigators to evaluate in human subjects the integrity of mesocortical and mesolimbic dopamine circuits implicated in preclinical studies of addiction that are difficult to image directly. A logical application of these functional imaging methods is to evaluate whether this hypothesis applies to cannabis. The rationale for neuroimaging studies of the dopamine system in cannabis-dependent subjects is further strengthened by the neurobiological evidence for the neuromodulatory role of cannabinoids with respect to dopamine (Gardner 2005).
Previous neuroimaging studies suggest that brain metabolic activity is decreased in prefrontal areas and cerebellum in chronic cannabis users compared to normal controls after short periods of abstinence less than 1 month (Amen and Waugh 1998; Block et al. 2000; Lundqvist et al. 2001; Volkow et al. 1996a). However, the pattern of cerebral metabolism after prolonged periods of abstinence (more than 1 month) has not been studied in cannabis-use disorders. Previous studies have reported reduced D2/D3 receptor availability measured with [11C]-raclopride in subjects with cocaine- (Volkow et al. 1993; 1997), opiate-(Wang et al. 1997), and alcohol- (Volkow et al. 1996b) use disorders. There do not appear to be any published imaging studies evaluating dopamine D2/D3 receptor availability in cannabis-use disorders after prolonged periods of abstinence.
Thus, the purpose of this pilot study was to examine cerebral D2/D3 receptor availability and glucose metabolism in young adults within a narrow age range (18–21-year olds) who have a history of prolonged exposure to cannabis and who have been abstinent for a period of time long enough to avoid imaging the acute withdrawal effects of cannabis. We postulated that a history of prolonged cannabis exposure in adolescence is associated with persistent (1) decreased striatal D2/D3 receptor availability and (2) decreased glucose metabolism in regions that comprise the dopaminergic brain reward circuits compared to control subjects even after prolonged periods of abstinence.
Materials and methods
Subject screening and selection
Male subjects with cannabis dependence in early full remission were recruited from a therapeutic community through referrals from clinicians and counselors. During their stay, they were closely monitored for any psychoactive substance use, and the residential program included regular and random urine toxicology. Male comparison subjects were recruited through advertisements in local community centers to obtain subjects with demographic characteristics similar to those of subjects in the cannabis group.
After a complete description of the study to the subjects, written informed consent was obtained according to procedures established by the Institutional Review Board and the Radiation Safety Committee of the North Shore–Long Island Jewish Health System. Then, subjects underwent laboratory testing, toxicology screening, psychiatric and substance abuse assessments, and Magnetic Resonance Imaging (MRI) Scan (GE 1.5T). Subjects were excluded based upon a history of current and past psychiatric, neurological, or significant medical illness, abnormal laboratory testing (including blood glucose level higher than 115 mg/dl), and abuse of prescription or over the counter medications (e.g., antihistamines, cold medications) or herbal supplements with central nervous system effects within the past month. Inclusion criteria for the cannabis group were (1) a Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) diagnosis of cannabis dependence in early full remission, (2) no current or past dependence on any other psychoactive drugs other than cannabis including alcohol (subjects with caffeine and nicotine dependence were eligible for this study), (3) cannabis use for at least 1 year before age 18, and (4) an average of three or more joints (i.e., the equivalent of 1.5 grams or more of cannabis) smoked per week, (5) sober for any substance of abuse for more than 1 month, (6) no current DSM-IV axis I disorder besides substance abuse.
Behavioral and imaging assessments
Assessments were conducted in three sessions. The first session included an interview with the Structural Clinical Interview for DSM-IV-TR Axis I Disorders (SCID), the Comprehensive Addiction Severity Inventory for Adolescents (CASI-A; Meyers et al. 1995), and an analog scale assessing craving. Based on the interview, the rater determined the age of first cannabis use and onset of DSM-IV criteria for cannabis dependence. Subjects had blood pressure, height, weight, and laboratory tests taken (routine blood work, urine analysis, and toxicology). All participants had a Urine Multidrug Screen Test Kit (detecting THC, opiates, amphetamine and metamphetamine, PCP, and cocaine). During the second session, subjects had a brain MRI to define regions of interest that were copied to PET images. During the third session, subjects had urine toxicology, craving, withdrawal symptoms, mood, and anxiety ratings with visual analog scales used in previous studies (Wang et al. 1999), depression rating with the Hamilton Rating Scales for Depression and PET scans.
Clinical assessments for substance-use disorders included available medical records, review of socioeconomic and familial background, self-report, and collateral information (health care providers, case managers, family members, friends, and housemates when available), and assessments of substance use using the substance-use module of the SCID and CASI-A interviews. To assess extent of cannabis use, the age of onset, duration and frequency of use, number of joints smoked per week, and cannabis gram–years (calculated as the product of the average daily cannabis use and duration of cannabis use since onset of cannabis dependence) were documented.
Neuroimaging procedures
The PET scans were performed in the Functional Brain Imaging Laboratory at North Shore University Hospital. In one scan session, the subjects underwent one scan to measure D2/D3 receptor availability using [11C]-raclopride and one scan to measure cerebral glucose metabolism using [18F]-2-deoxy-2-fluoro-D-glucose ([18F]-FDG). We measured [11C]-raclopride binding potential and glucose metabolism during resting state to avoid the potential variability related to the degree of subject engagement during an activation task. The radiotracers were synthesized according to published methods (Chaly et al. 1990; Farde et al. 1988). The General Electric (GE) Advance tomograph was used for the PET scans. The performance characteristics of the scanner have been determined previously (Dhawan et al. 1998). Subjects were instructed not to eat, smoke, or drink caffeinated beverages after midnight of the day preceding the PET scan procedures and had urine toxicology before PET scanning.
For the [11C]-raclopride scan, subjects were positioned in the GE Advance scanner after placement of an intravenous line in an antecubital vein. A 10-min transmission scan was obtained. Then, a 60-min dynamic PET data acquisition began upon intravenous injection of [11C]-raclopride (15 mCi±10%). The scan protocol for [11C]-raclopride involved five scans of 1 min, followed by seven scans of 5 min and two scans of 10 min. Then, the patient was taken out of the scanner. After a break at the end of the first scan to permit for adequate decay of the carbon-11 (at least five half lives of the carbon-11), the [18F]-FDG study began. Five millicurie (±10%) of [18F]-FDG was injected as an intravenous bolus to measure glucose metabolism. During the uptake interval, the subject sat in a darkened quiet room with eyes open and ears unoccluded. A single venous blood glucose sample was obtained at 20 min post-injection from an antecubital vein in the arm opposite the injection site for [18F]-FDG quantification and to scale a population-derived blood curve (0–50 min), according to published methods (Takikawa et al. 1993). Twenty-five minutes after radiotracer injection, the subject was positioned in the GE Advance Tomograph. A 10-min transmission scan was obtained, and then a static emission scan began at 40 min after radiotracer injection and lasted for 20 min. At the end of the PET scan, the subjects were removed from the scanner and the intravenous lines removed.
PET data and image analysis
For the [11C]-raclopride studies, analyses with both volume of interest (VOI) and voxel-based brain mapping were performed. The dynamic frames were realigned to each other and a summed image was generated for VOI drawing. All of the frames were averaged and four central slices that cover the striatum (total thickness=4×4.25=17 mm) were chosen for VOI identification. VOIs were then placed bilaterally on the caudate nucleus, putamen and occipital cortex using an automated algorithm. The time–activity curves from the PET scan were analyzed and a receptor binding parameter—distribution volume ratio (DVR)—was calculated using the occipital time–activity curve as the input function as described by Logan et al. (1996). DVR is a measure of binding potential that gauges the capacity of receptor occupancy. Previously (Asanuma et al. 2005; Ma et al. 2002), we have shown with [11C]-raclopride data that the use of the occipital input function is equivalent in revealing group differences as compared to the use of cerebellar input function that was validated in many studies (e.g., Lammertsma et al. 1996; Logan et al. 1996). Maps of binding potential were also created on a voxel basis and analyzed using SPM99.
Glucose metabolism was calculated by a modification of the method by Phelps et al. (1979) with minimal blood sampling to scale a population-derived standardized input function (Takikawa et al. 1993). It has been determined that after 15 min post-injection, the arterial and venous samples are linear with respect to count rates and glucose concentrations. For the [18F]-FDG studies, glucose metabolic rates were calculated on a pixel by pixel basis as described previously (Smith et al. 2002; Takikawa et al. 1993). Data processing was performed using the statistical parametric mapping program (SPM99; Wellcome Department of Cognitive Neurology, London, UK; Friston et al. 1995). The PET scans from each subject were non-linearly warped into Talairach space and proportionately scaled. The images were smoothed with an isotropic Gaussian kernel (Full width at half maximum 8 mm for all directions). Metabolic values were normalized by the global mean of each individual subject. Differences between groups were compared using the independent sample t-test option in SPM99 and were considered significant at a t threshold greater than 4.14 (p<0.001; uncorrected for multiple independent comparisons). Only regions that were significant at the cluster level (p<0.05 corrected for multiple comparisons) are reported. Complementary VOI analysis was also carried out post-hoc by averaging voxel values on the normalized and smoothed FDG images over spheres (6 mm diameter) that were centered at the peak coordinate of each reported cluster. These values were compared both before (absolute metabolism) and after (relative metabolism) normalization by the global mean of each individual subject. Furthermore, the relationship between [11C]-raclopride binding and normalized glucose metabolism was also investigated by performing correlation analysis in two different ways (1) glucose metabolic images were correlated on a voxel basis with striatal [11C]-raclopride binding values (2) glucose metabolism and [11C]-raclopride binding values were correlated in each of the VOIs used for post-hoc FDG analysis noted above.
Results
Demographics and clinical data
Six male subjects with cannabis dependence in early full remission (CD) and six age- and sex-matched healthy controls (HC) participated in this study. The mean age in both groups was 20±1 (standard deviation) years old (range=18–21). The CD group had a lower mean level of education compared to the HC group (10±1 years vs. 12± 1 years; t=−6.2, p<0.001) but similar cognitive abilities, as measured with the Wide Range Achievement Test-third edition (Nelson and O’Connell 1978). There was a higher proportion of cigarette smokers in the CD group compared to the HC group (Fisher’s Exact Test, p<0.04). However, none of the subjects had a current DSM-IV diagnosis of nicotine dependence. All CD subjects were in a residential program for more than 3 months, had limited time for smoking, and had no difficulty refraining from smoking 10 h before and during PET scan procedures. The day of the PET scan, CD and HC subjects were assessed for craving and withdrawal symptoms using visual analog scales, and no subject reported craving or withdrawal symptoms related to drugs, alcohol, or nicotine. Subject characteristics are reported in Table 1. There were no differences between groups for levels of subjective ratings of depression, anxiety, or happiness.
Table 1.
Demographics and clinical data of subjects with cannabis dependence in early full remission (CD) and healthy controls (HC)
| CD subjectsa (n=6) | HC subjectsa (n=6) | |
|---|---|---|
| Age | 20±1 (SD) | 20±1 (SD) |
| Race, number African American/Hispanic | 3/3 | 6/0 |
| Education | 10±1 | 12±1* |
| Wide Range Achievement Test-third edition | 95±8 | 96±11 |
| Handedness (right/left handed) | 5/1 | 4/2 |
| Smokers/non smokers | 5/1 | 1/5** |
| Age at onset of cannabis use | 12±2 (range 11–13) | |
| Years of cannabis use | 7±1 (6–9) | |
| Age at onset of cannabis dependence | 14±2 (12–17) | |
| Years of cannabis dependence | 5±2 (2–7) | |
| Amount of daily marijuana use (grams)b | 16±12 (4–32) | |
| Weeks of sobriety before PET scans | 15±5 (12–25) |
p<0.01,
p<0.05
Continuous variables are mean values±standard deviation
Daily use of marijuana in grams was estimated based on the following equivalencies: one joint=0.5 g; one blunt=2 g
Cerebral D2/D3 receptor availability
Using SPM99 and a mask for the striatal region (uncorrected p<0.01; extent threshold k=0 voxels), there were no differences between groups for striatal [11C]-raclopride binding potential. In addition, anatomical VOI analysis did not reveal significant differences between groups. Distribution of binding data was very homogenous in each group, and effect sizes (Cohen 1988) were small (Table 2). A power analysis shows that an increase in sample size from 6 to 30 in each group would not give more than 28% power to detect a difference in means between groups using a two-sample t-test with a 0.050 two-sided significance level.
Table 2.
VOI analysis of striatal [11C]-raclopride binding potentials in subjects with cannabis dependence in early full remission (CD) and healthy controls (HC)
| CD subjects (n=6) | Healthy subjects (n=6) | Effect size (Cohen’s d) | |
|---|---|---|---|
| Left caudate | 3.35±0.31 | 3.33±0.29 | 0.06 |
| Right caudate | 3.20±0.22 | 3.30±0.31 | 0.36 |
| Left putamen | 3.49±0.27 | 3.51±0.35 | 0.06 |
| Right putamen | 3.48±0.29 | 3.45±0.24 | 0.11 |
Cerebral glucose metabolism
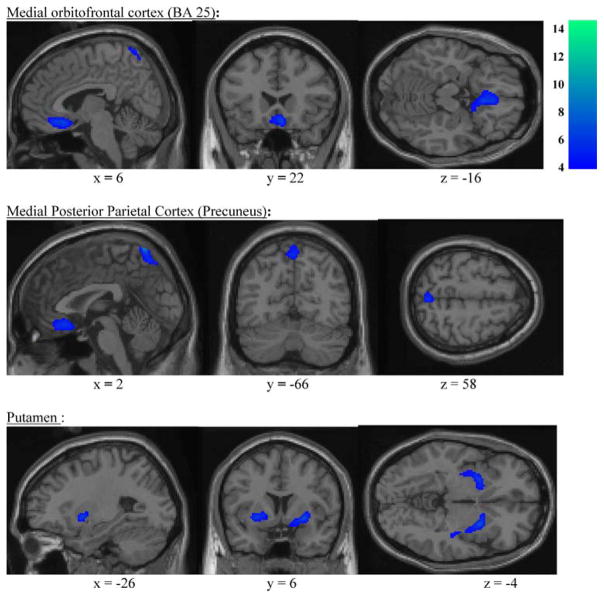
Using SPM99 (uncorrected p<0.001; extent threshold= 230 voxels), CD subjects demonstrated lower normalized glucose metabolism in several regions of the brain compared to HCs. Table 3 shows the coordinates of the regions that differed significantly between groups. SPM analysis revealed decreased normalized glucose metabolism in the right orbitofrontal cortex extending from gyrus rectus to anterior cingulate [Brodmann’s area 25] and the right medial posterior parietal cortex as well as the putamen, bilaterally (Fig. 1). In the cerebellum, normalized glucose metabolism was only decreased in a very small region of the right cerebellum (Montreal Neurological Institute coordinates 34, −30, −34, k=16 voxels, p<0.0005). In addition, VOI analysis revealed that absolute glucose metabolism in the CD subjects were lower in these regions but did not reach statistical significance due possibly to small sample size. However, the globally normalized regional values were reduced significantly (p<0.0005) by 7–14% with respect to the HC subjects (Table 4). This resulted from the reduced variability following normalization by global mean metabolism. The SPM analyses showed no brain region with higher normalized glucose metabolism in the CD subjects compared to HC subjects. There was a negative correlation between normalized glucose metabolism in the right posterior parietal cortex and cannabis gram–years (Pearson correlation coefficient −0.85, p<0.05). There were no significant correlations between normalized glucose metabolism in other areas of the brain or striatal [11C]-raclopride binding potential and cannabis gram–years.
Table 3.
Brain regions with decreased normalized glucose metabolism in subjects with cannabis dependence in early full remission (CD) compared to healthy controls (HC)
Fig. 1.
Decreased normalized glucose metabolism in subjects with cannabis dependence in early full remission (CD) compared to healthy controls (HC) in the right medial orbitofrontal cortex and medial posterior parietal cortex (voxel level two-tailed t scores)
Table 4.
VOI analysis of normalized glucose metabolism in subjects with cannabis dependence in early full remission (CD) compared to healthy controls (HC)
| Brain region | CD subjects (n=6) | HC subjects (n=6) | Percentage reduction* |
|---|---|---|---|
| Striatum, putamen left | 1.771±0.049 (SD) | 1.909±0.029 | −7.264 |
| Right | 1.723±0.047 | 1.873±0.026 | −7.997 |
| Orbitofrontal cortex | 1.280±0.052 | 1.495±0.063 | −14.366 |
| Parietal cortex, precuneus | 1.496±0.032 | 1.657±0.061 | −9.672 |
p<0.0005
Correlation between normalized glucose metabolism and D2/D3 receptor availability
There were no correlations between striatal [11C]-raclopride binding potential and normalized glucose metabolism in any region with decreased metabolism from voxel-based SPM analysis.
Discussion
We did not find any differences between groups in striatal D2/D3 receptor availability with either VOI or voxel-wise analysis methods. As predicted, we found decreased normalized glucose metabolism in the region of the right orbitofrontal region and striatum bilaterally. Our findings are in agreement with some previous neuroimaging studies showing decreased cerebral metabolic activity in the ventromedial or orbito-prefrontal cortex in cannabis users after periods of sobriety of 5 days or less (Amen and Waugh 1998; Block et al. 2000; Lundqvist et al. 2001). In a PET study, to measure cerebral blood flow with [15O]-water, Block et al. (2000) compared 17 frequent marijuana users abstinent for at least 26 h and 12 non-using controls. Compared to controls, marijuana users had lower normal blood flow in the ventral prefrontal cortex, posterior cerebellum, and vermis bilaterally. In a study measuring regional cerebral blood flow using 133Xe inhalation technique, Lundqvist et al. (2001) found decreased perfusion in right prefrontal, superior frontal, and central cortical areas in 12 male long-term cannabis users who were sober for 1 to 5 days compared to 14 age- and sex-matched normal controls. Volkow et al. (1996a) compared eight subjects with cannabis dependence who were sober for 72 h with eight non-users. They found decreased cerebral glucose metabolism in frontal and cerebellar regions, but post-hoc t-tests were only significant for the cerebellum. In a Blood Oxygen Level Dependent functional MRI study of non-verbal visual attention tasks, Chang et al. (2006) found that abstinent marijuana users had less activation in several areas of the attention network (right and left prefrontal cortex, right dorsal medial parietal cortex, and cerebellum) compared to normal controls. We found a decreased cerebral metabolism in the posterior medial parietal cortex, which was inversely related to the extent of cannabis exposure. Except for a very small region of the right cerebellum, we did not observe a significant decreased resting-state activity in the cerebellum. Our negative cerebellar findings are in contrast with other PET studies (Block et al. 2000; Volkow et al. 1996a) and may be due to an earlier age at onset of cannabis use, shorter periods of cannabis exposure, and longer duration of abstinence in our subjects with cannabis dependence compared to cannabis users in other studies.
The present study is limited in several respects. The sample size was small, which may explain our negative findings for striatal D2/D3 receptor availability. However, as described in Table 3, the distribution of D2/D3 receptor availability in caudate and putamen was very homogenous in each group. As both groups were matched in age and gender, it is unlikely that differences in striatal D2/D3 receptor availability would be revealed with a greater number of subjects. Effect sizes were small, and a power analysis indicates that an increase in sample size would still result in a low power to detect differences between groups. We cannot rule out that our cannabis-dependent subjects had a decreased brain glucose metabolism prior to cannabis use. Similar to previous studies (Lundqvist et al. 2001), there was a higher proportion of cigarette smokers in the CD group compared to the healthy subject group and changes in glucose metabolism may be related to cigarette withdrawal or craving (Brody et al. 2002). However, our CD subjects were not nicotine dependent, had no difficulty refraining from smoking for at least 10 h before and during PET scan procedures, and did not report craving or withdrawal symptoms for nicotine at the time of the PET scan. Finally, one cannabis user was not matched for handedness. It is difficult to estimate the impact of the difference in handedness. To our knowledge, there are no reports that differences in handedness are related to asymmetries in glucose metabolism or [11C]-raclopride binding in the inactivated state.
In summary, abstinent young adults with cannabis dependence in early full remission do not differ from controls in striatal D2/D3 receptor availability but demonstrate low normalized glucose metabolism in the right orbitofrontal region, putamen bilaterally, and precuneus. Decreased glucose metabolism after more than 12 weeks of sobriety suggests that our findings are not related to a residual effect of cannabis or an acute withdrawal effect. Future studies will evaluate the acute effects of cannabis exposure as well as the time course of abstinence with respect to the functional neuroanatomic changes associated with persistent deficits, as well as compensatory processes.
Acknowledgments
This work was supported by the National Institute Health, grants K23 DA015541 (SS), K02 MH01621, MH 64823 (GS), M01 RR018535, and a Faculty Award from the Feinstein Institute for Medical Research (Manhasset, NY) (SS). David Bjelke, CNMT and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies. We thank Terry Goldberg, PhD for his review of the manuscript, Steve Grant, PhD of the National Institute on Drug Abuse and Barbara Napolitano, MS for their helpful comments.
Footnotes
Financial disclosures Drs. Sevy, Smith, Ma, Dhawan, Chaly, Kingsley, Kumra, Mr. Abdelmessih, and Dr. Eidelberg reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Serge Sevy, The Zucker Hillside Hospital, North Shore–Long Island Jewish Health System, 75-59 263rd Street, Glen Oaks, NY 11004, USA. The Albert Einstein College of Medicine, Bronx, NY, USA.
Gwenn S. Smith, Centre for Addiction and Mental Health, University of Toronto, Toronto, Canada
Yilong Ma, The Feinstein Institute for Medical Research, Manhasset, NY, USA.
Vijay Dhawan, The Feinstein Institute for Medical Research, Manhasset, NY, USA.
Thomas Chaly, The Feinstein Institute for Medical Research, Manhasset, NY, USA.
Peter B. Kingsley, North Shore University Hospital, Manhasset, NY, USA
Sanjiv Kumra, University of Minnesota, Minneapolis, MN, USA.
Sherif Abdelmessih, The Zucker Hillside Hospital, North Shore–Long Island Jewish Health System, 75-59 263rd Street, Glen Oaks, NY 11004, USA.
David Eidelberg, The Feinstein Institute for Medical Research, Manhasset, NY, USA.
References
- Amen DG, Waugh M. High resolution brain SPECT imaging of marijuana smokers with AD/HD. J Psychoactive Drugs. 1998;30:209–214. doi: 10.1080/02791072.1998.10399692. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypo-activity in frequent marijuana users. Neuroreport. 2000;11:749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Chaly T, Mattacchieri R, Velez JW, Dahl JR, Margouleff D. A large scale production of 18F-FDG using a synthetic unit made of sterile disposable components and operated by a slave manipulator. Appl Radiat Isot. 1990;41:29–34. [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. LEA; Mahwah: 1988. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Dhawan V, Kazumata K, Robeson W, Belakhlef A, Margouleff C, Chaly T, Nakamura T, Dahl R, Margouleff D, Eidelberg D. Quantitative Brain PET. Comparison of 2D and 3D Acquisitions on the GE Advance Scanner. Clin Positron Imaging. 1998;1:135–144. doi: 10.1016/s1095-0397(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Farde L, Pauli S, Hall H, Eriksson L, Halldin C, Hogberg T, Nilsson L, Sjogren I, Stone-Elander S. Stereoselective binding of 11C-raclopride in living human brain—a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology (Berl) 1988;94:471–478. doi: 10.1007/BF00212840. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Bran Mapping. 1995;2:189–210. [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P. Neurocircuitry of addiction. In: Davis K, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. ACNP; Nahville: 2002. pp. 1357–1366. [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol. 2001;23:437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Ma Y, Smith G, Dhawan V, Chaly T, Pollock B, Eidelberg D. Selection of reference regions in the analysis of D2 receptor binding data with [11C]raclopride: cerebellum versus occipital cortex. NeuroImage. 2002;16:S46. [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett. 1992;148:173–176. doi: 10.1016/0304-3940(92)90832-r. [DOI] [PubMed] [Google Scholar]
- Meyers K, McLellan AT, Jaeger JL, Pettinati HM. The development of the Comprehensive Addiction Severity Index for Adolescents (CASI-A). An interview for assessing multiple problems of adolescents. J Subst Abuse Treat. 1995;12:181–193. doi: 10.1016/0740-5472(95)00009-t. [DOI] [PubMed] [Google Scholar]
- Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2005 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville: 2006. [Google Scholar]
- Smith GS, Ma Y, Dhawan V, Gunduz H, Carbon M, Kirshner M, Larson J, Chaly T, Belakhleff A, Kramer E, Greenwald B, Kane JM, Laghrissi-Thode F, Pollock BG, Eidelber D. Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse. 2002;45:105–512. doi: 10.1002/syn.10088. [DOI] [PubMed] [Google Scholar]
- Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl R, Margouleff D, Eidelberg D. Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology. 1993;188:131–136. doi: 10.1148/radiology.188.1.8511286. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996a;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996b;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]