Abstract
Objectives
To determine whether children with type 1 diabetes mellitus (T1DM) have evidence of increased aortic stiffness or early atherosclerosis as measured by magnetic resonance imaging (MRI).
Background
T1DM increases risk for cardiovascular disease in adults but whether this process starts in childhood is unknown.
Subjects
A total of 54 T1DM patients (15.4 ± 2.6 yr) and 30 age-matched controls (14.8 ± 2.7 yr) participated.
Methods
MRI was performed to assess aortic arch pulse wave velocity (PWV), strain, and distensibility of the ascending and descending thoracic aorta and measures of atherosclerosis.
Results
Groups were well-matched for age, pulse pressure, and gender. Low-density lipoprotein-cholesterol (LDL-C) was higher in T1DM (119.3 ± 50 vs. 76.1 ± 13.5 mg/dL, p < 0.0001). There was a trend toward decreased strain and distensibility in T1DM vs. controls in the ascending (distensibility: T1DM 62.2 ± 19.9 kPa−1 ×10−3, control 71.6 ± 26.4 kPa−1 ×10−3, p = 0.08) and descending aorta (strain: T1DM 25.8 ± 6.2% vs. control 28.3 ± 6.8%, p = 0.09). There was no difference in arch PWV. Advancing age and male gender was negatively associated with aortic stiffness. Hemoglobin A1c (HbA1c) was inversely related to descending aorta strain and distensibility (p < 0.05). Children with diabetes in the lowest two tertiles of insulin sensitivity demonstrated thoracic descending aortas with significantly lower strain (p = 0.027) and distensibility (p = 0.039) and increased measures of wall irregularity (p = 0.005). There were no differences in measurements of atherosclerosis between the two groups.
Conclusions
Adolescents with T1DM, especially those with lower insulin sensitivity, demonstrated a trend toward stiffer, less compliant thoracic aortas, which was inversely associated with diabetes control. These data suggest large vessel aortopathy starts early in T1DM.
Keywords: adolescents, cardiovascular diseases, type 1 diabetes mellitus, insulin resistance, magnetic resonance imaging
Heart disease is a leading cause of death in patients with type 1 diabetes mellitus (T1DM). Conventional cardiovascular risk factors, including dyslipidemia, dysglycemia, central adiposity, and insulin resistance worsen these risks and are strongly associated with premature development of microvascular disease and advanced atherosclerosis, both in the coronary arteries and abdominal aorta (1–4). These observations support the American Diabetes Association guidelines for the treatment of dyslipidemia in children and adolescents even in the absence of clinical trials demonstrating efficacy (5).
Pediatric investigators have used peripheral vessel measurement of pulse wave velocity (PWV), a measure of aortic stiffness, and carotid intima-media thickness (CIMT) by ultrasound, a measure of vascular thickening, to document subclinical atherosclerosis in children and adolescents with diabetes. In longitudinal data from the SEARCH CVD (cardiovascular disease) study, PWV in the carotid-femoral segment was increased in those with T1DM and increased during follow up with the principal determinants of aortic stiffness being waist circumference, increased blood pressure, body mass index (BMI), higher low-density lipoprotein-cholesterol (LDL-C), and poorer glucose control (6, 7). Studies have been inconsistent with regard to the presence of increased CIMT in T1DM although most show an increase in adults (8). Conversely, however, several studies have demonstrated aortic intima-media thickness (AIMT) to be a better marker of preclinical atherosclerosis than CIMT in the pediatric population (9, 10). The large SEARCH CVD study showed increased CIMT only in the carotid bulb of those with T1DM (mean age 18 yr) and this was related to poor diabetes control and not other cardiovascular risk factors (11).
To date, magnetic resonance imaging (MRI) has been less well studied than ultrasound- or pressure-based modalities in the assessment of subclinical atherosclerosis and arterial stiffness but offers several theoretical advantages. One is the ability to assess the entire aorta, including both the ascending segment and abdominal aorta. This allows a determination of PWV between any two points in addition to other robust measurements of local arterial stiffness such as strain and distensibility. MRI’s ability to obtain high spatial resolution imaging in any plane can produce cross sectional analysis of vessel walls as opposed to ultra sound’s unidimensional intima media thickness (IMT) measurement.
Using an insulin sensitivity score (ISS) that employed hemoglobin A1c (HbA1c), triglyceride (TG) concentrations, and waist circumference that were validated against hyperinsulinemic euglycemic clamp data, investigators observed that scores of high insulin resistance (low ISS) showed a linear correlation with traditional atherogenic risk factors (12). Thus, low ISS may be a useful parameter to help identify those individuals with insulin resistance.
We hypothesized that increased vascular stiffness and early atherosclerotic changes are related to T1DM, insulin sensitivity, and dyslipidemia. Using a standardized MRI protocol that included assessment of regional (arch PWV) and local (strain and distensibility) aortic stiffness and measures of atherosclerosis, we compared MRI changes in pediatric patients with T1DM with and without dyslipidemia to those of age-matched, non-diabetic, non-dyslipidemic healthy controls. Insulin resistance severity (ISS) and dysglycemia (HbA1c) were examined as potential predictors of differences between children with diabetes and controls. Subjects for this study were recruited from within a larger multi-institutional, double-blinded, randomized, placebo-controlled trial of statin intervention in adolescents with T1DM and dyslipidemia (Clinical-trials.gov NCT01236365).
Methods
Recruitment of study subjects between 10 and 20 yr of age occurred in the Nemours Children’s Clinics in Jacksonville, FL, Philadelphia, PA and Wilmington, DE after review and approval of the Nemours institutional review board, with reciprocity from Jefferson Medical College, and in accordance with the Declaration of Helsinki. Children with T1DM had to meet the following inclusion criteria: diagnosis for at least 1 yr, on stable insulin therapy, BMI < 95th percentile and normal thyroid function. Exclusion criteria were severe dyslipidemia [LDL-C > 160 mg/dL (4.14 mmol/L) and TGs > 400 mg/dL (4.48 mmol/L)], history of tobacco use, pregnancy and current use of anti-inflammatory, immunomodulatory, or lipid lowering drugs. Healthy control subjects were recruited from extensive institutional review committee-approved advertising and from the pediatric endocrinology and cardiology clinics. Age-matched healthy control subjects had similar inclusion criteria as those with T1DM except for a normal fasting blood glucose and HbA1c and LDL-C < 100 mg/dL (2.59 mmol/L).
All subjects underwent a single blood draw for HbA1c, glucose, and lipoprotein samples. If laboratory inclusion criteria were met, subjects returned for a history and physical examination, including vital signs, height, body weight, BMI, waist circumference, and resting blood pressures. LDL-C was calculated by the formula of Friedewald et al. for individuals with TG levels <400 mg/dL (4.48 mmol/L) (12). An ISS was determined using the SEARCH ISS model:
This model has been validated by the hyperinsulinemic–euglycemic clamp procedure (R2 = 0.74), which is considered the gold-standard measure of insulin sensitivity (13).
MRI protocol
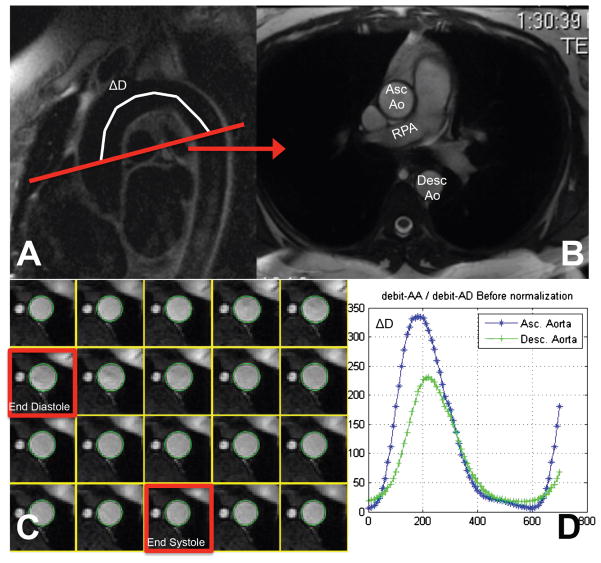
Control subjects and patients with T1DM underwent an MRI using a GE SIGNA 1.5T magnet at either Wolfson Children’s Hospital in Jacksonville, FL or AI duPont Hospital for Children in Wilmington, DE. All studies were performed using an eight channel cardiac coil and identical protocols. Images were centrally read by a single observer (MM). After scout images were obtained, a stack of breath-held, non-ECG gated steady state free precession (SSFP) images were obtained in the axial plane from the mid abdomen to the superior aspect of the thorax. These images were used to prescribe oblique, sagittal plane images of the aortic arch using an ECG-gated, breath-held, double inversion recovery (IR) ‘black blood’ sequence [6 mm thick, TE 36 ms, 32 cm field of view (FOV) with 0.75 phase FOV, 256 × 256 matrix, echo train length 24 and bandwidth 62.5 Hz; Fig. 1A]. The aortic arch image was in turn used to prescribe ascending and descending aorta images by transecting the aortic arch at the level of the right pulmonary artery (8 mm thick, 36 cm FOV with 0.75 phase FOV, 224 × 160 matrix, flip angle 45°, bandwidth 125 Hz with 20 phases and 10 views per segment; Fig. 1B). A phase contrast (PC), breath-held, ECG-gated sequence was then obtained at the same location (8 mm thick, 36 cm FOV with 0.75 phase FOV, 256 × 128 matrix, flip angle 20°, bandwidth 15.63 Hz with 30 phases and six views per segment with velocity encoding at 250 cm/s). Two sets of six breath-held, ECG-gated double IR sequences were obtained transecting the ascending and descending portion of the thoracic aorta starting immediately inferior to the lesser curvature of the aortic arch using the identical sequence prescription described above. This was repeated in the abdominal aorta between the diaphragm and celiac artery. Immediately following the study, while the patient remained in the supine position, three blood pressures were obtained from the left arm and an average of the second and third measurements were used for calculations of pulse pressure.
Fig. 1.
(A) Double inversion recovery (IR) sequence of the aortic arch from which cine steady state free precession (SSFP) and Phase Contrast (PC) sequences are prescribed and distance of flow propagation measured (D). (B, C) Result of the automated segmentation process and determination of percent change in area of ascending and descending aorta used to determine strain and distensibility (strain/pulse pressure). (D) Time of flow propagation (Δt) from ascending to descending aorta measured and along with D, used to calculate pulse wave velocity (D/Δt).
Image analysis
Aortic arch PWV was calculated using the transit time (Δt) of the flow curves and the distance (D) between the ascending and descending aortic locations of the phase contrast acquisition (Fig. 1A, C). Transit time was calculated using the semi-quantitative software ARTFUN (INSERM LIB, Paris, France) which utilizes the least squares estimate between all data points on the normalized systolic up-slopes of both the ascending and descending aortic flow curves as previously described (14). The distance traveled by the pulse wave was measured as the centerline of the aorta using 8–10 markers on the aortic arch double IR view with the first and last markers corresponding to the ascending and descending aorta in the plane used for the phase contrast acquisition.
Strain, defined as the percent area change:
was calculated as previously described (14). Distensibility, a measurement of strain ‘indexed’ to a patient’s pulse pressure in kPa−1 ×10−3 was also calculated.
All measurements used to calculate strain [maximum and minimum areas (cm2) of both ascending and descending aorta] and PWV [D (cm) and Δt (ms)] were performed three times by the same person (MM) and demonstrated excellent reproducibility [(average value ± standard deviation, SD); ascending aorta max (4.87 ± 0.07 cm2), ascending aorta minimun (Amin) (3.47 ± 0.08 cm2), descending aorta maximun (Amax) (2.61 ± 0.03 cm2), descending aorta min (2.07 ± 0.03 cm2), D (87.3 ± 0.67 cm), and Δt (31.0 ± 0.9 ms)].
In order to estimate degree of atheromatous plaque formation, double IR cross sectional sequences of the thoracic descending and abdominal aorta were analyzed using the software QPlaque (Medis, Leiden, The Netherlands). The internal and external vessel wall circumferences were manually traced for every 6 mm thick slice of adequate image quality (Fig. 2). This analysis produced an average and maximum wall thickness (mm), standard deviation of the wall thickness and wall area (mm2) for each slice. Abdominal wall volume (mm3) for each slice was calculated as the product of vessel area and slice thickness (6 mm). We considered increased average wall thickness, maximum wall thickness, wall area, standard deviation of the wall thickness, and wall volume as surrogate measures of atherosclerosis development.
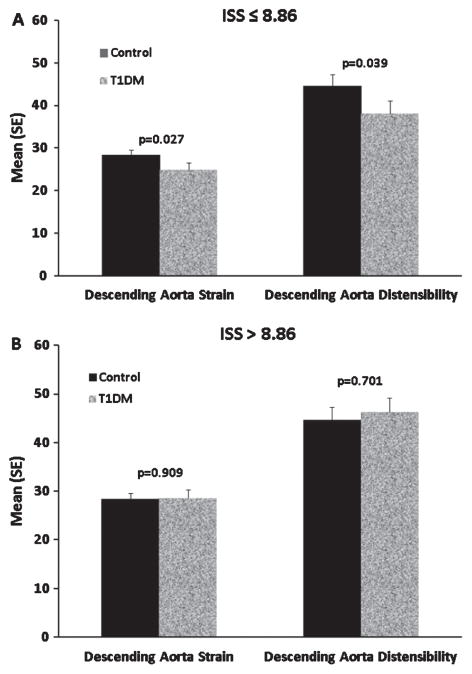
Fig. 2.
(A) Patients with diabetes and the lowest insulin sensitivity demonstrated significantly lower strain and distensibility of the descending aorta. (B) Patients with diabetes and the highest insulin sensitivity showed no significant differences.
A human phantom was scanned at both sites, 5 d apart, using identical protocols with excellent reproducibility (ascending aorta strain 11.2 vs. 15.3% and descending aorta strain 16.2 vs. 15.5%).
Statistical analysis
A two-sample t-test was used to compare the mean of quantitative variables and a chi-squared test was used to compare the distribution of categorical variables between controls and T1DM. Analysis of covariance (ANCOVA) was used for further comparisons after adjustment for age, gender, baseline BMI, and blood pressure percentiles. Multiple regression analyses were conducted to determine the association between each of the MRI measurements and the different indices of dysglycemia, dyslipidemia, and anthropometrics. Two-sample t-test and ANCOVA were used for further comparisons of means of MRI variables between control and T1DM patients in the lowest two tertiles of insulin sensitivity (ISS ≤ 8.86). Model and test assumptions were checked before analyses and an appropriate transformation was taken if needed. All tests were two-tailed with the level of significance of 0.05. SPSS (version 22, SPSS IBM, New York, NY, USA), and SAS (version 9.3, the SAS institute, Cary, NC, USA) softwares were used for the analyses.
Results
Cohort demographics for controls and subjects with diabetes are shown in Table 1. The groups were similar with regard to gender, age, pubertal Tanner staging, and pulse pressure. As anticipated, LDL-C concentrations for the T1DM group were significantly higher than controls as were the apolipoprotein B levels. Average HbA1c levels were consistent with suboptimal glycemic control.
Table 1.
Cohort demographics
| Controls | T1DM | p Value | |
|---|---|---|---|
| Number (N/%) | 30 (35.7) | 54 (64.3) | |
| Male (N/%) | 16/53 | 27/50 | 0.77 |
| Age (yr) | 14.8 ± 2.7 | 15.4 ± 2.7 | 0.368 |
| Tanner stage (%I–III/IV–V) | 40/60 | 20/80 | 0.20 |
| I (N/%) | 4/7 | 3/10 | |
| II (N/%) | 3/6 | 3/10 | |
| III (N/%) | 4/7 | 6/20 | |
| IV (N/%) | 13/24 | 4/13 | |
| V (N/%) | 30/56 | 14/47 | |
| BMI (kg/m2) | 19.9 ± 2.5 | 22.4 ± 3.7 | 0.001 |
| Waist circumference (cm) | 70.0 ± 7.8 | 77.2 ± 9.1 | 0.0007 |
| Systolic blood pressure (percentile) | 40.5 ± 23.7 | 55.5 ± 30.1 | 0.024 |
| Diastolic blood pressure (percentile) | 50.9 ± 21 | 61.4 ± 20.8 | 0.028 |
| Pulse pressure (kPa−1 × 10−3) | 6.5 ± 1.01 | 6.5 ± 1.1 | 0.954 |
| Total cholesterol | |||
| mg/dL | 145.1 ± 18.8 | 194.2 ± 36.7 | <0.0001 |
| mmol/L | 3.8 ± 0.5 | 5.0 ± 1.0 | |
| LDL-C | |||
| mg/dL | 76.1 ± 13.5 | 119.3 ± 50 | <0.0001 |
| mmol/L | 2.0 ± 0.4 | 3.1 ± 1.3 | |
| HDL-C | |||
| mg/dL | 56.3 ± 9.9 | 59.8 ± 15.2 | 0.255 |
| mmol/L | 1.5 ± 0.3 | 1.6 ± 0.4 | |
| Triglycerides | |||
| mg/dL | 62.9 ± 29 | 106.1 ± 117.9 | 0.052 |
| mmol/L | 0.7 ± 0.3 | 1.2 ± 1.3) | |
| Apolipoprotein B | |||
| ng/dL | 57.2 ± 9.3 | 87.3 ± 24.2 | <0.0001 |
| g/L | 5.7e−7 ± 9.3e−8 | 8.7e−7 ± 2.4e−7 | |
| ISS | 13.4 ± 2.2 | 7.4 ± 2.5 | <0.0001 |
| HbA1c | |||
| % | 5.1 ± 0.02 | 9.2 ± 1.9 | <0.0001 |
| mmol/mol | 32 ± 0.2 | 77 ± 20.8 | |
| Years with T1DM | NA | 7.8 ± 4.6 | NA |
BMI, body mass index; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein-cholesterol; ISS, insulin sensitivity score; LDL-C, low-density lipoprotein-cholesterol; NA, Not Applicable; T1DM, type 1 diabetes mellitus.
All data are expressed as mean ± SD.
T1DM subjects demonstrated a trend toward decreased strain and distensibility throughout the thoracic aorta as compared with healthy, age-matched controls (Table 2). Though these trends did not reach statistical significance in two-tailed analysis in our relatively small sample size, they suggest that the T1DM subjects in our study likely had stiffer, less compliant aortas. Lipid levels did not impact stiffness in those with diabetes (data not shown).
Table 2.
MRI comparison
| Controls | T1DM | p Value | |
|---|---|---|---|
| Ascending aorta strain (%) | 44.8 ± 12.9 | 40.1 ± 12.8 | 0.12 |
| Descending aorta strain (%) | 28.3 ± 6.8 | 25.8 ± 6.2 | 0.09 |
| Ascending aorta distensibility (kPa−1 × 10−3) | 71.6 ± 26.4 | 62.2 ± 19.9 | 0.08 |
| Descending aorta distensibility (kPa−1 × 10−3) | 44.7 ± 13.7 | 40.2 ± 11.8 | 0.13 |
| PWV (m/s) | 3.1 ± 1.4 | 3.2 ± 1.6 | 0.71 |
| Thoracic descending aorta volume (mm3) | 727 ± 36.2 | 802 ± 28.4 | 0.11 |
| Thoracic descending aorta wall thickness (SD) | 0.36 ± 0.1 | 0.41 ± 0.1 | 0.03 |
MRI, magnetic resonance imaging; PWV, pulse wave velocity; T1DM, type 1 diabetes mellitus.
All data are expressed as mean ± SD.
When these data were analyzed further and adjusted for age, gender, baseline BMI, and blood pressure percentiles, advancing age was negatively correlated with measurements of aortic strain [ascending aorta regression coefficient: (SE) = −1.74 (0.78), p = 0.03; descending aorta regression coefficient (SE) = −0.71 (0.34), p = 0.042]; male gender was associated with lower measurements of both distensibility (ascending aorta: male 62.9 ± 3.3 vs. female 72 ± 4.3, p = 0.015 and descending aorta: male 37.9 ± 1.9 vs. female 47.1 ± 1.9, p = 0.001) and ascending aorta strain (male 25.4 ± 1.0 vs. female 29 ± 1.0, p = 0.013). There was no significant difference in arch PWV between groups (Table 2).
MRI studies demonstrated no evidence of frank atherosclerotic plaque. Unadjusted analysis demonstrated no significant difference in average thickness, maximal thickness, average area, or average volume between the groups in either the abdominal or thoracic descending aorta though there was a non-significant trend toward increased thoracic descending aorta volume for the T1DM subjects. Wall thickness SD, an estimate of wall thickness irregularity, however, was significantly increased in the thoracic descending aorta of T1DM subjects (Table 2); when these data were adjusted for gender, study age, baseline BMI, and blood pressure percentiles, this difference disappeared. Lipid levels were unrelated to the atherosclerosis measures.
Regression analyses were conducted within the diabetic subgroups to determine if diabetes control (HbA1c) or lipid levels [LDL-C, apolipoprotein B, high -density lipoprotein-cholesterol (HDL-C), TG/HDL ratio] correlated with MRI measurements. HbA1c was inversely related to descending aorta strain and distensibility (p < 0.05). Additionally, Fig. 2 demonstrates that T1DM patients in the lowest two tertiles of insulin sensitivity (ISS ≤ 8.86) demonstrated thoracic descending aortas with significantly lower measures of strain (controls 28.3 ± 6.7% vs. T1DM 24.8 ± 5.9%, p = 0.03) and distensibility (controls 44.7 ± 13.7 kPa−1 ×10−3 vs. T1DM 38.2 ± 11.7 kPa−1× 10−3, p = 0.04) and had significantly more irregular wall thickness (controls 0.36 ± 0.06 mm vs. T1DM 0.40 ± 0.07 mm, p = 0.005). Our control patients demonstrated ISS values similar to the non-T1DM patients in Specht et al. (12) and did not demonstrate an association between ISS and compliance of the descending aorta (strain p = 0.9; distensibility p = 0.7).
Discussion
We have demonstrated the feasibility of aortic MRI for the measurement of cardiovascular risk in children with T1DM compared with healthy controls. T1DM patients with lower insulin sensitivity and poor glycemic control exhibited significant differences in aortic stiffness as assessed by MRI. Functional measurements of thoracic aorta stiffness showed a trend toward decreased compliance across the entire T1DM cohort and were inversely related to HbA1c levels and were worse in those with low ISS. These results are important and clinically relevant as they included all subjects with T1DM, those with high (>90 mg/dL) (2.33 mmol/L) and low LDL-C concentrations as well as subjects with both poor and excellent diabetes control.
Non-invasive assessment for early atherosclerotic plaque development has long been performed in clinical and epidemiological studies in adults. Data that incorporate risk measured in youth and subclinical atherosclerosis measures in adulthood have suggested the importance of youth risk to adult disease (15). Several studies, however, have recently demonstrated significant limitations of the unidimensional imaging modalities such as AIMT and CIMT to predict long-term cardiovascular outcomes (16–18). In the PDAY study, non-HDL cholesterol was related to early abdominal atherosclerosis (American Heart Association grades 1–3) but not raised plaque (American Heart Association grades 4–5) in 15–19 yr olds. Lesions at this early stage may not be thick enough to be seen by MRI techniques and this, plus the fact that those with LDL cholesterol levels above 160 mg/dL (4.14 mmol/L) were excluded, may explain our negative findings related to lipid levels (19).
Alternatively, functional evaluations of arterial stiffness such as strain, distensibility, and PWV have proven to be reproducible, non-invasive, and easy to perform tests of pre-atherosclerotic changes that may allow for earlier intervention, though these measurements have only recently been tested in children with diabetes. In the SEARCH CVD study, Alman et al. (2, 4, 20) demonstrated ultrasound measurements of arterial stiffness in adolescents with T1DM are inversely related to increasing numbers of healthy cardiovascular metrics (BMI <85th percentile, exercise, no smoking, normal lipid panel profile, and normotension). Similarly, Tryggestad et al. (21) found measures of large and small artery compliance significantly decreased after 16 yr of age in adolescents with type 2 diabetes mellitus. Such findings suggest these measurements might be used to monitor disease regression following medication or lifestyle interventions.
Using the same MRI protocol as described herein, Redheuil et al. (14) demonstrated a progressive decline with age in strain and distensibility values and increase in PWV velocities in healthy subjects from 20 to 84 yr of age. This age-related trend was reproduced by Voges et al. in a significantly younger group of healthy subjects ranging between 2 and 28 yr of age (22). Our measurements in the control population are consistent with the age-specific ranges reported in these studies.
Validation of this technique and understanding the effects of aging has prompted several studies to assess MRI vascular stiffness measures in association with known cardiovascular risk factors. Turkbey et al. (23) measured ascending aorta distensibility in adults within the Epidemiology of Diabetes Interventions and Complications Trial (EDICT), a long-term observational cohort study of T1DM patients enrolled as early as 13 yr of age. With an average follow up of 22 yr, multiple linear regression modeling demonstrated significant correlations between decreasing aortic distensibility values and known cardiovascular disease risk factors such as smoking history, mean HbA1c levels, hypertension, age, male gender, and mean LDL. Within the SEARCH for Diabetes in Youth Study, Urbina et al. (2) used sphygmomanometer estimates of central (aorto-femoral) PWV and peripheral (brachial artery) distensibility in T1DM adolescents with HbA1c and LDL-C profiles similar to our patient population. They found significantly higher PWV and lower distensibility measurements when compared with healthy, age-matched controls, and also demonstrated that indicators of vascular stiffness were markedly more common peripherally (33%) than centrally (9.9%) in the T1DM patients.
Normal-weight adolescents with T1DM have been reported to have lower ISS than do BMI-matched control subjects (24, 25). Low ISS is associated with coronary artery calcifications (25), a surrogate marker for coronary artery disease predictive of future cardiovascular disease outcomes. Adolescent studies show that decreased ISS is strongly associated with reduced exercise capacity (24), a cardiovascular functional marker and predictor of mortality. In a similar patient population, van Elderen et al. (26) also found a strong association between increasing aortic PWV and both small vessel cerebrovascular changes and worsening left ventricular systolic function. We were able to stratify our diabetic cohort into tertiles of ISS and compare the two lowest abnormal tertiles to the normal ISS group and showed vascular stiffness changes and possible atherosclerotic changes were present in the more abnormal ISS groups. Our subgroup ISS values were similar to those of Sprecht et al. (12) in which they validated the measurement.
To our knowledge, our study is the first MRI assessment of vascular stiffness comparing children and adolescents with T1DM to healthy age-matched controls. The measurement of ascending aorta distensibility of our control cohort was very similar to that reported for the entire healthy pediatric cohort within Voges et al.’s (22) study and the 20–29 years old group in Redheuil et al.’s (14) study (72 ± 26 vs. 74 ± 23 kPa−1 ×10−3). Our adolescent T1DM cohort’s measurements were much closer to that of Redheuil’s 30–39 years old group (62 ± 20 vs. 61 ± 23 kPa−1 ×10−3) suggesting large vessel stiffness of older individuals. There was a significant reciprocal association between aortic distensibility or strain and the degree of diabetes control as measured by HbA1c or risk severity as assessed by low ISS, supporting the role of dysglycemia, dyslipidemia, and adiposity in this process.
Limitations of our study include a relatively small sample size and the exclusion of both obese and severely dyslipidemic (LDL-C >160 mg/dL) T1DM patients who are at the extremes of CVD risk distribution. However, the fact that we were able to detect these differences despite the sample size suggests that the technique may offer promise in the detection of aortic stiffness. The lack of a difference in our measurements of atherosclerotic plaque formation may be secondary to these exclusions or may simply indicate this is a later development. In an attempt to control for pubertal maturity, we recruited age-matched control subjects. As anticipated in this age range, there was a relatively wide range of pubertal staging though it proved to be statistically non-significant. Lastly, the relatively long temporal resolution (approximately 20 ms assuming a heart rate of 100 beats per minute) may have limited the ability to detect a difference in PWV’s between groups. A larger scale study to follow up this pilot project may help address these issues.
In summary, even though we saw no early MRI-visible findings of atherosclerosis, this study shows a clinically meaningful trend toward early increases in MRI-based measures of large vessel stiffness in patients with T1DM. These results may reflect the relatively short duration of T1DM in our cohort with limited time to develop atherosclerosis. Aortic MRI offers promise in the measurement of early abnormalities in distensibility and strain in children with T1DM and may help the clinician determine which of these high-risk patients should receive more aggressive glucose control or statin therapy. The reciprocal association between distensibility/strain and the degree of diabetes control requires further study.
Acknowledgments
We are grateful to Carol Prospero, Karen Kowal, and Tina Ewen for their research assistance; to Dennis Mullen, RT R MR and Alisa Glenn, RT R MR of the AI duPont Hospital for Children and The Department of Pediatric Imaging at Wolfson Children’s Hospital for technical support for the MRI; and to the children and their families who participated in these studies. The study was funded by Nemours Research Programs and collaborative support from Pfizer, Medtronics, and Quest Diagnostics (NM – PI). SSG had a research grant from Glaxo Smith Kline. There are no other relevant disclosures.
References
- 1.McMahan CA, Gidding SS, Fayad ZA, et al. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165:883–890. doi: 10.1001/archinte.165.8.883. [DOI] [PubMed] [Google Scholar]
- 2.Urbina EM, Wadwa RP, Davis C, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156:731–737. 737.e1. doi: 10.1016/j.jpeds.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 4.Alman AC, Talton JW, Wadwa RP, et al. Cardiovascular health in adolescents with type 1 diabetes: the SEARCH CVD Study. Pediatr Diabetes. 2014;15:502–510. doi: 10.1111/pedi.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care. 2003;26:2194–2197. doi: 10.2337/diacare.26.7.2194. [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Talton JW, D’Agostino R, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:3938–3943. doi: 10.2337/dc13-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalla Pozza R, Beyerlein A, Thilmany C, et al. The effect of cardiovascular risk factors on the longitudinal evolution of the carotid intima medial thickness in children with type 1 diabetes mellitus. Cardiovasc Diabetol. 2011;10:53. doi: 10.1186/1475-2840-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamotte C, Iliescu C, Libersa C, Gottrand F. Increased intima-media thickness of the carotid artery in childhood: a systematic review of observational studies. Eur J Pediatr. 2011;170:719–729. doi: 10.1007/s00431-010-1328-y. [DOI] [PubMed] [Google Scholar]
- 9.Järvisalo MJ, Jartti L, Näntö-Salonen K, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 10.Harrington J, Peña AS, Gent R, Hirte C, Couper J. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr. 2010;156:237–241. doi: 10.1016/j.jpeds.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Urbina EM, Dabelea D, D’Agostino RB, et al. Effect of type 1 diabetes on carotid structure and function in adolescents and young adults: the SEARCH CVD study. Diabetes Care. 2013;36:2597–2599. doi: 10.2337/dc12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162:297–301. doi: 10.1016/j.jpeds.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabelea D, D’Agostino RB, Mason CC, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54:78–86. doi: 10.1007/s00125-010-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson JD, Sonka M, Blecha MB, Lin W, Davis PH. Risk factors associated with aortic and carotid intima-media thickness in adolescents and young adults: the Muscatine Offspring Study. J Am Coll Cardiol. 2009;53:2273–2279. doi: 10.1016/j.jacc.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 17.Den Ruijter HM, Peters SAE, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–2062. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahan CA, Gidding SS, Malcom GT, et al. Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics. 2006;118:1447–1455. doi: 10.1542/peds.2006-0970. [DOI] [PubMed] [Google Scholar]
- 20.Donaghue KC, Wadwa RP, Dimeglio LA, et al. Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2014;15(Suppl 20):257–269. doi: 10.1111/pedi.12180. [DOI] [PubMed] [Google Scholar]
- 21.Tryggestad JB, Thompson DM, Copeland KC, Short KR. Arterial compliance is increased in children with type 2 diabetes compared with normal weight peers but not obese peers. Pediatr Diabetes. 2013;14:259–266. doi: 10.1111/pedi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voges I, Jerosch-Herold M, Hedderich J, et al. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross-sectional study. J Cardiovasc Magn Reson. 2012;14:77. doi: 10.1186/1532-429X-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkbey EB, Redheuil A, Backlund JY, et al. Aortic distensibility in type 1 diabetes. Diabetes Care. 2013;36:2380–2387. doi: 10.2337/dc12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeau KJ, Regensteiner JG, Bauer TA, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Elderen SGC, Brandts A, Westenberg JJM, et al. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol. 2010;20:1132–1138. doi: 10.1007/s00330-009-1655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]