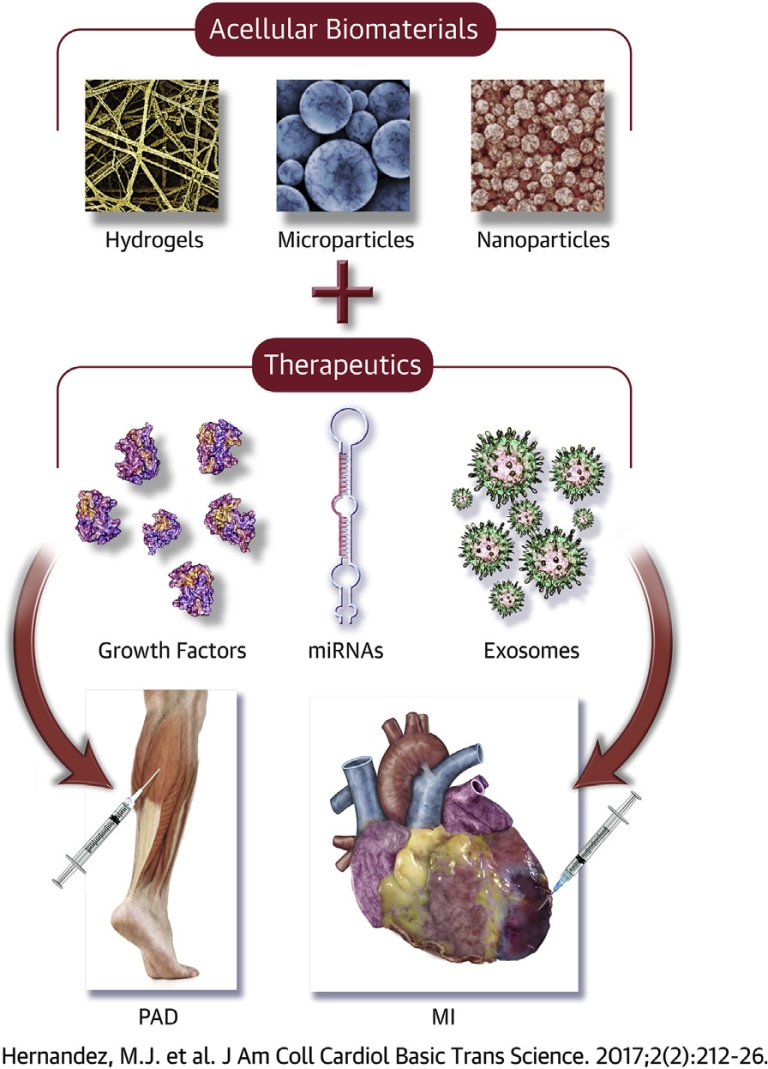
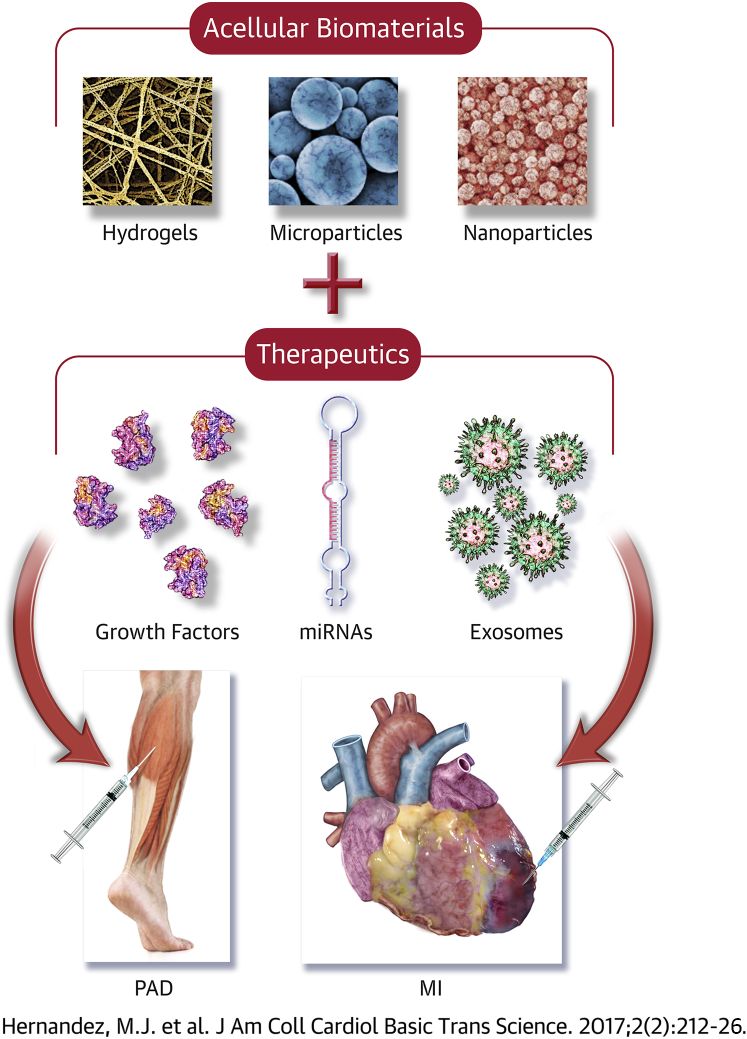
Central Illustration
Key Words: biomaterial, injectable, myocardial infarction, peripheral artery disease
Abbreviations and Acronyms: bFGF, basic fibroblast growth factor; CLI, critical limb ischemia; CVD, cardiovascular disease; ECM, extracellular matrix; FGF, fibroblast growth factor; HA, hyaluronic acid; HF, heart failure; HGF, hepatocyte growth factor; LV, left ventricular; MI, myocardial infarction; miRNA, microribonucleic acid; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; VEGF, vascular endothelial growth factor
Summary
As the number of global deaths attributed to cardiovascular disease continues to rise, viable treatments for cardiovascular events such as myocardial infarction or conditions like peripheral artery disease are critical. Recent studies investigating injectable biomaterials have shown promise in promoting tissue regeneration and functional improvement, and in some cases, incorporating other therapeutics further augments the beneficial effects of these biomaterials. In this review, we aim to emphasize the advantages of acellular injectable biomaterial-based therapies, specifically material-alone approaches or delivery of acellular biologics, in regard to manufacturability and the capacity of these biomaterials to regenerate or repair diseased tissue. We will focus on design parameters and mechanisms that maximize therapeutic efficacy, particularly, improved functional perfusion and neovascularization regarding peripheral artery disease and improved cardiac function and reduced negative left ventricular remodeling post–myocardial infarction. We will then discuss the rationale and challenges of designing new injectable biomaterial-based therapies for the clinic.
Cardiovascular disease (CVD) has long been the leading cause of death worldwide. In 2013, CVD accounted for 31% of all deaths (1), representing a 41.7% increase since 1990 (2). Of the conditions classified as CVD, myocardial infarction (MI) and peripheral artery disease (PAD) are associated with significant morbidity and mortality. In the United States alone, approximately 8.5 million individuals are afflicted by PAD (3), and an estimated 660,000 individuals experience a new MI and 305,000 have a recurrent MI annually (4). The resulting negative left ventricular (LV) remodeling and heart failure (HF) or critical limb ischemia (CLI) and potential limb amputation that occurs in MI and PAD patients, respectively, significantly reduces the life expectancy of these individuals. Therefore, treatments for repairing ischemic damage and restoring muscle function for MI and PAD are needed.
Although current medical interventions mitigate some symptoms, they fail to prevent HF post-MI or remain unavailable for many patients with PAD. The current gold standard for MI relies on percutaneous coronary intervention (PCI) or a coronary artery bypass graft to alleviate the occluded coronary artery. However, the ischemic damage is not addressed, which subsequently leads to negative LV remodeling and ultimately HF. For PAD, stenting and balloon angioplasty treat severe conditions, but restenosis often occurs. In fact, restenosis rates for stenting and balloon angioplasty with optional stenting were both over 45% 2 years after the initial intervention (5). Similar to MI patients, the extent of occlusions and resulting ischemic damage fluctuates greatly among PAD patients, ranging from intermittent claudication to CLI. This variability contributes to difficulties with identifying a widespread treatment, demonstrated by only 40% of individuals being eligible for existing surgical procedures (6). Ultimately, new medical interventions must be developed to overcome the limitations of current approaches for MI and PAD.
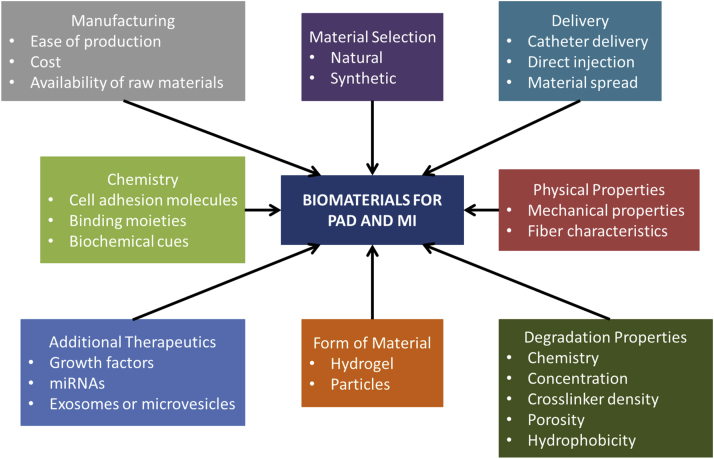
Within the past 15 years, biomaterials have emerged as a therapeutic approach to fill the existing gaps in treatments for MI and PAD (Table 1). To maximize therapeutic efficacy, biomaterials should be engineered according to specific design criteria, including material selection, mechanical properties, chemical properties, and so on. Design parameters and accompanying modifications are shown in Figure 1. This review will highlight design criteria and mechanisms of actions for biomaterial applications in PAD and MI patients and will discuss the progress toward engineering effective biomaterial-based therapies. These biomaterial applications will include material-alone approaches, as well the use of biomaterials as delivery vehicles for acellular biologics (Central Illustration).
Table 1.
Acellular Injectable Biomaterial Applications for MI and PAD
| Material | MI/PAD | Material Form | Biologics Delivered | Modifications | Ref. # |
|---|---|---|---|---|---|
| Alginate | MI | Hydrogel | HGF, IGF-1; PDGF-BB, VEGF-A | Conjugation with cell adhesion peptides; sulfation; copolymerization with fibrin | 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 61, 65 |
| PAD | Hydrogel; microspheres | IGF-1, VEGF; HGF; VEGF-F; SDF-1 | Combination with poly(d,l-lactide-co-glycolide) microspheres; sulfation; combination with collagen hydrogel | 44, 106, 109, 113 | |
| Chitosan | MI | Hydrogel | bFGF; FGF-2 | Introduction of azide | 60, 70 |
| PAD | Hydrogel | FGF-2 | Combination with lactose moieties and a periodate-oxidized IO4 heparin solution | (100) | |
| Collagen | MI | Hydrogel | N/A | N/A | 20, 22 |
| PAD | Hydrogel; microsponges; microspheres | SDF-1; bFGF; bFGF, HGF | Combination with alginate microspheres | 103, 106, 111 | |
| Decellularized myocardial ECM | MI | Hydrogel | N/A | N/A | 28, 30 |
| Decellularized pericardial ECM | MI | Hydrogel | bFGF; HGF | N/A | 67, 68 |
| Decellularized skeletal muscle ECM | PAD | Hydrogel | N/A | N/A | 85, 93 |
| Decellularized small intestine submucosa ECM | MI | Particles; hydrogel | N/A | N/A | 25, 34 |
| Dextran | MI | Microparticles | HGF | Acetalated | (69) |
| PAD | Nanoparticles | VEGF | Copolymerization with gelatin | (114) | |
| Fibrin | MI | Hydrogel | bFGF | Delivery with heparin-conjugated PLGA nanospheres; copolymerization with alginate | 13, 18, 19, 22, 63 |
| PAD | Hydrogel; particles | FGF-2 | Conjugation with heparin | 91, 92, 115 | |
| Fucoidan | PAD | Hydrogel | FGF-2 | N/A | (110) |
| Gelatin | MI | Microspheres | bFGF; IGF-1, VEGF | N/A | 59, 62, 64, 66 |
| PAD | Microspheres; hydrogel | FGF-4; bFGF; FGF-2; G-CSF | Crosslinking with poly-L-glutamic acid, crosslinking with poly-L-lysine | 97, 99, 101, 102, 104, 105, 107, 108, 112, 116 | |
| Hyaluronic acid | MI | Hydrogel | rTIMP-3 | Methacrylation; crosslinking with hydroxyethyl methacrylate; acrylation, crosslinking with PEG tetra-thiol | 23, 32, 33, 71 |
| Keratin | MI | Hydrogel | N/A | N/A | (29) |
| Matrigel | MI | Hydrogel | N/A | N/A | 22, 26 |
| PEG based | MI | Hydrogel | VEGF; HGF, VEGF; HGF, IGF-1; EPO | Crosslinking with amide- succinimidyl glutarate; crosslinking with succinimidyl glutaramide or amine; derivatization with vinyl sulfone; copolymerization with polycaprolactone; copolymerization with poly(δ-valerolactone); functionalization with cell adhesion peptides; coupling with UPy units; combination with α-cyclodextrin and copolymerization with polycaprolactone | 17, 21, 27, 31, 72, 73, 74 |
| Peptide nanofibers | MI | Hydrogel | VEGF; IGF-1; FGF-2, PDGF-BB | Biotinylation of peptides | 76, 80, 82 |
| PLGA based | MI | Microparticles, nanoparticles | NRG-1, FGF-1; VEGF; IGF-1 | Copolymerization with poly[(D,L-lactide-co-glycolide)-co-PEG] | 75, 77, 78, 83 |
| PAD | Nanoparticles | FGF-2 | N/A | (98) | |
| PNIPAAm based | MI | Hydrogel | bFGF | Combination with dextran chains and poly(ε-caprolactone)-2-hydroxylethyl methacrylate; copolymerization with acrylic acid and hydroxyethyl methacrylate-poly(trimethylene carbonate); copolymerization with propylacrylic acid and butyl acrylate | 35, 36, 79 |
| UPy | MI | Hydrogel | HGF, IGF-1 | N/A | (81) |
bFGF = basic fibroblast growth factor; ECM = extracellular matrix; EPO = erythropoietin; FGF = fibroblast growth factor; G-CSF = granulocyte-colony stimulating factor; HGF = hepatocyte growth factor; IGF = insulin-like growth factor; MI = myocardial infarction; NRG = neuregulin; PAD = peripheral artery disease; PDGF-BB = platelet-derived growth factor BB; PEG = polyethylene glycol; PLGA = poly(lactic-co-glycolic acid); PNIPAAm = poly(N-isopropylacrylamide); rTIMP = recombinant tissue inhibitor of matrix metalloproteinase-3; SDF-1 = stromal cell-derived factor; UPy = ureidopyrimidinone; VEGF = vascular endothelial growth factor.
Figure 1.
Design Variables to Be Considered When Developing Biomaterial Applications for MI and PAD
To successfully translate biomaterials to the clinic, specific design criteria must be considered to ensure that the final product remains biocompatible while maintaining its full therapeutic efficacy. Extensive engineering of a biomaterial can maximize therapeutic benefits, but these benefits must counterbalance accompanied costs and manufacturing difficulties. MI = myocardial infarction; miRNA = microribonucleic acid; PAD = peripheral artery disease.
Central Illustration.
Acellular Biomaterial Therapeutics for Repairing Ischemic Damage From MI and PAD
Preclinical studies have currently been investigating biomaterial-alone therapies or biomaterials loaded with therapeutics as potential treatment options for myocardial infarction (MI) and peripheral artery disease (PAD). Other therapeutics, like microribonucleic acids (miRNAs) or exosomes, also show promise as factors to be delivered with a biomaterial. However, the success of these therapies largely depends on satisfying specific design criteria.
Designing Acellular Injectable Biomaterial Therapies for MI
Biomaterials alone
In general, biomaterials designed for MI have demonstrated capabilities to prevent negative LV remodeling, including increasing infarct wall thickness and decreasing LV volume, fibrosis, and infarct size 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36. Additionally, many biomaterials also promote other beneficial processes like neovascularization. Since the first papers published in 2004 18, 19, studies in this area have significantly increased; a detailed coverage of individual studies can be found in other reviews 37, 38, 39.
For treating MI, important design criteria include material spread, delivery, and material selection, which affects chemical, mechanical, and degradation properties. Beginning with material selection, biomaterials can be divided into 2 classes: natural and synthetic biomaterials. Natural biomaterials can be derived from biological sources like alginate from brown algae 9, 11, 15, 16, collagen from connective tissue 20, 22, 40, or decellularized extracellular matrix (ECM) isolated from various tissues and organs 28, 41, 42. Several synthetic polymers like variations of poly(N-isopropylacrylamide) 35, 36 and synthetically modified naturally derived materials such as methacrylated hyaluronic acid (HA) have also been tested 23, 32. For eventual translation into MI patients, factors like biocompatibility, manufacturing ease, and cost must be considered. For naturally derived biomaterials, 2 main advantages include the ability to mimic native biochemical cues and potentially more cost-effective manufacturing by avoiding complex chemical synthesis. However, naturally derived materials can suffer from batch-to-batch variability due to variations in biological sources. With synthetic biomaterials, the material properties can be customized more extensively, and there are fewer issues with limited availability of raw materials. Conversely, disadvantages include potential biocompatibility issues and difficulty replicating the complex native tissue structure. Regardless of these advantages and disadvantages, most research for treating MI has utilized naturally derived biomaterials or synthetically modified derivatives 37, 38, 39.
Other factors to be considered for material selection include chemistry and mechanical properties, which can affect important cellular processes upon injection. These cellular responses, forming the basis for 1 proposed mechanism of action, include neovascularization, shifts in inflammatory/immune cells, decreases in cell death, changes in fibroblast activity (i.e. matrix production), and/or recruitment and differentiation of stem or progenitor cells. Figure 2 displays the cellular response of varying biomaterials upon injection 16, 23, 28, 35, 43, 44. Although the exact material properties leading to these outcomes are still unknown, chemical properties can be engineered accordingly, or biomaterials with the necessary properties should be selected. Naturally derived biomaterials, like collagen or decellularized ECM, have adhesion proteins, including fibronectin, fibrinogen, laminin, or collagen, to promote cell attachment. Using collagen and fibrin glue injections, neovascularization processes were stimulated 19, 22, while porcine-derived myocardial ECM hydrogels promoted infiltration of endothelial cells, smooth muscle cells, and progenitor cells 30, 41. However, for biomaterials lacking adhesion peptides, cell adhesion peptide sequences, like arginine-glycine-asparagine, can be added to improve cell adhesion, as was done by Yu et al. (16) with alginate. This modification resulted in significantly increased arteriole density relative to phosphate-buffered saline and unmodified alginate control subjects after 5 weeks post-treatment in a rat MI model.
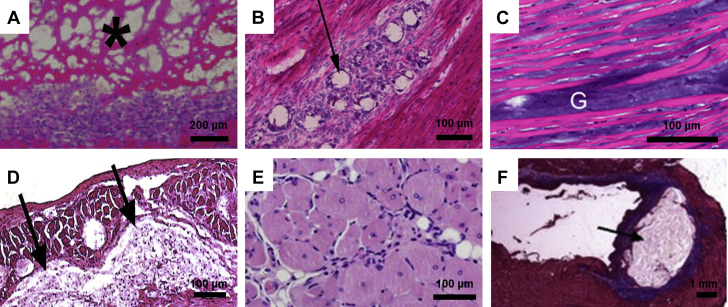
Figure 2.
Cellular Responses to Injected Biomaterials
Upon injection of a biomaterial, the resulting cellular responses can largely affect eventual tissue regeneration. Hematoxylin and eosin images are shown for (A) a decellularized myocardial extracellular matrix hydrogel (3 days post-injection), (B) acetalated dextran microparticles (7 days post-injection), (C) a methacrylated hyaluronic acid hydrogel (1 day post-injection), (D) a poly(N-isopropylacrylamide)-based synthetic hydrogel (8 weeks post-injection), and (E) an alginate hydrogel (14 days post-injection). (F) Masson’s trichrome staining shows residual alginate in a heart section 5 weeks post-injection. All tissue sections are from the heart except for (E), which is skeletal muscle. Black arrows, asterisks, and “G” denote the biomaterial.
Reproduced with permission from Seif-Naraghi et al. (28), Suarez et al. (43), Ifkovits et al. (23), Fujimoto et al. (35), Borselli et al. (44), and Yu et al. (16).
Another mechanism of action focuses on the mechanical support provided by biomaterial scaffolds, which may reduce wall stress according to LaPlace’s law. Mechanical properties are well known to affect cell fate 45, 46 and have been shown to affect outcomes in the heart (23). However, recent studies suggest that injectable materials in the heart predominantly act through their bioactivity and/or cell response rather than a mechanical support 27, 47. Moreover, many injected materials showing improvements in cardiac function are weak hydrogels with stiffnesses significantly lower than the myocardium, likely providing minimal mechanical support. One study modulating mechanical properties of a HA-based hydrogel resulted in differences in infarct size (23), showing that mechanical properties of a material are indeed important; however, these results could be related to the corresponding cellular response. Although not typically a concern with naturally derived injectable hydrogels, one should also ensure that a material is not too stiff so that it negatively affects diastolic function.
Similar to chemistry and mechanical properties, degradation properties can be significantly affected by poor material choice. Variables like the pH (which is acidic in an acute infarct [48]), temperature, and mechanical environment of the implanted biomaterial can also affect the degradation time. For most biomaterials tested to date, degradation times were inherent; however, it is possible to modify degradation rate though modulating chemistry, concentration, crosslinker density, porosity, and hydrophobicity. Burdick et al. (49) investigated the effects of HA macromer concentration, whereas Lee et al. (50) demonstrated the effect of crosslinking on degradation rates. Altogether, the inherent chemical, mechanical, and degradation properties of the chosen biomaterial can greatly influence the long-term therapeutic efficacy.
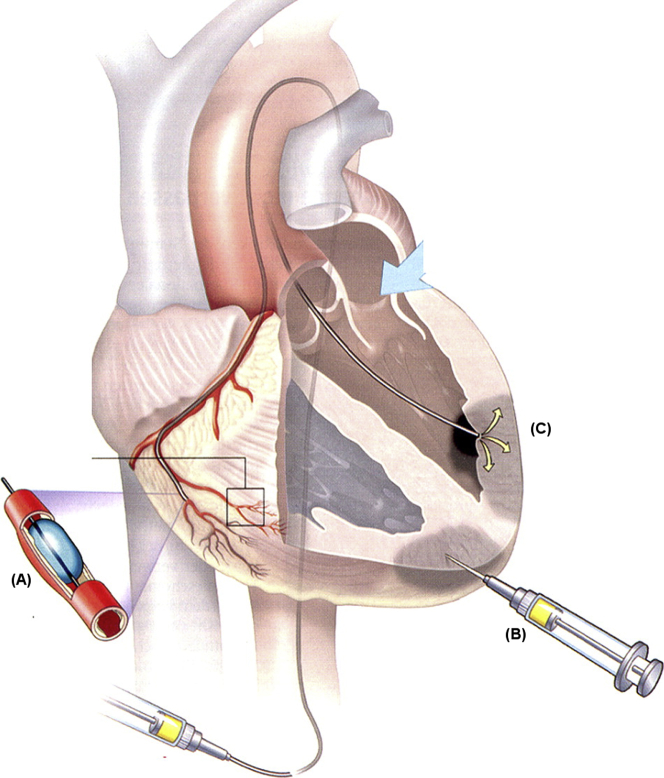
One critical, but often overlooked, design criterion is the delivery method for biomaterial therapies. Although injectable biomaterial-alone approaches and biomaterial patches 40, 42, 51 have gained attention for their therapeutic benefits, injectable versions have the advantage of a minimally invasive delivery route (52). Current delivery approaches include intracoronary infusion via a balloon infusion catheter, direct epicardial injection with a single- or double-barrel syringe, or transendocardial injection via a catheter (Figure 3) (53). Epicardial injections have the most control over delivery but often require an invasive surgical procedure, which is likely to complicate widespread use. Although the 2 remaining methods are minimally invasive, they have disadvantages, including relying on leaky acute MI vasculature for intracoronary delivery and needing specialized training for transendocardial delivery. Obviating the need for an invasive surgery with general anesthesia has, however, resulted in the majority of injectable therapeutics, including biomaterials, being delivered via catheter-based approaches in clinical trials. For catheter delivery, the material must be hemocompatible given embolization risks since the material is injected into a coronary with an infusion approach and is known to leak into the LV chamber with transendocardial injections. In addition, the material must be designed to have appropriate gelation kinetics to travel through a long, small-diameter catheter (typically 27-gauge) and gel in the infarct, but not in the blood stream. The rapid gelation and/or lack of hemocompatibility have prevented most injectable biomaterials from being delivered with these more translationally relevant methods. For instance, an alginate formulation that is being studied in HF patients was not initially designed for catheter delivery and, therefore, must be delivered via an invasive surgical approach 7, 12. In particular, multiple injections required with transendocardial injections create a unique design constraint not common with other injectable biomaterial applications.
Figure 3.
Delivery Methods for Biomaterial-Based Applications in MI
When designing a biomaterial approach for MI, the importance of the delivery route is often underestimated. (A) Intracoronary infusion via a balloon infusion catheter relies on leaky acute MI vasculature for delivery, whereas (C) transendocardial injection via a catheter requires specialized training. However, neither of these minimally invasive methods requires an invasive surgery, unlike (B) direct epicardial injections.
Reproduced with permission from Stamm et al. (53).
Upon injection, other variables, like material spread, have also been studied to avoid dangerous side effects like arrhythmias. A study by Suarez et al. (31) looked at the effects of interstitial spread with poly(ethylene glycol)-based hydrogels and did not discover changes to action potential propagation with high spreading materials. However, significant delays as well as a reduction in gap junction density were found with materials that were quick gelling and formed a bolus, suggesting that they may be a potential substrate for arrhythmias (31). This study was only done in rats, though, and additional studies are needed in large animals, as well as with different biomaterials.
By harnessing the potential of naturally derived biomaterials, several studies have progressed into clinical trials (Table 2). Using calcium crosslinked alginate hydrogels, the PRESERVATION I (IK-5001 for the Prevention of Remodeling of the Ventricle and Congestive Heart Failure After Acute Myocardial Infarction) trial (NCT01226563) included patients who had experienced a large MI and underwent successful primary PCI within 48 h of symptom onset, whereas the AUGMENT-HF (A Randomized, Controlled Study to Evaluate Algisyl-LVR as a Method of Left Ventricular Augmentation for Heart Failure) trial (NCT01311791) included HF patients, with approximately one-half of the participants having experienced a previous MI. PRESERVATION I participants received 4-ml infusions of either saline or the alginate solution 2 to 5 days post-PCI into the occluded artery via catheter-based intracoronary infusion (14). Despite observing prevention and a reversal of LV dilation and increased scar thickness in a swine acute MI model (11) and conservation of the LV end-diastolic volume index, LV end-systolic volume index, and ejection fraction (EF) with 2-ml infusions of the alginate hydrogel in the Phase I trial (8), no differences were seen between the treated and control groups in terms of the LV end-diastolic volume index after 6 months. It has been suggested that this was a result of too little material being utilized for larger infarcts, timing of treatment administration, and/or the inability of the material to reach the infarct zone due to microvascular occlusions. Another significant possibility is that cells do not adhere readily to alginate, and thus it has minimal bioactivity, which could have resulted in a lack of improvements. In the AUGMENT-HF trial, significantly increased peak VO2 levels and 6-min walk test distance were seen at 3, 6, and 12 months after intervention via 10 to 19 0.3-ml intramyocardial injections through a limited left thoracotomy approach compared with standard medical therapy alone 7, 12. However, compared to improved LV function and quality of life with alginate administration during cardiac bypass surgery or valve replacement/repair in phase I studies (10), no significant changes in the EF, LV end-diastolic diameter, or LV end-systolic diameter were seen in phase II. The latest biomaterial-alone therapy to advance into clinical trials is based on the preclinical studies done by Seif-Naraghi et al. (28) with an injectable decellularized myocardial ECM hydrogel in a porcine MI model. At 3 months post-injection, pigs treated with the ECM hydrogel showed an increase in global and regional cardiac function compared with control animals. In addition, the myocardial matrix hydrogel increased cardiac muscle compared with noninjected and saline-injected animals. Currently in a phase I clinical trial (NCT02305602), VentriGel (Ventrix, Inc., San Diego, California) is being delivered via transendocardial injections with a MyoStar catheter (Biosense Webster, Diamond Bar, California) in patients who experienced a previous MI (60 days to 3 years since the event).
Table 2.
Clinical Trials for Injectable Biomaterials in MI and PAD
| Material | Product Name (Identifier #) | Trial Phase | MI/PAD | Study Design |
Results | Ref. # | |||
|---|---|---|---|---|---|---|---|---|---|
| Design | Control | Patient Population | Delivery | ||||||
| Gelatin microspheres with bFGF | N/A | N/A | PAD | Nonrandomized | None | Patients with CLI, no option of medical or surgical treatment (7 total) | Single intramuscular injection (200 μg) | Significant improvements in 6-min walk distance, blood perfusion, transcutaneous oxygen pressure, and rest pain scale compared with pre-treatment values | (112) |
| Alginate | Algisyl-LVR (NCT00847964) | I | MI | Nonrandomized | None | HF patients (9 total) | Intramyocardial injections during cardiac bypass surgery or valve replacement/repair (9–15 injections, 0.25–0.35 ml each) | Improved LV function and quality of life | (10) |
| Algisyl-LVR (NCT01311791) | II (AUGMENT-HF) | MI | Randomized, single-blind | Standard medical therapy alone | HF patients, approximately one-half with previous MI (n = 78) | Intramyocardial injections via limited left thoracotomy (10–19 injections, 0.3 ml each) | Significant increases in peak VO2 levels and 6-min walk test distance, no changes in EF, LV end-diastolic diameter, or LV end-systolic diameter | 7, 12 | |
| Alginate | BL-1040 (NCT00557531) | I | MI | Nonrandomized | None | Experienced moderate to large MI, underwent successful primary PCI (n = 27) | Catheter-based intracoronary infusion (2 ml) | Preserved LVEDV index, LVESV index, and LVEF | (8) |
| IK-5001 (NCT01226563) | II (PRESERVATION I) | MI | Randomized, double-blind | Placebo (saline) | Experienced large MI, underwent successful primary PCI (n = 303) | Catheter-based intracoronary infusion (4 ml) | No differences in terms of LVEDV index | (14) | |
| Decellularized myocardial ECM hydrogel | VentriGel (NCT02305602) | I | MI | Nonrandomized | None | Experienced previous MI, 60 days to 3 years since event (18 patients projected) | Transendocardial delivery via MyoStar catheter | Ongoing | (28) |
CLI = critical limb ischemia; EF = ejection fraction; HF = heart failure; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; PCI = percutaneous coronary intervention; other abbreviations as in Table 1.
Compared with other emerging therapeutics for MI, biomaterials also represent a potentially more promising approach in terms of translation and commercialization (54). Biomaterial hydrogels allow for more precise treatment since the material remains localized upon injection unlike small molecule or protein therapeutics, which rapidly diffuse away from the injection site 55, 56, or cell injections, which also migrate and have poor survival 57, 58. Lastly, biomaterials alone represent a more cost-effective option since incorporation of additional therapeutics can dramatically increase expenses. By incorporating and understanding some of the design criteria described in the previous text, several biomaterial-alone approaches have yielded positive results, leading to a few clinical trials. To advance the field and progress into greater numbers of clinical trials, it is imperative that researchers consider these design criteria from the beginning. It will also be important to elucidate the mechanisms of action of these materials, which will lead to improved material generation.
Biomaterials and growth factors
In addition to utilizing biomaterial-alone approaches, codelivery with additional acellular biologics, predominantly growth factors, has been employed (Table 1) 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83. A major challenge for growth factor therapeutics has been rapid diffusion upon delivery, but biomaterial delivery vehicles can prolong the release rate and improve localization by selecting or designing biomaterials to elicit the desired release profile. Additionally, using biomaterials can decrease the costs of incorporating growth factors since smaller quantities are required. Generally, the material form, degradation properties, and chemistry are paramount for enhancing rate of release and localization; therefore, material selection and additional modifications must be analyzed to identify the optimal delivery vehicle.
For physical and degradation properties, biomaterials must retain and then gradually release growth factors upon delivery. The microscale or nanoscale architecture of hydrogels, microparticles, or nanoparticles significantly contributes to this tunable release (Figure 4) 29, 67, 78, 84, 85, 86; results for individual studies can be found in another review (87). With hydrogels and particles, the architecture provides small pores for slow growth factor release, and gradual degradation of the material contributes to a more complete release. Through the gelation of hydrogels or targeted delivery with particles, growth factors are localized to a region of interest and cannot diffuse rapidly upon delivery. Additionally, regulated delivery prevents systemic side effects due to uncontrolled diffusion, demonstrated by Lin et al. (82), with significantly reduced vascular leakage resulting from codelivery of high doses of vascular endothelial growth factor (VEGF) with self-assembling peptide nanofibers. Although a longer release may seem favorable, this is not true for all growth factors. In fact, complex processes, like angiogenesis, require precise timing and order for the delivery of therapeutics 84, 88. Suarez et al. (69) demonstrated this importance with an engineered hepatocyte growth factor (HGF) fragment diffusing at varying rates from acetalated dextran microparticles in a rat MI model due to different degradation profiles, showing that it was most effective when delivered over 3 days compared with 1.5 or 2.5 weeks.
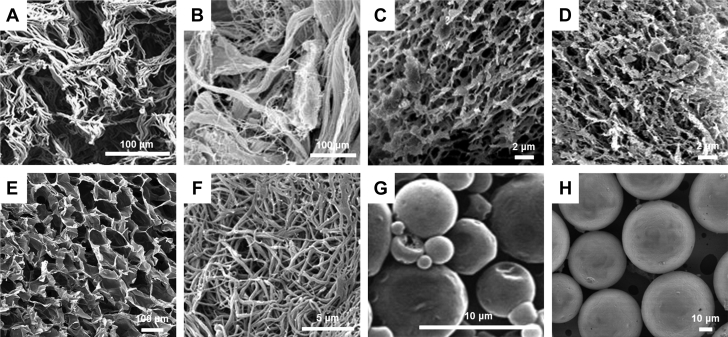
Figure 4.
Structures of Biomaterials for MI and PAD Applications
Biomaterial structures dictate important parameters including degradation and controlled release of therapeutics. The architecture, shown by scanning electron micrographs, varies among hydrogels, such as (A) keratin, (B) porcine-derived skeletal muscle extracellular matrix (ECM), (C) porcine-derived pericardial ECM, (D) collagen, (E) alginate, or (F) fibrin. Additionally, hydrogel architecture differs from particles like (G) poly(lactic-co-glycolic acid) microparticles or (H) acetalated dextran microparticles. Reproduced with permission from Shen et al. (29), DeQuach et al. (85), Seif-Naraghi et al. (67), Freeman et al. (84), Losi et al. (86), Formiga et al. (78), and Suarez et al. (43). Abbreviations as in Figure 1.
Instead of solely relying on the architecture of the biomaterial to control the retention and release, biomaterials can also be modified to encourage binding and retention of growth factors. This can be achieved through the presence of sulfated glycosaminoglycans or sulfation of a biomaterial to contribute to a slower release profile. Binding of growth factors can also yield higher therapeutic efficacy by increasing stability and activity, as was reviewed by Zisch et al. (89) for the release of angiogenic growth factors. Ruvinov et al. (65) also showed this with an affinity-binding alginate biomaterial consisting of insulin-like growth factor-1 and HGF bound by alginate-sulfate interactions in a rat MI model. After 4 weeks, the fibrotic area was significantly reduced and the relative scar thickness, blood vessel density, and average individual blood vessel area were significantly increased. With decellularized ECM hydrogels, however, sulfation is unnecessary because sulfated glycosaminoglycans are still present after decellularization (90). In 2 studies by Seif-Naraghi et al. (67) and Sonnenberg et al. (68), decellularized pericardial ECM hydrogels were mixed with basic fibroblast growth factor (bFGF) and an engineered HGF fragment, respectively. Both studies showed significantly increased arteriole density in animals treated with the ECM hydrogels and growth factors over growth factors delivered in saline, whereas Sonnenberg et al. (68), also observed an increased fractional area change.
Research conducted on growth factors encapsulated in biomaterials for treating MI has made several advances into large animal models. Liu et al. (64) utilized bFGF incorporated into gelatin microspheres in a pig infarct model, which yielded increases in EF and vascular density. Mentioned earlier, Lin et al. (82) injected self-assembling peptide nanofibers mixed with VEGF into a porcine MI model and saw improvements in fractional shortening and capillary and arteriole density and a decrease in infarct size. Finally, Koudstaal et al. (81) investigated a combination of growth factors, insulin-like growth factor-1 and HGF, incorporated into a synthetic hydrogel known as ureidopyrimidinone. Diffusion of the growth factors from the ureidopyrimidinone hydrogel resulted in increased cardiac function, capillary density, and cardiac progenitor cell migration in a porcine MI model. These recent advancements have provided a strong foundation for growth factor delivery in biomaterials, but translation into the clinic has been limited, potentially due to the high cost of incorporating growth factors.
Designing Acellular Injectable Biomaterial Therapies for PAD
Biomaterials alone
Current experimental treatments for PAD, including stem cells and growth factors, have not been entirely successful for many of the same reasons as MI therapeutics. Consequently, an effective, minimally invasive treatment that improves perfusion and repairs ischemic tissue damage is still needed. Biomaterial-alone therapies have shown considerable promise for repairing ischemic muscle by encouraging reperfusion and neovascularization 85, 91, 92, 93, but the success of these biomaterials as a stand-alone approach for PAD lies in satisfying particular design constraints (Table 1, Figure 1). Although many of the important design properties are similar to those mentioned earlier for MI, including material selection, physical properties, and degradation properties, the design criteria vary for PAD and may fluctuate depending on the disease spectrum of the patient (i.e., intermittent claudication vs. CLI).
Similar to MI, material selection is extremely important for PAD to encourage perfusion restoration and muscle regeneration. Each biomaterial must be engineered or evaluated to promote cell infiltration and proliferation/differentiation to treat both the ischemia and muscle atrophy associated with PAD (94). As such, the materials must allow for cell adhesion and have appropriate pore size for cell migration. Several preclinical studies have investigated naturally derived biomaterials like fibrin 91, 92 and decellularized ECM hydrogels 85, 93, but synthetic biomaterials have yet to be studied in detail. Fibrin is well known to encourage vascularization and has likewise been shown in rabbit hindlimb ischemia models to increase perfusion 91, 92; however, only ECM hydrogels have been evaluated for muscle repair (93). Chekanov et al. (91) utilized a fibrin sealant and observed significant increases in collateral vessel development and the area occupied by capillaries compared with no treatment or saline alone. Similarly, fibrin particles used by Fan et al. (92) yielded significantly augmented capillary density and perfusion recovery compared with control subjects. DeQuach et al. (85) utilized an injectable porcine-derived skeletal muscle ECM hydrogel in a rat hindlimb ischemia model and showed an increase not only in vascular cells, but also in proliferating muscle cells and muscle progenitor cells. Even after selecting a naturally derived material, however, the source for that material must still be chosen. With decellularized ECM hydrogels, for example, the tissue source can affect therapeutic outcomes. In a study conducted by Ungerleider et al. (93), 2 different decellularized ECM hydrogels, a porcine-derived skeletal muscle ECM and human umbilical cord ECM, were assessed in a rat hindlimb ischemia model. Although improvements in perfusion were seen for both hydrogels, the muscles injected with the skeletal muscle ECM hydrogel resembled the healthy morphology more closely than those injected with the human umbilical cord matrix, suggesting that tissue-specific cues may be important for regeneration.
The last 2 design criteria to be discussed for a biomaterial-alone approach in PAD are degradation properties and delivery. The main factor to be considered for degradation properties is whether the biomaterial will yield sufficient therapeutic improvements before it completely degrades. Because PAD most often affects the lower limbs, the mechanical environment caused by a load-bearing region can cause biomaterials to degrade more quickly. As a result, appropriate animal models must be used to generate results that are representative of the human mechanical environment. For delivery of these therapeutics, direct intramuscular injections should be utilized (ideally ≤26-gauge for patients); however, the number and timing of these injections must be determined. Due to the large surface area of the lower limbs, multiple injections of the biomaterials will be necessary. Results from small animal studies can provide insight for the appropriate concentration and required volume of injections, but these results must then be scaled up for larger animals and clinical studies. To date, limited work has been performed on developing a suitable large animal model, although a few recent studies suggest that this may be forthcoming 95, 96. Overall, there is still a great deal of research to be done for biomaterial- alone approaches in PAD; however, it is a promising approach that should be pursued.
Biomaterials and growth factors
Although biomaterial-alone approaches have not been extensively investigated for PAD, biomaterials have been utilized to deliver growth factors, as shown in Table 1 44, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116. To maximize therapeutic efficacy of a biomaterial and growth factor complex, similar design criteria to MI should be applied, including selecting or engineering materials based on physical form, chemistry, and degradation properties. Physical form, such as selecting particles as opposed to hydrogels, can alter the delivery method due to the ability to engineer particles for targeting. For chemical properties, modifications like binding moieties for growth factors, such as sulfate groups, can be added to encourage longer retention. Lastly, degradation plays an equally important role in controlling retention and release since rapid degradation will lead to a similar release rate for the therapeutic payload.
By incorporating these design principles, researchers have advanced some therapies into pre-clinical studies with rabbits and larger animal models and even 1 clinical trial. An early study conducted by Kasahara et al. (104) utilized gelatin microparticles to deliver fibroblast growth factor (FGF)-4 in a rabbit hindlimb ischemia model. Under vasodilatory conditions, the perfusion levels and angiographic scores were significantly higher in the gelatin/FGF-4 complex compared with gelatin or FGF-4 alone. In another study by Doi et al. (99), gelatin hydrogels encapsulated with bFGF were injected intramuscularly in Japanese white rabbits 2 weeks post-hindlimb ischemia surgery. Animals treated with the gelatin and bFGF hydrogel had significantly higher perfusion levels and vascular density compared with no treatment or gelatin alone at 4 weeks post-injection. A large animal study performed in mongrel dogs by Zhao et al. (116) studied bFGF encapsulated in gelatin microspheres. Significantly higher capillary densities and numbers of mature vessels were observed with the gelatin microspheres and bFGF-treated group relative to bFGF alone and empty microspheres. The final study to be mentioned includes the findings of a phase I to IIa clinical trial (Table 2). In a rabbit hindlimb ischemia model, Hirose et al. (101) injected gelatin hydrogel microspheres containing bFGF and saw increased perfusion, capillary density, and collateral vessel development compared with a no treatment control group. This led to an investigation by Marui et al. (112) in which biodegradable gelatin hydrogels loaded with bFGF were administered with a single intramuscular injection in patients with CLI; no controls were used for this study. At 4 and 24 weeks post-treatment, improvements were seen in the perfusion compared with values prior to treatment. By utilizing biomaterials as delivery vehicles, the growth factor release can be precisely controlled, and biomaterials can prevent degradation of growth factors to fully harness their therapeutic potential.
Designing Biomaterials as Delivery Vehicles for Emerging Therapeutics
Although numerous studies presented in this review demonstrate the efficacy of utilizing biomaterials alone or a combination of growth factors and biomaterials for treating MI and PAD, growth factors are not the only acellular therapeutic that should be considered for biomaterial-based therapies. Biologics like erythropoietin and recombinant tissue inhibitor of matrix metalloproteinase-3 have been studied 71, 73, yet emerging therapeutics, such as exosomes or microribonucleic acids (miRNAs), may also enhance the beneficial properties of biomaterials, while avoiding obstacles plaguing cellular-based treatments. Previous studies investigated the efficacy of these therapeutics in MI 117, 118, 119, 120, 121, 122, 123, 124 and PAD preclinical models 117, 125, 126, 127, 128, 129, but limited research has been conducted to optimize delivery. For exosomes, microvesicles, and miRNAs, maximized therapeutic efficiency has been hindered by poor retention upon injection. Because these therapeutics are typically injected alone, they rapidly diffuse from the injection site, similar to growth factors, therefore leading to minimal improvements in the targeted region. One study by Hinkel et al. (123) revealed the effect of catheter-based delivery compared with systemic delivery with antagomir-92a in a porcine MI model. Using a regional delivery approach, decreased infarct size and apoptosis were seen, and EF was improved compared with the systemic delivery.
This study emphasizes the importance of local delivery, but utilizing a biomaterial as a delivery vehicle is likely to further improve results. Because miRNAs are quickly degraded after injection due to the large amount of RNases circulating throughout the body, biomaterials can provide a shielded environment to maximize therapeutic effects. Additionally, the controlled release provided by biomaterials can also contribute to improved efficacy. Based on the current advancements in biomaterial-based therapies, many of the design principles discussed earlier could overcome these obstacles.
The physical form and chemistry of a biomaterial can significantly contribute to slower release kinetics in addition to providing a protected environment from degradation. By changing the physical form of the biomaterial, the release can be tuned for the specific payload, and targeting can also be incorporated with particles. Additionally, altering the biomaterial’s concentration often leads to changes in pore size, which can be utilized to change the release profile. The same microscale or nanoscale architecture being modified for desired release kinetics can also be used to protect the payload from degradation. In terms of the chemistry, modifications can be made to allow for better retention of additional therapeutics or release only upon cell infiltration.
Although no biomaterial-based therapies have been published for microvesicle or miRNA delivery in MI or PAD, there are a few studies related to other applications, which validate the use of biomaterials for the delivery of these newer therapeutics. For bone repair, a miR-29a inhibitor, intended to increase ECM deposition, was delivered with gelatin nanofibers (130). The investigators demonstrated the feasibility of this approach, as well as efficacy in terms of a slow release profile and sustained bioactivity of the miRNA inhibitor once released compared with a scrambled miRNA control. The Burdick lab has also begun investigating a hydrogel system for small interfering ribonucleic acid delivery (131), but further studies are still ongoing. Therefore, this represents a promising area of research for improving the delivery of the next generation of therapeutics and should be explored for MI and PAD.
Finding the Optimal Therapy for MI and PAD Patients: Balancing Therapeutic Potential and Commercialization Challenges
Extensive research has validated the use of acellular biomaterials, but difficulties must still be overcome before implementation into the clinic. When considering incorporation of additional factors, an acellular approach is optimal for multiple reasons. Including cells dramatically reduces shelf life due to instability and significantly increases manufacturing expenses for a large-scale setting. The addition of growth factors encompasses many of these same issues, including reduced shelf life and high cost, but new manufacturing methods are being studied to overcome these obstacles. For example, Cochran and colleagues 68, 132 have developed an engineered HGF fragment with increased stability and lower cost of manufacturing, while maintaining its therapeutic effects. With new methods being optimized for growth factor delivery, these lower-cost options could result in more feasible biomaterial-based treatments for MI and PAD patients.
As discussed earlier, biomaterials may also be delivered alone and have produced significant improvements in animal models of MI and PAD. From a manufacturing perspective, a biomaterial-alone approach is the preferred method, as the increased costs and manufacturing time associated with additional therapeutics are negated. However, several studies previously mentioned suggest that a combinatorial approach may be more effective. Although current growth factor therapies may not be ideal for eventual translation to the clinic, due to difficult and expensive manufacturing, less expensive, engineered growth factors, like the one mentioned previously, or other therapeutics may augment the benefits of injectable biomaterials. The studies utilizing microvesicles, exosomes, or miRNAs alone have also demonstrated substantial therapeutic efficacy in MI and PAD animal models, but more research must be done to optimize the delivery of these factors. In conclusion, research must be conducted to investigate the delivery of additional therapeutics with biomaterials, but the added therapeutic efficacy must outweigh the additional costs.
Conclusions
Acellular biomaterial-based therapies may be a solution for many patients experiencing MI and PAD. By harnessing the ability to engineer these biomaterials and employing the minimally invasive nature of many of these therapies, patients may soon receive treatments designed to stimulate tissue regeneration and improved muscle function. Although further research must be conducted to develop optimal biomaterial strategies, and manufacturing expenses must be carefully considered, the field is rapidly progressing toward identifying new treatments for MI and PAD patients.
Acknowledgments
The authors thank Raymond Wang, Jessica Ungerleider, and Vivienne Gunadhi for providing feedback and editing for this review.
Footnotes
Funding was provided in part by the National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL113468 and R01HL117326 (to Dr. Christman). Ms. Hernandez was supported by National Institutes of Health, National Heart, Lung, and Blood Institute funded training grant T32HL105373. Dr. Christman is a cofounder of, consultant for, and board member of Ventrix, Inc.; and holds equity interest in Ventrix, Inc.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Bloom D.E., Cafiero E.T., Jané-Llopis E. World Economic Forum; Geneva: 2011. The Global Economic Burden of Non-communicable Diseases. [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison M.A., Ho E., Denenberg J.O. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 5.Schillinger M., Sabeti S., Dick P. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 6.Dormandy J., Heeck L., Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. [PubMed] [Google Scholar]
- 7.Anker S.D., Coats A.J., Cristian G. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial) Eur Heart J. 2015;36:2297–2309. doi: 10.1093/eurheartj/ehv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey N., Linke A., Suselbeck T. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv. 2014;7:806–812. doi: 10.1161/CIRCINTERVENTIONS.114.001478. [DOI] [PubMed] [Google Scholar]
- 9.Landa N., Miller L., Feinberg M.S. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 10.Lee R.J., Hinson A., Bauernschmitt R. The feasibility and safety of Algisyl-LVR as a method of left ventricular augmentation in patients with dilated cardiomyopathy: initial first in man clinical results. Int J Cardiol. 2015;199:18–24. doi: 10.1016/j.ijcard.2015.06.111. [DOI] [PubMed] [Google Scholar]
- 11.Leor J., Tuvia S., Guetta V. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Mann D.L., Lee R.J., Coats A.J. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail. 2016;18:314–325. doi: 10.1002/ejhf.449. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee R., Zavadzkas J.A., Saunders S.M. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann Thorac Surg. 2008;86:1268–1276. doi: 10.1016/j.athoracsur.2008.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao S.V., Zeymer U., Douglas P.S. Bioabsorbable intracoronary matrix for prevention of ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2016;68:715–723. doi: 10.1016/j.jacc.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 15.Tsur-Gang O., Ruvinov E., Landa N. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials. 2009;30:189–195. doi: 10.1016/j.biomaterials.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Yu J., Gu Y., Du K.T., Mihardja S., Sievers R.E., Lee R.J. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009;30:751–756. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 17.Bastings M.M., Koudstaal S., Kieltyka R.E. A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv Healthc Mater. 2014;3:70–78. doi: 10.1002/adhm.201300076. [DOI] [PubMed] [Google Scholar]
- 18.Christman K.L., Fok H.H., Sievers R.E., Fang Q., Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 19.Christman K.L., Vardanian A.J., Fang Q., Sievers R.E., Fok H.H., Lee R.J. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Dai W., Wold L.E., Dow J.S., Kloner R.A. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 21.Dobner S., Bezuidenhout D., Govender P., Zilla P., Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009;15:629–636. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Huang N.F., Yu J., Sievers R., Li S., Lee R.J. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 23.Ifkovits J.L., Tous E., Minakawa M. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107:11507–11512. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X.J., Wang T., Li X.Y. Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction. J Biomed Mater Res A. 2009;90:472–477. doi: 10.1002/jbm.a.32118. [DOI] [PubMed] [Google Scholar]
- 25.Okada M., Payne T.R., Oshima H., Momoi N., Tobita K., Huard J. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010;31:7678–7683. doi: 10.1016/j.biomaterials.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Ou L., Li W., Zhang Y. Intracardiac injection of matrigel induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J Cell Mol Med. 2011;15:1310–1318. doi: 10.1111/j.1582-4934.2010.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rane A.A., Chuang J.S., Shah A. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PLoS One. 2011;6:e21571. doi: 10.1371/journal.pone.0021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seif-Naraghi S.B., Singelyn J.M., Salvatore M.A. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen D., Wang X., Zhang L. The amelioration of cardiac dysfunction after myocardial infarction by the injection of keratin biomaterials derived from human hair. Biomaterials. 2011;32:9290–9299. doi: 10.1016/j.biomaterials.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 30.Singelyn J.M., Sundaramurthy P., Johnson T.D. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez S.L., Rane A.A., Munoz A. Intramyocardial injection of hydrogel with high interstitial spread does not impact action potential propagation. Acta Biomater. 2015;26:13–22. doi: 10.1016/j.actbio.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tous E., Ifkovits J.L., Koomalsingh K.J. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011;12:4127–4135. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon S.J., Fang Y.H., Lim C.H. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater. 2009;91:163–171. doi: 10.1002/jbm.b.31386. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z.Q., Puskas J.D., Xu D. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of C-kit cells, myofibroblasts, and macrophages after myocardial infarction. J Am Coll Cardiol. 2010;55:1250–1261. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto K.L., Ma Z., Nelson D.M. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T., Wu D.Q., Jiang X.J. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail. 2009;11:14–19. doi: 10.1093/eurjhf/hfn009. [DOI] [PubMed] [Google Scholar]
- 37.Tous E., Purcell B., Ifkovits J.L., Burdick J.A. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011;4:528–542. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 38.Rane A.A., Christman K.L. Biomaterials for the treatment of myocardial infarction: a 5- year update. J Am Coll Cardiol. 2011;58:2615–2629. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Grover G.G., Christman K.L. Imperial College Press; Hackensack, NJ: 2016. Injectable hydrogels for regenerative engineering. [Google Scholar]
- 40.Serpooshan V., Zhao M., Metzler S.A. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34:9048–9055. doi: 10.1016/j.biomaterials.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singelyn J.M., DeQuach J.A., Seif-Naraghi S.B., Littlefield R.B., Schup-Magoffin P.J., Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perea-Gil I., Prat-Vidal C., Gálvez-Montón C. A cell-enriched engineered myocardial graft limits infarct size and improves cardiac function: pre-clinical study in the porcine myocardial infarction model. J Am Coll Cardiol Basic Trans Science. 2016;1:360–372. doi: 10.1016/j.jacbts.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suarez S., Grover G.N., Braden R.L., Christman K.L., Almutairi A. Tunable protein release from acetalated dextran microparticles: a platform for delivery of protein therapeutics to the heart post-MI. Biomacromolecules. 2013;14:3927–3935. doi: 10.1021/bm401050j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borselli C., Storrie H., Benesch-Lee F. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Young J.L., Engler A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGarvey J.R., Pettaway S., Shuman J.A. Targeted injection of a biocomposite material alters macrophage and fibroblast phenotype and function following myocardial infarction: relation to left ventricular remodeling. J Pharmacol Exp Ther. 2014;350:701–709. doi: 10.1124/jpet.114.215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burdick J.A., Chung C., Jia X., Randolph M.A., Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee K.Y., Bouhadir K.H., Mooney D.J. Controlled degradation of hydrogels using multi- functional cross-linking molecules. Biomaterials. 2004;25:2461–2466. doi: 10.1016/j.biomaterials.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 51.Callegari A., Bollini S., Iop L. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials. 2007;28:5449–5461. doi: 10.1016/j.biomaterials.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Johnson T.D., Christman K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv. 2013;10:59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 53.Stamm C., Nasseri B., Choi Y.H., Hetzer R. Cell therapy for heart disease: great expectations, as yet unmet. Heart Lung Circ. 2009;18:245–256. doi: 10.1016/j.hlc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Ungerleider J.L., Christman K.L. Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med. 2014;3:1090–1099. doi: 10.5966/sctm.2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laham R.J., Post M., Rezaee M. Transendocardial and transepicardial intramyocardial fibroblast growth factor-2 administration: myocardial and tissue distribution. Drug Metab Dispos. 2005;33:1101–1107. doi: 10.1124/dmd.104.002774. [DOI] [PubMed] [Google Scholar]
- 56.Anitua E., Sanchez M., Orive G., Andia I. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008;29:37–41. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M., Methot D., Poppa V., Fujio Y., Walsh K., Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 58.Muller-Ehmsen J., Whittaker P., Kloner R.A. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 59.Cittadini A., Monti M.G., Petrillo V. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail. 2011;13:1264–1274. doi: 10.1093/eurjhf/hfr143. [DOI] [PubMed] [Google Scholar]
- 60.Fujita M., Ishihara M., Morimoto Y. Efficacy of photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 in a rabbit model of chronic myocardial infarction. J Surg Res. 2005;126:27–33. doi: 10.1016/j.jss.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 61.Hao X., Silva E.A., Mansson-Broberg A. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Iwakura A., Fujita M., Kataoka K. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels. 2003;18:93–99. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 63.Jeon O., Kang S.-W., Lim H.-W., Hyung Chung J., Kim B.-S. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(l-lactide-co- glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27:1598–1607. doi: 10.1016/j.biomaterials.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y., Sun L., Huan Y., Zhao H., Deng J. Effects of basic fibroblast growth factor microspheres on angiogenesis in ischemic myocardium and cardiac function: analysis with dobutamine cardiovascular magnetic resonance tagging. Eur J Cardiothorac Surg. 2006;30:103–107. doi: 10.1016/j.ejcts.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 65.Ruvinov E., Leor J., Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32:565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 66.Sakakibara Y., Tambara K., Sakaguchi G. Toward surgical angiogenesis using slow-released basic fibroblast growth factor. Eur J Cardiothorac Surg. 2003;24:105–111. doi: 10.1016/s1010-7940(03)00159-3. discussion 112. [DOI] [PubMed] [Google Scholar]
- 67.Seif-Naraghi S.B., Horn D., Schup-Magoffin P.J., Christman K.L. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomaterialia. 2012;8:3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonnenberg S.B., Rane A.A., Liu C.J. Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative LV remodeling post-myocardial infarction. Biomaterials. 2015;45:56–63. doi: 10.1016/j.biomaterials.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suarez S.L., Muñoz A., Mitchell A.C. Degradable acetalated dextran microparticles for tunable release of an engineered hepatocyte growth factor fragment. ACS Biomater Sci Eng. 2016;2:197–204. doi: 10.1021/acsbiomaterials.5b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H., Zhang X., Li Y. Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive chitosan hydrogel. J Heart Lung Transplant. 2010;29:881–887. doi: 10.1016/j.healun.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Eckhouse S.R., Purcell B.P., McGarvey J.R. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med. 2014;6:223ra21. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salimath A.S., Phelps E.A., Boopathy A.V. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS One. 2012;7:e50980. doi: 10.1371/journal.pone.0050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang T., Jiang X.-J., Lin T. The inhibition of postinfarct ventricle remodeling without polycythaemia following local sustained intramyocardial delivery of erythropoietin within a supramolecular hydrogel. Biomaterials. 2009;30:4161–4167. doi: 10.1016/j.biomaterials.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 74.Wu J., Zeng F., Huang X.-P. Infarct stabilization and cardiac repair with a VEGF- conjugated, injectable hydrogel. Biomaterials. 2011;32:579–586. doi: 10.1016/j.biomaterials.2010.08.098. [DOI] [PubMed] [Google Scholar]
- 75.Chang M.Y., Yang Y.J., Chang C.H. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J Control Release. 2013;170:287–294. doi: 10.1016/j.jconrel.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 76.Davis M.E., Hsieh P.C., Takahashi T. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Formiga F.R., Pelacho B., Garbayo E. Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia-reperfusion model. J Control Release. 2010;147:30–37. doi: 10.1016/j.jconrel.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 78.Formiga F.R., Pelacho B., Garbayo E. Controlled delivery of fibroblast growth factor-1 and neuregulin-1 from biodegradable microparticles promotes cardiac repair in a rat myocardial infarction model through activation of endogenous regeneration. J Control Release. 2014;173:132–139. doi: 10.1016/j.jconrel.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 79.Garbern J.C., Minami E., Stayton P.S., Murry C.E. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32:2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J.H., Jung Y., Kim S.-H. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self- assembling peptides. Biomaterials. 2011;32:6080–6088. doi: 10.1016/j.biomaterials.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Koudstaal S., Bastings M.M., Feyen D.A. Sustained delivery of insulin-like growth factor-1/hepatocyte growth factor stimulates endogenous cardiac repair in the chronic infarcted pig heart. J Cardiovasc Transl Res. 2014;7:232–241. doi: 10.1007/s12265-013-9518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Y.D., Luo C.Y., Hu Y.N. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med. 2012;4:146ra109. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]
- 83.Simon-Yarza T., Tamayo E., Benavides C. Functional benefits of PLGA particulates carrying VEGF and CoQ10 in an animal of myocardial ischemia. Int J Pharm. 2013;454:784–790. doi: 10.1016/j.ijpharm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Freeman I., Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 85.DeQuach J.A., Lin J.E., Cam C. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur Cell Mater. 2012;23:400–412. doi: 10.22203/ecm.v023a31. discussion 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Losi P., Briganti E., Magera A. Tissue response to poly(ether)urethane- polydimethylsiloxane-fibrin composite scaffolds for controlled delivery of pro- angiogenic growth factors. Biomaterials. 2010;31:5336–5344. doi: 10.1016/j.biomaterials.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 87.Suarez S., Almutairi A., Christman K.L. Micro- and nanoparticles for treating cardiovascular disease. Biomater Sci. 2015;3:564–580. doi: 10.1039/C4BM00441H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tengood J.E., Ridenour R., Brodsky R., Russell A.J., Little S.R. Sequential delivery of basic fibroblast growth factor and platelet-derived growth factor for angiogenesis. Tissue Eng Part A. 2011;17:1181–1189. doi: 10.1089/ten.tea.2010.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zisch A.H., Lutolf M.P., Hubbell J.A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 90.Ungerleider J.L., Johnson T.D., Rao N., Christman K.L. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods. 2015;84:53–59. doi: 10.1016/j.ymeth.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chekanov V.S., Rayel R., Nikolaychik V. Direct fibrin injection to promote new collateral growth in hind limb ischemia in a rabbit model. J Card Surg. 2002;17:502–511. doi: 10.1046/j.1540-8191.2002.01006.x. discussion 512. [DOI] [PubMed] [Google Scholar]
- 92.Fan C.L., Gao P.J., Gu Y.J. Therapeutic angiogenesis by intramuscular injection of fibrin particles into ischaemic hindlimbs. Clin Exp Pharmacol Physiol. 2006;33:617–622. doi: 10.1111/j.1440-1681.2006.04416.x. [DOI] [PubMed] [Google Scholar]
- 93.Ungerleider J.L., Johnson T.D., Hernandez M.J. Extracellular matrix hydrogel promotes tissue remodeling, arteriogenesis, and perfusion in a rat hindlimb ischemia model. J Am Coll Cardiol Basic Trans Science. 2016;1:32–44. doi: 10.1016/j.jacbts.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Regensteiner J.G., Wolfel E.E., Brass E.P. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation. 1993;87:413–421. doi: 10.1161/01.cir.87.2.413. [DOI] [PubMed] [Google Scholar]
- 95.Long C.A., Sweet M., Chadid T. A novel large-animal model of peripheral arterial disease. J Vasc Surg. 2016;63:293–294. [Google Scholar]
- 96.Stacy M.R., Yu da Y., Maxfield M.W. Multimodality imaging approach for serial assessment of regional changes in lower extremity arteriogenesis and tissue perfusion in a porcine model of peripheral arterial disease. Circ Cardiovasc Imaging. 2014;7:92–99. doi: 10.1161/CIRCIMAGING.113.000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arai Y., Fujita M., Marui A. Combined treatment with sustained-release basic fibroblast growth factor and heparin enhances neovascularization in hypercholesterolemic mouse hindlimb ischemia. Circ J. 2007;71:412–417. doi: 10.1253/circj.71.412. [DOI] [PubMed] [Google Scholar]
- 98.Chappell J.C., Song J., Burke C.W., Klibanov A.L., Price R.J. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small. 2008;4:1769–1777. doi: 10.1002/smll.200800806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doi K., Ikeda T., Marui A. Enhanced angiogenesis by gelatin hydrogels incorporating basic fibroblast growth factor in rabbit model of hind limb ischemia. Heart Vessels. 2007;22:104–108. doi: 10.1007/s00380-006-0934-0. [DOI] [PubMed] [Google Scholar]
- 100.Fujita M., Ishihara M., Shimizu M. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non- anticoagulant heparin hydrogel in a rat hindlimb ischemia model. Wound Repair Regen. 2007;15:58–65. doi: 10.1111/j.1524-475X.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 101.Hirose K., Fujita M., Marui A. Combined treatment of sustained-release basic fibroblast growth factor and sarpogrelate enhances collateral blood flow effectively in rabbit hindlimb ischemia. Circ J. 2006;70:1190–1194. doi: 10.1253/circj.70.1190. [DOI] [PubMed] [Google Scholar]
- 102.Huang Y., Marui A., Sakaguchi H. Sustained release of prostaglandin E1 potentiates the impaired therapeutic angiogenesis by basic fibroblast growth factor in diabetic murine hindlimb ischemia. Circ J. 2008;72:1693–1699. doi: 10.1253/circj.cj-07-0960. [DOI] [PubMed] [Google Scholar]
- 103.Kanematsu A., Marui A., Yamamoto S. Type I collagen can function as a reservoir of basic fibroblast growth factor. J Controlled Release. 2004;99:281–292. doi: 10.1016/j.jconrel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Kasahara H., Tanaka E., Fukuyama N. Biodegradable gelatin hydrogel potentiates the angiogenic effect of fibroblast growth factor 4 plasmid in rabbit hindlimb ischemia. J Am Coll Cardiol. 2003;41:1056–1062. doi: 10.1016/s0735-1097(02)03007-3. [DOI] [PubMed] [Google Scholar]
- 105.Kawamura I., Takemura G., Tsujimoto A. Treatment of leg ischemia with biodegradable gelatin hydrogel microspheres incorporating granulocyte colony- stimulating factor. J Cardiovasc Pharmacol. 2011;57:416–423. doi: 10.1097/FJC.0b013e31820c9776. [DOI] [PubMed] [Google Scholar]
- 106.Kuraitis D., Zhang P., Zhang Y. A stromal cell-derived factor-1 releasing matrix enhances the progenitor cell response and blood vessel growth in ischaemic skeletal muscle. Eur Cell Mater. 2011;22:109–123. doi: 10.22203/ecm.v022a09. [DOI] [PubMed] [Google Scholar]
- 107.Layman H., Spiga M.-G., Brooks T., Pham S., Webster K.A., Andreopoulos F.M. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model. Biomaterials. 2007;28:2646–2654. doi: 10.1016/j.biomaterials.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Layman H., Sacasa M., Murphy A.E., Murphy A.M., Pham S.M., Andreopoulos F.M. Co- delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta Biomaterialia. 2009;5:230–239. doi: 10.1016/j.actbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 109.Lee J., Bhang S.H., Park H., Kim B.S., Lee K.Y. Active blood vessel formation in the ischemic hindlimb mouse model using a microsphere/hydrogel combination system. Pharm Res. 2010;27:767–774. doi: 10.1007/s11095-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 110.Luyt C.E., Meddahi-Pelle A., Ho-Tin-Noe B. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J Pharmacol Exp Ther. 2003;305:24–30. doi: 10.1124/jpet.102.046144. [DOI] [PubMed] [Google Scholar]
- 111.Marui A., Kanematsu A., Yamahara K. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J Vasc Surg. 2005;41:82–90. doi: 10.1016/j.jvs.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 112.Marui A., Tabata Y., Kojima S. A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ J. 2007;71:1181–1186. doi: 10.1253/circj.71.1181. [DOI] [PubMed] [Google Scholar]
- 113.Ruvinov E., Leor J., Cohen S. The effects of controlled HGF delivery from an affinity- binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials. 2010;31:4573–4582. doi: 10.1016/j.biomaterials.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 114.Xie J., Wang H., Wang Y. Induction of angiogenesis by controlled delivery of vascular endothelial growth factor using nanoparticles. Cardiovasc Ther. 2013;31:e12–e18. doi: 10.1111/j.1755-5922.2012.00317.x. [DOI] [PubMed] [Google Scholar]
- 115.Yang H.S., Bhang S.H., Hwang J.W., Kim D.I., Kim B.S. Delivery of basic fibroblast growth factor using heparin-conjugated fibrin for therapeutic angiogenesis. Tissue Eng Part A. 2010;16:2113–2119. doi: 10.1089/ten.TEA.2009.0673. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Y., Liu Z., Pan C. Preparation of gelatin microspheres encapsulated with bFGF for therapeutic angiogenesis in a canine ischemic hind limb. J Biomater Sci Polym Ed. 2011;22:665–682. doi: 10.1163/092050610X489880. [DOI] [PubMed] [Google Scholar]
- 117.Bonauer A., Carmona G., Iwasaki M. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 118.Lai R.C., Arslan F., Lee M.M. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Fiedler J., Jazbutyte V., Kirchmaier B.C. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 120.Wang J., Huang W., Xu R. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med. 2012;16:2150–2160. doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arslan F., Lai R.C., Smeets M.B. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Meloni M., Marchetti M., Garner K. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013;21:1390–1402. doi: 10.1038/mt.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hinkel R., Penzkofer D., Zuhlke S. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013;128:1066–1075. doi: 10.1161/CIRCULATIONAHA.113.001904. [DOI] [PubMed] [Google Scholar]
- 124.Vicencio J.M., Yellon D.M., Sivaraman V. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 125.Ranghino A., Cantaluppi V., Grange C. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012;25:75–85. doi: 10.1177/039463201202500110. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H.C., Liu X.B., Huang S. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hazarika S., Farber C.R., Dokun A.O. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127:1818–1828. doi: 10.1161/CIRCULATIONAHA.112.000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Endo-Takahashi Y., Negishi Y., Nakamura A. Systemic delivery of miR-126 by miRNA-loaded Bubble liposomes for the treatment of hindlimb ischemia. Scientific Reports. 2014;4:3883. doi: 10.1038/srep03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu G.W., Li Q., Niu X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.James E.N., Delany A.M., Nair L.S. Post-transcriptional regulation in osteoblasts using localized delivery of miR-29a inhibitor from nanofibers to enhance extracellular matrix deposition. Acta Biomater. 2014;10:3571–3580. doi: 10.1016/j.actbio.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang L.L., Sloand J.N., Gaffey A.C. Injectable, guest-host assembled polyethylenimine hydrogel for siRNA delivery. Biomacromolecules. 2017;18:77–86. doi: 10.1021/acs.biomac.6b01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu C.J., Jones D.S., 2nd, Tsai P.C., Venkataramana A., Cochran J.R. An engineered dimeric fragment of hepatocyte growth factor is a potent c-MET agonist. FEBS Lett. 2014;588:4831–4837. doi: 10.1016/j.febslet.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]