Abstract
Dogs used for dogfighting often receive minimal preventive health care, and the potential for spread of infectious diseases is high. The purpose of this study was to describe the prevalence of infectious diseases in dogs rescued from fighting operations to guide medical protocols for their immediate and long-term care. A total of 269 pit bull-type dogs were seized in a multi-state investigation. Fleas were present on most dogs, but few ticks were observed. Testing performed at intake included packed cell volume (PCV), serology and PCR for vector-borne pathogens, and fecal analysis.
The most common infections were Babesia gibsoni (39%), ‘Candidatus Mycoplasma haematoparvum’ (32%), Mycoplasma haemocanis (30%), Dirofilaria immitis (12%), and Ancylostoma (23%). Anemia was associated with B. gibsoni infection (63% of infected dogs, Odds ratio=2.5, P<0.001), but not with hemotropic mycoplasmas or Ancylostoma. Pit bull heritage and dogfighting are known risk factors for B. gibsoni infection, possibly via blood transmission from bites and vertical transmission. Hemotropic mycoplasmas have a similar risk pattern. Empirical care for dogs from dogfighting cases should include broad-spectrum internal and external parasiticides and monitoring for anemia. Dogfighting case responders should be prepared for mass screening and treatment of B. gibsoni and heartworm infections and should implement protocols to prevent transmission of infectious and zoonotic diseases in the shelter and following adoption. Former fighting dogs and dogs with possible dog bite scars should not be used as blood donors due to the risk of vector-borne pathogens that can escape detection and for which curative treatment is difficult to document.
Keywords: Anemia, Babesia gibsoni, Canine, Dogfighting, Hemotropic mycoplasma, Pit bull
Introduction
Although dogfighting is a felony offense in all 50 states and under federal law, this cruel bloodsport is still entrenched in communities across the United States. Growing public awareness of these crimes has led to more frequent seizures of dogs by law enforcement agencies (Lockwood, 2011, 2013). These agencies often partner with humane organizations to provide care for seized dogs, which are considered legal evidence. Dogs may be housed for months in traditional or temporary animal shelters while the legal cases proceed (Lockwood, 2013).
Fighting dogs typically receive minimal preventive care and are kept chained outside in poor conditions. Multiple dogs are housed in each ‘dog yard’ and are exposed to other fighting dogs during breeding, training, and fights. The potential for spread of infectious diseases is high. Since dogfighting is an underground illegal activity, little is known about the infectious diseases carried by dogs from organized fighting rings.
Historically, dogs seized in animal fighting investigations were routinely euthanized due to the belief that their heritage and training made them unsafe. Recently, however, rescue organizations have begun assessing their health, behavior, and suitability for adoption in response to widespread rehoming interest. Rescue operations often transport dogs around the country for adoption. While this lifesaving trend is laudable, there is potential risk for sending dogs harboring infectious diseases to unsuspecting owners or to regions where the infections are not currently endemic. The purpose of the study reported here was to describe the prevalence of infectious diseases in dogs rescued from fighting operations. This information will support an evidence-based foundation of medical protocols for biosecurity, disease screening, treatment, and long-term follow-up care for this unique population.
Materials and methods
Animals
The study included 269 dogs seized from eight scenes in four states (Alabama, Georgia, Mississippi, and Texas) during a federal dogfighting investigation in August and September, 2013. The dogs were pit bull-type phenotypes, only one of which had cropped ears. Ages estimated based on dentition included neonates (eight dogs, <6 weeks), juveniles (65 dogs, 6 weeks to 5.9 months), and adults (196 dogs, ≥6 months). A total of 52% of the dogs were female and 48% were male. The dogs were triaged at the scenes, vaccinated with a SC commercial rabies vaccine, and transported to Florida. They were housed in a climate-controlled warehouse that served as a temporary animal shelter until the legal cases were completed. Fleas were present on most dogs, but few ticks were observed. Ticks were not collected for species identification. Upon intake to the temporary shelter, dogs received intranasal vaccines containing modified-live Bordetella bronchiseptica, parainfluenza, and adenovirus-2, subcutaneous vaccines containing modified-live distemper virus, adenovirus-2, parainfluenza, and parvovirus, and topical moxidectin/imidacloprid for internal and external parasitism. Adult dogs were housed individually in chain-link portable kennels. Puppies were housed with littermates and nursing dams were housed with litters. Adoptable dogs diagnosed with B. gibsoni were treated with atovaquone (13.4 mg/kg orally q 8 h with a fatty meal) compounded into capsules (Wedgewood Pharmacy) and azithromycin (10 mg/kg orally q 24 h) for 10 days as previously described (Kirk, 2014). Dogs diagnosed with dirofilariasis were treated with a macrocyclic lactone monthly, an oral doxycycline regimen consisting of 1 month on and 2 months off throughout their custody, and with melarsomine following release from legal custody (Kirk, 2014).
Sample collection
Blood for routine health screening was collected by jugular or cephalic venipuncture into two EDTA tubes, one serum separator tube, and two heparinized microhematocrit tubes during examination the first week in custody. Serum was harvested by centrifugation. Fecal samples were collected after defecation within 4 days of intake. Use of surplus blood and feces following routine health screening was approved by the University of Florida Institutional Animal Care and Use Committee (Protocol 201308177, November 11, 2013).
Sample analysis
One set of EDTA blood samples was tested onsite for Dirofilaria immitis (heartworm) antigen and for antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii (SNAP 4Dx Plus Test, IDEXX Laboratories).
Serum was tested for B. gibsoni antibodies by ELISA as described (Goo et al., 2008) with slight modifications. In brief, 96-well plates were coated with full-length rBgTRAP with 6×His-tag. The plates were incubated with serum samples diluted to 1:200, followed by color development with horseradish peroxidase-conjugated anti-dog IgG and optical density measured at 650 nm. Samples were considered positive if the optical density was greater than the mean plus 3 standard deviations of the values from control samples collected from an Alaskan population of dogs determined to be free of B. gibsoni by PCR.
The packed cell volume (PCV) was determined by centrifugation of the microhematocrit tubes. For the purposes of this study, anemia was defined as PCV < 26% in neonates, PCV < 31% in juveniles, and PCV < 37% in adults (Hoskins, 2001).
The second set of EDTA blood samples was tested at a commercial reference laboratory for vector-borne pathogens by real-time PCR (Tick/Vector Comprehensive RealPCR Panel Canine, IDEXX Laboratories), including Anaplasma phagocytophilum (msp2 [p44], DQ519570), A. platys (groEL, heat shock protein, AY848753), Babesia spp. (ssrRNA, AF271082), Bartonella spp. (citrate synthase gene, AJ439406), Mycoplasma haemocanis (ribosomal RNA ssrRNA, AF197337), ‘Candidatus Mycoplasma haematoparvum’ (ribosomal RNA ssrRNA, AY383241), Ehrlichia canis (disulfide oxidoreductase (dsb) gene, AF403710), E. ewingii (disulfide oxidoreductase (dsb) gene, AY428950), E. chaffeensis (disulfide oxidoreductase (dsb) gene, AF403711), Hepatozoon canis (ssrRNA, AF176835), H. americanum (ssrRNA, AF176836), Leishmania spp. (glycoprotein gp63, YO8156), Neorickettsia risticii (ribosomal RNA 16S RNA, AF184082), and Rickettsia rickettsia (GroEL heat shock protein, AJ293326). Real-time PCR was performed with six quality controls, including quantitative PCR-positive controls, PCR-negative controls, negative extraction controls, quantitative DNA internal sample quality control targeting the host 18S rRNA gene complex, an internal positive control spiked into the lysis solution, and an environmental contamination monitoring control. Samples positive by PCR for Babesia spp. were submitted for species-specific real-time PCR testing, including B. canis (heat shock protein 70, AB248735), B. canis vogeli (heat shock protein 70, EF527401), B. canis rossi (heat shock protein 70, AB248738), B. felis (ITS-2, AY965742), B. gibsoni (heat shock protein 70, AB248731), and B. conradae (ITS-2, AY965742. All assays were designed and validated according to industry standards1.
Fecal samples were processed by zinc sulfate centrifugation to screen for ova and parasites at a commercial laboratory (IDEXX Laboratories) and tested for Giardia spp. antigen by ELISA (SNAP Giardia Test, IDEXX Laboratories).
Statistical analysis
Statistical analysis was conducted in two phases. In phase 1, two authors (SC, JL) calculated the prevalence of vector-borne and intestinal pathogens. For the purposes of statistical analysis, dogs positive for Babesia gibsoni by either PCR or serology were considered infected. Descriptive statistics for PCV and anemia were calculated, and asymptotic Χ2 tests were used to test for unadjusted bivariate associations between the presence of anemia and positive results for Babesia gibsoni, hemotropic mycoplasmas, or Ancylostoma. A value of P <0.05 was considered significant. All calculations were made with statistical software (SigmaStat for Windows 3.5, Systat Software).
Because the prevalence of vector-borne pathogens and intestinal parasitism varied among the eight investigation scenes, further analysis of PCV was conducted to control for the cluster effect of scene by a third author (JS). The Mantel-Haenszel method (Mantel and Haenszel, 1959) was used to calculate the odds ratio (OR) and 95% confidence interval (95% CI) for anemia, defined as the ratio of the odds of anemia when the vector-borne pathogen was present to that if it was absent. This also provided a P-value adjusted for clusters. Multiple linear regression was used to compare PCV results by calculating the average difference (PCV when pathogen was present minus PCV when pathogen was absent), adjusted for scene and for co-infections with any of three other major pathogens. A value of P <0.05 was considered significant. All calculations in this phase were conducted with statistical software (Statistical Analysis System (SAS) software version 9.3, SAS Institute).
Results
Pathogens
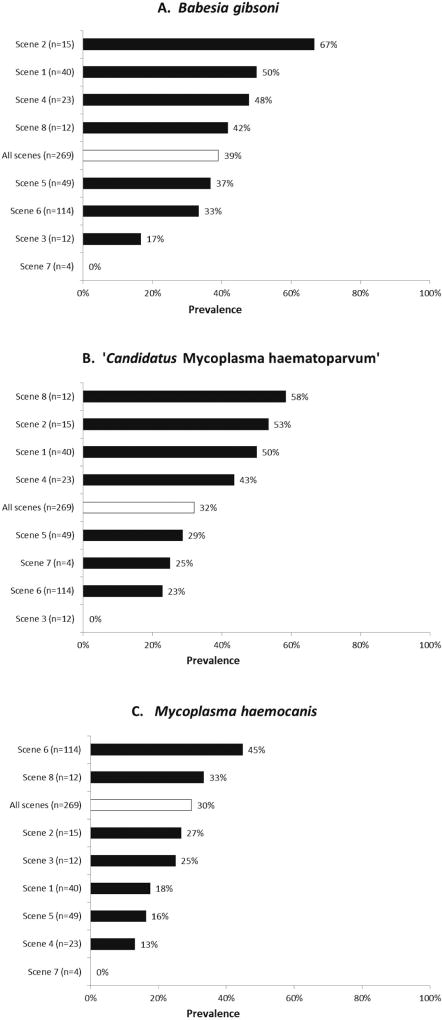
Vector-borne pathogens were present in most dogs (164/269, 61%; Table 1). The most common vector-borne pathogens were B. gibsoni and hemotropic mycoplasmas, the prevalence of which varied widely across the eight investigation scenes (Fig. 1). No dogs had evidence of infection with Anaplasma spp., Bartonella spp., Ehrlichia spp., Hepatozoon spp., Leishmania spp., Neorickettsia risticii, Rickettsia rickettsii, or Babesia spp. other than B. gibsoni.
Table 1.
Prevalence of vector-borne pathogens in 269 dogs seized from eight scenes in a federal dogfighting investigation.
| Test method | Dogs tested (n) |
Dogs positive (n) |
Percent positive |
|
|---|---|---|---|---|
| Babesia gibsoni | Serology | 261 | 102 | 38 |
| Babesia gibsoni | PCR | 269 | 86 | 32 |
| ‘Candidatus Mycoplasma haematoparvum’ | PCR | 269 | 86 | 32 |
| Mycoplasma haemocanis | PCR | 269 | 80 | 30 |
| Dirofilaria immitis1 | Serology | 196 | 23 | 12 |
| Anaplasma spp. | PCR | 269 | 0 | 0 |
| Anaplasma spp. | Serology | 269 | 0 | 0 |
| Bartonella spp. | PCR | 269 | 0 | 0 |
| Ehrlichia spp. | PCR | 269 | 0 | 0 |
| Ehrlichia spp. | Serology | 269 | 0 | 0 |
| Hepatozoon spp. | PCR | 269 | 0 | 0 |
| Leishmania spp. | PCR | 269 | 0 | 0 |
| Neorickettsia risticii | PCR | 269 | 0 | 0 |
| Rickettsia spp. | PCR | 269 | 0 | 0 |
| Borrelia burgdorferi | Serology | 269 | 0 | 0 |
Testing for D. immitis was performed only on adult dogs 6 months and older.
Fig. 1.
Prevalence of Babesia gibsoni (A), ‘Candidatus Mycoplasma haematoparvum’ (B), and Mycoplasma haemocanis (C) infection in dogs from each of the eight scenes in a federal dogfighting investigation, and prevalence from all scenes combined. (n = number of dogs seized from each scene)
Results of PCR and serologic testing for B. gibsoni were in agreement for 93% of dogs, including 84 that were positive by both PCR and serology and 165 that were negative by both PCR and serology. Two dogs were PCR-positive and serology-negative, and 18 were PCR-negative and serology-positive. For the purposes of statistical analysis, dogs that were positive by either test (104 dogs; 39%) were considered infected. One dog that was PCR-negative and serology-positive later gave birth to B. gibsoni-infected puppies. This dam was retested via PCR after diagnosis in her puppies and she tested positive for B. gibsoni.
Co-infections were more common than isolated infections. A total of 76/269 (28%) dogs were co-infected with both B. gibsoni and one or both canine hemotropic mycoplasmas (Table 2). There was a strong association between infection with B. gibsoni and infection with ‘Candidatus Mycoplasma haematoparvum’ (P <0.0001; OR=7.3, 95% CI=3.9–13.6).
Table 2.
Babesia gibsoni and hemotropic mycoplasma co-infections in 269 dogs seized from eight scenes in a federal dogfighting investigation. Babesia gibsoni infection includes dogs that had positive PCR and/or serology test results (n=104).
| Dogs positive (n) | Percent positive | |
|---|---|---|
| MHc only | 37 | 14 |
| Bg only | 28 | 10 |
| CMhp only | 15 | 6 |
| Bg + CMhp | 41 | 15 |
| Bg + MHc + CMhp | 22 | 8 |
| Bg + MHc | 13 | 5 |
| MHc + CMhp | 8 | 3 |
MHc, Mycoplasma haemocanis; Bg, Babesia gibsoni; CMhp, ‘Candidatus Mycoplasma haematoparvum’
Enteric pathogens were also commonly identified in dogs from which feces were obtained; 71/189 (37%) of dogs harbored at least one parasite (Table 3).
Table 3.
Prevalence of intestinal parasites in 189 dogs seized from eight scenes in a federal dogfighting investigation.
| Test method | Dogs tested (n) |
Dogs positive (n) |
Percent positive |
|
|---|---|---|---|---|
| Giardia spp. | ELISA | 162 | 22 | 14 |
| Ancylostoma spp. | Zinc sulfate centrifugation | 189 | 44 | 23 |
| Isospora spp. | Zinc sulfate centrifugation | 189 | 9 | 5 |
| Trichuris vulpis | Zinc sulfate centrifugation | 189 | 6 | 3 |
| Toxocara spp. | Zinc sulfate centrifugation | 189 | 3 | 2 |
Anemia
The PCV at intake ranged from 21 to 28% in neonates (median 26%), from 14 to 45% in juveniles (median 29%), and from 10 to 52% in adults (median 38%). In this population, 2/8 (25%) neonates, 48/65 (74%) juveniles, and 82/196 (42%) adults were anemic. One emaciated young adult dog had a PCV of 10% at intake. The dog, which had Ancylostoma on fecal analysis but was negative for vector-borne pathogens, was clinically stable. One week after intake and treatment with moxidectin/imidacloprid, the dog’s PCV increased to 15%. Another dog infected with B. gibsoni developed a hemolytic crisis and clinical deterioration requiring a blood transfusion when PCV fell from 29% on intake to 13% 3 weeks later.
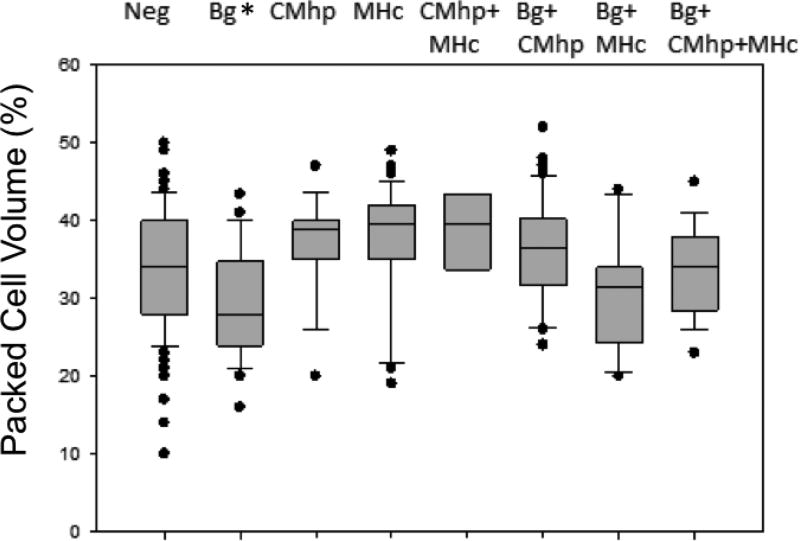
The presence of anemia was significantly associated with B. gibsoni infection in the unadjusted analysis (P = 0.007; Table 4) and remained significant (P <0.001) when adjusted for the cluster effect of investigation scene (Table 5). Anemia was not significantly associated with hemotropic mycoplasmas (P ≥ 0.3) or Ancylostoma spp. (P = 0.6). Median PCV was significantly lower in dogs infected solely with B. gibsoni (PCV 28%, range 16–43%) than in dogs free of vector-borne pathogens (PCV 34%, range 10–50%; P = 0.007; Fig. 2). Median PCVs in dogs infected with hemotropic mycoplasmas or with co-infections of B. gibsoni and hemotropic mycoplasmas were not significantly lower than those in dogs free of these infections (P >0.05; Fig. 2). When adjusted for investigation scene clusters and the presence of other vector-borne pathogens, PCV was significantly decreased by a mean of 3.4% (standard error 1.4%, P = 0.01) in dogs infected with B. gibsoni, and PCV was significantly increased by 2.5% (standard error 1.2%, P = 0.03) in dogs infected with Mycoplasma haemocanis.
Table 4.
Anemia in dogs with Babesia gibsoni, Ancylostoma, or hemotropic mycoplasma. For the purposes of this study, anemia was defined as packed cell volume (PCV) < 26% in neonates, PCV < 31% in juveniles, and PCV < 37% in adults.
| Pathogen | Infection status | n (%) | Anemia n (%) |
Median PCV (%) |
PCV range (%) |
P |
|---|---|---|---|---|---|---|
| Babesia gibsoni | Uninfected | 165 (61) | 67 (41) | 37 | 10–50 | |
| Infected | 104 (39) | 65 (63) | 34 | 16–52 | 0.0007 | |
| Ancylostoma spp. | Uninfected | 145 (77) | 62 (43) | 37 | 14–52 | |
| Infected | 44 (23) | 23 (52) | 35 | 10–47 | 0.3014 | |
| ‘Candidatus Mycoplasma haematoparvum’ | Uninfected | 183 (68) | 90 (49) | 34 | 10–50 | |
| Infected | 86 (32) | 42 (49) | 37 | 20–52 | 1.000 | |
| Mycoplasma haemocanis | Uninfected | 189 (70) | 98 (52) | 34 | 10–52 | 0.1829 |
| Infected | 80 (30) | 34 (43) | 38 | 19–49 |
Table 5.
Risk of anemia in dogs with Babesia gibsoni, Ancylostoma spp., or hemotropic mycoplasmas, adjusted for investigation scene.
| Estimated OR |
95% CI |
P (two-sided) |
|
|---|---|---|---|
| Babesia gibsoni | 2.5 | 1.5–4.3 | <0.001 |
| Ancylostoma spp. | 1.5 | 0.8–3.1 | 0.23 |
| ‘Candidatus Mycoplasma haematoparvum’ | 0.9 | 0.5–1.5 | 0.63 |
| Mycoplasma haemocanis | 0.7 | 0.4–1.3 | 0.26 |
OR, odds ratio for anemia, present:absent; CI, confidence Interval
Fig. 2.
Packed cell volume (PCV) in dogs with and without vector-borne pathogen infections. Boxes represent the 25th and 75th percentiles, lines indicate the medians, whiskers represent the 10th and 90th percentiles, and circles indicate outliers. The number of dogs in each column corresponds to the data presented in Table 2. Median PCV in dogs solely infected with B. gibsoni (Bg) was significantly lower than in dogs free of infections (Neg).
Neg, negative for B. gibsoni and hemotropic mycoplasmas; Bg, Babesia gibsoni; MHc, Mycoplasma haemocanis; CMhp, ‘Candidatus Mycoplasma haematoparvum;’ *P=0.007.
Discussion
Dogs seized simultaneously from eight different scene investigations in four states during a federal investigation of organized dogfighting commonly had infectious diseases, particularly B. gibsoni (39%), hemotropic mycoplasmas (51%), Ancylostoma spp. (23%), Giardia spp. (14%), and Dirofilaria immitis (12%)
Most dogs kept in fighting operations in the USA are pit bull-type dogs (Lockwood, 2013). Although there is no universally accepted definition of a pit bull, many are derived from the American Pit Bull Terrier, American Staffordshire terrier, Staffordshire bull terrier, American bulldog, and other guarding breeds. Most dogs reported to be infected with B. gibsoni in the USA are pit bull-type dogs or have a history of fighting with a pit bull. Of 131 B. gibsoni-infected dogs across the USA, 122 (93%) were reported to be American Pit bull terriers (Birkenheuer et al., 2005). Of 15 B. gibsoni-infected dogs of other breeds, 9/12 had a history of having been recently bitten by another dog, all of which were described as American Pit bull terriers. In a study of B. gibsoni infection in kennels, 14/29 (48%) American Pit bull terriers or American Staffordshire terriers from breeding operations were infected, compared to only 3/28 (11%) shelter dogs of other breeds when tested by PCR (Birkenheuer et al., 2003). Similarly, 18/33 (55%) American Pit bull terriers from two kennels in the Southeastern USA were positive for B. gibsoni by PCR, whereas none of the 87 dogs of other breeds from a veterinary hospital, a shelter, or a Foxhound breeding kennel in the same region were infected (Macintire et al., 2002). In another study, the prevalence of B. gibsoni infection in pit bull-type dogs confiscated from dogfighting operations and housed in shelters was 34% (Yeagley et al., 2009), similar to the 39% prevalence in the current study. The dogs with scars on the head and front limbs, which are common in dogs used for organized dogfighting, were 5.5 times more likely to be infected with B. gibsoni than were dogs without scars. Internationally, B. gibsoni is also found almost exclusively in breeds used for fighting. In Romania, a total of 13/14 (93%) infected dogs were American Pit bull terriers or American Staffordshire terriers (Imre et al., 2013), and in Japan, 32/35 (91%) infected dogs were Tosa dogs or American Pit bull terriers (Miyama et al., 2005). The known competent tick vectors (Haemaphysalis longicornis and Haemaphysalis bispinosa) for B. gibsoni in those countries are not endemic in the USA; thus, proposed mechanisms of transmission include other undefined biological vectors, horizontal transmission through blood and saliva exchange during fighting (Ayoob et al., 2010; Birkenheuer et al., 2005; Jefferies et al., 2007), and vertical transmission from infected dams to puppies (Abu et al., 1973; Fukomoto et al., 2005; Itoh and Itoh, 1990).
Approximately one-third of the dogs in this study were positive for canine hemotropic mycoplasmas by PCR. The prevalence of hemotropic mycoplasmas varies widely in different regions of the world. Prevalence has been reported at 0.6% for Mycoplasma haemocanis and 0.8% for ‘Candidatus Mycoplasma haematoparvum’ in the USA (Compton et al., 2012); 15.4% for hemoplasma in France (Wengi et al., 2008); 1.2% for hemoplasma in Switzerland (Wengi et al., 2008); and, 5.1% for Mycoplasma haemocanis and 1.8% for a ‘Candidatus Mycoplasma haemominutum-like’ organism in Brazil (de Faria Valle et al., 2014). Hemotropic mycoplasma infection was not associated with anemia, age, or gender in one study (Wengi et al., 2008), whereas the presence of biological vectors, old age, dog bite wounds, and neoplastic diseases were identified as risk factors in another study (de Faria Valle et al., 2014). Co-infection of hemotropic mycoplasmas and B. gibsoni was reported in pit bull-type dogs rescued following Hurricane Katrina (Levy et al., 2011). A study conducted in Japan revealed 4.1% of dogs were PCR positive for Mycoplasma haemocanis. Of the 913 dogs included, 73 were Tosa dogs, and 25 (34.2%) of those were positive for Mycoplasma haemocanis (Sasaki et al., 2008). These two studies combined with the current study suggest that breeds used for fighting are at increased risk for hemotropic mycoplasma infection, which may share similar modes of transmission with B. gibsoni. Because the incidence of clinical disease attributed to hemotropic mycoplasma infections is low in immunocompetent animals, and because treatment has not been shown to eliminate infection (Hulme-Moir et al., 2010; Sykes et al., 2004), the dogs in this study population were not treated for hemotropic mycoplasma infections.
A total of 23 of the adult dogs (12%) had heartworm infection, which is consistent with reports of prevalence among stray dogs in this region (Bowman et al., 2009; Levy et al., 2011; Tzipory et al., 2010; Wang et al., 2014). The presence of heartworm infection created a risk for transmission to other dogs housed in the temporary shelter or in future receiving shelters or adoptive homes, so all dogs were treated monthly with chemoprophylaxis. Because legal custody of the dogs was not transferred to the receiving organization until after legal proceedings were completed, adulticide therapy was not administered at the time of diagnosis. Instead, doxycycline was administered daily for 1 month on and 2 months off throughout their custody.
Approximately half of the dogs in this population were anemic at intake. The presence of anemia was significantly associated with B. gibsoni infection, but not with hemotropic mycoplasmas or Ancylostoma. In addition to the pathogens evaluated, PCV could be affected by other unmeasured factors, such as external parasitism and nutritional deficiencies. Treatment at intake with moxidectin/imidacloprid for flea control may have resulted in reduced detection of parasites in fecal analyses, resulting in an underestimation of the impact of intestinal parasitism on PCV. Despite the presence of B. gibsoni or hemotropic mycoplasmas in 61% of dogs, only one dog, a lactating female, developed a hemolytic crisis requiring a blood transfusion. This dog was emaciated on intake, and was actively nursing three puppies. Three weeks after admission into the temporary shelter, the dog was lethargic, anorexic, and had pale mucous membranes. A complete blood count and differential revealed regenerative anemia with PCV of 13% (PCV at intake was 29%); no blood parasites were observed microscopically, although this dog was positive for B. gibsoni by both PCR and serology at intake.
Results of infectious disease screening of this population indicate that dogs seized in dogfighting investigations are at increased risk of infection with Babesia gibsoni and hemotropic mycoplasmas. In addition, fleas, dirofilariasis, Giardia, and Ancylostoma were also common. Thus, caregivers in dogfighting cases should be alerted to risk of infections, observe dogs for clinical signs, and consider mass screening for vector-borne pathogens. Pregnant dogs, lactating dogs, and puppies are often seized during dogfighting investigations; these populations may be at greater risk of exhibiting clinical signs from infections and thus warrant closer monitoring for anemia. Dogs seized from dogfighting investigations should not be used as blood donors due to high risk of subclinical infections with vector-borne pathogens. Blood donor candidates that have suspected fighting breed heritage or scars from dog bites should receive repeated screening by both PCR and serology to rule out B. gibsoni in addition to routine disease screening panels (Wardrop et al., 2005) prior to donating blood.
Conclusions
Pit bull heritage and dogfighting are known risk factors for B. gibsoni infection, possibly via blood or saliva transmission from bites and vertical transmission. Hemotropic mycoplasmas appear to follow a similar risk pattern, suggesting a common mode of transmission. Mild to moderate anemia was common in seized dogs, but many anemia cases were not associated with documented infections or parasitism other than fleas. Empirical care for all dogs seized in dogfighting investigations should include broad-spectrum internal and external parasiticides, routine vaccinations, and monitoring for anemia. Dogfighting case responders should be prepared for mass screening and treatment of B. gibsoni and heartworm infections and should implement protocols to prevent transmission of infectious and zoonotic diseases in the shelter and following adoption. Former fighting dogs and dogs with possible dog bite scars should not be used as blood donors due to the risk of vector-borne pathogens that can escape detection and for which curative treatment is difficult to document.
Highlights.
Babesia gibsoni and hemotropic mycoplasmas are common in dogs in fighting operations.
Anemia is common and is most strongly associated with B. gibsoni infection.
Fighting dogs should not be blood donors due to the risk of transmitting pathogens.
Acknowledgments
This study was supported by grants from Maddie’s Fund and by the Lois Kugler Small Animal Research Trust. PCR testing, B. gibsoni serology, and SNAP 4DxPlus test kits were contributed by IDEXX Laboratories. This work was supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See: Applied Biosystems, User Bulletin #3 http://tools.thermofisher.com/content/sfs/manuals/cms_041001.pdf (Accessed 26 February, 2016)
Conflict of interest statement
Christian Leutenegger, Jiayou Liu, and Ramaswamy Chandrashekar are employed by IDEXX Laboratories. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper.
This material was presented in part and published as an abstract at the 2014 American College of Veterinary Internal Medicine (ACVIM) Forum, Nashville, Tennessee, USA, 4–7 June 2014.
References
- Abu M, Hara I, Naito I, Shibauchi O. Babesia infections in puppies probably due to transplacental transmission. Journal of Veterinary Medicine. 1973;609:203–206. [Google Scholar]
- Ayoob AL, Hackner SG, Prittie J. Clinical management of canine babesiosis. Journal of Veterinary Emergency and Critical Care. 2010;20:77–89. doi: 10.1111/j.1476-4431.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003) Journal of the American Veterinary Medical Association. 2005;227:942–947. doi: 10.2460/javma.2005.227.942. [DOI] [PubMed] [Google Scholar]
- Birkenheuer AJ, Levy MG, Stebbins M, Poore M, Breitschwerdt E. Serosurvey of antiBabesia antibodies in stray dogs and American pit bull terriers and American staffordshire terriers from North Carolina. Journal of the American Animal Hospital Association. 2003;39:551–557. doi: 10.5326/0390551. [DOI] [PubMed] [Google Scholar]
- Bowman D, Little SE, Lorentzen L, Shields J, Sullivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: Results of a national clinic-based serologic survey. Veterinary Parasitology. 2009;160:138–148. doi: 10.1016/j.vetpar.2008.10.093. [DOI] [PubMed] [Google Scholar]
- Compton SM, Maggi RG, Breitschwerdt EB. ‘Candidatus Mycoplasma Haematoparvum’ and Mycoplasma haemocanis infections in dogs from the United States. Comparative Immunology, Microbiology and Infectious Diseases. 2012;35:557–562. doi: 10.1016/j.cimid.2012.06.004. [DOI] [PubMed] [Google Scholar]
- de Faria Valle S, Messick JB, Pires dos Santos A, Kreutz LC, Blatt Duda NC, Machado G, Corbellini LG, Biondo AW, Diaz Gonzalez FH. Identification, occurrence and clinical findings of canine hemoplasmas in southern Brazil. Comparative Immunology, Microbiology and Infectious Diseases. 2014;37:259–265. doi: 10.1016/j.cimid.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Suzuki H, Igarashi I, Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. International Journal for Parasitology. 2005;35:1031–1035. doi: 10.1016/j.ijpara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Goo YK1, Jia H, Aboge GO, Terkawi MA, Kuriki K, Nakamura C, Kumagai A, Zhou J, Lee EG, Nishikawa Y, Igarashi I, Fujisaki K, Xuan X. Babesia gibsoni: Serodiagnosis of infection in dogs by an enzyme-linked immunosorbent assay with recombinant BgTRAP. Experimental Parasitology. 2008;118:555–560. doi: 10.1016/j.exppara.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Hoskins JD. Veterinary Pediatrics: Dogs and Cats from Birth to Six Months. 3. Saunders; Philadelphia, PA: 2001. p. 594. [Google Scholar]
- Hulme-Moir KL, Barker EN, Stonelake A, Helps CR, Tasker S. Use of real-time quantitative polymerase chain reaction to monitor antibiotic therapy in a dog with naturally acquired Mycoplasma haemocanis infection. Journal of Veterinary Diagnostic Investigation. 2010;22:582–587. doi: 10.1177/104063871002200413. [DOI] [PubMed] [Google Scholar]
- Imre M, Farkas R, Ilie MS, Imre K, Darabus G. Survey of babesiosis in symptomatic dogs from Romania: occurrence of Babesia gibsoni associated with breed. Ticks and Tick-borne Diseases. 2013;4:500–502. doi: 10.1016/j.ttbdis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Itoh N, Itoh S. A case of canine babesiosis possibly developed by transplacental infection. Journal of the Japanese Veterinary Medical Association. 1990;43:275–276. [Google Scholar]
- Jefferies R, Ryan UM, Jardine J, Broughton DK, Robertson ID, Irwin PJ. Blood, Bull Terriers, and Babesiosis: further evidence for direct transmission of Babesia gibsoni in dogs. Australian Veterinary Journal. 2007;85:459–463. doi: 10.1111/j.1751-0813.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- Kirk S. Efficacy of azithromycin and compounded atovaquone for treatment of Babesia gibsoni in a large-scale dogfighting case. Proceedings of the American College of Veterinary Internal Medicine (ACVIM) Forum; Nashville, Tennessee, USA. 4–7 June 2014.2014. [Google Scholar]
- Levy JK, Lappin MR, Glaser AL, Birkenheuer AJ, Anderson TC, Edinboro CH. Prevalence of infectious diseases in cats and dogs rescued following Hurricane Katrina. Journal of the American Veterinary Medical Association. 2011;238:311–317. doi: 10.2460/javma.238.3.311. [DOI] [PubMed] [Google Scholar]
- Lockwood R. Dogfighting Toolkit for Law Enforcement: Addressing Dogfighting in Your Community. U.S. Department of Justice's Office of Community Policing Services and American Society for Prevention of Cruelty to Animals (ASPCA) 2011 [Google Scholar]
- Lockwood R. Animal Fighting. In: Miller L, Zawistowski S, editors. Shelter Medicine for Veterinarians and Staff. 2. John Wiley & Sons, Inc; Ames, Iowa, USA: 2013. pp. 441–452. [Google Scholar]
- Macintire DK, Boudreaux MK, West GD, Bourne C, Wright JC, Conrad PA. Babesia gibsoni infection among dogs in the southeastern United States. Journal of the American Veterinary Medical Association. 2002;220:325–329. doi: 10.2460/javma.2002.220.325. [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–748. [PubMed] [Google Scholar]
- Miyama T, Sakata Y, Shimada Y, Ogino S, Watanabe M, Itamoto K, Okuda M, Verdida RA, Zuan Z, Nagasawa H, et al. Epidemiological Survey of Babesia gibsoni Infection in Dogs in Eastern Japan. The Journal of Veterinary Medical Science. 2005;67:467–471. doi: 10.1292/jvms.67.467. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ohta K, Matsuu A, Hirata H, Ikadai H, Oyamada T. A molecular survey of Mycoplasma haemocanis in dogs and foxes in Aomori Prefecture, Japan. The Journal of Protozoology Research. 2008;18:57–60. [Google Scholar]
- Sykes JE, Bailiff NL, Ball LM, Foreman O, George JW, Fry MM. Identification of a novel hemotropic mycoplasma in a splenectomized dog with hemic neoplasia. Journal of the American Veterinary Medical Association. 2004;224:1946–1951. doi: 10.2460/javma.2004.224.1946. [DOI] [PubMed] [Google Scholar]
- Tzipory N, Crawford PC, Levy JK. Prevalence of Dirofilaria immitis, Ehrlichia canis, and Borrelia burgdorferi in pet dogs, racing greyhounds, and shelter dogs in Florida. Veterinary Parasitology. 2010;171:136–139. doi: 10.1016/j.vetpar.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Wang D, Bowman DD, Brown HE, Harrington LC, Kaufman PE, McKay T, Nelson CT, Sharp JL, Lund R. Factors influencing U.S. canine heartworm (Dirofilaria immitis) prevalence. Parasites & Vectors. 2014;7:1–18. doi: 10.1186/1756-3305-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardrop KJ, Reine N, Birkenheuer A, Hale A, Hohenhaus A, Crawford C, Lappin MR. Canine and feline blood donor screening for infectious disease. Journal of Veterinary Internal Medicine. 2005;19:135–142. doi: 10.1111/j.1939-1676.2005.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengi N, Willi B, Boretti FS, Cattori V, Riond B, Meli ML, Reusch CE, Lutz H, Hofmann-Lehmann R. Real-time PCR-based prevalence study, infection follow up and molecular characterization of canine hemotropic mycoplasmas. Veterinary Microbiology. 2008;126:132–141. doi: 10.1016/j.vetmic.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Yeagley TJ, Reichard MV, Hempstead JE, Allen KE, Parsons LM, White MA, Little SE, Meinkoth JH. Detection of Babesia gibsoni and the canine small Babesia 'Spanish isolate' in blood samples obtained from dogs confiscated from dogfighting operations. Journal of the American Veterinary Medical Association. 2009;235:535–539. doi: 10.2460/javma.235.5.535. [DOI] [PubMed] [Google Scholar]