Abstract
IMPORTANCE
To our knowledge, no effective treatments exist for Alzheimer disease, and new molecules are years away. However, several drugs prescribed for other conditions have been associated with reducing its risk.
OBJECTIVE
To analyze the association between statin exposure and Alzheimer disease incidence among Medicare beneficiaries.
DESIGN, SETTING, AND PARTICIPANTS
We examined the medical and pharmacy claims of a 20% sample of Medicare beneficiaries from 2006 to 2013 and compared rates of Alzheimer disease diagnosis for 399 979 statin users 65 years of age or older with high or low exposure to statins and with drug molecules for black, Hispanic, and non-Hispanic white people, and men and women of Asian, Native American, or unkown race/ethnicity who are referred to as “other.”
MAIN OUTCOMES AND MEASURES
The main outcome was incident diagnosis of Alzheimer disease based on the International Classification of Diseases, Ninth Revision, Clinical Modification. We used Cox proportional hazard models to analyze the association between statin exposure and Alzheimer disease diagnosis for different sexes, races and ethnicities, and statin molecules.
RESULTS
The 399 979 study participants included 7794 (1.95%) black men, 24 484 (6.12%) black women, 11 200 (2.80%) Hispanic men, 21 458 (5.36%) Hispanic women, 115 059 (28.77%) white men, and 195 181 (48.80%) white women. High exposure to statins was associated with a lower risk of Alzheimer disease diagnosis for women (hazard ratio [HR], 0.85; 95%CI, 0.82–0.89; P<.001) and men (HR, 0.88; 95%CI, 0.83–0.93; P<.001). Simvastatin was associated with lower Alzheimer disease risk for white women (HR, 0.86; 95%CI, 0.81–0.92; P<.001), white men (HR, 0.90; 95%CI, 0.82–0.99; P=.02), Hispanic women (HR, 0.82; 95%CI, 0.68–0.99; P=.04), Hispanic men (HR, 0.67; 95%CI, 0.50–0.91; P=.01), and black women (HR, 0.78; 95%CI, 0.66–0.93; P=.005). Atorvastatin was associated with a reduced risk of incident Alzheimer disease diagnosis for white women (HR, 0.84, 95%CI, 0.78–0.89), black women (HR, 0.81, 95%CI, 0.67–0.98), and Hispanic men (HR, 0.61, 95%CI, 0.42–0.89) and women (HR, 0.76, 95%CI, 0.60–0.97). Pravastatin and rosuvastatin were associated with reduced Alzheimer disease risk for white women only (HR, 0.82, 95% CI, 0.70–0.95 and HR, 0.81, 95%CI, 0.67–0.98, respectively). High statin exposure was not associated with a statistically significant lower Alzheimer disease risk among black men.
CONCLUSIONS AND RELEVANCE
The reduction in Alzheimer disease risk varied across statin molecules, sex, and race/ethnicity. Clinical trials that include racial and ethnic groups need to confirm these findings. Because statins may affect Alzheimer disease risk, physicians should consider which statin is prescribed to each patient.
Commonly known as statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors are the most prescribed cholesterol-lowering medication in the United States.1 From 2003 to 2012, statin use increased from 17.8% to 25.9% among the population 40 years of age or older.1 Ninety-three percent of the more than40million people using cholesterol–lowering medication use statins.1 While all statins lower cholesterol levels, they differ in molecular structure, low-density lipoprotein cholesterol-lowering efficacy, pharmacokinetics, and cost.2 Clinically relevant differences may favor one statin over another.
Evidence shows that serum cholesterol levels link to β-amyloid deposition and Alzheimer disease (AD) pathology.3–8 Cholesterol’s role in β-amyloid processing has led to hypotheses that cholesterol-moderating drugs could influence AD onset and progression. Statins are hydrophilic or lipophilic, which can play a role in pharmacodynamics and pharmacokinetic behavior. Statins have other nonlipid effects that may influence AD onset and progression.2
Research on the link between statins and AD risk has shown a protective association, with shortcomings. Randomized clinical trials have not drawn sufficient conclusions owing to insufficient follow-up times, samples lacking minorities, and the removal of hyperlipidemic participants.3,9–14 Cohort studies have examined the relationship in relatively small samples and found negative associations between statin use and AD.15–19 Others have used large data sets of veterans that lack generalizability.20 Some studies have found that lipophilic statins have a stronger association with AD incidence,19 whereas others have found no difference.15 No studies to our knowledge have used large, longitudinal data with detailed demographic information to identify and quantify which statins have the greatest therapeutic efficacy for AD, and whether effects differ by sex or race/ethnicity. Since few therapeutics targeted toward AD are readily available, identifying more and targeting patient phenotypes could delay or prevent AD.
We used administrative claims data from a 20% sample of Medicare beneficiaries to analyze the association of statin use with AD onset. Demographic information linked to longitudinal pharmacy claims allowed us to estimate the differential effects of simvastatin and atorvastatin (lipophilic) and pravastatin and rosuvastatin (hydrophilic) across race/ethnicity and sex. We examined whether greater statin use was associated with lower AD incidence and compared AD risk of those with high and low exposure by statin type using Cox proportional hazard models matched according to age, sex, race/ethnicity, region, education, and comorbid conditions.
Methods
Data
Using a 20% sample of Medicare beneficiaries, we linked data on enrollment, demographics, vital status, and Parts A, B, and D claims. Part D data included key elements related to prescription drug events. Part A data listed hospital stays, including diagnostic codes. Part B data included reimbursable outpatient claims. Each claim contained diagnostic (International Classification of Diseases, Ninth Revision, Clinical Modification) and procedure (Current Procedural Terminology, Fourth Revision) codes, service dates, and demographics. Enrollment and claims data were supplemented with claims histories from the Chronic Conditions Data Warehouse. Informed consent was gained and internal review board approval was granted by the University of Southern California and the National Bureau of Economic Research.
Study Sample
The study sample consisted of statin users 65 years of age or older as of January 2006 who were continuously enrolled in Medicare fee for service and Part D for more than 2 years (Table 1). We excluded patients with an AD diagnosis prior to 2009. Diagnoses were determined by International Classification of Diseases, Ninth Revision, Clinical Modification code 331.0. We required an AD index diagnosis to be verified in a subsequent claim. The final study sample of 399 979 statin users consisted of 310 240 non-Hispanic white people, 32 658Hispanic people, 32 278 non-Hispanic black people, and 24 803 people of Asian, native American, or unknown race/ethnicity (ie, other race/ethnicity).
Table 1.
Sample Selection
| Restrictions | No. of People |
|---|---|
| 20% of 2006–2012 Medicare beneficiaries ≥65 y enrolled for >2 y in fee for service and Part D | 2 666 059 |
| Excluded if not a statin user | 1 223 641 |
| Excluded if AD diagnosed prior to 2009 | 1 162 648 |
| Continuously observed from 2006 to at least 2009 (final sample) | 399 979 |
Abbreviation: AD, Alzheimer disease.
Measuring Statin Exposure
We identified statins by selecting Part D event records (2006 and 2012) for simvastatin, atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, or rosuvastatin. We matched the corresponding National Drug Code to 3 drug databases (Rx-Norm, IMS Health, and First DataBank) and used the verified list to select beneficiaries with Part D claims.
We defined a statin user as anyone with at least 2 prescription fills of any statin between 2006 and 2012. Individuals categorized as having high exposure fell into at least the 50th percentile of days of filled prescriptions in a given year for at least 2 years during 2006, 2007, and 2008. Other users were categorized as having low exposure. Individuals with high and low exposure during the period from 2006 to 2008 generally continued at similar levels in 2009 to 2013. We assessed exposure to any statin and independently for the 4 most commonly prescribed: simvastatin, atorvastatin, pravastatin, and rosuvastatin.
Study Design
We compared AD diagnosis rates of Medicare beneficiaries with high and low exposure to statins. To mitigate concern that AD onset could lead to poor adherence or discontinuation of statins, we designated the period from2006 to 2008 the statin exposure period prior to our outcome period, which is from 2009 to 2013. Beneficiaries were followed up for an average of 7.2 years and the mean number of years between statin exposure and AD diagnosis was 5.4. We conducted analyses of the association of statin use and AD incidence for any statin and separately by specific statin.
Statistical Analyses
We examined the association of high vs low statin exposure from 2006 to 2008 with incident AD over the next 5 years (2009–2013). We matched the high- and low-exposure groups using coarsened exact matching to control for the potentially confounding influences of age, sex, race/ethnicity, region, years since hyperlipidemia diagnosis, education, and health status and to reduce imbalance between groups. Coarsened exact matching temporarily coarsens each variable into substantively meaningful groups, finds exact matches of treatments and controls, and retains the original (uncoarsened) values of the matched data for analysis.18–21 The statistical significance for P values was designated as less than .05. After matching, we used Cox proportional hazards models to analyze the relationship between statin use and AD diagnosis risk. Models included controls for age, sex, race/ethnicity, health, years since hyperlipidemic diagnosis, percentage of residents from a beneficiary’s zip code area who completed high school, and diagnoses prior to 2009 of non-AD dementia, acute myocardial infarction, atrial fibrillation, stroke, diabetes, and hypertension. We ran the models on the full sample and separately by sex and race/ethnicity. The proportional-hazards assumption was checked by examining Kaplan-Meier curves and with Schoenfeld residuals. Health status was measured using indicators for comorbid conditions and the Centers for Medicaid and Medicare Services–Hierarchical Condition Category (CMS-HCC), an index based on health status from diagnostic data and demographics in which higher numbers indicate worse health. The CMS uses the index to predict health care expenditures, and it highly correlates with mortality.22 We used year of hyperlipidemic diagnosis to control for unobserved statin use prior to the 2006 enactment of Part D. Race/ethnicity was determined using the beneficiary race code in CMS enrollment data and by applying an algorithm developed by the Research Triangle Institute that improves name-based identification of Hispanic and Asian people.23
We compared AD incidence of individuals with low or high exposure to statins because all statin users are similar across other dimensions. For example, average age difference between high-and low-exposure statin users is 0.6 years, and low- and high-exposure statin users have similar disease prevalence: non-AD dementia (9.7% of high-exposure usersand9.1% of low-exposure users), acute myocardial infarction (8.2% of high-exposure users and 6.6% of low-exposure users), diabetes (48.7% of high-exposure users and 45.6% of low-exposure users), stroke (18.7% of high-exposure users and 17.4% of low exposure users), hypertension (93.5% of high exposure users and and 90.7% of low exposure users), and atrial fibrillation (17.2% of high exposure users and 14.7% of low exposure users). The high- and low-exposure groups also had similar mean HCC comorbidity scores (0.95 and 0.91 for the high and low exposure groups, respectively).
Results
Unadjusted Rates of AD Incidence by Sex and Race/Ethnicity
From 2009 to 2013, 1.72% of women and 1.32% of men received a diagnosis of AD annually (Table 2). The incidence of AD was higher among Hispanic and black women (2.29% and 2.11%, respectively) than white women (1.64%). White men had the lowest incidence of AD (1.23%), lower than that of “other” race women (1.37%) and men (1.29%). The incidence of AD was 1.86% and 1.94% among Hispanic men and black men, respectively. The CMS-HCC was highest for black men. The mean annual number of days of statin use was lower for Hispanic and black people (254 days/year for Hispanic men and women and black men; 255 for black women) than for white people, with 282 and 284 days/year for white women and men, respectively. Simvastatin was the most commonly used statin (62% of women and 61% of men used it in our sample). About half of the men and women in our sample had used atorvastatin. Pravastatin and rosuvastatin were used less frequently (19% of women and 17% of men used pravastatin, and 18% of women and 16% of men used rosuvastatin). Rosuvastatin use was higher among Hispanic women (26%) and men (22%). Alzheimer disease and associated diseases were higher among black and Hispanic people than white people, while statin use was lower. We conducted separate analyses to compare high and low exposure to statins by race/ethnicity.
Table 2.
Descriptive Statistics of Sex and Race Subgroups
| % of Individuals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| All | White | Hispanic | Black | Othera | ||||||
|
|
|
|
|
|
||||||
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| AD per y (2009–2013) | 1.72 | 1.32 | 1.64 | 1.23 | 2.29 | 1.86 | 2.11 | 1.94 | 1.37 | 1.29 |
|
| ||||||||||
| Mean age, y | 76.4 | 75.1 | 76.5 | 75.1 | 75.7 | 75.1 | 76.2 | 74.3 | 75.9 | 75.8 |
|
| ||||||||||
| Mean % of high school graduates | 74 | 75 | 76 | 77 | 61 | 61 | 67 | 67 | 75 | 75 |
|
| ||||||||||
| HCC community score | 0.90 | 0.96 | 0.87 | 0.93 | 0.98 | 1.09 | 1.12 | 1.21 | 0.87 | 0.97 |
|
| ||||||||||
| Pre-2009 diagnosis | ||||||||||
|
| ||||||||||
| Non-AD dementia | 10 | 8 | 9 | 7 | 12 | 11 | 14 | 16 | 10 | 10 |
|
| ||||||||||
| AMI | 6 | 9 | 7 | 9 | 5 | 8 | 6 | 8 | 4 | 7 |
|
| ||||||||||
| Atrial fibrillation | 14 | 18 | 16 | 20 | 10 | 12 | 10 | 11 | 9 | 11 |
|
| ||||||||||
| Diabetes | 47 | 47 | 42 | 44 | 66 | 66 | 64 | 60 | 58 | 59 |
|
| ||||||||||
| Stroke | 18 | 17 | 18 | 17 | 19 | 20 | 23 | 25 | 15 | 18 |
|
| ||||||||||
| Hypertension | 93 | 91 | 91 | 90 | 95 | 93 | 98 | 96 | 93 | 93 |
|
| ||||||||||
| Years since hyperlipidemic diagnosis, mean, no. | 5.5 | 5.1 | 5.6 | 5.3 | 5.3 | 4.8 | 5.0 | 4.2 | 5.1 | 4.9 |
|
| ||||||||||
| Statin-days per y, mean no. | 277 | 280 | 282 | 284 | 254 | 254 | 255 | 254 | 284 | 280 |
|
| ||||||||||
| Use statins | ||||||||||
|
| ||||||||||
| Simvastatin | 62 | 61 | 61 | 60 | 69 | 67 | 66 | 66 | 64 | 62 |
|
| ||||||||||
| Atorvastatin | 49 | 50 | 48 | 50 | 52 | 52 | 48 | 47 | 55 | 57 |
|
| ||||||||||
| Pravastatin | 19 | 17 | 20 | 17 | 18 | 16 | 21 | 18 | 16 | 15 |
|
| ||||||||||
| Rosuvastatin | 18 | 16 | 17 | 16 | 26 | 22 | 17 | 14 | 21 | 21 |
|
| ||||||||||
| No. (% of sample) | 256 635 (64.2) | 143 344 (35.8) | 195 181 (48.8) | 115 059 (28.8) | 21 458 (5.4) | 11 200 (2.8) | 24 484 (6.1) | 7794 (1.9) | 15 512 (3.9) | 9291 (2.3) |
Abbreviations: AD, Alzheimer disease; AMI, acute myocardial infarction; HCC, Hierarchical Condition Category.
Asian, Native American, or unknown individuals.
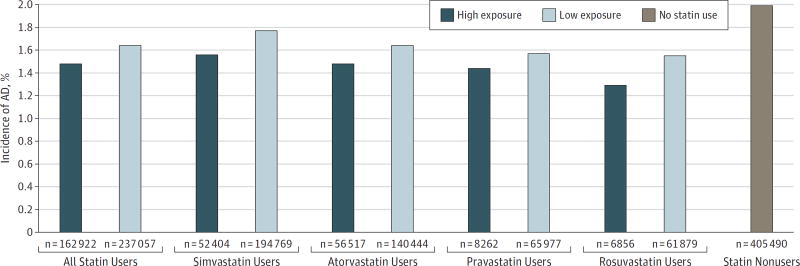
Beneficiaries exposed to higher levels of statins from 2006 to 2008 were 10% less likely to have an AD diagnosis in the subsequent 5 years than similar beneficiaries with lower statin exposure (Figure 1). Individuals with high exposure to statins had lower rates of AD compared with individuals with low exposure to statins across all 4 statin types. Those who did not use any statins (omitted from our analytic sample) had an AD incidence of 1.99% over the same period (Figure 1).
Figure 1. Incidence of Alzheimer Disease (AD) Among Statin Users (2009–2013).
The data on the incidence of AD among 399 979 statin users and 405 490 nonusers 65 years of age or older were obtained from Medicare claims data. High-exposure individuals are those with days of filled prescriptions in at least the 50th percentile of days in the mean statin-year in at least 2 years of the period from 2006 to 2008. The low-exposure groups used the designated statin, but for fewer days, or later in the sample period.
Hazard Models
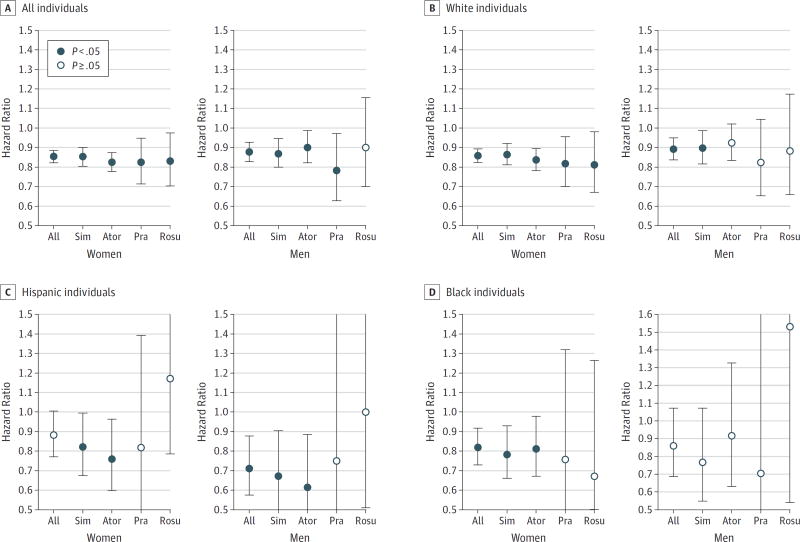
Figure 2 displays the hazard ratios (HRs) of AD incidence associated with high vs low exposure to statins. Hazard ratios, 95% CIs, and P values are reported in Table 3. High exposure was associated with decreased AD risk among women (HR, 0.85; 95%CI, 0.82–0.89; P<.001) and men (HR, 0.88; 95%CI, 0.83–0.93; P<.001). This represented a 15% and 12% reduction among women and men, respectively. The association varied across race/ethnicity and sex. The risk of AD was reduced for Hispanic men (HR, 0.71; 95%CI, 0.58–0.88; P=.001). White women and men also had a reduced AD risk (HR, 0.86; 95% CI, 0.82–0.89; P<.001 for women and HR, 0.89; 95%CI, 0.84–0.95; P<.001 for men), as did black women (HR, 0.82; 95%CI, 0.73–0.92; P<.001). Although the estimated HR was less than 1, no significant difference in AD risk was found for black men with high exposure to statins relative to low.
Figure 2. Incidence of Alzheimer Disease Associated With High vs Low Exposure to Statins.
Hazard ratios depicting the relative risk of Alzheimer disease incidence from 2009 to 2013 for those with high vs low exposure to statins. High-exposure individuals are those with days of filled prescriptions in at least the 50th percentile of days in the mean statin-year in at least 2 years of the period from 2006 to 2008. The low-exposure group used the designated statin, but for fewer days, or later in the sample period. Error bars indicate 95%CI (Table 3). Ator indicates atorvastatin; Pra, pravastatin; Rosu, rosuvastatin; and Sim, simvastatin.
Table 3.
Incidence of AD Associated With High vs Low Exposure to Statinsa
| Statin Type | Hazard Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| All | P Value | White | P Value | Hispanic | P Value | Black | P Value | |
| Women | ||||||||
| All | 0.85 (0.82–0.89) | <.001 | 0.86 (0.82–0.89) | <.001 | 0.88 (0.77–1.01) | .06 | 0.82 (0.73–0.92) | <.001 |
| Simvastatin | 0.85 (0.81–0.90) | <.001 | 0.86 (0.81–0.92) | <.001 | 0.82 (0.68–0.99) | .04 | 0.78 (0.66–0.93) | .01 |
| Atorvastatin | 0.82 (0.77–0.87) | <.001 | 0.84 (0.78–0.89) | <.001 | 0.76 (0.60–0.97) | .02 | 0.81 (0.67–0.98) | .03 |
| Pravastatin | 0.82 (0.71–0.95) | .01 | 0.82 (0.70–0.95) | .01 | 0.82 (0.48–1.39) | .46 | 0.76 (0.43–1.32) | .33 |
| Rosuvastatin | 0.83 (0.70–0.97) | .02 | 0.81 (0.67–0.98) | .03 | 1.17 (0.78–1.75) | .44 | 0.67 (0.36–1.26) | .22 |
| Men | ||||||||
| All | 0.88 (0.83–0.93) | <.001 | 0.89 (0.84–0.95) | <.001 | 0.71 (0.58–0.88) | .001 | 0.86 (0.69–1.07) | .18 |
| Simvastatin | 0.87 (0.80–0.94) | .001 | 0.90 (0.82–0.99) | .02 | 0.67 (0.50–0.91) | .01 | 0.77 (0.55–1.07) | .12 |
| Atorvastatin | 0.90 (0.82–0.99) | .02 | 0.92 (0.83–1.02) | .11 | 0.61 (0.42–0.89) | .01 | 0.92 (0.63–1.33) | .64 |
| Pravastatin | 0.78 (0.63–0.97) | .03 | 0.82 (0.65–1.04) | .11 | 0.75 (0.29–1.89) | .54 | 0.70 (0.25–1.95) | .50 |
| Rosuvastatin | 0.90 (0.70–1.16) | .40 | 0.88 (0.66–1.17) | .38 | 1.00 (0.51–1.97) | .99 | 1.53 (0.54–4.33) | .42 |
Abbreviation: AD, Alzheimer disease.
Hazard ratios depicting relative risk of AD incidence during 2009 to 2013 for those with high exposure to statins, compared with low exposure. High-exposure individuals are those with days of filled prescriptions in at least the 50th percentile of days in the mean statin-year in at least 2 years of 2006, 2007, and 2008. The low-exposure group used the designated statin, but for fewer days, or later in the sample period.
Associations by Statin Type
The association between statin use and AD incidence varied across statin type, race, and sex. Simvastatin was associated with a lower AD risk for white women (HR, 0.86; 95%CI, 0.81–0.92; P<.001), Hispanic women (HR, 0.82; 95%CI, 0.68–0.99; P=.04), and black women (HR, 0.78; 95%CI, 0.66–0.93; P=.01). White men (HR, 0.90; 95% CI, 0.82–0.99; P=.02) and Hispanic men (HR, 0.67; 95%CI, 0.50–0.91; P=.01) also had a reduced AD risk with high exposure to simvastatin. No statistically significant reduction in AD risk for black men was associated with any statin. Atorvastatin was associated with reduced AD risk among white women (HR, 0.84; 95%CI, 0.78–0.89; P<.001), Hispanic women (HR, 0.76; 95%CI, 0.60–0.96; P=.02), and Hispanic men (HR, 0.61; 95%CI, 0.42–0.89; P=.01). Pravastatin and rosuvastatin were only significantly associated with reduced AD risk for white women (HR, 0.82; 95%CI, 0.70–0.95; P=.01 for pravastatin and HR, 0.81; 95%CI, 0.67–0.98; P=.03 for rosuvastatin).
Discussion
We analyzed the association between statin use and AD incidence for men and women of different races/ethnicities across the 4 most commonly prescribed statins. Lipophilic statins cross the blood-brain barrier more readily, leading to hypotheses that they could have a stronger association with AD risk than hydrophilic statins.24 Our findings generally support this theory for Hispanic men and women, and for black women. But white women had a reduced AD risk from all statins tested, regardless of lipophilicity. Hydrophilic statins are less commonly used; thus, the sample sizes for these drugs are smaller than for lipophilic statins, and the estimates are less precisely measured as demonstrated by the large 95% CIs. The existing literature presents conflicting results on the associations between AD and lipophilic and hydrophilic statins,15,19 highlighting the need for a better understanding of how statins pass through the blood-brain barrier and affect the mechanisms underlying AD.
By decreasing cholesterol levels, statins may reduce the formation of β-amyloid peptide.7,25 Although clinical trials of statins for patients with AD found no evidence of clinical effectiveness, enrollment was not based on cholesterol or lipid levels, and the simvastatin trial excluded individuals with dyslipidemia,26 those most likely to benefit from lipid-lowering therapeutics. In population studies, statins’ effect on AD incidence and prevalence is mixed with studies reporting no effects,27 unclear effects,28 or beneficial effects,15–19 which could be attributable to differences in portioning statins into lipid or water compartments, sex differences, genetic variance, and differential response.
Prior studies compared statin users and nonstatin users. Nonusers are likely a weaker control group than statin users with low exposure in analyzing the association between statin use and AD incidence. Nonusers are individuals without a hyperlipidemia diagnosis or individuals with undiagnosed hyperlipidemia or diagnosed hyperlipidemia who are not being treated or not adhering to medication. In our sample, they are 2 years older than statin users and less likely than statin users to have acute myocardial infarction (4% vs 7.3%), diabetes (32.8% vs 46.9%), stroke (16.6% vs 17.9%), and hypertension (81.7% vs 91.8%), and slightly more likely to have atrial fibrillation (16.2% vs 15.7%). Estimates from models that compared statin users with nonusers are provided in eTable 1 in the Supplement and show lower HRs among women (HR, 0.79; 95%CI. .77–.82; P<.001) and men (HR, 0.79; 95%CI, 075–.82; P<.001) than the HRs that we estimated from our sample of statin users.
Our analysis indicated interactions of race/ethnicity, sex, and statin type in the association between statin use and AD incidence. Substantial differences in allele frequencies affect therapeutic response across races/ethnicities. For example, the clinical response to statins to reduce cholesterol and lipoproteins is variable and related to genetic heterogeneity.29 Of particular relevance to AD, variants in the APOE gene are associated with differential responses to statins.29 Variance in genetic coding for drug-metabolizing enzymes and transporters can underlie response and resistance.29 Two general classes of proteins—cytochrome c enzymes and ATP-binding cassette subfamily B transporters—are principal regulators of drug metabolism and clearance.29 African populations carrying CYP2C19 and CYP2D6 variants exhibit ultrarapid activity and are likely to exhibit lower systemic exposure to drugs.30 ATP-binding casette subfamily B member 1 is a major drug transporter that can contribute to resistance by influencing clearance.31 There is a positive selection for ATP-binding cassette subfamily B member 1 alleles in African populations compared with others.32
The risk of AD is higher among ethnic minorities,33 and thus these people from diverse racial and ethnic backgrounds face a high burden. Even modest advancements in the treatment and prevention of AD (eg, a drug that can delay the disease’s onset by 1 year) will result in a cost savings of $223 billion in 2050.34 The right type of statin, for the right person, at the right time may provide an inexpensive means to decrease the burden of AD.
Limitations
A strength of our analysis was the use of Medicare claims data. Medicare covers 93% of the US population 65 years of age or older and includes validated measures of race/ethnicity.35 The disadvantage of Medicare claims data is possible measurement errors in statin use due to censored earlier use before the enactment of Part D. We mitigate this bias by controlling for the year of hyperlipidemic diagnosis reported in the Chronic Conditions Data Warehouse and match our treatment and control groups according to years since hyperlipidemia diagnosis. Additional measurement errors in statin use could be introduced if people switched statins during the study period; however, switching in both the high- and low-exposure groups of statin users would bias our results toward zero or no association.
High-exposure statin users in the treatment group may have higher mortality risk than the low-exposure statin users. Increased mortality would cause this group to exit our sample at a higher rate and artificially deflate AD rates. To address this concern, we estimated models based on a sample of surviving participants from 2006 to 2013 (eTable 2 in the Supplement).
Genetic factors contributing to AD risk, such as the APOE4 allele, are unlikely to vary by statin exposure. Thus, while genetic factors may affect the efficacy of statins across race/ethnicity, they are unlikely to drive the AD reduction within a race/ethnicity. Other factors, such as education level, may differ for those with high vs low exposure. To address this, we link the zip codes of individuals in our sample to zip code–level data including variables for the percentage of residents who completed high school and include it as a control in the Cox regressions. Education also correlates with comorbid conditions, which we are able to control for using the CMS-HCC index and comorbidity indicators.
Our study was designed to reduce the chance that imminent AD affects statin use. However, imminent AD may be associated with lower exposure to statins in earlier years. We checked for this by removing low-exposure users and comparing the high-exposure group (at least 50th percentile of days in at least 2 years, 2006–2008) with a group with the same high exposure to statins (50th percentile of days), but in just 1 of the subsequent years (2009–2012), and thus lower exposure. As such, we compared high-exposure users with later users (fewer years of exposure) but not lesser users (eTable 3 in the Supplement), and confirmed our findings of reduced AD incidence associated with high exposure to statins. We also compared AD incidence between low and high statin users for AD onset that occurred at least 4 years later (2012 and 2013). This reduced our sample size (196 651 women and 109 404 men), so we estimated for all statins separately for men and women. Again, we found that high exposure to statins was associated with reduced AD risk, with a slightly smaller effect (eTable 4 in the Supplement).
Other robustness checks included testing a threshold of high exposure at the 75th percentileand25th percentile of days of statin prescription fills. The resulting HRs (eTable 5 in the Supplement) decreased monotonically with stricter thresholds. We constructed a statin-use measure that adjusted for variation across statins in dose and low-density lipoprotein lowering properties. Results were robust to this adjustment.
Conclusions
Our study identified the associations between AD incidence and statin use by statin type, sex, and race/ethnicity. This suggests that certain patients, facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce AD risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of AD.
Supplementary Material
Key Points.
Question
What is the association between statin use and the incidence of Alzheimer disease among male and female Medicare beneficiaries?
Findings
In this study, high vs low exposure to statins was associated with a lower incidence of Alzheimer disease for women and men, respectively. The reduction in incidence of Alzheimer disease varied across race/ethnicity and type of statin.
Meaning
Statins may potentially affect Alzheimer disease risk, so physicians should consider which statin is prescribed to their patients.
Acknowledgments
Funding/Support: This research was supported by the National Institute on Aging of the National Institutes of Health (awards 1RC4AG039036-01 and P30AG043073-01) and the University of Southern California Zumberge Research Fund (grant 1R34AG049652 [Dr Brinton]). Dr Barthold is supported through the Schaeffer-Amgen Fellowship Program funded by Amgen.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank Patricia St Clair, ScB, Brown University, and Bryan Tysinger, BS, Harvey Mudd College and the data core of the Leonard D. Schaeffer Center for Health Policy and Economics at the University of Southern California for administrative and data support. These individuals did not receive compensation for their contributions.
Footnotes
Author Contributions: Drs Zissimopoulos and Joyce had full access to all study data and take responsibility for the integrity of the data and accuracy of the data analysis.
Concept and design: Zissimopoulos, Brinton, Barthold.
Acquisition, analysis, or interpretation of data: Zissimopoulos, Barthold, Brinton, Joyce.
Drafting of the manuscript: Zissimopoulos, Barthold, Joyce.
Critical revision of the manuscript for important intellectual content: Zissimopoulos, Brinton, Barthold, Joyce.
Statistical analysis: Zissimopoulos, Barthold, Joyce.
Obtaining funding: Brinton, Joyce.
Study supervision: Zissimopoulos.
Conflict of Interest Disclosures: None reported.
Contributor Information
Julie M. Zissimopoulos, Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles.
Douglas Barthold, Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles.
Roberta Diaz Brinton, Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles; Center for Innovation in Brain Science, University of Arizona Health Sciences, Tucson.
Geoffrey Joyce, Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles.
References
- 1.Gu Q, Paulose-Ram R, Burt VL, Kit BK. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. [Accessed March 30, 2016]; https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Published December 2014. [PubMed]
- 2.Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001;111(5):390–400. doi: 10.1016/s0002-9343(01)00870-1. [DOI] [PubMed] [Google Scholar]
- 3.Kandiah N, Feldman HH. Therapeutic potential of statins in Alzheimer’s disease. J Neurol Sci. 2009;283(1–2):230–234. doi: 10.1016/j.jns.2009.02.352. [DOI] [PubMed] [Google Scholar]
- 4.Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Assocations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014;71(2):195–200. doi: 10.1001/jamaneurol.2013.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesser GT, Beeri MS, Schmeidler J, Purohit DP, Haroutunian V. Cholesterol and LDL relate to neuritic plaques and to APOE4 presence but not to neurofibrillary tangles. Curr Alzheimer Res. 2011;8(3):303–312. doi: 10.2174/156720511795563755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petanceska SS, DeRosa S, Olm V, et al. Statin therapy for Alzheimer’s disease: will it work? J Mol Neurosci. 2002;19(1–2):155–161. doi: 10.1007/s12031-002-0026-2. [DOI] [PubMed] [Google Scholar]
- 7.Refolo LM, Pappolla MA, LaFrancois J, et al. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2001;8(5):890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- 8.Petanceska SS, DeRosa S, Sharma A, et al. Changes in apolipoprotein E expression in response to dietary and pharmacological modulation of cholesterol. J Mol Neurosci. 2003;20(3):395–406. doi: 10.1385/JMN:20:3:395. [DOI] [PubMed] [Google Scholar]
- 9.McGuinness B, Passmore P. Can statins prevent or help treat Alzheimer’s disease? J Alzheimers Dis. 2010;20(3):925–933. doi: 10.3233/JAD-2010-091570. [DOI] [PubMed] [Google Scholar]
- 10.Caballero J, Nahata M. Do statins slow down Alzheimer’s disease? a review. J Clin Pharm Ther. 2004;29(3):209–213. doi: 10.1111/j.1365-2710.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 11.Jukema JW, Cannon CP, de Craen AJ, Westendorp RG, Trompet S. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60(10):875–881. doi: 10.1016/j.jacc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159(10):688–697. doi: 10.7326/0003-4819-159-10-201311190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II, review of human trials and recommendations. Arch Neurol. 2011;68(11):1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia [published online January 4, 2016] Cochrane Database Syst Rev. doi: 10.1002/14651858.CD003160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity: the Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80(1):13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 16.Hendrie HC, Hake A, Lane K, et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn Dis. 2015;25(3):345–354. doi: 10.18865/ed.25.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356(9242):1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Shofer JB, Rhew IC, et al. Age-varying association between statin use and incident Alzheimer’s disease. J Am Geriatr Soc. 2010;58(7):1311–1317. doi: 10.1111/j.1532-5415.2010.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin F-C, Chuang Y-S, Hsieh H-M, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine (Baltimore) 2015;94(47):e2143. doi: 10.1097/MD.0000000000002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolozin B, Wang SW, Li N-C, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5(1):20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwell M, Iacus S, King G, Porro G. Cem: coarsened exact matching in Stata. [Accessed April 25, 2016];Stata J. 2009 9(4):524–546. http://www.stata-journal.com/sjpdf.html?articlenum=st0176. [Google Scholar]
- 22.Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res. 2010;10(1):245. doi: 10.1186/1472-6963-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonito A, Bann C, Eicheldinger C, Carpenter L. Creation of New Race-Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries. Rockville, MD: Agency for Healthcare Research and Quality, and Center for Medicare and Medicaid Services; 2008. [Google Scholar]
- 24.Vuletic S, Riekse RG, Marcovina SM, Peskind ER, Hazzard WR, Albers JJ. Statins of different brain penetrability differentially affect CSF PLTP activity. Dement Geriatr Cogn Disord. 2006;22(5–6):392–398. doi: 10.1159/000095679. [DOI] [PubMed] [Google Scholar]
- 25.Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer’s disease: is there a link? Neurology. 2001;57(6):1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- 26.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77(6):556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zandi PP, Sparks DL, Khachaturian AS, et al. Cache County Study investigators. Do statins reduce risk of incident dementia and Alzheimer disease? the Cache County Study. Arch Gen Psychiatry. 2005;62(2):217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 28.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I, review of epidemiological and preclinical studies. Arch Neurol. 2011;68(10):1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangravite LM, Thorn CF, Krauss RM. Clinical implications of pharmacogenomics of statin treatment. Pharmacogenomics J. 2006;6(6):360–374. doi: 10.1038/sj.tpj.6500384. [DOI] [PubMed] [Google Scholar]
- 30.Warnich L, Drögemöller BI, Pepper MS, Dandara C, Wright GE. Pharmacogenomic research in South Africa: lessons learned and future opportunities in the rainbow nation. Curr Pharmacogenomics Person Med. 2011;9(3):191–207. doi: 10.2174/187569211796957575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung KL, Pan J, Ohnuma S, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014;74(2):598–608. doi: 10.1158/0008-5472.CAN-13-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Ding K, Zhang Y, Jin L, Kullo IJ, He F. Comparative and evolutionary pharmacogenetics of ABCB1: complex signatures of positive selection on coding and regulatory regions. Pharmacogenet Genomics. 2007;17(8):667–678. doi: 10.1097/FPC.0b013e328165249f. [DOI] [PubMed] [Google Scholar]
- 33.Tang M-X, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 34.Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer’s disease onset. Forum Health Econ Policy. 2014;18(1):25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health & Human Services USA. [Accessed April 21, 2016];A profile of older Americans: 2014. http://www.aoa.acl.gov/aging_statistics/profile/2014/docs/2014-profile.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.