Abstract
Background
The prevalence of atrial fibrillation in the human immunodeficiency virus (HIV)-infected population is growing, but the ability of the CHA2DS2-VASc score to predict thromboembolic (TE) risk is unknown in this population.
Setting
Within the Veterans Affairs HIV Clinical Case Registry, 914 patients had an atrial fibrillation diagnosis between 1997–2011 and no prior TE events.
Methods
We compared TE incidence by CHADS2VASc scores, and stratified by warfarin use. Using Cox proportional hazards regression with adjustment for competing risks, we modeled associations of CHADS2VASc scores and warfarin use with TE risk.
Results
At baseline, the distribution of CHA2DS2-VASc scores was 0 (n=208), 1 (n=285), and 2+ (n= 421); 34 patients developed 38 TE events during a median of 3.8 years follow-up. Event rates by CHA2DS2-VASc scores of 0, 1, and 2+ were 5.4, 9.3, and 8.1 per 1000 person years, respectively; multivariate adjusted hazards ratios (HRs) were 1.70 (95% confidence interval [CI] 0.65, 4.45) for CHA2DS2-VASc score 1 (p=0.28) and HR=1.34 (0.51, 3.48) for score 2+ versus 0 (p=0.55). Baseline warfarin use was associated with increased TE risk, though not statistically significant (HR 2.06 [0.86, 4.93], p=0.11) with similar results when modeled as time-updated use and duration of use.
Conclusion
In this national registry of HIV-infected veterans with atrial fibrillation, CHA2DS2-VASc scores were only weakly associated with TE risk. Furthermore, warfarin did not appear to be effective at preventing TE events. These results should raise concerns about the optimal strategy for TE prevention among HIV-infected persons with atrial fibrillation.
Keywords: atrial fibrillation, thromboembolic risk, HIV
Introduction
Human immunodeficiency virus (HIV) is a disease that affects approximately 1.2 million people in the United States and 36.9 million people worldwide.1,2 With the development of highly active antiretroviral therapy (ART), HIV-infected persons are living longer, shifting the health challenges from HIV-related to non-AIDS conditions. Several studies have shown that HIV infection is an independent risk factor for the development of cardiovascular (CV) disease, including arrhythmias.3–6 In the general population, atrial fibrillation is the most common arrhythmia that affects health. This is of particular concern in the HIV population as studies have shown not only a greater burden of cardiac arrhythmias, but also a higher prevalence of cerebrovascular events among HIV patients.7–9 In the setting of atrial fibrillation, anticoagulation is used clinically for the purpose of preventing thromboembolic (TE) events, but the decision to anticoagulate requires accurate assessment of the risks and benefits for individual patients.10 The CHA2DS2-VASc score is a widely used risk calculator to guide anticoagulation therapy among individuals with atrial fibrillation. It was developed in an older population without HIV infection, who have distinct risk profiles compared with individuals with HIV.11 Risk calculators for atherosclerotic CV disease, including the Framingham score, PROCAM score, and the 2013 ACC/AHA CV risk calculator, have previously been shown to underestimate risk in the HIV population.12–15 To our knowledge, the association of the CHA2DS2-VASc score with TE events in the HIV population has not been studied. Accordingly, the purpose of this study was to calculate the CHA2DS2-VASc score for HIV-infected individuals with atrial fibrillation using data in the Department of Veterans Affairs (VA) HIV Clinical Case Registry, a national clinical database. We compared observed TE event rates by CHA2DS2-VASc scores, and explored the potential effectiveness of warfarin therapy to prevent TE events.
Methods
The Department of VA HIV Clinical Case Registry is a national database of HIV-infected veterans; VA is the nation’s largest public integrated health care system and the largest single provider of health care to HIV-infected patients. This HIV registry contains demographic, clinical, laboratory, pharmacy, utilization, hospitalizations, inpatient diagnoses, and vital status information, which are included in the VA electronic medical record.16
We identified patients with a diagnosis of atrial fibrillation between January 1997 and December 2011 in the VA HIV Clinical Case Registry. Patients with a history of TE events prior to the diagnosis of atrial fibrillation were excluded in order to focus the analysis on patients at risk for an initial event due to arrhythmia. Patients entered the cohort on January 1, 1997 if they already had a diagnosis of atrial fibrillation or subsequently at their first known documentation of atrial fibrillation.
The primary outcome was incidence of TE events, defined by hospital diagnostic coding for strokes, pulmonary embolisms, and peripheral embolisms.11 In the primary analyses, we limited to ischemic TE events. In secondary analyses, we included hemorrhagic strokes in order to include potential adverse events from anticoagulation and to account for potential miscoding. TE events that occurred within one year after diagnosis of atrial fibrillation were excluded to ensure that all TE events occurred after the atrial fibrillation diagnosis and were not a result of delayed recording of TE events that may have triggered the initial diagnosis of atrial fibrillation.
The factors comprising the CHA2DS2-VASc score are heart failure, hypertension, diabetes, prior TE event, vascular disease, female sex, and age ≥ 65 years; 2 points are accorded for age ≥ 75 and prior TE event, and one point for each of the other factors.11 Anticoagulation status was assessed by warfarin use. To account for compliance, renewals, and duration of use, we determined warfarin status when patients were enrolled, and throughout follow up. In separate models, warfarin was defined as a baseline (at time of enrollment) and time-updated exposure.
Demographic (age, gender, and race) and clinical characteristics were included in our analyses along with the CHA2DS2-VASc factors listed above. We defined comorbid conditions based on a combination of physician problem lists, procedures, ambulatory diagnoses, hospitalization discharge diagnoses, laboratory results, and medication prescriptions. We applied previously validated algorithms to define the following conditions: diabetes, hypertension, hyperlipidemia, hepatitis B or C virus co-infection, illicit drug use, and smoking.17–20 A list of the ICD9 codes used to define events and co-morbidities was published previously (see Appendix Table 1 of Go et al NEJM 2004 and Choi et al AIDS 2011).17,18 HIV specific characteristics included serologic measures of CD4 cell count expressed in cells/mm3, viral load expressed in RNA copies/ml, and ART use. Other clinical characteristics included were body mass index, kidney function by estimated glomerular filtration rate (eGFR), and proteinuria (defined as 30mg/dL or greater on urinalysis). We defined continuous use of warfarin as a sequence of repeated medication renewals without a gap of longer than 6 months.
We first compared demographic and clinical characteristics, stratified by baseline CHA2DS2-VASc score categories (0, 1, or 2+) using the chi-square test for categorical variables and the Kruskal-Wallis tests for continuous variables. We compared incidence rates of TE events across CHA2DS2-VASc score categories using the log-rank test.
We used Fine-Gray models (accounting for the competing risk of mortality) to examine associations of demographics, baseline clinical characteristics, and warfarin use with risk of TE events.21 We modeled warfarin use in several ways: baseline status, time-updated, and current duration of use (in years).
We tested associations of warfarin use with TE events in both unadjusted and multivariable adjusted analyses. Multivariable adjusted models were constructed using Bayesian model averaging, retaining predictors of TE risk with posterior probabilities > 35%.22 We used marginal structural models as an alternate approach to estimate the association of warfarin use with TE events, to account for the fact that the decision to prescribe warfarin may be influenced by a patient’s covariates.23 Marginal structural models are a useful method to minimize drug channeling bias. We generated inverse probability of treatment weights for each patient by modeling warfarin exposure as a function of demographic and clinical characteristics. These weights were then applied to subsequent models evaluating the association of warfarin with TE events.
We evaluated predictors of warfarin use at baseline using Poisson relative risk regression with a robust variance estimator. To construct parsimonious models of warfarin use, we used Bayesian model averaging and retained predictors with posterior probabilities > 35%.
Bayesian model averaging was performed using the BMA package from the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were conducted using the SAS system, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Of the 914 HIV-infected veterans with atrial fibrillation who met the inclusion criteria for this study, 208 had a CHA2DS2-VASc score of 0, 285 had a score of 1, and 421 had a score of 2 or higher. The demographic and clinical characteristics of the patients at baseline, stratified by CHA2DS2-VASc score, are shown in Table 1. Patients with CHA2DS2-VASc scores of 2 or greater were notable for older age, higher prevalence of heart failure, diabetes, hypertension, and vascular disease. In addition, patients with scores of 2+ had lower eGFR. Patients with scores of 1 or 2+ had higher rates of smoking (Table 1). Patients with higher scores were also more likely to be on ART and to have a higher current CD4 T-cell count, and less likely to have an elevated HIV viral load as compared to patients with lower CHA2DS2-VASc scores.
Table 1.
Demographic and baseline clinical characteristics stratified by CHA2DS2-VASc Score
| CHA2DS2-VASc | CHA2DS2-VASc | CHA2DS2-VASc | |||
|---|---|---|---|---|---|
| Parameter | Overall N=914 |
0 N=208 |
1 N=285 |
2+ N=421 |
p-value |
| CHA2DS2-VASc Factors | |||||
| Age | 56 (49, 63) | 51 (44, 57) | 54 (48, 61) | 61 (53, 68) | <.0001 |
| Female | 16 (2%) | 0 | 7 (2%) | 9 (2%) | 0.086 |
| Diabetes | 196 (21%) | 0 | 21 (7%) | 174 (41%) | <.0001 |
| Hypertension | 466 (51%) | 0 | 154 (54%) | 312 (74%) | <.0001 |
| Vascular Disease | 275 (30%) | 0 | 40 (14%) | 235 (56%) | <.0001 |
| Heart Failure | 217 (24%) | 0 | 32 (11%) | 185 (44%) | <.0001 |
| Other | |||||
| Caucasian | 479 (52%) | 116 (56%) | 128 (45%) | 235 (56%) | 0.050 |
| Black | 418 (46%) | 89 (43%) | 151 (53%) | 178 (42%) | |
| Other Race | 17 (2%) | 3 (1%) | 6 (2%) | 8 (2%) | |
| Total Cholesterol (mg/dL) | 166 (138, 194) | 158 (136, 194) | 174 (148, 203) | 163 (135, 190) | 0.0039 |
| Low Density Lipoprotein (mg/dL) | 94 (72, 117) | 91 (76, 114) | 96 (73, 127) | 92 (70, 114) | 0.071 |
| High Density Lipoprotein (mg/dL) | 37 (29, 46) | 37 (28, 47) | 37 (30, 46) | 36 (29, 46) | 0.42 |
| Triglyceride (mg/dL) | 141 (98, 217) | 137 (94, 196) | 138 (96, 220) | 144 (101, 221) | 0.47 |
| Smoking | 349 (38%) | 62 (30%) | 110 (39%) | 177 (42%) | 0.012 |
| Illicit drug use | 400 (44%) | 81 (39%) | 119 (42%) | 200 (48%) | 0.090 |
| Alcoholism | 314 (34%) | 62 (30%) | 92 (32%) | 160 (38%) | 0.085 |
| Body Mass Index (kg/m2) | 25 (22, 28) | 24 (22, 28) | 25 (22, 29) | 25 (22, 28) | 0.32 |
| Serum Albumin (mg/dL) | 3.7 (3.2, 4.1) | 3.8 (3.1, 4.2) | 3.7 (3.3, 4.1) | 3.7 (3.2, 4.1) | 0.61 |
| Proteinuria | 289 (32%) | 60 (29%) | 81 (28%) | 148 (35%) | 0.10 |
| eGFR | 85 (65, 102) | 95 (78, 108) | 90 (69, 105) | 79 (58, 96) | <.0001 |
| HIV-Related Factors | |||||
| Current CD4 Count | 347 (179, 537) | 320 (107, 497) | 330 (165, 485) | 358 (209, 585) | 0.014 |
| Nadir CD4 | 205 (83, 360) | 216 (82, 378) | 199 (80, 356) | 202 (86, 360) | 0.95 |
| HIV Viral Load>1000 | 385 (43%) | 112 (55%) | 126 (45%) | 147 (35%) | <.0001 |
| ART use (current) | 622 (68%) | 116 (56%) | 187 (66%) | 319 (76%) | <.0001 |
| Hepatitis C | 320 (35%) | 70 (34%) | 92 (32%) | 158 (38%) | 0.32 |
| Hepatitis B | 146 (16%) | 26 (13%) | 43 (15%) | 77 (18%) | 0.16 |
Continuous variables reported as median (interquartile range). Categorical variables reported as N (%). ART = antiretroviral therapy; eGFR = estimated glomerular filtration rate; HIV = human immunodeficiency virus; IQR = interquartile range.
As shown in Figure 1, patients with a CHA2DS2-VASc score of either 1 or 2+ were about twice as likely to be on warfarin at baseline compared to patients with low CHA2DS2-VASc scores. At baseline, there were 199 warfarin users and 715 non-users. Of the users, 32% were still on warfarin at the end of the study, while of the non-users, 11% initiated warfarin during the study and 8% were on warfarin at the end of the study. Characteristics that were independently associated with higher prevalence of baseline warfarin use included vascular disease, heart failure, higher body mass index, higher serum albumin, and ART use (Table 1, Supplemental Digital Content).
Figure 1. Prevalence of baseline warfarin use by CHA2DS2-VASc score.
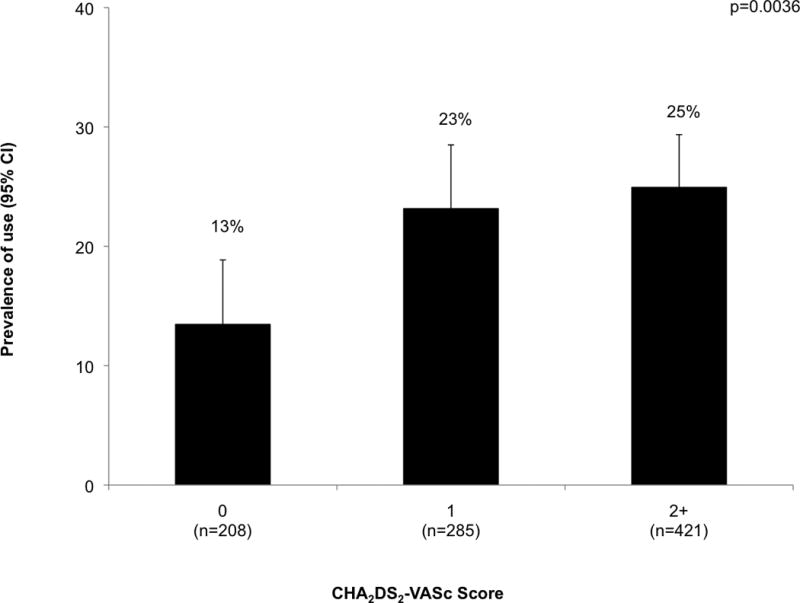
p-value denotes test for difference in rate of warfarin use by level of CHA2DS2-VASc score. CI = confidence interval.
During a median follow up of 3.8 years (interquartile range [IQR] 1.3–7.5 years), 34 patients developed a total of 38 TE events, which included 12 pulmonary embolisms, 3 peripheral embolisms, and 23 ischemic strokes. One ischemic stroke was also coded as a hemorrhagic stroke. The TE event rates by baseline CHA2DS2-VASc scores per 1000 person years (PY) were 5.4 (95% confidence interval [CI] 2.4, 12.0) for CHA2DS2-VASc score of 0; 9.3 (95% CI 5.5, 15.7) for score of 1; and 8.1 for score of 2+ (95% CI 4.8, 13.8) (Figure 2). In adjusted models that accounted for the competing risk of death, higher CHA2DS2-VASc scores appeared to be associated with increased risk of TE events, although the associations did not reach statistical significance. CHA2DS2-VASc score of 1 versus 0 had a hazard ratio (HR) of 1.70 (95% CI 0.65, 4.45; p = 0.28), while CHA2DS2-VASc score of 2+ versus 0 had a HR of 1.34 (95% CI 0.51, 3.48; p = 0.55). CHA2DS2-VASc score of 1+ versus 0 had a HR of 1.50 (95% CI 0.62, 3.63; p = 0.37).
Figure 2. Thromboembolic event rates stratified by baseline CHA2DS2-VASc score.
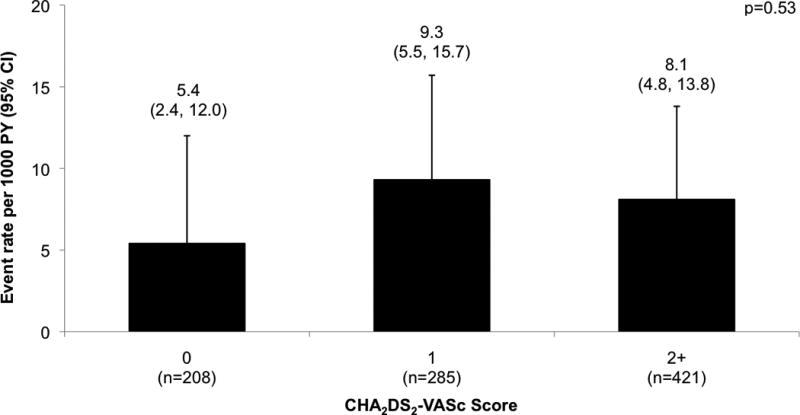
Comparison of thromboembolic event rates across baseline CHA2DS2-VASc scores reported as event rate per 1000 person years and 95% confidence interval. Log-rank test for difference in event rates across CHA2DS2-VASc categories showed a p-value of 0.53. Unadjusted HR (95% CI) of CHA2DS2-VASc score of 1 versus 0 is 1.70 (0.65, 4.45), p = 0.28. Unadjusted HR (95% CI) of CHA2DS2-VASc score of 2+ versus 0 is HR 1.34 (0.51, 3.48), p = 0.54. CI = confidence interval; HR = hazard ratio; PY = person years.
We next examined the association of individual demographic and clinical factors with TE event risk. None of the individual risk factors that comprised the CHA2DS2-VASc score showed a statistically significant association with TE event risk (Table 2), although a weak effect of hypertension or heart failure could not be ruled out. Results remained similar in sensitivity analyses excluding those taking warfarin at baseline and in analyses including hemorrhagic strokes as an outcome (Table 2 and 3, Supplemental Digital Content). Of note, the effect of gender could not be estimated as none of the women developed TE events. Non-white race was associated with higher risk for TE events in both unadjusted and adjusted models. Higher BMI was associated with higher risk for TE events in unadjusted models. Higher high density lipoprotein concentrations were associated with lower risk for TE events. There was little association of HIV-related factors with TE, although a protective effect of ART or HCV cannot be excluded. Sensitivity analyses excluding those taking warfarin at baseline and analyses including hemorrhagic strokes as an outcome showed similar results with the exception of black race no longer being statistically significantly associated with higher risk for TE events in those not taking warfarin at baseline (Table 2 and 3, Supplemental Digital Content).
Table 2.
Associations of demographic and baseline clinical characteristics with thromboembolic events
| Parameter | Level | N (%) | Event rate (95%CI) (per 1000 PY) |
Unadjusted | Adjusted | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||||
| CHA2DS2-VASc Factors | |||||||
| Age | per decade | 1.03 (0.77, 1.37) | 0.86 | ||||
| Gender | Female | 0 | 0 | na | |||
| Male | 34 (3.8%) | 8.0 (5.7, 11.2) | |||||
| Diabetes | Yes | 5 (2.6%) | 7.2 (3.0, 17.2) | 0.81 (0.31, 2.08) | 0.66 | ||
| No | 29 (4.0%) | 8.0 (5.5, 11.5) | reference | ||||
| Hypertension | Yes | 21 (4.5%) | 9.0 (5.8, 13.7) | 1.42 (0.71, 2.83) | 0.32 | ||
| No | 13 (2.9%) | 6.5 (3.8, 11.2) | reference | ||||
| Vascular Disease | Yes | 6 (2.2%) | 5.5 (2.5, 12.2) | 0.59 (0.25, 1.41) | 0.24 | ||
| No | 28 (4.4%) | 8.6 (6.0, 12.5) | reference | ||||
| Heart Failure | Yes | 24 (3.4%) | 12.3 (6.6, 22.9) | 1.46 (0.70, 3.03) | 0.32 | ||
| No | 10 (4.6%) | 6.8 (4.6, 10.1) | reference | ||||
| Other | |||||||
| Race | Black | 21 (5.0%) | 10.0 (6.5, 15.4) | 1.67 (0.83, 3.34) | 0.15 | 2.09 (1.02, 4.29) | 0.044 |
| Other | 0 | 0 | na | na | |||
| White | 13 (2.7%) | 5.9 (3.4, 10.2) | reference | reference | |||
| Total Cholesterol | Per 10 mg/dL | 1.00 (0.93, 1.07) | 0.90 | ||||
| Low Density Lipoprotein | Per 10 mg/dL | 1.03 (0.94, 1.12) | 0.55 | ||||
| High Density Lipoprotein | Per 10 mg/dL | 0.76 (0.62, 0.93) | 0.007 | 0.72 (0.57, 0.90) | 0.004 | ||
| Triglycerides | Per doubling | 1.11 (0.84, 1.47) | 0.46 | ||||
| Smoking | Yes | 11 (3.2%) | 8.6 (4.8, 15.5) | 1.04 (0.51, 2.13) | 0.91 | ||
| No | 23 (4.1%) | 7.5 (5.0, 11.3) | reference | ||||
| Illicit drug use | Yes | 13 (3.3%) | 7.7 (4.5, 13.3) | 0.89 (0.45, 1.77) | 0.75 | ||
| No | 21 (4.1%) | 7.9 (5.2, 12.1) | reference | ||||
| Alcoholism | Yes | 13 (4.1%) | 10.0 (5.8, 17.2) | 1.34 (0.68, 2.67) | 0.40 | ||
| No | 21 (3.5%) | 6.9 (4.5, 10.6) | reference | ||||
| Body Mass Index | Per kg/m2 | 1.05 (1.00, 1.11) | 0.03 | ||||
| <20 | 2 (1.6%) | 4.2 (1.1, 16.9) | reference | ||||
| 20–25 | 11 (3.2%) | 6.4 (3.5, 11.6) | 1.81 (0.40, 8.16) | 0.44 | |||
| 25–30 | 15 (5.4%) | 10.5 (6.4, 17.5) | 3.86 (0.91, 16.48) | 0.07 | |||
| >30 | 6 (3.8%) | 8.3 (3.7, 18.4) | 2.82 (0.58, 13.70) | 0.20 | |||
| Serum Albumin | Per 0.5 mg/dL decrease | 0.91 (0.73, 1.14) | 0.41 | ||||
| <3.5 | 8 (2.5%) | 6.8 (3.4, 13.6) | Reference | ||||
| 3.5–4.0 | 16 (4.9%) | 9.3 (5.7, 15.1) | 1.47 (0.64, 3.38) | 0.36 | |||
| >4.0 | 10 (3.7%) | 7.0 (3.8, 13.0) | 1.26 (0.51, 3.11) | 0.62 | |||
| Proteinuria | Yes | 8 (2.8%) | 8.4 (4.2, 16.7) | 0.83 (0.38, 1.82) | 0.65 | ||
| No | 26 (4.2%) | 7.7 (5.2, 11.3) | reference | ||||
| eGFR | per 10 ml/min decrease | 0.95 (0.86, 1.06) | 0.36 | ||||
| HIV-Related Factors | |||||||
| Current CD4 | Per doubling | 0.95 (0.83, 1.08) | 0.40 | ||||
| Nadir CD4 | Per doubling | 0.96 (0.80, 1.15) | 0.67 | ||||
| HIV Viral Load | Per 10-fold increase | 1.15 (0.91, 1.45) | 0.25 | ||||
| ART use (current) | Yes | 22 (3.2%) | 6.9 (4.5, 10.5) | 0.82 (0.41, 1.67) | 0.59 | ||
| No | 12 (5.6%) | 10.4 (5.9, 18.4) | reference | ||||
| Hepatitis C | Yes | 7 (2.2%) | 5.7 (2.7, 11.9) | 0.61 (0.27, 1.41) | 0.25 | ||
| No | 27 (4.6%) | 8.7 (6.0, 12.7) | reference | ||||
| Hepatitis B | Yes | 5 (3.4%) | 8.3 (3.4, 19.9) | 1.01 (0.39, 2.60) | 0.98 | ||
| No | 29 (3.8%) | 7.8 (5.4, 11.2) | reference | ||||
ART = antiretroviral therapy; CI =confidence interval; eGFR = estimated glomerular filtration rate; HIV = human immunodeficiency virus; HR = hazard ratio; na = not applicable; PY = person years.
Baseline warfarin users had a higher incidence of TE events than did non-users (11.1 [95% CI 6.0, 20.7] and 7.0 [95% CI 4.7, 10.4] per 1000 PY, respectively), although this difference was only marginally statistically significant in a stratified log-rank test that accounted for CHA2DS2-VASc score (p = 0.20). Unadjusted and adjusted Fine-Gray models were used to test for association between warfarin use and TE event risk. In adjusted models, marginal structural models were used to control for possible drug channeling bias. Baseline warfarin use was associated with a two-fold increased TE risk in the adjusted Fine-Gray model, though not statistically significant (HR 2.06 [95% CI 0.86, 4.93], p=0.11). In sensitivity analyses that included hemorrhagic strokes as an outcome, baseline warfarin use in both unadjusted and adjusted Fine-Gray models were associated with a two-fold increase in TE risk and reached statistical significance (HR 2.14 [95% CI 1.07, 4.26], p = 0.031; HR 2.49 [95% CI 1.12, 5.52], p = 0.025, respectively) (Table 4, Supplemental Digital Content). In both unadjusted and adjusted Fine-Gray models, warfarin use when modeled as both a time-updated predictor and current duration of use was not significantly associated with higher TE risk, but did have HRs > 1.0, which does not support a protective effect. Results remained similar in sensitivity analyses including hemorrhagic strokes as an outcome (Table 4, Supplemental Digital Content).
Discussion
As the HIV-infected population is growing older, the incidence of atrial fibrillation is expected to increase, and clinicians will be faced with the challenging decision of whether or not to anti-coagulate. In our study, the CHA2DS2-VASc score, which is the calculator of choice in the general population, was only weakly associated with a risk of TE events among HIV-infected individuals with atrial fibrillation. Most important, there was no evidence for a rising gradient of TE risk as CHA2DS2-VASc scores increased from 1 to 2+, or even as CHA2DS2-VASc scores increased from 0 to 1+. A CHA2DS2-VASc score of 2 or higher is the clinical cutpoint for anticoagulation in the general population, yet HIV-infected individuals with a CHA2DS2-VASc score of 2+ actually had slightly lower incidence of TE events than HIV-infected individuals with a CHA2DS2-VASc score of 1 in our study. Also, none of the individual components of the CHA2DS2-VASc score had a significant association with TE risk in this HIV population. These findings challenge not only the current practice of using a CHA2DS2-VASc score of 2 or higher as the cutoff for starting anticoagulation, but also the use of the CHA2DS2-VASc score as a TE risk calculator in the HIV-infected population. Finally, we found that warfarin use was not protective against TE risk, and appeared potentially to be associated with higher TE risk. This raises the possibility that warfarin may have a different effectiveness in the HIV-infected population with atrial fibrillation compared with the general population.
A previous study of atrial fibrillation in 30,533 HIV-infected veterans demonstrated that lower CD4 counts and higher viral loads were associated with increased incidence of atrial fibrillation.5 Thus, it is possible that the CHA2DS2-VASc score was not significantly associated with TE events in the HIV population because it does not include HIV-specific factors, such as CD4 counts and viral load suppression, which are related to atrial fibrillation.5
Multiple studies have shown ART use, CD4 count, and HIV viral load to be associated with cerebrovascular disease. Both the Data Collection on Adverse Events of Anti-HIV drugs study and the Strategies for Management of ART study showed that higher CD4 counts and lower viral loads were associated with lower risk.9,24 In the former study, longer ART exposure was associated with increased risk for cerebrovascular events and in the latter, continuous ART use was associated with fewer events than intermittent ART use.7,24 In our study, both higher CD4 count and ART use appeared to be associated with a decreased risk of TE events (Table 2).
Other factors may play a larger role in predicting TE events in the HIV population than in the general population. For example, we found higher levels of high density lipoprotein to be protective against TE events; poorly controlled HIV leads to decreased high density lipoprotein, while ART, especially non-nucleoside reverse transcriptase inhibitors, increases high density lipoprotein. Other lipid changes are also known to be a result of HIV infection itself and certain antiretrovirals. While dyslipidemia from ART and HIV infection may influence TE risk, triglycerides and low density lipoprotein were not significant predictors in our study.7,25–28 Concurrent chronic co-infections are important factors to consider as well. While our study found little association between hepatitis B or C co-infection with TE risk, multiple studies in the general population have found hepatitis C infection to be associated with increased stroke risk.29,30
There are several potential explanations for why warfarin did not appear to be effective in TE prevention in our study. First, chronic inflammation persists among effectively treated HIV-infected individuals, which may contribute to an increased thrombogenic state and which may not be modified by warfarin use.31–33 This heightened inflammation and thrombosis may underlie the high number of ischemic strokes and myocardial infarctions that have been reported in the HIV population.8,34 In fact, the SMART study found that higher levels of d-dimer were associated with increased risk for cardiovascular disease and mortality in HIV infection.35 There is accruing evidence that the pathophysiology underlying the increased rates of stroke seen in the HIV population involves the elevated levels of inflammation and endothelial damage that are observed with HIV infection.36 Second, stroke in HIV is a heterogenous condition, and not all strokes are from emboli among those with atrial fibrillation; other important etiologies include large artery atherosclerosis and vasculitis.37 Warfarin may not be effective for preventing strokes from these etiologies, yet would increase risk for hemorrhagic strokes.38 Third, warfarin use in the HIV population is more difficult and requires closer monitoring due to the drug-drug interactions between warfarin and numerous ART drugs. Protease inhibitors and nonnucleoside reverse transcriptase inhibitors, in particular, have been found to either inhibit or induce warfarin metabolism depending on the ART drug.39,40 In addition, aside from the drug-drug interactions between warfarin and numerous ART drugs, patients on warfarin, and especially HIV-infected patients on warfarin, often do not have international normalized ratios (INRs) consistently in therapeutic ranges.41,42 Warfarin would only be effective in decreasing TE risk if the INRs were monitored closely.
Another potential reason for why warfarin may not be effective in decreasing TE risk in HIV-infected individuals with atrial fibrillation is that the decision to anticoagulate high risk individuals is complex and also takes into account compliance, bleeding risks, and patient choice. As such, in our study, increased viral load and hepatitis C were associated with less use of warfarin, while higher CD4 counts and higher nadir CD4 counts were associated with more use of warfarin, suggesting that patients with less well-controlled HIV infections are less likely to get put on warfarin, and warfarin treatment appears to be provided to patients who are most likely to be compliant with this potentially dangerous medication.
The observed TE event rates in patients with a CHA2DS2-VASc score of 1 or higher were lower than predicted rates for the general population based on CHA2DS2-VASc scores.10 We believe this is because our study population differs in age substantially from the study population in the original CHA2DS2-VASc study. Namely, the mean age of our study population was 56 years versus 66 years in the original study, which is reflective of the younger age of the HIV population versus the population of atrial fibrillation patients in the general population.11 It is also possible that TE events occurring outside the VA were not captured and documented in our database. However, our registry includes both inpatient and outpatient diagnoses and the observed TE event rate in our CHA2DS2-VASc score group of 0 was very similar to the event rates of stroke in the HIV population reported by other large-scale studies.7,8 Therefore, our study strongly suggests that the CHA2DS2-VASc score is not well calibrated for the HIV-infected population.
Our findings challenge how we currently should assess TE risk in atrial fibrillation patients with HIV, as well as whether warfarin is an effective or even safe treatment for TE prevention in this population. Clinicians must decide how to weigh our observational findings with a relatively small number of events versus the clinical trials of stroke prevention that did not include patients with HIV infection. However, our initial findings suggest there is an urgent need for additional studies to confirm our results, to better understand the mechanism of TE events in HIV, and ultimately to develop a more accurate TE risk calculator for the HIV atrial fibrillation population. There are TE risk factors specific to the HIV population that our findings suggest should be part of a TE risk calculator that can be reliably used for the HIV atrial fibrillation population, and further studies will be needed to assess which risk factors should be incorporated and how much weight each of those risk factors should have.
This study had several important limitations. Overall, the number of TE events (including strokes) that were observed in our study was small, which limited precision and power. Also, since this was a cohort of veterans, the vast majority of patients were male. Another limitation was that this study looked only at warfarin use in terms of oral anticoagulation, and did not include novel oral anticoagulants (NOACs), which were not on VA formulary during the study period and were only on the United States market for about year during our study period.43 However, a very small number of the patients who were grouped as warfarin non-users might have been on a NOAC that they received outside the VA system. Warfarin non-users may also have been on aspirin, which may have provided some protective effect against TE events, and aspirin use could not be included in the study because it is typically not obtained from VA pharmacy.44 Also, the VA registry that we used did not have INR values, and so we were unable to assess for warfarin compliance. However, we did model warfarin use in multiple ways to account for these limitations and to our knowledge these are the only analyses to have addressed anticoagulation effectiveness on TE event prevention in this setting.
In conclusion, this is the first study to our knowledge to look at the ability of the CHA2DS2-VASc score to predict TE events in the HIV-infected population with atrial fibrillation. Our results show that the CHA2DS2-VASc score was only weakly associated with risk of TE events among HIV-infected individuals with atrial fibrillation, and warfarin did not appear to be effective at preventing TE events in this population and was potentially harmful. Atrial fibrillation is not only prevalent among HIV patients, but this population is at especially high risk of TE events, and so having an accurate TE risk calculator for HIV patients is clinically imperative. Further investigation is needed to confirm our findings and should include additional prospective studies that include more females and also evaluate NOAC use. Ultimately, identification of factors that are more predictive of TE events among HIV-infected individuals can be used to develop an HIV-specific calculator to guide anticoagulation.
Supplementary Material
Table 3.
Association of warfarin use with thromboembolic events, with and without adjustment for CHA2DS2-VASc score
| Parameter | Level | Unadjusted HR (95%CI) |
p-value | Multivariable Adjusted Marginal Structural Model | |
|---|---|---|---|---|---|
| HR (95%CI) | p-value | ||||
| Baseline warfarin use | User vs. non-user | 1.77 (0.85, 3.70) | 0.13 | 2.06 (0.86, 4.93) | 0.11 |
| Time-updated warfarin use | Yes vs. no | 1.05 (0.45, 2.41) | 0.92 | 1.13 (0.45, 2.87) | 0.80 |
| Current warfarin durationa | Per year of exposure | 2.00 (0.55, 7.24) | 0.29 | 1.27 (0.27, 5.91) | 0.76 |
Variables adjusted for include all factors that were found to be significantly associated with either warfarin use or thromboembolic risk, which were high density lipoprotein, race, heart failure, body mass index, and albumin.
Defined as duration of use among current warfarin users. CI = confidence interval; HR = hazard ratio; vs. = versus.
Acknowledgments
Source of Funding: 2R01AG034853 (source of funding). Dr. Scherzer received an honorarium from Merck in June 2014 for participating in a HIV Renal Expert Input Forum; this honorarium was donated to NCIRE to support kidney research. Dr. Shlipak is on the Scientific Advisory Boards of Tai Diagnostics and Cricket Health.
Footnotes
Meetings Presented At: American Heart Association Scientific Sessions, New Orleans November 2016
Conflicts of Interest:
The remaining authors have no disclosures.
References
- 1.Hall QA H Irene, Tang Tian, Song Ruiguang, Chen Mi, Green Timorthy, Kang Jian. Prevalence of Diagnosed and Undiagnosed HIV Infection — United States, 2008–2012. Morbidity and Mortality Weekly Report. 2015;64(24):657–662. [PMC free article] [PubMed] [Google Scholar]
- 2.HIV/AIDS. [Accessed May 313, 2016]; [Accessed May 13, 2016];2015 Nov; Available at: http://www.who.int/mediacentre/factsheets/fs360/en/ Available at: http://www.who.int/mediacentre/factsheets/fs360/en/
- 3.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. The New England journal of medicine. 2003 Nov 20;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 4.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008 Jul 8;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu JC, Li Y, Marcus GM, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. Journal of the American College of Cardiology. 2013 Jun 4;61(22):2288–2295. doi: 10.1016/j.jacc.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. Journal of the American College of Cardiology. 2012 May 22;59(21):1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.d'Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS (London, England) 2004 Sep 3;18(13):1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 8.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. Journal of acquired immune deficiency syndromes (1999) 2012 Aug 1;60(4):351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS (London, England) 2014 Nov 13;28(17):2573–2577. doi: 10.1097/QAD.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014 Dec 2;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 12.Schambelan M, Wilson PW, Yarasheski KE, et al. Development of appropriate coronary heart disease risk prediction models in HIV-infected patients. Circulation. 2008 Jul 8;118(2):e48–53. doi: 10.1161/CIRCULATIONAHA.107.189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law MG, Friis-Moller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV medicine. 2006 May;7(4):218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 14.Nery MW, Martelli CM, Silveira EA, et al. Cardiovascular risk assessment: a comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. The Scientific World Journal. 2013;2013:969281. doi: 10.1155/2013/969281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanni MV, Fitch KV, Feldpausch M, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS (London, England) 2014 Sep 10;28(14):2061–2070. doi: 10.1097/QAD.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backus L, Mole L, Chang S, Deyton L. The Immunology Case Registry. Journal of clinical epidemiology. 2001 Dec;54(Suppl 1):S12–15. doi: 10.1016/s0895-4356(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010 Feb 9;121(5):651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes care. 2004 May;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 20.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Medical care. 2006 Aug;44(8 Suppl 2):S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. 1999/06/01. [Google Scholar]
- 22.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial. Statistical science. 1999:382–401. [Google Scholar]
- 23.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology (Cambridge, Mass.) 2000 Sep;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 24.El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 25.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. Jama. 2003 Jun 11;289(22):2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 26.Fellay J, Boubaker K, Ledergerber B, et al. Prevalence of adverse events associated with potent antiretroviral treatment: Swiss HIV Cohort Study. Lancet. 2001 Oct 20;358(9290):1322–1327. doi: 10.1016/s0140-6736(01)06413-3. [DOI] [PubMed] [Google Scholar]
- 27.Purnell JQ, Zambon A, Knopp RH, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS (London, England) 2000 Jan 7;14(1):51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 28.Grunfeld C, Kotler DP, Arnett DK, et al. Contribution of metabolic and anthropometric abnormalities to cardiovascular disease risk factors. Circulation. 2008 Jul 8;118(2):e20–28. doi: 10.1161/CIRCULATIONAHA.107.189623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He H, Kang R, Zhao Z. Hepatitis C virus infection and risk of stroke: a systematic review and meta-analysis. PloS one. 2013;8(11):e81305. doi: 10.1371/journal.pone.0081305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PloS one. 2012;7(2):e31527. doi: 10.1371/journal.pone.0031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013 Oct 17;39(4):633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PloS one. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsue PY, Scherzer R, Grunfeld C, et al. HIV infection is associated with decreased thrombin generation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Apr;54(8):1196–1203. doi: 10.1093/cid/cis014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vittecoq D, Escaut L, Chironi G, et al. Coronary heart disease in HIV-infected patients in the highly active antiretroviral treatment era. AIDS (London, England) 2003 Apr;17(Suppl 1):S70–76. doi: 10.1097/00002030-200304001-00010. [DOI] [PubMed] [Google Scholar]
- 35.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. The Journal of infectious diseases. 2010 Jun 15;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. The Lancet. Neurology. 2012 Oct;11(10):878–890. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007 Apr 17;68(16):1257–1261. doi: 10.1212/01.wnl.0000259515.45579.1e. [DOI] [PubMed] [Google Scholar]
- 38.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 39.Liedtke MD, Rathbun RC. Warfarin-antiretroviral interactions. The Annals of pharmacotherapy. 2009 Feb;43(2):322–328. doi: 10.1345/aph.1L497. [DOI] [PubMed] [Google Scholar]
- 40.Liedtke MD, Rathbun RC. Drug interactions with antiretrovirals and warfarin. Expert opinion on drug safety. 2010 Mar;9(2):215–223. doi: 10.1517/14740330903493458. [DOI] [PubMed] [Google Scholar]
- 41.Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008 Nov 11;118(20):2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 42.Anderson AM, Chane T, Patel M, Chen S, Xue W, Easley KA. Warfarin therapy in the HIV medical home model: low rates of therapeutic anticoagulation despite adherence and differences in dosing based on specific antiretrovirals. AIDS patient care and STDs. 2012 Aug;26(8):454–462. doi: 10.1089/apc.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Affairs USDoV. Cost-Effectiveness of Dabigatran and Warfarin for Veterans with AFib: Pilot Study. [Accessed April, 2017]; https://www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141703253.
- 44.Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015 May 12;84(19):1933–1940. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


