Abstract
Neurotrophic factors, a family of secreted proteins that support the growth, survival and differentiation of neurons, have been intensively studied for decades due to the powerful and diverse effects on neuronal physiology, as well as their therapeutic potential. Such efforts have led to detailed understanding on molecular mechanisms of neurotrophic factor signaling. One member, brain-derived neurotrophic factor (BDNF) has drawn much attention due to its pleiotropic role in the central nervous system (CNS) and implications in various brain disorders. In addition, recent advances linking the rapid-acting antidepressant, ketamine to BDNF translation and BDNF-dependent signaling, has re-emphasized the importance of understanding the precise details of BDNF biology at the synapse. While substantial knowledge related to the genetic, epigenetic, cell biological, and biochemical aspects of BDNF biology has now been established, certain aspects related to the precise localization and release of BDNF at the synapse have remained obscure. A recent series of genetic and cell biological studies have shed light on the question -- the site of BDNF release at the synapse. In this Perspectives article, these new insights will be placed in the context of previously unresolved issues related to BDNF biology, as well as how BDNF may function as a downstream mediator of newer pharmacological agents currently under investigation for treating psychiatric disorders.
Introduction
Over 25 years ago, Hans Thoenen, one of the scientists who, along with Yves Barde, discovered brain-derived neurotrophic factor (BDNF), concluded a review article with a set of future questions that needed to be addressed to fully understand the biological function of BDNF. He noted that a key issue was to identify the precise location of BDNF secretion at the level of the synapse - whether BDNF “was released by dendrites and/or axon terminals only. These problems, although extremely challenging experimentally, must be addressed in order to test current speculations in the involvement of neurotrophic molecules in synaptic plasticity and processes related to memory”.1 While significant progress has been made in the subsequent decades on many aspects of BDNF-dependent molecular signaling pathways and biological functions, especially with relevance to neuropsychiatric disorders, this basic question about BDNF has not been fully clarified.
A remarkable series of studies over the past few years have shed light on this question -- the site of BDNF release at the synapse, which has now been established to comprise neuronal, astrocytic and microglial components.2 In this Perspectives article, these recent genetic and cell biological studies3–6 will be placed in the context of what had been established about BDNF localization, and how these new insights can inform previously unresolved issues related to BDNF biology, as well as how BDNF may function as a downstream mediator of newer pharmacological agents currently under investigation for treating psychiatric disorders.
BDNF is a member of a unique family of polypeptide growth factors, neurotrophins, which influence the proliferation, differentiation, survival and death of neuronal and non-neuronal cells. 7, 8 While the biological roles for neurotrophins were initially characterized during development of the nervous system, it is now clear that BDNF, in particular, has multiple roles in the adult nervous system, such as regulating synaptic connections, synapse structure, neurotransmitter release, and synaptic plasticity. BDNF is secreted into the synapse leading to the activation of tropomyosin receptor kinase B (TrkB) and its downstream signaling cascades that contribute to gene transcriptional events critical for synaptic plasticity and cognitive function.
It has been established that BDNF is initially synthesized in the endoplasmic reticulum as a precursor protein, a pre-proBDNF, which, in neurons is then selectively sorted to the regulated secretory pathway and ultimately packaged into dense core vesicles (DCV).9 Trafficking of BDNF into the biosynthetic pathway is a complex, highly regulated process, the precise mechanisms of which remain unclear. However, two sorting molecules have been identified as required for optimal sorting of newly synthesized BDNF to the regulatory secretory pathway - a VPS10 domain protein, sortilin, which has been demonstrated to interact with the BDNF prodomain, and carboxypepitdase E (CPE), which interacts with the BDNF mature domain.10, 11 At the presynaptic terminal, synaptotagmin-IV12, and SNAREs, Syb2, SNAP25 and SNAP4713 have all be demonstrated to regulate the activity-dependent vesicular release of BDNF. Cleavage and processing of pre-proBDNF can occur at multiple sites including the Golgi apparatus by furin, the DCV by proconvertases, and extracellularly by plasmin generated by TPA or selective matrix metalloproteinases including MMP3 and MMP7.14
Thus, it has been traditionally assumed that BDNF synthesis and secretion occur from presynaptic neuronal sites. In vitro evidence for presynaptic storage and release had been shown in cultured neurons through co-localization of ectopically expressed and endogenous BDNF with vesicular markers such as synaptotagmin and secretogranin II.10, 12, 15 However, localization of endogenous BDNF protein in the intact brain has been difficult to demonstrate due to the low basal levels of BDNF, the variability in the available peptide-based polyclonal BDNF antibodies, as well as lack of age-matched genetic knock-out (KO) controls (as the global BDNF KO mice die in early postnatal life) to validate the immunoreactivity. In vivo genetic evidence has supported the role of presynaptic BDNF when it was first demonstrated that conditional BDNF knock-out mice lacking cortical BDNF (Emx-BDNFKO) displayed preferential neuronal loss in the striatum, a region that expresses minimal BDNF and had been assumed to receive neurotrophic support via anterograde delivery of BDNF from regions including the cortex.16
Presynaptic BDNF
A recent landmark study by Yves Barde utilized a series of newly developed reagents including a monoclonal BDNF antibody, an epitope-tagged BDNF knock-in mouse line (BDNF-Myc), as well as a conditional BDNF KO mouse line (cBDNF-ko) to identify its subcellular localization in the mature mouse brain.3 They focused on the hippocampal granule cell mossy fiber projections to the CA3 – one of the brain regions with the highest BDNF levels, as well as the CA3 Schaffer collaterals to the CA1. They determined using both immnuohistochemical techniques and immuno-electron microscopy (EM) that BDNF was almost exclusively localized to presynaptic DCV. Strikingly, almost no BDNF immunoreactivity was observed in post-synaptic compartments. Interestingly, the DCV subtype that BDNF was stored in was distinct from those that contained other neuropeptides such as cholecystokinin and Met-enkephalin, suggestive of selective segregation of BDNF into a subpopulation of secretory vesicles. In addition, using separate antibodies for the prodomain and mature domains, they determined that mature BDNF and the cleaved BDNF prodomain co-localized in these DCV. They also reported co-secretion of the mature BDNF along with the cleaved prodomain, which has subsequently been confirmed by others.17
These findings are significant as they provide rigorous evidence supporting the notion that newly synthesized BDNF is localized to the presynaptic compartment. These findings also support the numerous electrophysiological studies implicating the requirement of presynaptic BDNF release in synaptic plasticity events, especially in hippocampal circuits. Of note, endogenous BDNF has been implicated in enhancing calcium transients in CA3 dendrites.18 In addition, it has also been shown that BDNF is necessary for LTP induction at hippocampal CA3-CA1 synapses.19, 20 In particular, region-specific BDNF KO mice also specifically implicated a requirement for presynaptic BDNF in LTP induction at the CA3-CA1 synapse.21
BDNF from other Synaptic Sites
However, recently, accumulating evidence has suggested that BDNF is capable of being synthesized and secreted from other components of the synapse including astrocytes 22, microglia4, and post-synaptic dendrites9 (Figure 1). One of the key lines of support for additional sites of BDNF localization was subsequent immune-EM studies analyzing another epitope-tagged BDNF knock-in mouse line (BDNF-HA) that demonstrated endogenous BDNF localization presynaptically (axons, terminals) and postsynaptically (dendrites, spines) in vesicular compartments in the mature hippocampus.5 One interpretation of these divergent results may be due to the higher sensitivity of the anti-HA antibody, as compared to the monoclonal BDNF antibody, allowing for detection of the lower levels of BDNF in the other compartments. In this context, while the predominant mode of BDNF secretion is from presynaptic sites, local effects at individual synapses may be modulated by lower levels of BDNF secreted from alternative sites.
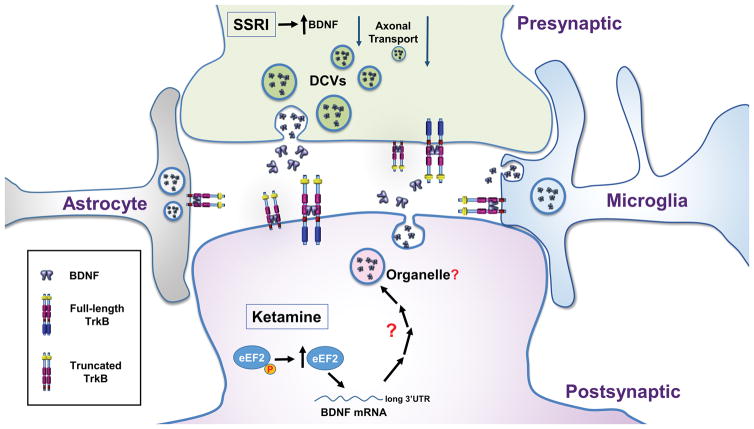
Figure 1. BDNF and its receptors at the mature synapse.
Schematic of synapse showing localization of BDNF and its receptors in presynaptic, postsynaptic neuronal compartments, as well as from astrocytes and microglia. BDNF is stored in dense core vesicles (DCV) in the presynaptic compartment. The storage organelle for BDNF in the other synaptic components has not been identified. Conventional antidepressants (such as SSRI) elevate presynaptic BDNF, while ketamine has been postulated to enhance postsynaptic BDNF protein translation. While neurons express full-length TrkB as well as truncated TrkB with developmental and pathologically changing levels, non-neuronal cells mainly exhibit expression of truncated TrkB.76–78
It is now well accepted that glial cells are active components contributing to multiple aspects of synapse physiology, including clearance of extracellular ions and neurotransmitters, initial synapse formation and maintenance.2 In response to neuronal activity, microglia, a population of brain resident macrophage, are activated and release so-called “gliotransmitters” to modulate neuronal communication. It had been previously been shown that BDNF is synthesized and secreted from microglia23–25, and involved in pain modulation26. A recent study by Wenbiao Gan demonstrated the direct contribution of BDNF secreted from microglia on synaptic remodeling associated with another function - learning and memory.4 The study showed that depletion of microglia leads to deficits in behavioral performance in multiple learning paradigms ranging from motor skill learning to fear conditioning and novel object recognition, as well as learning-induced remodeling of excitatory synapses. Using an epitope-tagged BDNF knock-in mouse line (BDNF-HA), they were able to detect endogenous BDNF in the microglia. Intriguingly, loss of microglial BDNF recapitulates the defects observed in microglia-depleted mice, suggesting that BDNF from non-neuronal sources regulates important functions related to learning and memory at the synapse.
With regards to BDNF synthesis and secretion from postsynaptic sites, there exists a long-standing series of studies suggesting its localization to this component of the synapse. For example, a number of in vitro studies in cultured neurons have demonstrated localization of ectopically expressed BDNF protein in dendrites.12, 13, 27–29 In vitro studies that overexpressed fluorescence-tagged BDNF constructs have also demonstrated activity-dependent BDNF secretion from dendrites utilizing similar regulators of vesicular secretion as used for presynaptic BDNF secretion (synaptotagmin-IV, Syb2, SNAP25 and SNAP47)12, 13, as well as from post-synaptic endocytic compartments that were regulated by synaptotagmin-6 and complexin30. In addition, in situ hybridization (ISH) studies have reliably detected endogenous Bdnf mRNA in neuronal dendrites in both cultured neurons and the intact brain; this expression increases following induction of neuronal activity.31, 32 The Bdnf gene has nine unique promoters that drive transcription of at least twenty different Bdnf transcripts that encode an identical BDNF protein.33–36 Each variant consists of a 5′ untranslated region (UTR) exon that is alternatively spliced to a downstream common coding exon. The existence of multiple Bdnf splice variants has led to the spatial code hypothesis, which posits that differential expression of Bdnf transcripts enables local spatial, temporal, and stimulus-specific BDNF production.37 Indeed, Bdnf mRNA variants show activity-dependent targeting to dendrites, especially in the hippocampus.32, 34, 38, 39 Upon activation of both cortical and hippocampal neurons, exon 1 and 4-containing Bdnf transcripts are localized to the cell body and proximal dendrites, while exon 2 and 6-containing Bdnf transcripts are found in distal dendrites.40–42 Supporting these findings, silencing exon 1 and 4-containing Bdnf transcripts in cultured hippocampal neurons reduces proximal dendrite number, while silencing exon 2 and 6-containing Bdnf transcripts alters distal dendrite morphology.40 Recent studies confirmed that loss of exon 2 and 6-containing Bdnf splice variants impacts dendritic structure and spine morphology in CA1 and CA3 pyramidal cells of the intact hippocampus.43 In addition to differential localization by the 5′UTR, Bdnf transcripts with short versus long 3′ untranslated regions (3′ UTRs) exhibit distinct subcellular localization to dendrites as well as cell bodies.44 Alternative polyadenylation of Bdnf transcripts also differentially impacts spine morphology and synaptic plasticity in CA1 hippocampal neurons.44 Existence of Bdnf transcripts in dendrites suggests local production of BDNF protein, and several lines of evidence support the existence of BDNF protein as well as a functional role for it in the dendritic compartment. Indeed, disruption of dendritic localization of Bdnf transcripts leads to deficiencies in dendritic spine morphology and long-term potentiation.44 In addition, EM studies in intact brain localized endogenous BDNF to post-synaptic compartments5, 45, although a caveat to the earlier EM studies was that appropriate age matched BDNF KO controls could not validate the specificity of the immunoreactivity. Finally, a series of electrophysiological studies in dissociated neuronal cultures, as well as hippocampal slices have implicated that postsynaptic BDNF release is required for certain synaptic events including enhancement of presynaptic glutamate release, as well as homeostatic synaptic plasticity.46–48
In this context, a recent study by James McNamara’s and Ryohei Yasuda’s laboratories elegantly demonstrated a role for newly synthesized BDNF in postsynaptic dendritic spines in a form of neuronal plasticity termed structural long-term potentiation (sLTP). While previously BDNF had been implicated in this form of structural plasticity involving rapid enlargement of dendritic spines, the source of BDNF was not specified.49 Utilizing a series of live cell-imaging techniques, as well as a series of BDNF and TrkB receptor knock-in and knock-out mice, they were able to demonstrate the role of postsynaptically synthesized BDNF in eliciting autocrine BDNF/TrkB signaling in individual dendritic spines. They utilized two-photon fluorescence lifetime imaging combined with fluorescence resonance energy transfer (FRET)-based sensor for TrkB receptors to assess BDNF-dependent receptor activation after sLTP induction by single spine glutamate–uncaging.5 They detected, rapid onset of TrkB receptor activation, as well as BDNF release from spines utilizing BDNF that was fused to pH-sensitive fluorophore (superecliptic pHluorin; SEP). The study also directly demonstrated by immune-EM the existence of endogenous BDNF protein in an epitope-tagged BDNF knock-in mouse line (BDNF-HA) in the intact mature hippocampus. The HA-immunoreactivity was found both presynaptically (axons, terminals) and postsynaptically (dendrites, spines) in vesicular compartments. In addition, selectively knocking out BDNF in post-synaptic CA1 neurons, led to deficits in sLTP, which could be rescued by addition of BDNF.
These findings do come with caveats, such as that ectopic overexpression of pHluorin-fused BDNF has the limitation of possibly not representing endogenous conditions. Also, the subcellular localization findings are different than those reported from the previous BDNF EM study3, which did not detect BDNF immunoreactivity in dendrites or spines. As noted above, localization of endogenous BDNF to both pre and postsynaptic compartments may be due to differences in sensitivity of the antibodies utilized (anti-HA vs monoclonal anti-BDNF). Even with these caveats, the latter study is noteworthy as it demonstrates the contribution of postsynaptic BDNF on structural and functional plasticity. It is intriguing to speculate that postsynaptically localized Bdnf transcripts would produce BDNF protein that would be packaged into vesicles for release from dendrites that were separate in class from presynaptic DCV’s containing BDNF. In the hippocampus, it has been demonstrated that other neuropeptides, such as dynorphin can be secreted from postsynaptic sites in a manner distinct from presynaptic dynorphin secretion.50, 51 However, in neuronal dendrites there is sparse distribution of DCV’s, suggesting that there may also be specialized structures for postsynaptic BDNF protein production, packaging, and release. Finally, another study from this same group demonstrated that the postsynaptically released BDNF from one dendritic spine is capable of priming neighboring dendritic spines for subsequent structural plasticity – a finding that suggests that postsynaptically released BDNF can have a different impact than presynaptically released BDNF on synaptic plasticity within a set of synapses.6
Clinical Implications
BDNF signaling has been linked to the etiology of depression and implicated in the action of traditional antidepressant therapies (including SSRIs, TCAs, and ECT). Importantly, several recent reports have established that BDNF plays a critical role in contributing to the synaptic plasticity mechanisms underlying the fast-acting antidepressant effects of ketamine and other NMDA receptor antagonists.52–54 Decreases in BDNF expression have been identified in a number of brain regions, including the prefrontal cortex and hippocampus, of individuals with mood disorders.55–59 Moreover, the expression of BDNF is increased in depressed patients after administration of antidepressants.57, 60 Deletion of the Bdnf gene in a number of mouse models attenuates behavioral responses to antidepressants and infusion of exogenous BDNF into the ventricles or the hippocampus has antidepressant effects.61
The recent studies discussed above highlight the possibility that BDNF biosynthesis and secretion occurs at different synaptic sites thereby allowing for multiple levels of regulation by a single growth factor. In this context, the precise subcellular localization of BDNF has significant implications for multiple classes of antidepressant treatments in which elevating BDNF levels is considered a main downstream mechanism of action.62–67 As such, these recent studies open up new questions about which pools of BDNF, and from which cell types, are being affected by different antidepressants treatments. For example, postsynaptic BDNF biosynthesis may be particularly noteworthy in the quest to elucidate the mechanism of action of the glutamatergic receptor modulator, ketamine, an NMDA receptor antagonist, which has been demonstrated to lead to rapid and sustained antidepressant responses. By blocking post-synaptic NMDA receptors, it has been shown that ketamine deactivates a kinase (eEF2 kinase), which alleviates a block on BDNF translation, resulting in rapid (within 30 minutes) BDNF protein synthesis.52 Of note, a recent preclinical study in mice showed that metabolites of ketamine have rapid antidepressant effects that are independent of the NMDA receptor. Specifically, they report that the (2R,6R)-hydroxynorketamine metabolite has antidepressant effects that do not require NMDARs, but do involve alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionic (AMPA) receptor activation as well as rapid upregulation of BDNF expression.68 However, whether the antidepressant activity of (2R,6R)-hydroxynorketamine requires BDNF has not yet been tested. Moreover, the challenge to the view that the primary mechanism by which ketamine mediates its antidepressive qualities is via NMDAR antagonism, has fueled substantial debate in the field.69–71 Definitive answers to these important questions, including the extent to which BDNF is required for the behavioral effects of ketamine and its metabolites, will require further investigations.
Since it was traditionally assumed that ketamine acts on postsynaptic NMDA receptors, these recent BDNF studies discussed here suggest that pharmacological agents used to alleviate symptoms of depression, including ketamine, could also be selectively enhancing the rapid synthesis of a postsynaptic pool of BDNF. It is tempting to speculate that the rapid, yet sustained, effects of glutamatergic modulators, including ketamine, may differ from conventional antidepressants in that the pool (or pools) of BDNF that are being upregulated may lead to enhancement of BDNF-TrkB signaling at separate synaptic sites. Expression of dendritic BDNF may be particularly important in the context of mechanisms underlying rapid acting antidepressants because it has been demonstrated that BDNF application to isolated dendrites results in increased local translation in dendrites, a process which is critical for spine remodeling and synthesis, synaptogenesis and induction of synaptic plasticity.72 Thus, future antidepressant therapeutic development efforts may involve strategies to selectively elevate these separate pools of BDNF at the synapse (neuronal, astrocytic, microglia) that may lead to distinct functional outcomes. Given that individual Bdnf transcripts may differentially contribute to separate pools of BDNF at alternate synaptic sites, the unique Bdnf promoters as well as the 5′ and 3′ UTR sequences themselves are potentially promising candidates.
Conclusion
These recent series of studies highlight how BDNF biosynthesis and secretion can occur at different synaptic sites thereby allowing for multiple levels of regulation by a single growth factor. In particular, the studies highlight one theme in which even though the majority of BDNF that can be detected using current methodologies is stored and secreted from presynaptic sites, microglia, astrocytes and neuronal dendritic spines can produce and secret BDNF at lower levels that can have qualitatively significant functional outcomes. Future studies undoubtedly will address the main questions in determining the precise mechanism on biosynthesis and release of BDNF, and relative impact of BDNF from these alternate synaptic sites in terms of in vivo consequences. Equally important will be a renewed focus on the downstream effects of secreted BDNF interacting with not only the selective populations of TrkB receptors, which are localized on the various constituents of the synapse (Figure 1), but also the array of other BDNF receptors and co-receptors (truncated TrkB, p75, SorCS2, and Slitrk5), which are also present at the synapse.17, 73–75 As example, as noted above, it has been shown that postsynaptically released BDNF from one dendritic spine can interact with postsynaptic TrkB receptors in neighboring dendritic spines to mediate heterosynaptic plasticity.6 Thus, understanding the basic cell biology of BDNF and its receptors at the synapse will continue to provide insights into the myriad of functions of this neurotrophin in both normal and pathological conditions.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 2.Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18(11):1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196(6):775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538(7623):99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature. 2016;538(7623):104–108. doi: 10.1038/nature19784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews Neuroscience. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 8.Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001;11(3):281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 9.Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neuroscience research. 2009;65(1):11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(26):6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45(2):245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12(6):767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando R, 3rd, Polleux F, et al. SNAREs Controlling Vesicular Release of BDNF and Development of Callosal Axons. Cell reports. 2015;11(7):1054–1066. doi: 10.1016/j.celrep.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hempstead BL. Deciphering proneurotrophin actions. Handb Exp Pharmacol. 2014;220:17–32. doi: 10.1007/978-3-642-45106-5_2. [DOI] [PubMed] [Google Scholar]
- 15.Berg EA, Johnson RJ, Leeman SE, Boyd N, Kimerer L, Fine RE. Isolation and characterization of substance P-containing dense core vesicles from rabbit optic nerve and termini. J Neurosci Res. 2000;62(6):830–839. doi: 10.1002/1097-4547(20001215)62:6<830::AID-JNR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(17):4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. 2013;4:2490. doi: 10.1038/ncomms3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang SB, Stein V, Bonhoeffer T, Lohmann C. Endogenous brain-derived neurotrophic factor triggers fast calcium transients at synapses in developing dendrites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(5):1097–1105. doi: 10.1523/JNEUROSCI.3590-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 21.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39(6):975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 22.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63(1–2):83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Goncalves N, et al. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(11):3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miwa T, Furukawa S, Nakajima K, Furukawa Y, Kohsaka S. Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. J Neurosci Res. 1997;50(6):1023–1029. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1023::AID-JNR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 27.Adachi N, Kohara K, Tsumoto T. Difference in trafficking of brain-derived neurotrophic factor between axons and dendrites of cortical neurons, revealed by live-cell imaging. BMC Neurosci. 2005;6:42. doi: 10.1186/1471-2202-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, et al. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(45):14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brigadski T, Hartmann M, Lessmann V. Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(33):7601–7614. doi: 10.1523/JNEUROSCI.1776-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong YH, Lee CM, Xie W, Cui B, Poo MM. Activity-dependent BDNF release via endocytic pathways is regulated by synaptotagmin-6 and complexin. Proc Natl Acad Sci U S A. 2015;112(32):E4475–4484. doi: 10.1073/pnas.1511830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baj G, Del Turco D, Schlaudraff J, Torelli L, Deller T, Tongiorgi E. Regulation of the spatial code for BDNF mRNA isoforms in the rat hippocampus following pilocarpine-treatment: a systematic analysis using laser microdissection and quantitative real-time PCR. Hippocampus. 2013;23(5):413–423. doi: 10.1002/hipo.22100. [DOI] [PubMed] [Google Scholar]
- 32.Tongiorgi E, Righi M, Cattaneo A. Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(24):9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10(3):475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 35.Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain research. 2006;1067(1):1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90(3):397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tongiorgi E. Activity-dependent expression of brain-derived neurotrophic factor in dendrites: facts and open questions. Neuroscience research. 2008;61(4):335–346. doi: 10.1016/j.neures.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Tongiorgi E, Armellin M, Giulianini PG, Bregola G, Zucchini S, Paradiso B, et al. Brain-derived neurotrophic factor mRNA and protein are targeted to discrete dendritic laminas by events that trigger epileptogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(30):6842–6852. doi: 10.1523/JNEUROSCI.5471-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sathanoori M, Dias BG, Nair AR, Banerjee SB, Tole S, Vaidya VA. Differential regulation of multiple brain-derived neurotrophic factor transcripts in the postnatal and adult rat hippocampus during development, and in response to kainate administration. Brain research Molecular brain research. 2004;130(1–2):170–177. doi: 10.1016/j.molbrainres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Baj G, Leone E, Chao MV, Tongiorgi E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci U S A. 2011;108(40):16813–16818. doi: 10.1073/pnas.1014168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Molecular and cellular neurosciences. 2005;28(3):556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Chiaruttini C, Sonego M, Baj G, Simonato M, Tongiorgi E. BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Molecular and cellular neurosciences. 2008;37(1):11–19. doi: 10.1016/j.mcn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Maynard KR, Hobbs JW, Sukumar M, Kardian AS, Jimenez DV, Schloesser RJ, et al. Bdnf mRNA splice variants differentially impact CA1 and CA3 dendrite complexity and spine morphology in the hippocampus. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134(1):175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, et al. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59(3):454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 46.Gubellini P, Ben-Ari Y, Gaiarsa JL. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(24):5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(52):13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, et al. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68(6):1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319(5870):1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, et al. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14(6):3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14(6):1265–1272. doi: 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 52.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunham JS, Deakin JF, Miyajima F, Payton A, Toro CT. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. Journal of psychiatric research. 2009;43(14):1175–1184. doi: 10.1016/j.jpsychires.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. The American journal of psychiatry. 2012;169(11):1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4):260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 58.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of general psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 59.Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatric disease and treatment. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23(34):10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain research. 2003;974(1–2):228–235. doi: 10.1016/s0006-8993(03)02584-8. [DOI] [PubMed] [Google Scholar]
- 67.Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28(1):53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 68.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. (R)-Ketamine Shows Greater Potency and Longer Lasting Antidepressant Effects Than Its Metabolite (2R,6R)-Hydroxynorketamine. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 70.Abdallah CG. What’s the Buzz About Hydroxynorketamine? Is It the History, the Story, the Debate, or the Promise? Biol Psychiatry. 2017;81(8):e61–e63. doi: 10.1016/j.biopsych.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collingridge GL, Lee Y, Bortolotto ZA, Kang H, Lodge D. Antidepressant Actions of Ketamine Versus Hydroxynorketamine. Biol Psychiatry. 2017;81(8):e65–e67. doi: 10.1016/j.biopsych.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 72.Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(44):9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dorsey SG, Renn CL, Carim-Todd L, Barrick CA, Bambrick L, Krueger BK, et al. In vivo restoration of physiological levels of truncated TrkB.T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron. 2006;51(1):21–28. doi: 10.1016/j.neuron.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 74.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8(8):1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 75.Song M, Giza J, Proenca CC, Jing D, Elliott M, Dincheva I, et al. Slitrk5 Mediates BDNF-Dependent TrkB Receptor Trafficking and Signaling. Dev Cell. 2015;33(6):690–702. doi: 10.1016/j.devcel.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biffo S, Offenhauser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121(8):2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 77.Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61(4):647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- 78.Gomes RA, Hampton C, El-Sabeawy F, Sabo SL, McAllister AK. The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(44):11487–11500. doi: 10.1523/JNEUROSCI.2364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]