Abstract
Aims
Small studies using ultrasensitive C-peptide assays suggest endogenous insulin secretion is frequently detectable in patients with long standing type 1 diabetes (T1D) but these studies do not use representative samples. We aimed to use stimulated Urine C-peptide Creatinine Ratio (UCPCR) to assess C-peptide in a large cross-sectional, population-based study of patients with T1D.
Methods
We recruited 924 patients from primary and secondary care in 2 UK centres with a clinical diagnosis of T1D, diagnosed under 30 years, and diabetes duration >5 years. Median(IQR) age of diagnosis 11(6-17)y, duration 18(11-26)y. All provided a home post-meal UCPCR, which was measured using a Roche electrochemiluminescence assay.
Results
80% (740/924) had detectable endogenous C-peptide (UCPCR >0.001 nmol/mmol). Most, 52% (483/924), had very low historically undetectable levels (UCPCR 0.0013-0.03 nmol/mmol). 8% (70/924) had UCPCR≥0.2 nmol/mmol, equivalent to serum levels associated with reduced complications and hypoglycaemia. Absolute UCPCR levels fell with duration. Age of diagnosis and duration were independent predictors of C-peptide in multivariate modelling.
Conclusions
This population based study shows the majority of long duration T1D patients have detectable urine C-peptide. While the majority are insulin microsecretors, some maintain clinically relevant endogenous insulin secretion for many years after diagnosis of diabetes. Understanding this may lead to a better understanding of pathogenesis in T1D and open new possibilities for treatment.
Background
Recent studies have challenged the traditional view of type 1 diabetes leading to absolute insulin deficiency. Sensitive C-peptide assays have shown that 43-74% people with longstanding (> 5 years) type 1 diabetes (T1D) are microsecretors of endogenous insulin(1; 2) with C-peptide levels in a range not detected by previous assays (1-30 pmol/L). Importantly, we showed that these low levels increased during a mixed meal suggesting that there are a very small number of functional beta-cells(1). The implications of these studies could be important, as if beta cells remain in most people with T1D, then they are either regenerating or evading immune attack. Either of these possibilities might open up new avenues of treatment in T1D.
The studies to date have not been able to give an accurate estimate of the prevalence of very low level C-peptide secretion in long duration T1D. There have only been two small studies using sufficiently sensitive C-peptide assays from clinic based populations (n=74(1) and 182 (144 > 5years duration)(2)). The 382 Joslin Medallists studied by Keenan and colleagues were defined on the basis of their long term survival (>50 years): the high prevalence of retained C-peptide (67% > 30 pmol/L) is likely to reflect survival bias because complications risk is reduced in patients with maintained endogenous C-peptide(3). There are no large community based studies examining low-level insulin production in T1D.
Measurement of urine post meal C-peptide creatinine ratio (UCPCR) is an alternative to serum C-peptide testing(4–7). UCPCR involves a single, spot urine measurement and has the advantage of long term stability (3 days) at room temperature which facilitates large scale community studies as samples can be posted. We have shown that a home UCPCR correlates with 90 minute serum C-peptide in the mixed meal tolerance test (MMTT)(6; 7). UCPCR and serum C-peptide identified similar long duration patients with T1D as having detectable C-peptide in a MMTT(1).
We aimed to assess the prevalence of detectable endogenous C-peptide using urine C-peptide creatinine ratio in a large non-selected, population based, study of T1D and assess its clinical associations.
Methods
Study Participants
We recruited 924 patients, with T1D for 5 or more years, from primary and secondary care in the catchment area of two UK hospitals in Tayside (Ninewells hospital Dundee, UK, n=474) and Devon (Royal Devon and Exeter Hospital, Exeter, Devon, UK, n=450). These patients were recruited as part of the UNITED (Using pharmacogeNetics to Improve Treatment in Early onset Diabetes) study. All patients were diagnosed with diabetes before 30 years and were aged under 50 years at recruitment. Type 1 patients were included on the basis of a clinical diagnosis of T1D, diagnosis under 30 years, and being treated with insulin since diagnosis. To exclude monogenic diabetes, patients with a UCPCR >0.2 nmol/mmol(8) who did not have GAD or IA2 antibodies, were tested for monogenic diabetes as previously described(9). To avoid inadvertent inclusion of patients with young-onset type 2 diabetes, patients with a UCPCR >0.2 nmol/mmol who were GAD and IA2 autoantibodies negative were excluded if their BMI was greater than 30 kgm-2. 97% of participants were white European. Over 60% of eligible participants were recruited.
Informed consent was obtained from all participants and the study was approved by the National Research Ethics Service Committee South West and the East of Scotland Research Ethics Committee (references 10/H0106/63 and NRS10/DI33). Clinical and demographic data were collected at the time of consent.
C-peptide assessment
We assessed C-peptide using a home post-meal urine C-peptide creatinine ratio (UCPCR). Participants voided their bladder before their largest (highest carbohydrate content) meal of the day, and collected a urine sample 2 hours after the meal in a sample pot containing boric acid preservative. As in previous validation studies(6,7) the content of the meal was not specified and the patients took their normal basal and prandial insulin(10). Patients returned the sample to the laboratory within 36 hours usually by post. Samples were analysed within 36 hours (on the same day or subsequent day). C-peptide analysis was performed using the Roche electrochemiluminescence assay on the Roche E170 analyser (Roche, Mannheim, Germany) in the Royal Devon and Exeter Hospital Blood Sciences Laboratory as previously described(4).
C-peptide thresholds
We considered a UCPCR >0.001 nmol/mmol to have analytically detectable UCPCR, this reflected being able to detect a urine C-peptide concentration of >3.3 pmol/L in the 10x diluted urine(4). In addition, we analysed two other thresholds: UCPCR≥0.03 nmol/mmol and ≥0.2nmol/mmol, which are equivalent to serum C peptide of 30 pmol/L (a common historical limit of detection(3; 11; 12)) and 200 pmol/L (a clinically defined level associated with reduced microvascular complications and hypoglycaemia(13)). The UCPCR equivalent cut offs were derived using linear regression calculated UCPCR values from previous studies comparing UCPCR and serum C-peptide measurements(6; 7; 14).
Statistical Analysis
We tested the independence of effects of age of diagnosis and duration on UCPCR with a logistic regression model using either analytically detectable UCPCR or UCPCR≥0.2 nmol/mol as the outcome variable. Age of diagnosis and duration were treated as continuous predictor variables. The model fit was assessed using a Hosmer-Lemeshow goodness-of-fit test. We assessed the impact of age of diagnosis and diabetes duration on retained endogenous C-peptide production by comparing proportions of detectable and undetectable UCPCR across duration and age of diagnosis quintiles. We used a Kruskall-Wallis test and a non-parametric trend test as age of diagnosis and duration data were non-normally distributed.
Differences in HbA1c, BMI and insulin dose between groups defined by UCPCR C-peptide values were assessed using a t-test. A linear regression was used to test the independence of UCPCR to predict insulin dose allowing for BMI, age of diagnosis, duration and HbA1c. For all pediatric patients we calculated a BMI z score relative to the 1990 UK reference population(15). We then calculated a BMI adjusted to age 22 for all pediatric patients, and this was included in reported values of BMI, and used for any analysis involving BMI.
All statistical analysis was performed using STATA version 12.1 (StataCorp, Texas, USA). All confidence intervals reported are 95% confidence intervals.
Results
The clinical characteristics of patients recruited are given in Table 1.
Table 1.
Clinical characteristics of cohort. * BMI results for pediatric patients adjusted to equivalent BMI for age 22. CSII = continuous subcutaneous insulin infusion.
| Median (IQR) unless otherwise specified |
|
|---|---|
| Total number | n=924 |
| Gender | 492 male (53%) |
| Age diagnosis (years) | 11 (6-17) |
| Duration Diabetes (years) | 18.6 (11.2-26.7) |
| BMI (kgm-2) * | 24.8 (23.1-27.6) |
| HbA1c (%) | 8.7 (7.9-9.8) |
| HbA1c (mmol/mol) | 72 (63-84) |
| Insulin dose (units/Kg/24 hours) | 0.78 (0.60-0.97) |
| CSII use (%) | 13 |
| Post meal UCPCR (nmol/mmol) | 0.012 (0.004-0.036) |
Prevalence of detectable C-peptide
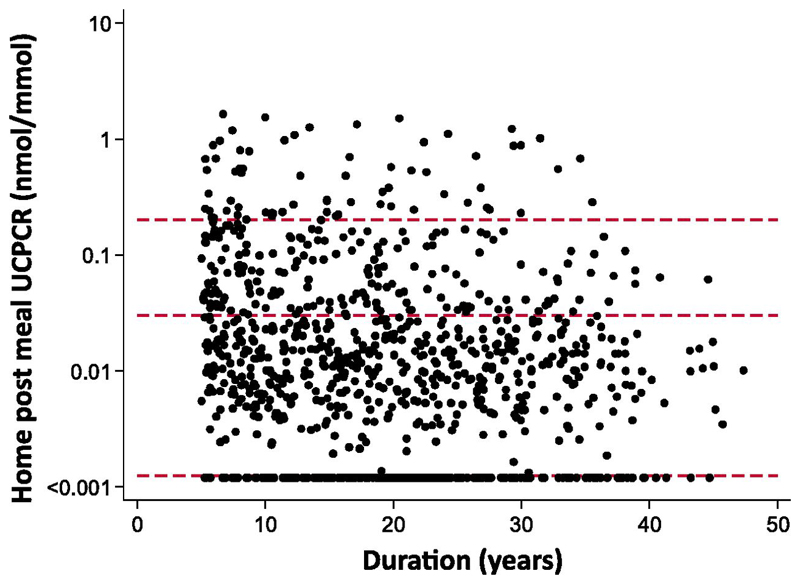
80% (740/924, CI 77-83%) of participants had detectable C-peptide (UCPCR >0.001 nmol/mmol). The majority of patients 52% (483/924, CI 49-55%) had a UCPCR between 0.001 and 0.03 nmol/mmol, (Figure 1). 20% (187/924, CI 18-23%) had a UCPCR between 0.03 nmol/mmol and 0.2 nmol/mmol and 8% (70/924, CI 6-9%), had a UCPCR above 0.2 nmol/mmol.
Figure 1.
Scatterplot of UCPCR against duration. Red dashed reference lines at UCPCR=0.2 nmol/mmol - equivalent to stimulated serum C-peptide of 200 pmol/L, and UCPCR=0.03 - equivalent to serum values of 30 pmol/L, the lower limit of many historical assays, and UCPCR=0.001 nmol/mmol – effective lower limit of detection of this assay). UCPCR values are plotted on a log scale to allow separation of the range of low levels found.
Associations of Detectable C-peptide
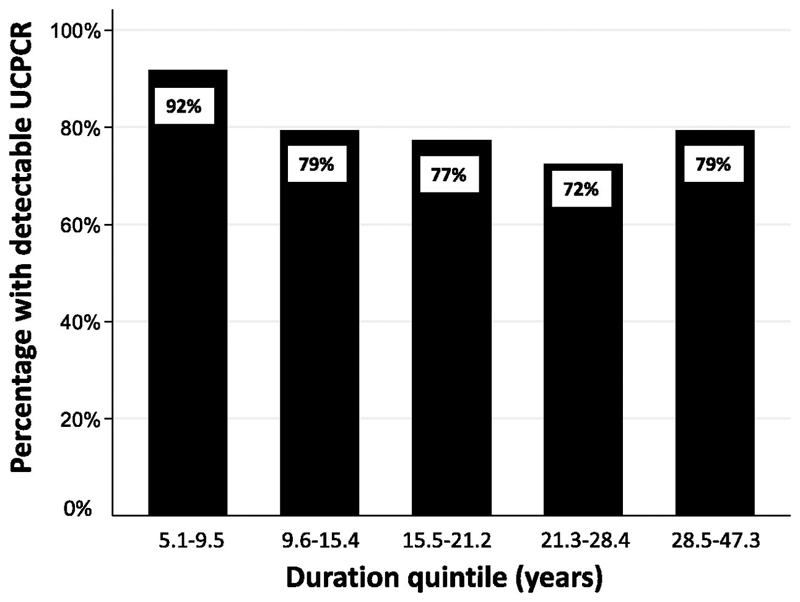
The presence of detectable C-peptide was inversely associated with shorter duration of diabetes but was unrelated to age of diagnosis or BMI. Patients with detectable UCPCR (>0.001 nmol/mmol) had a shorter diabetes duration than those without (17.8 years v 20.9 years, p=0.0003, Table 2). The percentage of patients with detectable UCPCR within each quintile of duration of T1D is given in Figure 2. There was a trend for decreasing prevalence of detectable C peptide with duration quintile (p<0.0001). The apparent increase between the fourth (72% (CI 66-78%)), and fifth decide(79% (CI 73-85%)) was not significant (p=0.1).
Table 2.
Table showing clinical characteristics across groups defined by UCPCR result. *BMI results for pediatric patients adjusted to equivalent BMI for age 22. Data are presented as median (IQR). **Kruskall-Wallis test. CSII = continuous subcutaneous insulin infusion.
| UCPCR GROUP (nmol/mmol) |
<0.001 | ≥0.001 - <0.03 | ≥0.03 – <0.2 | ≥0.2 | p-value** |
|---|---|---|---|---|---|
| Number | 184 | 483 | 187 | 70 | |
| Age diagnosis (years) | 10 (6-16) | 11 (6-16) | 12 (8-21) | 16 (13-21) | 0.0001 |
| Duration (years) | 20.9 (14.9-26.9) | 19.1 (11.5-27.7) | 15.0 (8.2-23.4) | 13.9 (7.9-21.6) | 0.0001 |
| Insulin dose (uKg-124hr-1) | 0.77 (0.61-0.93) | 0.78 (0.60-0.97) | 0.77 (0.60-1.00) | 0.74 (0.55-1.01) | 0.9 |
| CSII use (%) | 16 | 15 | 9 | 10 | 0.2 |
| HbA1c (%) | 8.9 (7.8-10.2) | 8.6 (7.9-9.7) | 8.7 (7.9 – 9.8) | 9.1 (7.6-10.3) | 0.3 |
| HbA1c (mmol/mol) | 74 | 70 | 72 | 76 | |
| BMI (Kgm-2)* | 24.0 (22.6-26.5) | 24.8 (23.2-27.7) | 25.3 (23.2-27.8) | 24.8 (23.0-26.6) | 0.7 |
Figure 2.
Bar chart of proportion of subjects with UCPCR detectable (>0.001 nmol/mmol) against duration quintile. P<0.0001 for trend of decreasing proportion across duration groups.
In logistic regression with duration of diabetes, age of diagnosis and BMI as covariates only diabetes duration was associated with presence of detectable C-peptide (supplementary table 1)
Associations of higher levels of C-peptide
Patients with a UCPCR ≥0.2 nmol/mmol had a shorter diabetes duration than those without (13.9 years v 18.9 years, p<0.0001, and were diagnosed older 16 v 11, p<0.0001,Table 2). In a logistic regression with duration of diabetes, age of diagnosis and BMI as covariates, diabetes duration and age of diagnosis were both associated with a UCPCR ≥0.2 nmol/mmol (Supplementary table 2, multivariate logistic regression). The odds of having a UCPCR≥0.2 nmol/mmol increased by 7% (OR 1.07, CI 1.04-1.11, P<0.0001) for each increase in year of age of diagnosis and decreased by 4% (OR 0.96, CI 0.92-0.99, p=0.01) for each year increase in duration.
Association with insulin dose and glycaemia
Insulin dose and glycaemia was similar in those with and without detectable C-peptide (insulin dose 0.77 v 0.78 ukg-124hours-1, Table 1, HbA1c 8.7 vs 8.9% (72 v 74 mmol/mol), Table 2). There was no association between UCPCR level and either HbA1c or insulin dose in univariate or multivariate regression.
Patients on CSII had better glycaemic control (HbA1c 8.2% IQR(7.5-9.2) V 8.8% (7.9-9.9), p <0.001 and lower doses of insulin (0.66 (0.53-0.86) v 0.79 (0.60-0.98) U/kg/day p <0.001) but did not have higher values of UCPCR (0.01 (0.004-0.04) v 0.01 (0.003-0.02)nmol/mmol, p =0.1).
Discussion
This large study, using home post-meal UCPCR, found 80% of all people with T1D for 5 or more years, had measurable endogenous C-peptide. Across the range of durations in the study, the prevalence did not fall below 72%. These findings provide strong evidence that most people with T1D do not develop complete beta-cell loss, and will continue to secrete low levels of insulin for decades after diagnosis. These results support the histological data(3; 16) that occasional insulin-producing beta cells are visible in most histological pancreas samples of people with long duration T1D.
The 2 previous smaller studies using sensitive C-peptide assays in T1D support the high prevalence in our study. Our initial study on 74 people (median duration 30 years) showed 73% had detectable post mixed meal serum C-peptide (1). The study by Wang et al found a lower proportion (43% of 182 patients (median duration 15 years) (2) which probably reflected the use of a fasting sample and that the ELISA used was less sensitive than the chemiluminesence assay used in our two studies (1). The absolute levels of C-peptide in our study are lower than those seen in the very long duration participants (>50 years) in the Joslin Medallists, where 67% had serum C-peptide above 30 pmol/L(3). Only 28% of our participants had a UCPCR equivalent or above this level. This probably reflects increased survival of those with retained C-peptide at the longer durations found in the Joslin medallists. The failure to find an increase in C-peptide secretion in those of very long duration our study probably reflects that we did not recruit patients over 50 years duration as recruitment was limited to patients aged under 50 years.
Even after 5 years of diabetes, duration of diabetes is a predictor of both C-peptide level and the presence of detectable C-peptide. There was a decline in absolute C-peptide levels and in likelihood of detectable C-peptide with longer duration diabetes. Age of diagnosis was not associated with whether C-peptide was detectable but did associate with higher C-peptide levels. Age of diagnosis is associated with HLA risk and may reflect the strength and intensity of the underlying autoimmune process(17). This may explain the relationship seen in our data and numerous other studies(3; 18; 19). If age of diagnosis is a marker of the rate of immune destruction of the beta cells, then the lack of association between age of diagnosis and detection of low level C-peptide may suggest other factors are more important in determining whether a few functional beta cells remain or it may reflect that the impact of age of diagnosis is small and not detectable by our sample size.
Some participants in our study continue to make relatively large amounts of C-peptide despite long duration T1D. 8% of participants in our study with a duration over 5 years had UCPCR≥0.2 nmol/mmol, and another 20% had UCPCR ≥0.03 pmol/L. This is similar to the 8% of adults over 5 years post diagnosis who had a stimulated serum C-peptide >200 pmol/L when screened for DCCT(20). However it is important to recognise that these levels are considerably lower than seen in Type 1 diabetes in the first year following diagnosis. Median post-meal UCPCR was 1.04 IQR (0.44-2.3)nmol/mmol in 100 individuals within the first year from diagnosis of type 1 diabetes (unpublished data from the UNITED study) and median post OGTT (n=38) UCPCR was 3.8 (IQR 2.4-7.0) in 38 non-diabetic controls (21). The high level of C-peptide was unlikey to be an incorrect diagnosis of T1D in a patient with T2D or monogenic diabetes as these were aggressively excluded from this study. It is not known why some patients with T1D retain relatively high endogenous insulin for so long: potential explanations include that these individuals have a less aggressive autoimmune process leading to slower beta cell destruction, that the autoimmune process has subsided through ‘burnout’, or that beta cells in these individuals have a greater ability to regenerate.
We did not find an association of persistent C-peptide secretion with either insulin dose or HbA1c. This is in contrast with studies of a recent analysis of DCCT by Lachin et al (12) that demonstrates a continuous relationship between stimulated serum C-peptide and HbA1c and insulin dose, but only in the patients assigned to intensive therapy. The difference probably reflects 1. that our study was cross sectional so only a proportion will be having intensive glycaemic management and 2. the low cut off we used means the majority of the patients in our study also had very low levels of C-peptide, which are unlikely to have a clinically significant effect.
There are some limitations in our study. The home post meal UCPCR does not involve a fixed high carbohydrate meal, does involve taking prandial insulin, and is not supervised so is likely to be less sensitive than a formal mixed meal tolerance test assessment of serum C-peptide performed in previous studies. However a home UCPCR is highly correlated with MMTT serum C-peptide in T1D (6) and both urine and serum were equally sensitive in detecting very low level C peptide in long standing T1D(1). Any bias is small from the variable meal(6) or insulin administration(10) and would only result in C-peptide being less likely to be detected. We did not assess renal function in this study. Urine C-peptide levels are lower in those with CKD so this could lead to an underestimate of the prevalence of patients with retained endogenous insulin secretion. Participants in this study were mainly white European, and our results may not be generalizable to other racial groups and other geographical regions.
The presence of a spectrum of endogenous insulin production at all durations of T1D is relevant to the study and treatment of the disease process in T1D. A pressing question is why some patients still have significant levels of endogenous insulin many years from diagnosis. The factors that cause the variation from undetectable or very low levels in most, to very high levels in a few may inform ongoing attempts to prevent, halt, or reverse the pathological process in T1D. Given these individuals with higher levels of C-peptide are in a minority, large studies such as UNITED may be required to identify enough patients for future study. Identifying outliers with the highest or lowest levels of endogenous insulin will allow study of their immunology, genetics and clinical phenotype in more detail. This may provide valuable insights into the biology of disease progression in T1D.
In conclusion, this population-based study confirms that the majority of people with long duration type 1 diabetes are insulin microsecretors and have detectable endogenous C-peptide. The presence of detectable C-peptide in most people with type 1 diabetes may have important clinical and scientific implications and warrants further investigation.
Acknowledgements
The UNITED team includes all authors of the paper, the nursing staff in Exeter (Tina Sanders, Sarah Tiley) and Dundee (Emma Gellatly, Lynsey Beall, Bridget Shepherd), the Dundee T1D Bioresouce (PI Professor Helen Colhune), the UNITED database manager Keith Milburn, and the genetic testing team Kev Colclough and Professor Sian Ellard.
Funding
This work was supported by a grant from the Health Innovation Challenge Fund (HICF), a parallel funding partnership between the Wellcome Trust and the Department of Health (Grant number 091985/HICF 1009-041). This work was supported by the National Institute for Health Research (NIHR), through the Primary Care Research Network and the NIHR Exeter Clinical Research Facility through funding for ATH, BMS, MS, SH, MH and general infrastructure. ATH is supported by a WellcomeTrust Senior Investigator award. ERP is supported by a Wellcome Trust New Investigator award. RAO is supported by a Diabetes UK Clinical Training Fellowship. TJM is supported by an NIHR CSO Clinical Scientist Fellowship. The views expressed are those of the authors and not necessarily those of the funders.
Footnotes
Contribution statement
RAO, TJM and ATH designed the study. MS and SH recruited patients and collected samples. TJM performed all biochemical analysis. RAO, TJM, BMS, MMH, ERP and ATH performed data analysis and interpretation. RAO wrote first draft of manuscript and all authors read and modified the manuscript.
The Authors have no relevant declarations of interest. ATH is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes care. 2012;35:465–470. doi: 10.2337/dc11-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald TJ, Knight BA, Shields BM, Bowman P, Salzmann MB, Hattersley AT. Stability and reproducibility of a single-sample urinary C-peptide/creatinine ratio and its correlation with 24-h urinary C-peptide. Clinical chemistry. 2009;55:2035–2039. doi: 10.1373/clinchem.2009.129312. [DOI] [PubMed] [Google Scholar]
- 5.Bowman P, McDonald TJ, Shields BM, Knight BA, Hattersley AT. Validation of a single-sample urinary C-peptide creatinine ratio as a reproducible alternative to serum C-peptide in patients with Type 2 diabetes. Diabet Med. 2012;29:90–93. doi: 10.1111/j.1464-5491.2011.03428.x. [DOI] [PubMed] [Google Scholar]
- 6.Besser RE, Ludvigsson J, Jones AG, McDonald TJ, Shields BM, Knight BA, Hattersley AT. Urine C-peptide creatinine ratio is a noninvasive alternative to the mixed-meal tolerance test in children and adults with type 1 diabetes. Diabetes care. 2011;34:607–609. doi: 10.2337/dc10-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AG, Besser RE, McDonald TJ, Shields BM, Hope SV, Bowman P, Oram RA, Knight BA, Hattersley AT. Urine C-peptide creatinine ratio is an alternative to stimulated serum C-peptide measurement in late-onset, insulin-treated diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2011;28:1034–1038. doi: 10.1111/j.1464-5491.2011.03272.x. [DOI] [PubMed] [Google Scholar]
- 8.Besser RE, Shepherd MH, McDonald TJ, Shields BM, Knight BA, Ellard S, Hattersley AT. Urinary C-peptide creatinine ratio is a practical outpatient tool for identifying hepatocyte nuclear factor 1-{alpha}/hepatocyte nuclear factor 4-{alpha} maturity-onset diabetes of the young from long-duration type 1 diabetes. Diabetes care. 2011;34:286–291. doi: 10.2337/dc10-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, Houghton JA, Shepherd M, Hattersley AT, Weedon MN, Caswell R. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013;56:1958–1963. doi: 10.1007/s00125-013-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser RE, Jones AG, McDonald TJ, Shields BM, Knight BA, Hattersley AT. The impact of insulin administration during the mixed meal tolerance test. Diabetic medicine : a journal of the British Diabetic Association. 2012;29:1279–1284. doi: 10.1111/j.1464-5491.2012.03649.x. [DOI] [PubMed] [Google Scholar]
- 11.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 12.Lachin JM, McGee P, Palmer JP. Impact of C-Peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes. 2014;63:739–748. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30:803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Mayer-Davis EJ, Andrews JS, Dolan LM, Pihoker C, Hamman RF, Greenbaum C, Marcovina S, Fujimoto W, Linder B, Imperatore G, et al. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2012;55:3359–3368. doi: 10.1007/s00125-012-2719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besser RE, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed-meal tolerance test: use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes care. 2013;36:195–201. doi: 10.2337/dc12-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker A, Lauria A, Schloot N, Hosszufalusi N, Ludvigsson J, Mathieu C, Mauricio D, Nordwall M, Van der Schueren B, Mandrup-Poulsen T, Scherbaum WA, et al. Age-dependent decline of beta-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes, obesity & metabolism. 2014;16:262–267. doi: 10.1111/dom.12216. [DOI] [PubMed] [Google Scholar]
- 20.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 21.Oram RA, Rawlingson A, Shields BM, Bingham C, Besser RE, McDonald TJ, Knight BA, Hattersley AT. Urine C-peptide creatinine ratio can be used to assess insulin resistance and insulin production in people without diabetes: an observational study. BMJ open. 2013;3:e003193. doi: 10.1136/bmjopen-2013-003193. [DOI] [PMC free article] [PubMed] [Google Scholar]