Abstract
Background
Associations between certain environmental and lifestyle factors and Parkinson’s disease (PD) have been reported in several studies, but information on these factors and Parkinson’s Disease (PD) in South Asia, is limited.
Objective
To determine associations between lifestyle factors and PD in an urban clinic-based study in Sri Lanka.
Methods
In this case-control study, demographic and lifestyle factor data (including diet, coffee/tea drinking, smoking, alcohol status) was collected from an unselected cohort of PD patients and age and gender-matched controls attending clinics in Greater Colombo, Sri Lanka. Associations between lifestyle factors and PD status were assessed using Logistic Regression analysis, while links with age of PD onset were explored with Kaplan Meier and Cox Regression survival analyses. Results with p<0.05 were considered to be statistically significant.
Findings
Of 229 patients with parkinsonism, 144 had Idiopathic PD using standard diagnostic criteria. Controls numbered 102. Coffee drinkers and smokers were significantly less likely to have PD (coffee, p<0.001; Odds Ratio (OR)=0.264; smoking, p=0.043; OR=0.394). Coffee drinkers were older at PD onset (p<0.001). Similar trends seen with tea drinking were not statistically significant.
Conclusions
This is the first formal study of PD and these lifestyle factors in South Asia. It demonstrates an inverse association between coffee drinking, smoking and PD, and an association between coffee drinking and later age of PD onset. This is in line with other studies done worldwide, suggesting biological associations with global relevance.
Keywords: Parkinson’s disease, lifestyle, coffee, smoking, Sri Lanka
Introduction
Parkinson’s Disease (PD) has a multifactorial aetiology, with a complex interaction of genetic and environmental risk factors. Several environmental and lifestyle factors have been associated with PD and especially smoking, coffee and tea intake have been linked to reduced risk[1][2]. Meta-analyses of global studies have confirmed a decreased relative risk for smoking (pooled Relative Risk(RR) 0.59) and coffee intake (pooled RR 0.75)[2]. Further dose dependent effects on delaying the age of PD onset have been seen with coffee intake, while tea intake has also been associated with decreased risk and delayed age of onset [3].
Few studies have investigated these associations in South Asian populations, which have different genetic and lifestyle exposure profiles compared to Western and East Asian populations. Studies on PD in Indian and other South Asian populations have also found that PD patients indigenous to this part of the world have some phenotypic differences compared to Caucasian populations. In particular, an increased proportion of patients have levodopa hypo-responsiveness, bradykinesia-dominant disease and early cognitive dysfunction. This difference in phenotype has been suggested to be linked to higher rates of diabetes and hypertension in the populations along with dietary and genetic factors [4]. Considering these differences, few studies have investigated associations between PD and lifestyle factors in South Asia to ascertain if these are different compared to those established in Caucasian and other populations. Studies in India have found associations between increased PD risk and non-smoking status, well water drinking and pesticide use [5][6] but none have investigated factors such as coffee and tea intake.
Sri Lanka is a multi-ethnic country with an agricultural background and one of the fastest ageing populations in South Asia. Neurodegenerative diseases such as PD and Alzheimer’s disease (AD) are thus becoming an increasingly important healthcare issue, but few formal studies have been performed. A recent neuropathological study on neurodegenerative pathology in elderly brains found more PD related Lewy-body pathology in Sri Lankan subjects compared to Indian subjects, while AD related pathology was similar in both groups, potentially indicating variability in neurodegenerative disease pathology even among South Asian countries[7].
There is currently limited data available on PD and no data on lifestyle risk factors and PD, in Sri Lanka. Due to cultural reasons, the lifestyle factor profiles in South Asian countries can be very different from those seen in Western countries. For example in Sri Lanka, smoking and alcohol intake have a strong male predominance. Furthermore, most Sri Lankans drink tea regularly, while coffee intake is much lower than in the West. Therefore, this study aimed to investigate, for the first time, the association between lifestyle factors and PD in a clinic based population in Colombo, Sri Lanka.
Methods
This was a retrospective case-control study. Patients were recruited from the Neurology and Movement Disorders clinics in hospitals in Greater Colombo, Sri Lanka over a 4 year period. All patients who had been clinically diagnosed with Parkinson’s disease (PD) or parkinsonism by a certified Neurologist were approached regarding the study and all consenting patients (~80%) were recruited. Retrospective detailed case note review of recruited patients was conducted in order to identify those who fulfilled UK Queen Square Brain Bank criteria for Idiopathic PD, and only those fulfilling these criteria were included in the final study. Controls consisted of patients without neurological disorders recruited from non-neurological clinics or relatives of PD patients, who had no neurological complaints. Control status was considered to be the absence of a diagnosis of PD up to the point of assessment.
The study was performed with appropriate approval from the University of Sri Jayewardenepura Ethics Committee (No. 427/09) and informed consent was obtained from all participants.
Demographic, lifestyle and medical history information was collected using a pro-forma data sheet. This included information on province of birth, ethnicity, education level, diet, smoking, alcohol, coffee and tea intake, pesticide exposure and presence of medical co-morbidities (ischaemic heart disease, diabetes and hypertension). Details of the patients’ neurological history and examination were obtained from their medical records.
Variables were further categorised as appropriate prior to analysis. Due to the location of the clinics most participants were from the Western province. Therefore ‘province of birth’ was categorised into Western Province and non-Western Province. Education level was categorised as ‘below A-Level’ (i.e. left school before the age of 18) and ‘A-Level and above’. Coffee drinking was categorised as “coffee drinkers” and “coffee non-drinkers”, where “coffee non-drinkers” were considered to be those who drank <2 cups/month. As all participants drank tea, tea intake was divided into “standard intake” (</=3 cups/day) and “high intake” (>3 cups /day). Presence of pesticide exposure was considered to involve regular occupational spraying of pesticides. Other data was categorised as follows -: diet (vegetarian/non-vegetarian); smoking (ever-smokers/never-smokers); alcohol (ever-drinkers/never-drinkers); ischaemic heart disease, diabetes, hypertension (presence/absence).
Statistical analyses were performed using SPSS version 21. Between-group comparisons were performed on percentage numbers, using T tests and Chi-squared tests. Logistic Regression analysis was performed to determine the effect of the demographic and lifestyle factors on the risk of PD, with PD status as the dependent variable. Kaplan Meier survival analysis and Cox Regression analysis were performed to assess the effect of these factors on the age of onset of PD. Results with a p value <0.05 were considered to be statistically significant.
Results
Demographics
229 patients with parkinsonism were recruited. Of these, 144 fulfilled UK Queen Square Brain Bank Criteria for Idiopathic PD based on retrospective case note review. An age and gender matched group of 102 controls were also recruited.
No significant differences in age, gender, ethnicity and province of birth were seen between the patient and control groups (Table 1). Education level was higher amongst the PD group, which may be related to higher educated PD patients being more likely to attend clinic.
Table 1.
Key demographics and summary of the measured lifestyle factors and co-morbidities
| Demographics | Patients | Controls | p value |
|---|---|---|---|
| Total Number | 144 | 102 | |
| Gender % (M:F) | 59.7 : 40.3 | 54.9 : 45.1 | 0.260 |
| Age at Assessment (Average ± SD) (y) | 61.8 ± 8.2 | 61.7 ± 7.9 | 0.950 |
| Ethnicity % (Burgher: Muslim : Sinhala : Tamil) | 0.7 : 7.1 : 87.3 : 4.9 | 1.0 : 4.9 : 89.2 : 4.9 | 0.914 |
| Province of Birth % (Western Province : non Western Province) | 72.9 : 27.1 | 65.0 : 35.0 | 0.187 |
| Education level % (Below A Level : A Level and above) | 73.6 : 26.4 | 86.1 : 13.9 | 0.022* |
| Lifestyle Factors and Co-morbidities | |||
| Diet (% Vegetarian) | 11.3 | 9.8 | 0.701 |
| Smoking status (% ever smokers) | 28.1 | 44.1 | 0.010* |
| Alcohol status (% ever drinkers) | 35.0 | 44.1 | 0.151 |
| Coffee drinkers (%) | 34.8 | 67.6 | <0.001** |
| Tea drinking (% high intake) | 14.8 | 24.5 | 0.060 |
| Ischaemic Heart Disease (%) | 6.0 | 7.8 | 0.571 |
| Diabetes (%) | 20.6 | 17.64 | 0.570 |
| Hypertension (%) | 27.6 | 30.4 | 0.640 |
| Pesticide exposure history (%) | 3.6 | 4.9 | 0.624 |
(M:F- Male:Female; SD-Standard Deviation; *p<0.05; **p<0.01);
Lifestyle Factors and PD
Simple group comparison analyses suggested that a significantly smaller percentage of PD patients were coffee drinkers or ex /current smokers (Table 1). Logistic regression analysis, with PD versus control status as the dependent variable and age, gender and above stated lifestyle factors/comorbidities as predictor variables, indicated a highly significant negative association between coffee drinking and PD (p<0.001, Odds Ratio(OR) 0.264; 95% Confidence Interval (CI) 0.14–0.499) (Table 2). There was also a significant negative association between ‘ever smoking status’ and PD (p=0.043, OR 0.394; 95% CI0.159–0.972). The negative association between high tea intake and PD did not reach statistical significance (p=0.106, OR=0.523; 95% CI0.238–1.148).
Table 2.
Summary table displaying the results of the Logistic Regression analysis of factors contributing to the risk of PD.
| Variable | B coefficient | p value | Odds Ratio-Exp (B) | 95% Confidence Intervals for Exp(B) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | -1.079 | 0.015 | 0.340* | 0.142 | 0.813 |
| Age At Assessment | -0.005 | 0.810 | 0.995 | 0.957 | 1.035 |
| Diet | -0.212 | 0.671 | 0.809 | 0.304 | 2.151 |
| Smoking | -0.932 | 0.043 | 0.394* | 0.159 | 0.972 |
| Alcohol | -0.619 | 0.194 | 0.539 | 0.212 | 1.370 |
| Coffee | -1.330 | 0.000 | 0.264** | 0.140 | 0.499 |
| Tea | -0.649 | 0.106 | 0.523 | 0.238 | 1.148 |
| Education | 0.653 | 0.109 | 1.921 | 0.864 | 4.272 |
| IHD | -1.084 | 0.143 | 0.338 | 0.079 | 1.443 |
| Diabetes | -0.222 | 0.611 | 0.801 | 0.341 | 1.881 |
| Hypertension | -0.263 | 0.472 | 0.769 | 0.375 | 1.576 |
| Pesticide Exposure | -0.683 | 0.373 | 0.505 | 0.112 | 2.272 |
| Constant | 4.676 | 0.014 | 107.305 | ||
p<0.05;
p<0.01
Considering the significant effect of gender, the data was also analysed separately for males and females. The association between decreased PD and smoking was more significant in males (p=0.019, OR 0.313; 95% CI 0.118 – 0.826). Coffee drinking and decreased PD were more significantly associated in females (p<0.001, OR 0.096; 95% CI0.031–0.299).
Lifestyle Factors and PD Age of Onset
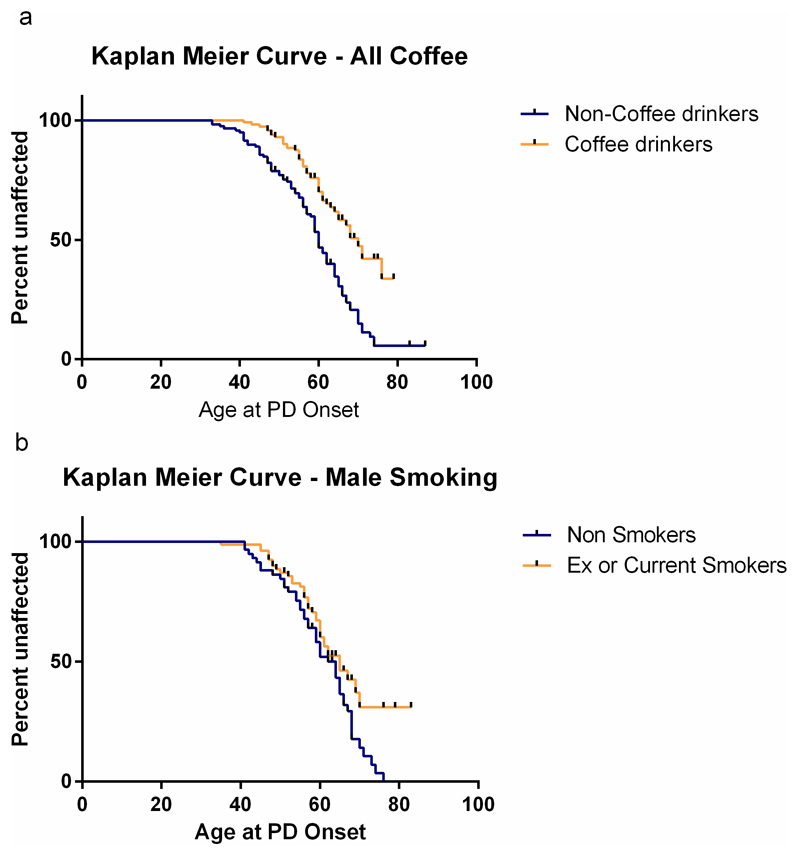
Survival analysis with gender stratification was performed to investigate the relationship between each of the lifestyle factors and age of onset of PD symptoms. Kaplan Meier survival analysis demonstrated that regular coffee drinking was associated with a later age of onset of PD (overall, p<0.001; females, p<0.001; males, p= 0.013; Log Rank (Mantel-Cox)), with the significance withstanding Bonferroni correction for multiple testing for the population as a whole and also for female participants (Figure 1a). In males, ever-smoking was associated with delayed PD onset (p=0.048; Log Rank (Mantel-Cox)) (Figure 1b).
Figure 1. Kaplan Meier curves of age of onset of PD in different patient groups.
(a) Coffee drinkers versus non-drinkers
(b) Male smokers versus non-smokers
Higher education level was associated with earlier PD onset (p=0.01; Breslow (Generalised Wilcoxon), which may indicate that those with increased education were more aware of the significance of early PD symptoms. There was no significant effect with other variables.
Cox regression analysis confirmed the significant association between coffee intake and delayed PD onset following adjustment for age, gender and all measured variables (p<0.001; Exp(B) 2.229) (Table S1).
Conclusions and Discussion
This is the first study evaluating associations between lifestyle factors and PD in Sri Lanka. It indicates a significant association between coffee drinking and a decreased prevalence (˜70% less) and delayed age of onset of PD. It also demonstrates an association between smoking and non-PD status. These findings are in keeping with other studies from around the world[2] and support the contention that these factors are strongly associated with the development of PD regardless of genetic and ethnic background.
Tea intake has been associated with a decreased risk of PD[2]. The association between high tea intake and decreased PD status seen in this study was not statistically significant. This may be due to the relatively small sample size and the lack of non-tea drinking participants.
There are many possible theories to explain the biological basis of the effects seen with caffeine intake, smoking/nicotine and PD. In the past, these associations have been considered to be due to a lack of addictive behaviours in the premorbid personalities of patients likely to develop PD but subsequent research has shown dose dependent protective effects on risk and age of onset, with beneficial effects of caffeine in therapeutic trials, possibly through its action as an Adenosine A2 receptor antagonist[2][8][9]. Several studies have also demonstrated evidence for the neuroprotective effects of coffee and caffeine in other neurodegenerative diseases, including in the progression from Mild Cognitive Impairment to AD dementia [10]. The effect of caffeine on the gut microbiota has also been suggested to play a role[11].
Nicotine may also have some neuroprotective effects, acting via nicotinic acetylcholine receptors in neurones and glia[12]. However, PD patients have also been found to have less difficulty quitting smoking than those without PD, suggesting that decreased responsiveness to nicotine could be a prodromal feature of PD itself. This potentially indicates a role for reverse causation in explaining the association between smoking and decreased PD - namely people are less likely to smoke regularly because they are more likely to develop PD [13].
This is the first study investigating these lifestyle factors and PD in Sri Lanka, but it has some limitations. Due to the recruitment strategy, most of the participants are from regions around the capital, Colombo, which may not be fully representative of the population of the entire country. Smoking and alcohol intake are generally very low among females in Sri Lanka for cultural reasons, which may attenuate the overall effects seen, although the separate analysis for males and females does take account of this.
The lack of detailed information on the lifestyle factors (e.g. age at start of regular coffee intake, number of years of intake and number of cups/day), is a further limitation. Thus, it is not possible to determine if the exposure started prior to the onset of PD symptoms (which would support a more causative role) or afterwards (which would simply indicate a correlation). Finally, the study relies on patient reported information and patients’ interpretation of lifestyle factor associated questions which can sometimes be less reliable.
Nevertheless, the results from this unique Sri Lankan study are in keeping with more detailed prospective studies from around the world, suggesting that the findings are likely to be valid. Details on the phenotypes of the PD patients in this study were not available, but considering the previously observed phenotypic differences in South Asian PD patients[4], this study may provide some indication that the observed lifestyle factor associations have global, biological relevance to all phenotypes of Idiopathic PD. However, more detailed, larger studies will be required to confirm the observed associations between PD, coffee intake and smoking, as well as to further probe other potential associations (eg. with tea intake) in Sri Lanka. Investigation of the biological mechanisms underlying these associations will be valuable in identifying potential therapeutic and preventative strategies.
Supplementary Data
Acknowledgements
The authors thank all the participants in this study. The authors also thank Dr. R.P.P. Dinalee for her contribution to data collection.
Financial support –:
University of Sri Jayewardenepura and The World Health Organisation, Sri Lanka.
NIHR Cambridge Biomedical Research Centre and MRC-Wellcome Trust Cambridge Stem Cell Institute, UK.
Abbreviations
- PD
Parkinson’s Disease
- OR
Odds Ratio
- CI
Confidence Interval
- SD
Standard Deviation
Footnotes
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.de Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Wirdefeldt K, Adami H-O, Cole P, et al. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 3.Tan EK, Tan C, Fook-Chong SMC, et al. Dose-dependent protective effect of coffee, tea and smoking in Parkinson’s disease: A study in ethnic Chinese. J Neurol Sci. 2003;216:163–7. doi: 10.1016/j.jns.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Hu MTM, Chaudhuri KR, Jarosz J, et al. An imaging study of parkinsonism among African-Caribbean and Indian London communities. Mov Disord. 2002;17:1321–8. doi: 10.1002/mds.10261. [DOI] [PubMed] [Google Scholar]
- 5.Behari M, Srivastava AK, Das RR, et al. Risk factors of Parkinson’s disease in Indian patients. J Neurol Sci. 2001;190:49–55. doi: 10.1016/s0022-510x(01)00578-0. http://www.sciencedirect.com/science/article/pii/S0022510X01005780. [DOI] [PubMed] [Google Scholar]
- 6.Das K, Ghosh M, Nag C, et al. Role of familial, environmental and occupational factors in the development of Parkinson’s disease. Neurodegener Dis. 2011;8:345–51. doi: 10.1159/000323797. [DOI] [PubMed] [Google Scholar]
- 7.Wijesinghe P, Shankar SK, Chickabasaviah YT, et al. Cytoskeletal Pathologies of Age-Related Diseases between Elderly Sri Lankan (Colombo) and Indian (Bangalore) Brain Samples. Curr Alzheimer Res. 2016;13:268–80. doi: 10.2174/156720501303160217121210. [DOI] [PubMed] [Google Scholar]
- 8.Postuma RB, Lang AE, Munhoz RP, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79:651–8. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarzschild MA, Xu K, Oztas E, et al. Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson’s disease. Neurology. 2003;61:S55–61. doi: 10.1212/01.WNL.0000095214.53646.72. [DOI] [PubMed] [Google Scholar]
- 10.Cao C, Loewenstein Da, Lin X, et al. High blood caffeine levels in mci linked to lack of progression to dementia. J Alzheimer’s Dis. 2012;30:559–72. doi: 10.3233/JAD-2012-111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derkinderen P, Shannon KM, Brundin P. Gut feelings about smoking and coffee in Parkinson’s disease. Mov Disord. 2014;29:976–9. doi: 10.1002/mds.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quik M, Perez Xa, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27:947–57. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz B, Lee P-C, Lassen CF, et al. Parkinson disease and smoking revisited Ease of quitting is an early sign of the disease. Neurology. 2014:1396–402. doi: 10.1212/WNL.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.