Abstract
Previous research has identified patterns of cognitive deficits in patients with Alzheimer disease (AD), but little is known about their pattern of daily functional impairment. A total of 49 patients with AD and 52 healthy elderly controls were administered neuropsychological tests as well as the Direct Assessment of Functional Status (DAFS) test, an observation-based test of activities of daily living (ADLs). In this project, we assessed 14 separate tasks assessed by the DAFS. To analyze the data, 4 cognitive domains were created using neuropsychological composite z scores (means and standard deviation obtained from control data) for patients with AD. Results revealed that patients with AD performed worse on the memory, language, and visual-spatial relative to the executive domain. Additionally, patients with AD performed poorer than the controls on nearly all 14 DAFS tasks, with their worse performance being on the shopping-related tasks which, in part, requires memory skills. Logistic regression revealed better specificity than sensitivity classifications based on the DAFS tasks, and stepwise regression analyses indicated that cognitive domains predicted specific aspects of functional abilities. These findings suggest that patients with AD display a distinct pattern of ADLs performance, that traditional neuropsychological tests are useful in predicting daily functioning, and the DAFS has some strengths and weaknesses in classifying AD and controls.
Keywords: Alzheimer disease, functional status, neuropsychological testing, dementia
Introduction
Alzheimer disease (AD) is a neurodegenerative disorder that is characterized by progressive cognitive deterioration and associated decline in activities of daily living (ADLs). The hallmark of AD is impairment in episodic memory, evidenced by studies demonstrating inadequate encoding and retrieval of verbal and nonverbal information,1–3 even in the very mild stages of the illness.4 However, from decades of research, it is clear that multiple cognitive domains are affected as the disease progresses. Research has documented significant deficits in visual-spatial5 and language.4,6 To the degree that executive skills are affected in AD is somewhat equivocal. Some studies suggest that this domain is relatively preserved during the early stages of the disease,5,7 while others demonstrate equal or greater impairment of this cognitive domain relative to the others.8,9
It has been helpful to identify specific patterns of cognitive impairment in AD as the diagnosis of this disorder still remains one of exclusion. Patterns of deficits, such as documenting greater impairment in the domain of memory versus executive functioning, are essential for differential diagnostic purposes as well as for treatment planning. For these same reasons, it would be advantageous to identify patterns of daily functioning deficits in this group of patients, particularly those in the early stages. It would be specifically helpful to identify, of the many daily functioning skills, which skill in patients with early stage AD is likely to initially display the greatest difficulty with and whether the domain that shows the greatest impairment corresponds to a domain-specific cognitive deficit observed on neuropsychological tests.
The presence of deficits in daily functional abilities has been mostly demonstrated with the use of informant-rated instrumental-activities-of-daily-living measures.10,11 However, studies have revealed that caregivers tend to over- or underestimate the patient’s capabilities when using rating measures primarily due to the nature of the relationship and/or the level of burden being experienced by the caregiver.12–14 For this reason, observation-based daily functional tasks are likely to be more accurate. There is some evidence to suggest that these measures adequately correlate with neuropsychological tests and that in fact, some neuropsychological tests effectively predict specific types of daily functioning.15–17 For example, in a study by Farias et al16 various domains of cognitive dysfunction in patients with AD was examined in relation to domains of daily functioning using an observation-based ADLs tasks. They found that the neuropsychological test scores of patients with AD accounted for approximately 50% of the overall variability found in an observation-based daily functional measure. More specifically, they found that performance on specific cognitive domains, such as visual-spatial and executive skills, best predicted certain daily tasks, such as balancing a checkbook. In a recent study,15 we also demonstrated that some executive functioning tests (eg, Wisconsin Card Sorting Test) better predicted certain aspects of daily functioning (eg, communication such as preparing a letter to be mailed).
While the studies mentioned above have found relationships between cognitive and daily functional abilities in patients with AD, it is not clear whether the domains of functioning and magnitude of ADLs deficits are similar to those demonstrated on cognitive tasks. Additionally, while cognitive tasks are administered in order to assess dementia and/or specific deficits in cognitive domains, very few clinicians actually assess daily functioning. In a recent survey of almost 750 members of neuropsychological societies, Rabin et al18 found that only approximately 35% of the sample actually used a form of ecologically oriented functional measure and of those, very few appeared to use a comprehensive, performance-based measure. This suggests that if we understand the pattern of daily functional performance of patients with AD and how neuropsychological measures predict such functioning, clinicians who do not administer such tasks routinely can at least draw on empirical data to predict the level of daily dysfunction.
Thus, the present study had the following aims. First, we were interested in examining the pattern of domain-specific and severity of deficits on a daily functional task as compared with the pattern and severity deficits in specific neurocognitive domains in a group of patients with mild-stage AD. The purpose was to compare and contrast the cognitive and functional domains that displayed the earliest decline in a group of patients with mild AD. Second, we were interested in examining the extent to which particular cognitive deficits best predict specific domains of daily dysfunction in these patients with mild-stage AD in order to gain better understanding of the cognitive process required for specific daily functional tasks. Finally, we were interested in learning which specific functional tasks best differentiate dementia patients from normal controls.
Methods
Participants
A total of 49 patients with AD and 52 healthy age- and education-matched older adults (controls) participated. Patients with AD were recruited from 3 sites, including an Alzheimer’s Association Center, a hospital-based geriatric center, and a Veterans Administration healthcare center. All participants were referred to the study with a predetermined diagnosis of AD, based on clinical evaluation by their primary physician and/or neurologist using the the National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer’s Disease and Related Disorders Association (NINDC-ADRDA) criteria for probable AD.19
Healthy, age- and education-matched controls were also recruited in order to (1) use their neuropsychological test scores for computing composite scores for patients with AD, and 2) use for analysis to determine sensitivity and specificity classification rates for groups using subscales of the daily functional test. The controls were either partners of the patients with AD or recruited via newspaper advertisements, posted flyers, or by word-of-mouth. Controls and patients completed a comprehensive health questionnaire and were excluded if they had a history of significant, untreated medical conditions (e.g., diabetes, hypertension), psychiatric illness (e.g., depression), substance abuse, incidents of loss of consciousness for >5 minutes, and neurological disorders (with the exception of the diagnosis of AD for the patient group) that are known to affect cognitive functioning. Additionally, participants with major issues with mobility that would interfere with task performance were excluded from the study. All individuals were participating in a larger National Institutes of Health (NIH)-funded research study comparing functional status among older people with dementia and without dementia. As part of the larger study, participants completed approximately 3 hours of testing. Tests included observation-based ADLs tasks and a neuropsychological battery that included tests of memory, visual-spatial, abstract reasoning, and language skills.
The demographic information for the participants, including age, educational level, and Mini-Mental State Examination (MMSE) scores can be found in Table 1. As can be seen from this table, the participants were on average in their eighth decade of life and were relatively well educated. No significant differences were found between the groups on age, F(1, 99) = 2.42, P = .12, or education, F(1, 99) = .99, P = .32, but there were significantly larger number of male patients with AD (χ2 = 6.5, P < .01) and female normal controls (χ2 = 18.13, P < .001). Mini-Mental State Examination scores for the patients with AD were significantly lower than that of the normal controls, F(1, 99) = 54.59, P < .001. Nonetheless, the AD group, on average, was in the mild stages of dementia.
Table 1.
Mean (SD) and Range of Values for Demographic Information for AD and Controls
| Demographic Variable | AD | Control |
|---|---|---|
| Gender (M/F)* | 30/19 | 16/36 |
| Age | 74.41 (±8.53) | 71.42 (±10.57) |
| Range | 52–87 | 50–87 |
| Education | 15.37 (±3.11) | 14.76 (±2.66) |
| Range | 8–21 | 7–20 |
| MMSEa | 23.60 (±5.24) | 29.29 (±1.04) |
| Range | 17–30 | 26–30 |
Abbreviations: AD, Alzheimer disease; SD, standard deviation; MMSE, Mini-Mental State Examination.
Statistically significant difference (P < .05).
Measures
Daily Functional Measure
The Direct Assessment of Functional Status (DAFS)20 is a performance-based measure of daily activities, which was specifically designed to be used in patients with dementia. It is a well-validated measure of functional ability with high inter-rater and test–retest reliability.20 Briefly, the DAFS has demonstrated inter-rater reliabilities (κs) mostly in the mid .9 range, with the lowest for a particular subscale being .91. This test also shows high test–retest reliability with Cohen κs ranging from .57 to .92 for the individual subscales. Finally, good convergent and discriminative validities have been displayed when the DAFS was compared with brief functional screening tests (ie, r =−.59 when correlated with the Blessed Dementia Rating scale). This instrument assesses 7 functional domains, which then can be partitioned into more specific tasks: (1) time orientation assesses (a) ability to tell time using a clock (8 points) and (b) orientation to person, place, and date (8 points); (2) communication skills assesses (a) the ability to use a telephone (8 points) and (b) the ability to prepare and mail a letter (6 points); (3) transportation skills assesses (a) the ability to identify road signs (10 points) and knowing driving rules (3 points); (4) financial skills assesses (a) the ability to identify currency (7 points), (b) the ability to count currency (4 points), (c) the ability to write a check (4 points), (d) the ability to balance a checkbook (of 4 points); (5) shopping skills assesses (a) the ability to “shop” from a mock grocery store by freely recalling shopping items (6 points), (b) “shop” by recognizing items (6 points), and (c) “shop” with a list (4 points). The ability to make correct change (1 point) is also included on the shopping skills subscale, but for the purposes of this study and for analysis it was separated as its own subscale. Finally, the DAFS has 2 other scales that involve grooming and eating abilities, which most participants scored a near perfect score and thus were not included in the analyses. Examiners presented the specific tasks to the participants and rated their ability based on observed performance.
Neuropsychological Measures
Memory tests
The California Verbal Learning Test–Short Form (CVLT-SF)21 is a measure of rote verbal learning and memory in which a list of 9 words is presented over 4 trials and recalled after a 10-minute delay. This test produces a number of outcome measures, including total words recalled during the learning trials and items recalled after a 10-minute delay. For the purposes of this study, we calculated a “percentage savings score” outcome measure by dividing free recall of items after the 10-minute delay period by the number of items recalled during the last (fourth) learning trial and multiplying by 100.
The Rey-Osterrieth Complex Figure22 (ROCF) 30-minute delay is a test of visual memory in which participants are asked to reproduce from memory a complex design they were asked to copy 30 minutes prior. The Meyer and Meyer23 scoring method was used in which the complex drawing was divided into18 components and each component is given a score ranging from 0 to 2, for a total score of 36 points for a perfect drawing. Again, we were interested in the savings of information, thus a percentage savings score was calculated by dividing the points earned after the 30-minute delay portion by the points earned during the initial drawing of the design and this ratio was then multiplied by 100.
Executive functioning test
Wisconsin Card Sorting Test24 (WCST) is a complex test of cognitive flexibility and reasoning ability. Participants are required to match 64 stimulus cards to 4 key cards. The cards can be matched based on 3 abstract principles: color, shape, or number. The participant is provided with very little feedback and has to use abstract reasoning skills to complete the task accurately. The test was manually administered and computer scored to increase scoring accuracy. There are several outcome measures and for the purposes of this project the total correct categorical sorts, total errors committed, and the number of perseverative (repetitive) errors committed were used.
Controlled Oral Word Association25 (for letters F, A, and S). This is a test of the participant’s ability to produce words starting with the letters F, A, and S in 1 minute per letter. The total number of correct words produced for each letter served as the outcome measures.
Language tests
Boston Naming Test–Shortened (BNT).26 Of the original 60 BNT items, on this shortened version, participants were asked to name 15 line drawn objects. If participants were unable to name objects freely, they were given semantic cues (ie, given a cue regarding the category to which an object belongs) and/or phonemic cues (ie, provided with the first syllable of the object name). Participants were given 1 point for each correct response that was freely provided or recalled with semantic cueing, for a total of 15 possible points.
Category fluency27,28 is designed to test the participant’s ability to produce words from a given category (ie, animals). As such, it is believed to be a test of language production drawn from semantic knowledge. The total number of correct words produced for the category “Animals” in 60 seconds served as the outcome measure.
Visual-spatial test
ROCF22 Copy condition—this portion of the test is designed to measure the participant’s ability to copy a complex line design. The Meyer and Meyer23 scoring method was used so that participants were given full or partial credit for accurate recall and placement of 18 details of the design. A total score of 36 is possible.
Data Analyses
Composite scores for each neuropsychological domain were computed in the following way. First, using means and standard deviations from the controls, patient scores were converted into z scores. This way, the degree to which patients deviated from “normal” performance was captured. Second, composite scores were created by averaging z scores of tests that represent 4 cognitive domains: memory, executive, language, and visual-spatial. Compiling composite scores is a method used in previous research,4,5 as it allows for management of the large number of outcome variables in some neuropsychological domains, as well as rendering performance metrics across tests comparable. The composite score for the neuropsychological domains was calculated in the following way: (1) the memory domain was computed by averaging the z scores for percentage savings responses on the CVLT-SF and ROCF; (2) the executive domain was computed by averaging the z scores for the WCST categories achieved, total errors committed, and number of perseverative errors committed, as well as the number of words produced during the F, A, S task; (3) the language domain was computed by averaging the z scores for words produced during the category fluency task and the number of correctly identified items on the 15-item BNT; and (4) the visual-spatial domain was based on the z score value obtained for copying the ROCF design.
The neuropsychological composite z scores were then used as dependent variables so that performance of patients with AD across the various cognitive domains could be compared. It should be noted that z scores were adapted so that the greater the value, the better the performance.
To render performance metrics across the DAFS subscales comparable, subscale scores were converted into percentage correct responses by dividing the participant’s score obtained on a DAFS subscale by the total points possible on that sub-scale and multiplying by 100.
Results
Comparison of Neuropsychology Domains in AD
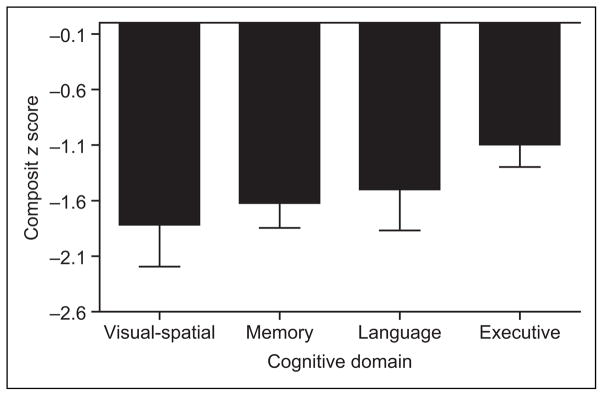
A multivariate analysis of variance (MANOVA) was performed in order to assess the pattern of domain-specific cognitive deficits in patients with AD. Composite z scores created for the memory, executive functioning, language, and visual-spatial domains were used as the dependent variables. The analysis revealed significant differences in performance by the patients with AD in the cognitive domains, Wilk Lambda F(3, 27) = 5.07, P < .0001. Least significant difference (LSD) post hoc analyses further revealed that patients with AD performed best in the executive functioning domain relative to all 3 other domains (P values < .05). While greater than nearly 1.5 standard deviation below normal controls, the AD group’s performance in the memory, language, and visual-spatial domains did not statistically differ. Figure 1 better illustrates this pattern of performance.
Figure 1.
Comparison of DAFS Subscales
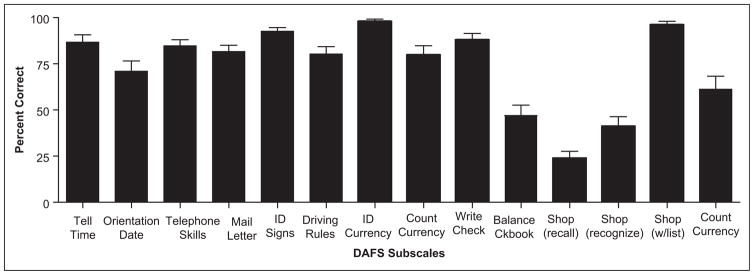
To better understand the pattern of test performance for patients with AD on the specific DAFS subscales, a repeated measures analysis of variance (ANOVA) was performed and the results revealed significant differences between the sub-scales, Wilk Lambda F(13, 37) = 33.44, P <.0001. Least significant difference post hoc analyses revealed multiple differences between the 14 subscales. The largest difference and most significant finding was that free recall shopping was the worse performed subscale relative to all of the others, followed by recognition of shopping items (which was performed worse than all other subscales except balancing a checkbook) and finally balancing a checkbook was performed worse than all other subscales (except for recognition of shopping items; all P values < .05). The data are presented in Figure 1 as well as Table 2. In Table 2, mean percentage correct values and 95% confidence intervals are presented for AD and controls. As can be seen, the controls performed at or near 100% on most subscales, with the shopping task being their worst performance. On the other hand, the patients with AD performed within the 90% accuracy range on only 3 subscales, with 6 subscales being performed at a 60% or less accuracy rate.
Table 2.
Average Percentage of Correct Responses on the DAFS Tasks for AD and Controls Sorted by Better-to-Worst Accuracy Ranges
| Accuracy Range | Patients With AD
|
Controls
|
||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| DAFS Task | Mean Accuracy | 95% CI | DAFS Task | Mean Accuracy | 95% CI | |
| 100–90% | ID currency | 98.25 | 96.26–100.00 | ID currency | 100.00 | 100.00–100.00 |
| Shop with a list | 96.43 | 93.15–99.71 | Shop with a list | 100.00 | 100.00–100.00 | |
| ID road signs | 92.65 | 88.64–96.66 | Count currency | 99.51 | 98.53–100.00 | |
| ID road signs | 98.82 | 97.61–100.00 | ||||
| Tell time | 98.04 | 95.57–100.00 | ||||
| Orientation to date | 97.55 | 94.85–100.00 | ||||
| Write check | 97.06 | 94.37–99.74 | ||||
| Mail letter | 96.08 | 92.89–99.27 | ||||
| Telephone skills | 95.10 | 93.10–97.10 | ||||
| Driving rules | 94.77 | 90.85–98.69 | ||||
| Make change | 92.45 | 85.10–99.80 | ||||
| 80% | Write check | 88.27 | 81.86–94.67 | Shopping (recognition) | 81.05 | 75.58–86.51 |
| Tell time | 86.73 | 78.83–94.64 | ||||
| Telephone skills | 84.69 | 77.89–91.50 | ||||
| Mail letter | 81.63 | 74.76–88.49 | ||||
| Count currency | 80.10 | 70.61–89.60 | ||||
| 70% | Driving rules | 80.27 | 72.23–88.31 | Balance checkbook | 74.51 | 65.90–83.12 |
| Orient to date | 70.92 | 59.63–82.21 | ||||
| 60% | Make change | 61.22 | 47.08–75.36 | Shopping (free recall) | 63.40 | 56.57–70.23 |
| 40% | Balance checkbook | 46.94 | 35.45–58.45 | |||
| Shopping (recognition) | 41.50 | 31.67–51.32 | ||||
| 20% | Shopping recall | 24.15 | 17.10–31.20 | |||
Abbreviations: AD, Alzheimer disease; CI, confidence interval; DAFS, Direct Assessment of Functional Status.
Classification Rate
In order to assess how well the DAFS subscales predict AD and control group membership, a series of logistic regression analyses were performed. The groups were designated as the dependent variable and each of the 14 DAFS subscales were entered individually in each analysis as the independent variable. The results for the analyses are presented in Table 3. Of the 14 DAFS subscales, all but the ability to identify currency and shopping with a list significantly classified AD and controls. Additionally, the specificity rate (classifying controls as “normal” or “healthy”) for most subscales was in the 80%, 90%, or 100% range. However, sensitivity rates (classification of AD as patients) were much lower. The tasks that were most impressive in accurately classifying patients were shopping from free recall and balancing a checkbook. Sensitivity for shopping within a recognition paradigm and writing a check were also adequate, but all other DAFS tasks classified patients with AD with approximately 50% or less accuracy.
Table 3.
Results of Logistic Regression Analyses
| DAFS Variables | Sensitivity | Specificity | Overall Classification | Odds Ratio | Wald χ2 | P Value |
|---|---|---|---|---|---|---|
| Tell time | 26.5% | 94.1% | 61.0% | 0.92 | 4.78 | .03 |
| Orientation to date | 42.9% | 94.1% | 69.0% | 0.96 | 9.48 | .002 |
| Telephone skills | 51.0% | 64.02% | 57.8% | 0.95 | 6.10 | .01 |
| Mailing a letter | 51.0% | 83.0% | 67.6% | 0.95 | 10.12 | .001 |
| ID road signs | 34.7% | 90.6% | 63.7% | 0.91 | 5.67 | .02 |
| Driving rules | 40.8% | 86.8% | 64.7% | 0.97 | 8.63 | .003 |
| ID currency | 8.2% | 100.0 | 55.9% | 0.24 | 0.01 | ns |
| Count currency | 34.7% | 98.1% | 6.6% | 0.91 | 5.73 | .02 |
| Write check | 75.0% | 77.4% | 76.2% | 0.96 | 5.76 | .02 |
| Balance checkbook | 81.3% | 75.5% | 78.2% | 0.98 | 9.82 | .002 |
| Shopping (free recall) | 81.6% | 83.0% | 82.4% | 0.95 | 29.59 | <.001 |
| Shopping (recognition) | 69.4% | 90.6% | 80.4% | 0.95 | 23.34 | <.001 |
| Shopping (with a list) | 14.3% | 100.0% | 58.8% | 0.92 | 3.49 | ns |
| Making change | 38.8% | 92.5% | 66.7% | 7.76 | 11.78 | .001 |
Abbreviations: ns, not statistically significant; AD, Alzheimer disease; DAFS, Direct Assessment of Functional Status.
Prediction of Daily Functioning in AD
Using the neuropsychological z score domains of the patients with AD as the independent variables, individual stepwise regression analyses were performed using the specific DAFS percentage correct subscales as the dependent variables. The results of the analyses are presented in Table 3. The visual-spatial domain was the single best predictor for the DAFS identification of road signs and the ability to count currency. The language domain was the single best predictor of DAFS subscales assessing the ability to use the telephone, mail a letter, identify currency, write a check, and make change. The memory domain was the best predictor of patient’s orientation to date, and executive functioning best predicted the ability to balance a checkbook on the DAFS. A few DAFS subscales were best predicted by multiple cognitive domains. While visual-spatial skills was the best predictor for the ability to tell time (accounted for 42% of the variability), but above and beyond this domain, language skills accounted for an additional 8% of the variability. Similarly, the ability to identify driving rules was best accounted for by visual-spatial skills (10%), but above and beyond this domain, memory skills accounted for an additional 11% of the variability. Shopping from memory (free recall) was best predicted by memory (accounted for 35% of the variability) and executive functioning (additional 8% of the variability). Shopping by recognition was best predicted by the memory domain (accounted for 41% of variability), followed by executive functioning (accounted for 24% of variability), and finally by visual-spatial (accounted for an additional 4% of variability).
Discussion
One of the purposes of the present study was to assess the pattern of deficits that mild-stage AD patients relative to controls display on an observation-based daily functional task and on traditional neuropsychological tests. Interesting and informative results were revealed. First, patients with AD performed poorest on memory, visual-spatial, and language cognitive domains, with relative sparing of the executive functioning domain. While our findings are in line with some studies demonstrating that in early AD, this pattern of cognitive decline is to be expected,5 other research has shown that executive dysfunction is not uncommon in patients with very-mild-to-mild AD.8,9 Neuropathological changes in AD, particularly to the bitemporal/parietal hypoperfusion and hippocampal atrophy, appear to be associated with particular impairment in memory (both verbal and nonverbal), visual perceptual/spatial deficits, and language impairment,28,29 while executive dysfunction may be a result of the early disconnection of the cortico-cortical tracts.30,31 Additionally, we might have expected greater impairment in memory functioning relative to the other domains. One explanation for why the memory domain was not more impaired relative to the others may be that we selected to include savings scores in our overall memory composite score. Due to initial learning deficits (both on CVLT and ROCF tasks), the deficits may have been attenuated.
Second, we were interested in learning the pattern of daily functional decline in patients with AD as compared to normal controls. The results revealed that patients with AD performed at a 60% or lower accuracy rate on 4 of the 14 subscale tasks. The shopping from memory (free recall) task was performed at an accuracy rate of only about 24%. Moreover, patients with AD had difficulty with this task even when they were provided with a recognition paradigm for selecting shopping items (approximately 41% accuracy rate). This pattern of functional impairment makes theoretical sense, given that memory deficits are the hallmark of AD,1,2 that memory was the most impaired cognitive domain in this sample of patients, and that these subscales rely heavily on memory skills as they require participants to encode and recall a set of 6 shopping items both freely and with recognition cueing (ie, to select the items from a mock grocery store). It is possible that atrophy found in temporal/parietal lobes of patients with AD contributes to their diminished ability to encode and retrieve the list of shopping items, as it does to their memory functioning on formal episodic memory tests. Of course, this hypothesis will need to be tested with formal brain imaging and/or neuropathological studies. Interestingly, patients with AD performed remarkably well (96.5% accuracy) when they were given a shopping list, which again suggests that they are likely to have difficulty with daily tasks that require strong episodic memory demands.32 Balancing a checkbook was also problematic for patients with AD as they were only able to obtain accuracy rates of about 46% on this task, and making correct change was challenging as they only obtained 60% accuracy scores.
Conversely, the normal controls performed at or better than an 80% accuracy rate on 12 out of the 14 subscales. Balancing a checkbook (75%) and shopping for items from free recall (63%) appeared to be most challenging for normal controls. However their accuracy rates were far greater than that of patients with AD. Further investigation into factors that pose difficulty for normal elderly individuals when performing these tasks is needed. It may be that the memory demands of the recalling 6 items for a 10-minute period and the sequential steps and mathematical skills needed to balance a checkbook is more problematic than other daily tasks for normal individuals. Additionally, the accuracy rate for balancing a checkbook may be an issue of task familiarity. These scores may be somewhat lower, given that the controls in this study were mainly comprised of females (eg, balancing a checkbook may not be a task that this elderly female cohort performs normally). Future studies are needed using a more balanced composition of males and females.
Of particular interest is the fact that patients with AD obtained relatively high scores on the transportation subscales. Understanding these findings is important given that driving abilities are frequently scrutinized and privileges often revoked in patients diagnosed with dementia. There is clear evidence in the literature to suggest that patients with AD commit greater number of errors on driving simulation tasks33,34 and on real-life driving tasks.33 It may appear counterintuitive that the patients with AD in our study performed better on these DAFS subscales relative to the others, given previous results about driving difficulties. However, in our study, the DAFS only assessed the ability to identify road signs and driving rules and not actual driving ability which is likely due to poor processing of visual sensory cues and attentional deficits.33–35 This may be a noteworthy shortcoming of the DAFS test that requires further investigation and possibly modification.
As rightly pointed out by previous researchers, better understanding the ability of functional tests in accurately classifying patients and controls would be most useful for clinicians.28 As such, the current study examined the sensitivity and specificity of each of the 14 DAFS tasks. The results revealed that while specificity rates for accurately classifying controls were relatively high for most of the DAFS tasks, sensitivity rates were rather low for patients with AD. It appears that the ability to shop from free recall, balance a checkbook, shop with recognition cueing, and write a check were the most sensitive in accurately classifying patients with AD. One reason the other tasks were not as sensitive may be due to the fact that patients with AD were in the mild stages of illness and that in the more advanced disease stage the DAFS may be capable of better differentiating AD from controls. To the best of our knowledge, this is the first study to assess the classification rates of patients with AD using a performance-based functional measure. Thus, further investigation using more severe stages of illness and perhaps other dementia groups is needed to better understand these findings. Additionally, the tasks that were best at differentiating patients with AD from controls seem to rely most heavily on memory and/or involve more task complexity.
The result of our study also directly addresses the ability of neuropsychological tests to predict everyday functional status in patients with mild AD. Our findings revealed that approximately 10% to 69% of the variability in functional performance was predicted by the cognitive domain studies in patients with AD. These findings are quite similar to those found by Farias and colleagues,16 but further expand on their results given that in our study we examined more detailed sub-scales of the DAFS measure and included a more comprehensive neuropsychological test battery. Similar to the findings of Farias et al,16 we found that from a theoretical standpoint the majority of the associations between neuropsychological and daily functional performance made sense. The language domain appeared best predict of daily functioning that required communication of information, such as making a phone call, mailing a letter, writing a check, and verbally identifying currency. Making correct change also depended on language skills, perhaps because on this task participants are not required to solve complex math problems, but the demand was to communicate the correct amount of change. Basic visual-spatial skills appeared to be the single most important factor needed in discriminating objects (such as road signs and counting currency), while memory was an important factor for recall of information, whether it related to orientation to time and place, or episodic memory for shopping items. Our findings also suggest that executive functioning best predicted activities such as shopping with a list, balancing a checkbook, and to some degree, shopping from free recall or within a recognition paradigm. This is not surprising as one major component of executive functioning is the ability to plan and to effectively carry out an action.36 Specifically, in planning an activity, one must be able to devise a strategy for the given task (eg, shopping, balancing a checkbook) and weigh the option and make choices to be made, while maintaining good impulse control. Taken together, our findings suggest that the DAFS tasks predicted by the executive functioning and memory domains were among the most complex, requiring sequential processing, planning, and retention of learned materials.
Better understanding which neuropsychological tests best predict functioning has important theoretical and clinical importance. Theoretically, it allows us to better understand the cognitive processes, such as planning, organization, and encoding, thought to best be assessed by tests of executive functioning and episodic memory, that underlie actual daily tasks. Similarly, since few clinicians, particularly neuropsychologist,37 report using actual performance-based daily tasks to assess patient’s functional abilities, it is important to understand the cognitive tasks and domains that best predict such functioning.32
The present study increases our understanding about the pattern of performance of patients with AD on neuropsychological and daily functional tests. Overall, these findings suggest that even in the mild stages of AD, functions such as shopping (particularly when performed from memory or by item recognition alone) are the first to be significantly impaired. Most often, neuropsychologists do not administer formal, standardized performance-based measures of daily functioning,16 thus the current study provides empirical findings regarding the pattern of functional decline that can be used by clinicians in planning treatment. Additionally, clinicians can similarly use the findings of the current study in predicting what daily tasks may be most impaired in patients when only formal neuropsychological test findings are available for patients.
It should be noted that there were some limitations to the current study. First, we understand that neuropsychological tests do not typically represent a single, distinct cognitive domain, but tap into multiple domains. This is, in some cases, evidenced by the multiple domains that predicted functioning in the regression model. However, for the purposes of this study, we needed to place tests into distinct cognitive domains. This placement was based on the domain that the test best represented based on the findings of previous research. Second, in the current study it happened that more male patients and more female controls volunteered. We understand that this is a limitation and recognize that replication of this study with a more balance gender composition is needed. Similarly, findings may differ for diverse ethnic and cultural groups and that future research is needed for those groups. Additionally, we studied a group of patients with AD in the mild stages of the disease. It is possible that the results and patterns of performance on neuropsychological and functional tests would change as a result of disease severity and/or type of dementia. This warrants further investigation. Another consideration is that this study did not collect detailed data on medication intake. Thus, how specific types and dosages of various medications may have affected the performance of the patients with AD or controls is not available and should be further examined in future studies. Finally, this study was conducted in a laboratory-like setting, and it should be noted that a host of intervening factors that were not systematically studied could affect daily functioning in patients with AD. As aptly detailed by Marcotte et al37 a host of variables, such as the testing environment, limited sampling of behavior, individualized approaches to problem solving, level of motivation, and specific compensatory strategies could mediate or intervene in the relationship between neuropsychological performance and daily functional abilities.
Figure 2.
Table 4.
Results of Stepwise Regression for Patients With AD Using Cognitive Domain Scores as the Independent Variable to Predict Various DAFS Subscale Scores
| DAFS Subscales | Predictor | Adjusted R2 | Standardized B | F | P Value |
|---|---|---|---|---|---|
| Telling time | Visual-spatial | .42 | .58 | 21.69 | <.0001 |
| Language | .49a | .31 | 4.94 | .035 | |
| Orientation to date | Memory | .29 | .56 | 12.83 | .001 |
| Telephone skills | Language | .31 | .58 | 14.09 | .001 |
| Mail letter | Language | .36 | .62 | 17.03 | <.0001 |
| Identify road signs | Visual-spatial | .23 | .51 | 9.84 | .004 |
| Driving rules | Visual-spatial | .10 | .36 | 4.38 | .04 |
| Memory | .21a | .36 | 4.64 | .04 | |
| Identify currency | Language | .15 | .42 | 6.06 | .02 |
| Count currency | Visual-spatial | .24 | .52 | 10.28 | .003 |
| Write check | Language | .35 | .61 | 16.37 | <.0001 |
| Balance checkbook | Executive function | .33 | .59 | 15.05 | .001 |
| Shopping (free recall) | Memory | .35 | .55 | 16.62 | <.0001 |
| Executive function | .43a | .32 | 5.17 | .03 | |
| Shopping (recognition) | Memory | .41 | .57 | 21.30 | <.0001 |
| Executive function | .65a | .45 | 20.19 | <.0001 | |
| Visual-spatial | .69a | .23 | 4.47 | .04 | |
| Shopping (with a list) | Executive function | .24 | .51 | 10.08 | .004 |
| Making change | Language | .17 | .44 | 6.78 | .015 |
Abbreviations: AD, Alzheimer disease; DAFS, Direct Assessment of Functional Status.
This value is the combined effect of the 2 predictors.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: NIGMS grant GM048680 to JR. Additional support for the project was provided by CSUN College of Social & Behavioral Sciences, NIGMS grant GM063787 (Minority Biomedical Research Support Program-Research Initiative for Scientific Enhancement) & NIMH T34 MH20023 (Career Opportunities in Research).
Footnotes
This study was conducted at California State University, Northridge (CSUN).
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- 1.Hodges J. The amnestic prodrome of Alzheimer’s disease [editorial] Brain. 1998;121(pt 9):1601–1602. doi: 10.1093/brain/121.9.1601. [DOI] [PubMed] [Google Scholar]
- 2.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 3.Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124(pt 1):96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- 4.Vliet EC, Manly J, Tang M, Marder K, Bell K, Stern Y. The neuropsychological profiles of mild Alzheimer’s disease and questionable dementia as compared to age-related cognitive decline. J Int Neuropsychol Soc. 2003;9(5):720–732. doi: 10.1017/S1355617703950053. [DOI] [PubMed] [Google Scholar]
- 5.Razani J, Boone K, Miller BL, Lee A, Sherman D. Neuropsychological performance of right- and left-frontotemporal dementia compared to Alzheimer’s disease. J Int Neuropsychol Soc. 2001;7(4):468–480. doi: 10.1017/s1355617701744037. [DOI] [PubMed] [Google Scholar]
- 6.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparison of verbal fluency tasks in the detection of dementia of the Alzheimer’s type. Arch Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 7.Lafleche G, Albert M. Executive function deficits in mild Alzheimer’s disease. Neuropsychology. 1995;9(3):313–320. [Google Scholar]
- 8.Baudic S, Barba GD, Thibaudet MC, Smagghe PR, Traykov L. Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol. 2006;21(1):15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Crowell TA, Luis CA, Vanderploeg RD, Schinka JA, Mullan M. Memory patterns and executive functioning in mild cognitive impairment and Alzheimer’s disease. Aging Neuropsychol Cogn. 2002;9(4):288–297. [Google Scholar]
- 10.Searight RH, Dunn EJ, Grisso T, et al. The relation of the Halstead-Reitan neuropsychological battery to rating of everyday functioning in a geriatric sample. Neuropsychology. 1989;3:135–145. [Google Scholar]
- 11.Dunn EJ, Searight HR, Grisso T, Margolis RB, Gibbons JL. The relation of the Haltead-Reitan neuropsychological battery to functional daily living skills in geriatric patients. Arch Clin Neuropsychol. 1990;5(2):103–117. [PubMed] [Google Scholar]
- 12.Razani J, Kakos B, Orieta-Barbalace, et al. Predicting caregiver burden from daily functional abilities of patients with mild dementia. J Am Geriatr Soc. 2007;55(9):1415–1420. doi: 10.1111/j.1532-5415.2007.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loewenstein DA, Arguelles S, Bravo M, et al. Caregiver’s judgments of the functional abilities of the Alzheimer’s disease patient: a comparison of proxy reports and objective measures. J Gerontol B Psychol Sci Soc Sci. 2001;56(2):P78–P84. doi: 10.1093/geronb/56.2.p78. [DOI] [PubMed] [Google Scholar]
- 14.La Rue A. Aging and Neuropsychological Assessment. New York, NY: Plenum Press; 1992. [Google Scholar]
- 15.Razani J, Casas R, Wong JT, Lu P, Alessi C, Josephson K. The relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl Neuropsychol. 2007;14(3):208–214. doi: 10.1080/09084280701509125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farias ST, Harrell CN, Neumann C, Houtz A. The relationship between neuropsychological performance and daily functioning in individuals with Alzheimer’s disease: ecological validity of neuropsychological tests. Arch Clin Neuropsychol. 2003;18(6):655–672. [PubMed] [Google Scholar]
- 17.Loewenstein DA, Rubert MP, Berkowitz-Zimmer N, Guterman A, Morgan R, Hayden S. Neuropsychological test performance and prediction of functional capacities in dementia. Behav Health Aging. 1992;2(3):149–158. [Google Scholar]
- 18.Rabin LA, Burton LA, Barr WB. Utilization rates of ecologically oriented instruments among clinical neuropsychologists. Clin Neuropsychol. 2007;21(5):727–743. doi: 10.1080/13854040600888776. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Loewenstein DA, Amigo E, Duara R, et al. A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. J Gerontol. 1989;44(4):P114–P121. doi: 10.1093/geronj/44.4.p114. [DOI] [PubMed] [Google Scholar]
- 21.Delis D, Kramer J, Kaplan E, et al. Adult Version-Manual. 2. San Antonio, TX: The Psych Corp; 2000. California Verbal Learning Test. [Google Scholar]
- 22.Osterrieth PA. Le test de copie d’une figure complexe. Arch de Psych. 1944;30:206–356. [Google Scholar]
- 23.Meyer JE, Meyer KR. In: A Training Manual for the Clinical Scoring of the Rey-Osterrieth Complex Figure and the Recognition Subtests. Meyers John E., editor. Marian Health Center, Department of Psychology; Sioux City, IA: 1992. [Google Scholar]
- 24.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test (WCST) Manual-Revised and Expanded. Odessa, FL: PAR; 1993. [Google Scholar]
- 25.Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 26.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- 27.Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neurospychol. 1987;9(5):479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- 28.Monsch AU, Bondi MW, Butters N, et al. A comparison of category and letter fluency in Alzheimer’s disease and Huntington’s disease. Neupsychology. 1994;8(1):25–30. [Google Scholar]
- 29.Smith GE, Bondi MW. Normal aging, mild cognitive impairment, and Alzheimer’s disease. In: Morgan JE, Ricker JH, editors. Textbook of Clinical Neuropsychology. New York, NY: Taylor & Francis; 2008. pp. 599–615. [Google Scholar]
- 30.Kessler J, Mielke R, Grond M, Herholz K, Heiss W. Frontal lobe tasks do not reflect frontal lobe function in patients with probable Alzheimer’s disease. Int J Neurosci. 2000;104(1–4):1–15. [PubMed] [Google Scholar]
- 31.Collette F, Delrue G, Van Der Linden M, Salmon E. The relationships between executive dysfunction and frontal hypometabolism in Alzheimer’s disease. Brain Cogn. 2001;47(1–2):272–275. [Google Scholar]
- 32.Lowenstein D, Acevedo A. The relationship between instrumental activities of daily living and neuropsychological performance. In: Marcotte TD, Grant I, editors. Neuropsychology of Everyday Functioning. New York, NY: Guilford Press; 2010. [Google Scholar]
- 33.Frittelli C, Borghetti D, Iudice G, et al. Effects of Alzheimer’s disease and mild cognitive impairment on driving ability: a controlled clinical study by simulated driving test. Int J Geriatr Psychiatry. 2009;24(3):232–238. doi: 10.1002/gps.2095. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo M, Reinach S, McGehee D, Dawson J. Simulated car crashes and crash predictors in drivers with Alzheimer’s disease. Arch Neurol. 1997;54(5):545–551. doi: 10.1001/archneur.1997.00550170027011. [DOI] [PubMed] [Google Scholar]
- 35.Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver landmark and traffic sign identification in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76(6):764–768. doi: 10.1136/jnnp.2004.049338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 37.Marcotte TD, Scott C, Kamat R, Heaton RK. Neuropsychology and the prediction of everyday functioning. In: Marcotte TD, Grant I, editors. Neuropsychology of Everyday Functioning. New York, NY: Guilford Press; 2010. pp. 39–61. [Google Scholar]