Abstract
Smoking-related biomarkers for lung cancer and other diseases are needed to enhance early detection strategies and to provide a science base for tobacco product regulation. An untargeted metabolomics approach by ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UHPLC-Q-TOF MS) totaling 957 assays was used in a novel experimental design where 105 current smokers smoked 2 cigarettes one hour apart. Blood was collected immediately before and after each cigarette allowing for within-subject replication. Dynamic changes of the metabolomic profiles from smokers’ four blood samples were observed and biomarkers affected by cigarette smoking were identified. Thirty-one metabolites were definitively shown to be affected by acute effect of cigarette smoking, uniquely including menthol-glucuronide, the reduction of glutamate, oleamide, and 13 glycerophospholipids. This first time identification of a menthol metabolite in smokers’ blood serves as proof-of-principle for using metabolomics to identify new tobacco-exposure biomarkers, and also provides new opportunities in studying menthol-containing tobacco products in humans. Gender and race differences also were observed. Network analysis revealed 12 molecules involved in cancer, notably inhibition of cAMP. These novel tobacco-related biomarkers provide new insights to the effects of smoking which may be important in carcinogenesis but not previously linked with tobacco-related diseases.
Keywords: Metabolomics, UHPLC-QTOF-MS, Smokers, Plasma, Menthol
Introduction
Lung cancer early detection and prevention strategies are limited because there are no validated biomarkers of lung cancer risk for smokers. The Food and Drug Administration (FDA) now has the authority to regulate new tobacco products design and constituents, assess health claims by manufactures for some products purportedly that reduce risk compared to conventional tobacco products, and to enact performance standards. To do this, the FDA will rely on biomarkers and clinical trials to provide a science base for FDA decision-making (1). One way to identify new biomarkers for tobacco-related disease is to study the global metabolic impact on smokers from cigarette smoke, which is poorly understood for both the chemical metabolites of smoke (i.e., biomarkers of exposure) and the metabolites produced by cellular and organ reactions in response to smoke constituents (i.e., biomarkers of effect). By using untargeted metabolomic profiling, perturbations of many detectable metabolites (metabolome) in response to a treatment or disease can be quantitatively measured (2). The metabolome consists of peptides, oligonucleotides, sugars, organic acids, ketones, aldehydes, amino acids, lipids, steroids, alkaloids from cell reactions and foreign chemicals (e.g., medicines, cigarette smoke, and environmental toxins). Levels of these compounds may be affected by genetics, gender, age, hormones, metabolic rate, environment, lifestyle, disease, and other xenobiotic exposures. Metabolomic profiles, therefore, represent phenotypic responses for both endogenous processes and exogenous exposures. Previously, we have validated the use of untargeted metabolomics in smokers’ blood (3), and also have shown that this methodology is highly reproducible in a large scale study that identified diagnostic and prognostic markers in non–small cell lung cancer (NSCLC) patients (4).
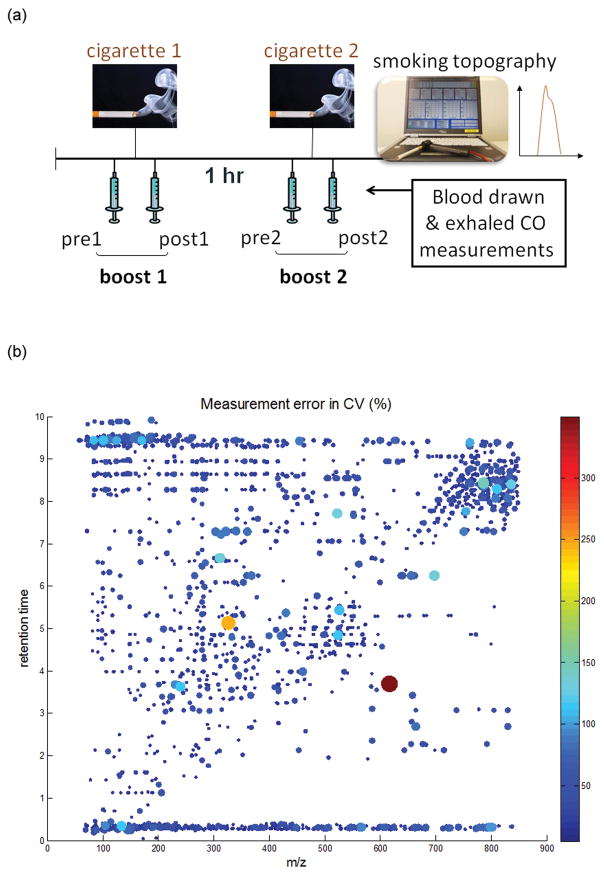
In order to assess the complex outcome of cigarette smoking on smokers’ metabolomes, we used blood from a cross-sectional epidemiological study of well-characterized smokers, where blood was collected immediately before and after smoking, done twice for smoking two cigarettes one hour apart (Fig. 1a). Given the study design, the results are not confounded by other exposures, for example, subjects were not eating, exposed to car exhaust or did any activities during the one hour interval other than answering questions. The comparison of results before and after the cigarette, with replication, therefore establishes unambiguously that the metabolomic phenotype measured herein is solely related to smoking. Experiments were conducted under various quality control procedures including an assessment for the coefficient of variation (%CV) on features from pooled samples (Fig. 1b, see additional details in Fig. S1–S2).
Fig. 1.
(a) Study design for the cross-sectional study of smokers. (b) Distribution of the estimated measurement error in CV (%) before filtering. Each dot represents a single peak. The dot size and color corresponds to the CV value: the larger the dot, and the brighter the color of the dot, the larger the CV value of the individual peak.
Materials and Methods
Study Participants
Plasma samples were obtained from a study of 105 smokers at the National Institute of Drug Abuse (NIDA) and Georgetown University (GU). Eligible subjects were at least 18 years old, current smoking > 10 cigarettes/day for at least 5 years and had a stable smoking pattern for at least one year. Exclusion criteria included active respiratory tract or oral cavity disease, prior history of cancer, recent general anesthesia, recent smoking cessation therapy or antidepressants, psychiatric disorder or any other reason that precludes understanding the informed consent, and pregnancy. Questionnaires included demographics, detailed smoking history, past medical history and behavior. This study was approved by the Institutional Review Boards of Georgetown University, The National Institute on Drug Abuse, and the Ohio State University.
Smoking Protocol and Biospecimen Collection
All participants smoked two cigarettes of their usual brand, one hour apart (Fig. 1a). The participants were asked to smoke one of their cigarettes naturally as the first cigarette and to smoke their second cigarette using the Clinical Research Support System Device (CReSS; Plowshare Technologies, Baltimore, MD) that measures puff topography. Carbon monoxide (CO) levels in the participant’s expired air were also determined before and five minutes after each cigarette by Vitalograph (Vitalograph Inc, Lenexa, KS). Blood was collected immediately before and two minutes after smoking each cigarette and heparin-containing green-top tubes were used in this study. All blood samples were processed immediately and stored in the −80°C. Nicotine, cotinine and trans-3′-hydroxycotinine levels in the blood samples were determined by gas chromatography and liquid chromatography–tandem mass spectrometry, as previously described (5, 6). During the 60 minutes between smoking, subjects completed in-person interviews. Cotinine levels, nicotine boosts (i.e., the level of increase before and after a cigarette) and CO boosts were determined to serve as an important validity check of exposure (Fig. S3a). Subjects with negative nicotine boost levels or with plasma cotinine levels less than 100 ng/ml were excluded to ensure subjects with active smoking history in the study (Fig. S3b).
UHPLC-QTOF-MS Analysis
Experiments were performed in positive and negative ion modes by Ultra-High Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-Q-TOF MS) for the four time points from 105 smokers for a total of 957 assays, including 117 controls (see QC section for more details). Sample aliquots were mixed with 195 μL of 66% acetonitrile (ACN) containing the internal standards debrisoquine and 4-nitrobenzoic acid (4-NBA). Following centrifugation, the supernatant was injected onto a reverse-phase 50 × 2.1 mm ACQUITY 1.7-μm C18 column (Waters, Milford, MA) using an ACQUITY UPLC® system (Waters, Milford, MA) with a gradient mobile phase consisting of 2% ACN in water containing 0.1% formic acid (A) and 2% water in ACN containing 0.1% formic acid (B). Each sample was resolved for 10 min at a flow rate of 0.5 ml/min. The gradient consisted of 100% A for 0.5 min and decreased to 40% A over 3.5 minutes, and then to 0% over the next 4 min, and held at 100% B for one additional minute. The column eluent was introduced directly into the mass spectrometer by electrospray. Mass spectrometry was performed on a Q-TOF Premier (Waters, Milford, MA) operating in negative-ion (ESI−) and positive-ion (ESI+) electrospray ionization modes with a capillary voltage of 3200 V and a sampling cone voltage of 20 V in negative mode and 35 V in positive mode. The desolvation gas flow was set to 800 liters/h and the temperature was set to 350°C. The cone gas flow was 25 liters/h, and the source temperature was 120°C. Data were acquired in centroid mode from 50 to 850 m/z in MS scanning. Raw data were converted to Network Common Data Format (NetCDF) files using MassLynx (Waters, Milford, MA). They were then preprocessed by XCMS online, a cloud-based metabolomic data processing platform (7), for feature detection, retention time correction, retention time alignment to obtain a list of features in which each feature is represented by its m/z value, retention time and intensities across samples.
Quality Control (QC)
Experimental reproducibility of the instrument was assessed over multiple days for replicates and quality control samples (Fig. S1 & S2), as previously reported (3). In order to ensure the quality and reproducibility of the data, pooled samples from seven nonsmoker subjects were placed as every tenth sample totaling 62 analyzes (3). Also, 10% of the subjects plasma (n=55) was repeated at the end of the experiment. A list of features was used to assess the variability among the pooled QC samples by comparing coefficient of variation (%CV) along with m/z values and retention times to detect the measurement error of the LC-MS system, if any (Fig. 1b). A total of 389 out of 4809 features with a greater than 15% CV were excluded for later analysis.
Data Analysis
Preprocessed data sets were analyzed using SIMCA (Umetrics Inc, Kinnelon, NJ), Matlab (MathWorks, Natick, MA), JMP (SAS, Cary, NC), Partek Genomics Suite (Partek Inc, St Louis, MO) and Metaboanalyst (www.metaboanalyst.ca). Significant features were searched against METLIN Metabolomics Database, Human Metabolome Database (HMDB), and LIPID MAPS Structure Database (LMSD) with the mass accuracy of ten parts per million to identify putative metabolite identifications.
To ensure that the data followed the assumptions of normality, data was log2 transformed. Since blood cotinine level is a more precise measure of nicotine consumption than self-reported usage (cigarette per day) (8), plasma cotinine levels were used in the analysis to represent smokers’ cigarette exposure. Paired t tests and linear mixed-effects models were performed on the relative intensity of the features based on a four-way analysis of covariance (ANCOVA) adjusted for gender, race and cotinine levels by using a residual maximum likelihood (REML) technique. In addition, Fisher’s Least Significant Difference (LSD) contrast method was used to determine pairwise differences in metabolite levels between post- to pre- cigarettes, gender and race for each cigarette. The results of specific comparisons were obtained using false discovery rate by the Benjamini-Hochberg procedure (FDR < 0.05) controlling procedures to correct for multiple testing. To identify the correlation between metabolites of interest, and the correlation of metabolites to known covariates including plasma cotinine levels, Pearson correlation or Spearman’s rank correlation were performed accordingly. The construction, interaction, and pathway analysis of potential biomarkers was performed by Ingenuity Pathways Analysis (IPA, Ingenuity Systems) tool in order to identify the biological functions, mechanisms, and pathways that are most relevant to the metabolites of interest.
Validation of Metabolites
HPLC with MS/MS was used to confirm the identity of detected metabolites (Phenomenex Luna NH2 column on a Dionex Ultimate 3000 HPLC system, coupled to a Bruker maXis 4G ESI Q-TOF), operated in positive and negative modes. The instrument was calibrated with Agilent Low-Concentration Tuning Mix (Agilent, Santa Clara, CA) before sample analysis, and the capillary voltage was set at 4500 V for positive mode and 4000 V for negative mode. The metabolite identifications were confirmed by matching the retention time, mass error and isotopic pattern of the parent ion, and tandem mass spectrum of the parent ion under the same collision energy from the biological sample to that of the commercially available standard metabolites.
Reagents and chemicals
All reagents and solvents were of HPLC grade. 4-NBA, debrisoquine sulfate, and oleamide were purchased from Sigma-Aldrich (St. Louis, MO); ACN and water were purchased from Fisher Optima grade (Fisher Scientific, Waltham, MA); L-glutamate was purchased from ChromaDex (Irvine, CA); lysoPC(14:0), lysoPC(15:0), lysoPC(16:0), lysoPC(18:0), lysoPE(18:0), lysoPC(18:1), lysoPC(19:0), and lysoPC(20:0) were purchased from Avanti (Alabaster, Alabama); menthol-glucuronide was purchased from Toronto Research Chemicals (North York, ON, Canada).
Results
Characteristics of smokers in the study
Participants in this study (n=105) were predominantly male (63.8%) and African-American (60%). Mean age of study participants was 43.0 (range 18–69, SD=9.8) years, with an average smoking history of 22.9 (range 5–50, SD=9.9) years. Participants reported smoking 19.9 (SD=8.4) cigarettes per day on a typical day, and 17.8 (SD=9.6) cigarettes in the previous 24 hours.
Untargeted metabolomics profiling
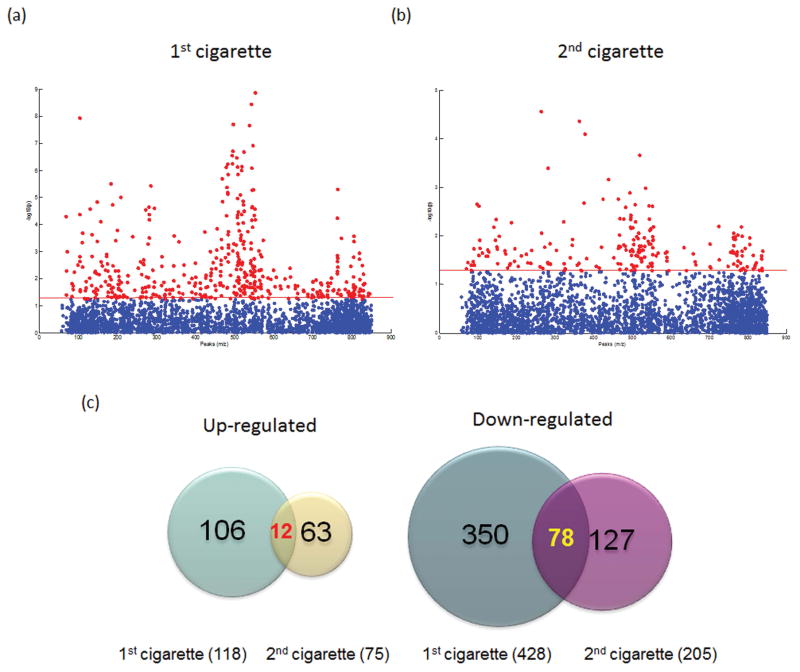
Pair-wise comparison of pre– and post– cigarette samples showed 546 features from the 1st cigarette and 280 features from the 2nd cigarette that were significantly increased or decreased when smoking the cigarette (p < 0.05), before correcting for multiple comparisons (Fig. 2a–b). Among those features, 12 were consistently increased and 78 were decreased across both cigarettes (Fig. 2c). A 4-way analysis of covariance (ANCOVA) model was used controlling for gender, race, and cotinine levels for effect of metabolite expression due to cigarette smoking, because the study did not include equal numbers of race and gender, and blood cotinine is a more accurate measure of nicotine consumption than self-reported usage reflecting the actual intake (8). The mean F-ratio for each factor was computed to show the significance of different sources of variation in the entire data in the ANCOVA model (Fig. S4). If the F-ratio of a factor is higher than the error, that factor contributes significant variation to the data versus random error across all the variables. Based on the ANCOVA model, all factors including cotinine, gender, race, and pre– & post– cigarettes contributed significant variation to the data across all the variables.
Fig. 2.
Scatter plots representation of significant features regulated by (a) the first cigarette, and (b) the second cigarette. Significant features were selected by paired t-tests with threshold 0.05. The red dots represent features above the threshold. All p values are transformed by -log10 so that the more significant features (with smaller p values) were plotted higher on the graph. (c) Venn diagram representing metabolites significantly increased or decreased after 1st and 2nd cigarette.
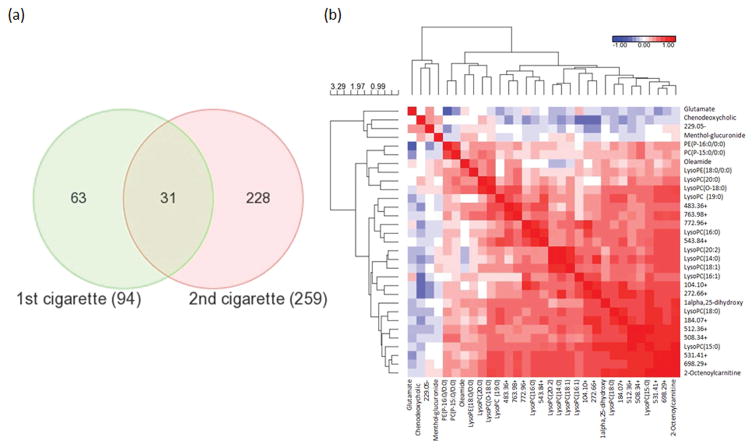
Correcting for multiple comparisons, there were 31 features that differed between pre-1 to post-1 paired samples of the first cigarette (FDR < 0.05) that also were significantly different (and in the same direction) for the second cigarette with a p value < 0.05 (Fig. 3a), where 27 decreased and 4 increased (Table S1). Table S2 provides the Spearman rank correlation coefficients of significant metabolites in the baseline to plasma cotinine levels. The main chemical taxonomy classes of those metabolites were putatively identified as glycerophospholipids (13), sterol lipids (2), amino acids, peptides, and analogues (1), fatty acid esters (1), fatty amides (1), and prenol lipids (1). Twelve features could not be mapped to current databases and therefore their identities are unknown. Metabolite identifications were confirmed using commercially available standards, and the structures and MS/MS spectra of representative metabolites and their respective authentic standard compounds are shown in Fig. S6. The confirmed identities were menthol-glucuronide, lysoPC(19:0), lysoPC(18:0), lysoPC(16:0), lysoPC(14:0), lysoPE(18:0/0:0), lysoPC(15:0), lysoPC(18:1), glutamate, lysoPC(20:0) and oleamide (Table S1). Covariances among 31 significant features on the baseline (pre-1 time point) were evaluated using a heat map (Fig. 3b). All glycerophospholipids were clustered together with positive correlations, indicating a more global effect on glycerophospholipids by smoking. Other known metabolites positively correlated with the glycerophopholipids included 1α,25-dihydroxy-11-(4-hydroxymethylphenyl)-9,11-didehydrovitamin D3, oleamide, and 2-octenoylcarnitine. Menthol-glucuronide, a menthol metabolite, increased the most robustly (p = 3.05E-09, FDR = 4.58E-08) among all metabolites of interests (Fig. S5). It was positively correlated with 229.05–, 2-octenoylcarnitine and glutamate, and negatively correlated with oleamide, lysoPC(16:1) and 508.34+ (p < 0.05, Table S3).
Fig. 3.
Significant metabolites and their putative identifications. (a) Venn-diagram represents the significant metabolites influenced by cigarette smoking from the ANCOVA model; (b) Pairwise correlations from pre-1 cigarettes for the significant metabolites. Abbreviations: phosphatidylethanolamine (PE), phosphatidylcholines (PC), lysophosphatidylcholines (LysoPC), lysophosphatidylethanolamine (LysoPE).
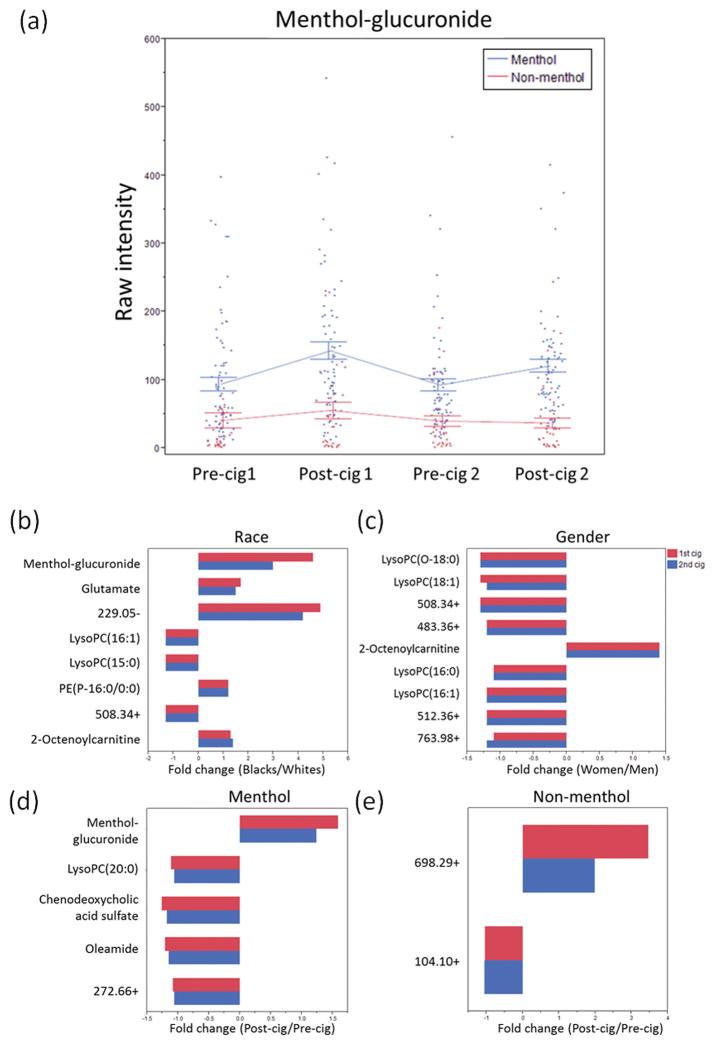
The levels of menthol-glucuronide for all time points were statistically higher in the menthol smokers than in the non-menthol smokers, independent of race (Fig. 4a). Menthol levels increased with smoking for the menthol smokers, but not nonsmokers, and the level decreased between cigarettes (Fig. 4a), consistent with the known half-life of 56.2 minutes (9). Menthol-glucuronide was positively correlated with baseline cotinine level in all smokers (Table S2, r=0.2; p=0.04) and menthol smokers (Table S2, r=0.31; p=0.008), and was positively correlated to nicotine boost (Table S4, r=0.26; p=0.04) and CO boost (Table S4, r=0.23; p=0.04) in all smokers.
Fig. 4.
(a) Levels of menthol-glucuronide across all time points. Blue dots and lines represent menthol smokers and red dots and lines represent non-menthol smokers. Each error bar was constructed using 1 standard error from the mean. Differences in (b) Race, (c) Gender, and among (d) Menthol cigarette smokers and (e) Non-menthol cigarette smokers on the significant metabolites after both cigarette smoking. All differences in fold change were significant at p < 0.05 level.
Race and menthol smoking effects
Random Forest analysis was used to perform supervised classifications for race differences on the pre–1 cigarette plasma. There was 81.7% accuracy for race (Fig. S7a). Principal component analysis (PCA) modeling of the significant metabolites demonstrated separation of metabolomic profiles between Black and White smokers (Fig. S8a). In order to determine the racial differences in the metabolic capacity impacted by cigarette smoking, ANCOVA model controlling for gender and cotinine levels was conducted and 8 features were significantly different between Blacks and Whites (p < 0.05, Fig. 4b). There were 5 metabolites significantly higher in Black smokers, namely menthol-glucuronide (fold change=3.0–4.6), glutamate (fold change=1.5–1.7), 229.05– (fold change=4.2–4.9), PE(P-16:0/0:0) (fold change=1.2), and 2-octenoylcarnitine (fold change=1.3–1.4). Metabolites higher in White smokers were lysoPC(16:1), lysoPC(15:0) and 508.34+ (fold change=1.3). Among the smokers in our study, 92% of the Black participants smoked menthol cigarettes compared to 31% of the White smokers. In order to determine the effect of the cigarette additive on the metabolomics profiles, smokers were stratified to menthol (n=71) and non-menthol (n=34) smokers, and 4-way ANCOVA models controlled for gender, race, and cotinine levels were used on both groups, separately. Significantly different features by the type of cigarette smoked overlapped from two cigarettes (FDR < 0.05 from the 1st cigarette and p < 0.05 from the 2nd cigarette) indicated five from the menthol smokers namely menthol-glucuronide, lysoPC(20:0), chenodeoxycholic acid sulfate, oleamide, 272.66+, and 698.29+ and 104.10+ from non-menthol smokers (Fig. 4d–e).
Gender effects
Random Forest analysis showed 87.3% accuracy for gender (Fig. S7b). PCA model of the significant metabolites demonstrated differential metabolomic profiles between male and female smokers (Fig. S8b). After adjustment for race and cotinine levels by ANCOVA model, 8 metabolites were significantly higher among men than women (p < 0.05) (Fig. 4c), namely lysoPC(O-18:0), lysoPC(18:1), 508.34+, 483.36+, lysoPC(16:0), lysoPC(16:1), 512.36+, and 763.98+. In contrast, 2-octenoylcarnitine was higher among women than men after smoking both cigarettes (p < 0.05, Fig. 4c).
Pathway analysis and networks affected
The thirty-one significant metabolites affected by cigarette smoking were analyzed by IPA, using the KEGG IDs, and projected onto Ingenuity’s knowledge-based networks. Considered in this analysis were direct and indirect relationships including endogenous chemicals, focusing on interaction networks observed from all data sources. There were total of five KEGG IDs associated with the identifiable metabolites, and three out of five were mapped by IPA for the analysis, namely 1-acylglycerophosphocholine (representing 13 glycerophospholipids in the study), l-glutamic acid, and oleamide. Carbohydrate metabolism, lipid metabolism, and small molecule biochemistry were the top three molecular and cellular functions affected (p < 0.05).
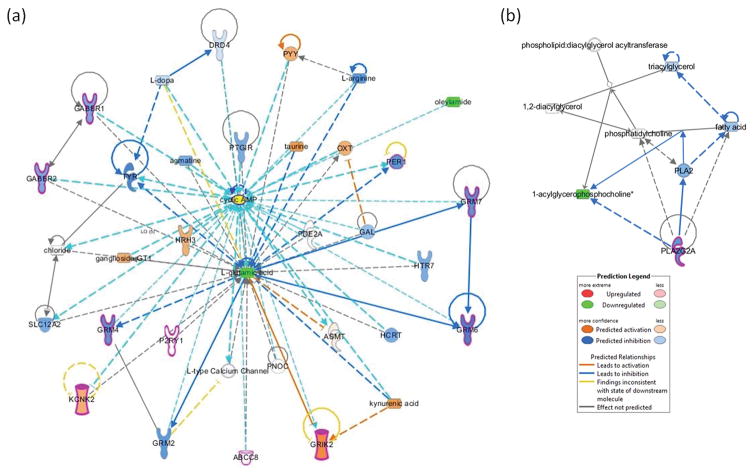
Network analysis was generated de novo based on the mapped metabolites to explore potential molecular events and mechanisms affected by cigarette smoking. It uses information obtained from the literature to assemble and extrapolate known interactions, signaling, as well as the relationships between these entities. Two networks affected with the associated functions of (1) Amino acid metabolism, small molecule biochemistry, cell-to-cell signaling and interaction, with 11 molecules involved in cancer; and (2) Cellular compromise, connective tissue development and function, and organ morphology, with 1 involved in cancer, were observed (Fig. 5a–b). The 12 molecules involved in cancer are ABCC8, cyclic AMP, GABBR1, GABBR2, GRIK2, GRM4, GRM6, GRM7, KCNK2, P2RY1, PER1, and PLA2G2A. Using the Molecule Activity Predictor within IPA to predict the in silico upstream and downstream effects in the network, IPA predicted that decreased level of L-glutamic acid, and oleamide in the first network will lead to the inhibition of the glutamate receptors GRIK2, GRM4, GRM6, and GRM7, and ultimately the inhibition of cyclic AMP (Fig. 5a). Cyclic AMP mediates a total of 33 relationships in this network. The decreased levels of glycerophospholipids in the second network were the result of the inhibition of calcium-dependent phospholipase A2 enzyme PLA2G2A (Fig. 5b).
Fig. 5.
Two networks associated with cigarette smoking. Green nodes represented metabolites decreased in level in our study. Twelve molecules known as biomarkers for cancer were outlined in magenta.
Discussion
In 2009, The United States Congress passed the Family Smoking Prevention and Tobacco Control Act (FSPTCA) authorizing the FDA to regulate the tobacco industry, including requiring performance standards that govern exposures and assessing products putatively developed to reduce exposure (and health claims associated with them). Critical to these efforts are human studies using biomarkers of exposure and harm in both observational studies and clinical trials, in lieu of long-term epidemiology studies. While there are chemically-specific biomarkers for smoke exposure that are being studied for risk, e.g., cotinine, tobacco-specific nitrosamines and 1-hydroxypyrene (10). These biomarkers have proved useful for estimates of tobacco exposure but other biomarkers are needed to understand the relation between tobacco exposure and biologic effect. Separately, new biomarkers of smoking-related disease are needed to improve the early detection of lung cancer and other diseases. For example, while low-dose CT screening is now recommended for lung cancer screening, biomarkers could be useful for identifying persons who would most benefit from screening, or assist in follow-up for detection lesions with better decision making about invasive procedures.
In this study, using a novel experimental design where an individual smoked two cigarettes allowing for within-subject replication, dynamic changes on the smoking profiles were observed and biomarkers affected by cigarette smoking were identified. Thirty-one metabolites were definitively shown to be affected by cigarette smoking, among which a menthol metabolite was the most significant. There are current considerations for menthol regulation by the Food and Drug Administration. This first identification of a menthol metabolite in smokers’ blood serves as a proof-of-principle for untargeted metabolomics to find new biomarkers of smoking, and also provides new opportunities for human studies of menthol tobacco products. For example, e-cigarette vapors do not have most of the tobacco toxicants, but some are mentholated and so the menthol-glucuronide might be useful for studies of e-cigarettes that need to assess exposure. Separately, the effects of menthol on smoking behavior and carcinogen exposure have been inconclusive (11), and so this marker could help better define exposure. Other metabolites were identified including the reduction of glutamate and oleamide, and the systematic reduction of 13 glycerophospholipids after cigarette smoking. The network analysis indicated multiple inhibitions to the glutamate receptors and the inhibition of cyclic-AMP, which may be important in carcinogenesis but not previously linked with tobacco-related carcinogenesis. Networks affected involved total of 12 known molecules for cancer.
The menthol glucuronide levels in this study rose with each cigarette, and the baseline level was correlated with the plasma cotinine levels in all smokers combined, and more so for menthol cigarette smokers. Menthol was detected in all smokers because menthol is contained in all cigarettes, including ones not marketed as containing menthol (12). While menthol glucuronide has never been identified before in the blood of smokers, in urine, it has been correlated with cigarettes per day, cotinine, and tobacco-smoke carcinogens (12). In this previous study, menthol glucuronide was measured in 54% of regular cigarette smokers, compared to 82% of menthol cigarette smokers. However, our study identified the menthol glucuronide in the blood of all smokers.
This study identified new endogenous biomarkers that were affected by smoking, namely a decrease in 13 glycerophospholipids, including 11 lysoPCs. These compounds, also known as phosphoglycerides, are important cell membrane constituents that maintain structural integrity and ion permeability, and in pulmonary surfactant to reduce surface tension during expiration (13). In animal models, consistent with our data, glycerophospholipids decreased with smoke exposure in liver (14) and with cigarette-containing naphthalene treatment in lung (15). These are biologically potent compounds presenting as minor phospholipids in the plasma (8–12%) and cellular membranes (≥ 3%) (16). They are involved in inflammation and immune responses (17), and assist cell differentiation (18). Patients with atherosclerotic diseases (19) have lower levels of plasma lysoPCs and these compounds induce inflammation of human coronary artery smooth muscle cells (20), while inhibiting endothelial cell migration and proliferation (21). In the lung, they are protective against lung vascular injury mediated by the inhibition of neutrophil NADPH oxidase activation through the elevation of intracellular cAMP (22). From the pathway analysis herein, decreased levels of glycerophospholipids were the result of the predicted inhibition of calcium-dependent phospholipase A2 enzyme PLA2G2A (Fig. 5b). Reduced expression of PLA2G2A has been associated with high-grade tumors, increased lymph node metastasis, increased venous invasion, lymphatic invasion in esophageal squamous cell carcinoma (ESCC) (23), and reduced in overall survival rate in ESCC (23) and gastric cancer (24). Thus, lower levels of lysoPCs may contribute to lung cancer, heart disease and lung disease.
Previous studies using untargeted metabolomics have also identified the effects of smoking on lipid metabolites, as reported herein. One studied 25 smokers and 25 non-smokers, who first identified differences in a number of fatty acids that were either higher or lower in smokers than non-smokers, including 3 lysoPCs that were increased and 5 that decreased (25). A second study of 28 smokers versus 101 nonsmokers also observed that some lysoPCs went up while others decreased, with glycerophospholipid changes for 30 subjects who quit smoking (26, 27). The identified changes, however, were different than those reported herein, but these studies have the potential for confounding due to their cross-sectional nature, while our study demonstrates conclusively the impact of smoking on glycerophospholipid levels. In our study, baseline levels of PE(P-16:0/0:0), PC(P-15:0/0:0), oleamide, lysoPE(18:0/0:0), lysoPC(20:0), lysoPC(O-18:0) and lysoPC(19:0) from both cigarettes were significantly lower than non-smokers (QCs) while lysoPC(16:1) and lysoPC(18:1) were higher than non-smokers (FDR < 0.05, Fig. S9). LysoPC(16:1) and lysoPC(18:1) of smokers both have greater dynamic range than non-smokers compare to other significant metabolites (Fig. S9). In addition, the level of lysoPC(16:1) was higher among Whites than Blacks (Fig. 4b), and the level of lysoPC(18:1) was higher among men than women (Fig. 4c). Importantly, lysoPC(16:0), lysoPC(18:0), and lysoPC(18:1) that are changed herein have been noted as lower in the plasma of lung cancer patients compared to controls (28). Concluding from the literature and our findings, decreased levels of lysoPC(16:0), lysoPC(18:0) and lysoPC(18:1) could be potential biomarker for early carcinogenesis influenced by cigarette smoking to be evaluated in future prospective studies.
Oleamide in this study was found to decrease with smoking. This is consistent with a previous report indicating that levels decrease in smokers and in lung cancer patients compared to controls (29). However, a mechanism of action for cancer is unclear, although it is widely used experimentally to inhibit gap junction intercellular communication, may serve as a proliferative agent for human lymphocytes and breast cancer cells, and derivatives of oleamide are being investigated as an antimetastatic agent (30, 31).
In the present study, plasma glutamate levels were significantly decreased after cigarette smoking. Glutamate is a crucial nutrient involved in more than 40 metabolic pathways, maintaining cellular functions and has an important role in amino acid and carbohydrate metabolism (33). Krebs cycle is the key metabolic pathway in the carbohydrate metabolism that’s altered by mainstream cigarette smoke in lung cells (34). The decrease of glutamate could be due to the deamination catalyzed by glutamate dehydrogenase to the production of α-ketoglutarate, an intermediate in the Krebs cycle, which plays an anaplerotic function in the carbohydrate metabolism (33). Together with l-cysteine and glycine, glutamate is also one of the precursors for the synthesis of glutathione, the major cellular protective antioxidant in the lungs (35). Low plasma glutamate and glutamine levels are often found in patients with chronic obstructive pulmonary disease (COPD) (36) and emphysema (37), and is associated with an increase in glycolytic metabolism within respiratory and peripheral skeletal muscle, and reduction of glutathione levels (37, 38). Xu et al. reported significant higher serum levels of glutamate among smokers compared to non-smokers in their cross-sectional study (27), while Mandal et al. reported higher plasma glutamate among smokers than non-smokers (39); neither reported results specific to smoking a cigarette. Here we also observed higher plasma levels of glutamate among smokers from the baseline compared to non-smokers (Fig. S10). The deprivation of glutamate found herein could be due to the up-regulation of glutathione synthesis, or an increased glutaminolysis rate, all in response to cigarette smoking. From the pathway analysis, decreased levels of plasma glutamate after cigarette smoke lead to the inhibition of glutamate receptors: GRM4, GRM6, GRM7 and GRIK2 (Fig. 5a). Glutamate receptors are present in the nervous system as well as peripheral organs including liver, kidney, lung, muscle, and blood cells. Decreased expression of metabotropic glutamate receptor 4 (encoded by GRM4) has been shown to increase cancer cell growth (40). While the mechanism behind the acute effect of cigarette smoking in decreasing plasma glutamate remains unexplored, more studies are needed to elucidate the role of different glutamate receptor subtypes in peripheral circulation influenced by cigarette smoking.
There is evidence that for a given level of smoking history, women may be at increased risk for lung cancer compared to men. Genetics and hormonal factors have been proposed as smoking-attributable risks for lung cancer in women (41). It is known that estrogen is involved as important regulators of plasma lipid metabolism and lipid profile. Women smokers are at higher risk developing dyslipidemia (42) and cardiovascular diseases (43). Aberrant plasma lipid profiles were often found in various cancers and are associated with cancer risk (44), and gender-specific lipid metabolites are also found in lung cancer patients (29). However, definitive mechanisms underlying this disparity are still unclear. In our study, 8 metabolites including 4 lysoPCs were lower, and 2-octenoylcarnitine is higher in women than men after smoking. It reveals gender differences in cigarette-influenced metabolites and suggests that attention should be paid for their application as biomarkers in studying gender-specific early carcinogenic events.
Lung cancer incidence and mortality rates are higher in Blacks than Whites, and Black smokers preferentially smoke menthol cigarettes compared to Whites (45). Differences in genetics, nicotine metabolism, and smoking behavior could all play a role on this health disparity. In our study, menthol-glucuronide, glutamate, 229.05-, PE(P-16:0/0:0), 2-octenoylcarnitine were higher among Blacks and lysoPC(16:1), lysoPC(15:0), 508.34+ were higher among Whites. These differences appear to be related to preferences for specific types of cigarettes, as we observed stronger preference for menthol cigarettes among Blacks (92%) than Whites (31%) in our study.
Perturbations in small molecules by wet total particulate matter and gas/vapor phase from mainstream whole smoke treated human alveolar epithelial carcinoma (A549) cells were investigated previously (34), and changes of small molecule metabolic pathways in amino acid, carbohydrate, lipid, energy, nucleotide, coenzyme, vitamin & others were found (34). The pathway analysis herein indicated that carbohydrate metabolism, lipid metabolism and small molecule biochemistry were the top three metabolic processes affected in smokers, all of which are known to be affected by smoking (46). The major network affected includes 12 known cancer molecules and among their biological functions are inhibition of second messenger cAMP, which in turn leads to many signal transduction cascades including the inhibition of proliferation, collagen synthesis, myofibroblast transformation and to mediate antiproliferative effects in lung fibroblasts (47). Inhibitors of a cAMP specific phosphodiesterase 4 (PDE4) preventing the breakdown of cAMP is used for the treatment of asthma (48) and COPD (49). Based on our pathway analysis, we suggest for the first time, that the acute effects of cigarette smoking could lead to a reduction of cellular cAMP level, provide proliferative advantage for DNA damaging cells, and thus create a microenvironment for tumor growth and respiratory disease.
There are strengths and limitations to our study. One important strength is the unique study design that assessed smokers immediately before and after smoking a cigarette. This allowed us to examine the dynamic changes on the plasma metabolome regulated by the acute effects of cigarette smoking without confounding by other exogenous exposures that would affect the metabolome (e.g., diet, medication, and lifestyle). Acute effects happen very rapidly after a single exposure has occurred, and chronic effects happen only after repeated long-term exposure due to numerous single exposures. Thus, single acute exposures, well documented by our methods, have direct relevance to chronic risk since they represent what happens for each and every cigarette smoked. Furthermore, oleamide is an endogenous substance occurs naturally in the body, and was found to decrease with smoking in this study. It has also been reported as slip agent from polymers along with a larger amide erucamide (32). However, no erucamide was found in the datasets and no signs of polymer contamination occurred during the experiment. Therefore, the results reported herein are reflecting dynamic oleamide changes in the metabolome, while contaminants would be either random or constant and would not pass the stringent statistical tests. Additionally, assessing the smokers for two cigarettes allowed for within-subject replication. There are some limitations of this study, such as low sample size and limited statistical power for some subset analyzes, and causal relationships cannot be established due to nature of the study. Another limitation was the choice of reversed-phase UHPLC column and separation methods, which were chosen to obtain the most abundant profile for our samples. Using this column, highly polar metabolites, such as sugars and amino acids are not retained sufficiently compared to hydrophobic compounds. This methodology also limits the ability to detect some carcinogen-metabolites normally bound to plasma proteins such as polycyclic aromatic hydrocarbons, benzo[a]pyrene, and NNAL. However, this is balanced by the opportunity to study cellular endogenous metabolites affected by smoking, providing some insight into possible disease mechanisms. The identification of unknown metabolites is a major bottleneck in the metabolomics field. It is a complex and costly process limited by the number of commercially available standards, with intensive effort required, and often results in a low yield of correctly characterized metabolites. Therefore, identification of the unknown metabolites was not carried out in the present manuscript. However, spectral interpretation and structural elucidation of the unknown features are needed in the future in order to identify and validate the unknown features in the study.
In summary, a metabolomics assessment was applied to a novel experimental design in smokers to evaluate the acute effects of cigarette smoking. The identification of particular metabolites (e.g., menthol-glucuronide, glutamate, oleamide, and glycerophospholipids) allows for future studies to assess smoking-related exposures affecting endogenous cellular mechanisms and accounting for exogenous smoke exposure that could assist the FDA’s regulation of tobacco products. The pathways identified herein provide insight into tobacco disease pathogenesis. These results, if indicative of cancer risk pathways, could enhance smoking risk assessment and improve early detection strategies.
Supplementary Material
Acknowledgments
Funding sources
Transdisciplinary Tobacco Use Research Center grant P50-CA84718 from the National Cancer Institute and the National Institute on Drug Abuse
American Lung Association Lung Health Dissertation Grant
Laboratory Assessment of Tobacco Use Behavior and Exposure to Toxins N01 PC64402 from National Cancer Institute
Method development and experiments were performed in the Proteomics and Metabolomics Shared Resource (PMSR) of the Lombardi Comprehensive Cancer Center in Georgetown University. Target identification was performed in the Proteomics Shared Resource in the Ohio State University Comprehensive Cancer Center supported by the Cancer Center Support Grants (CCSGs) for NCI-designated Cancer Centers (P30) and the NSF Award 1040302. The authors acknowledge the Center for Biostatistics at the Ohio State University Comprehensive Cancer Center for contributing in data analysis.
Definitions of abbreviations used
- UHPLC-QTOF-MS
ultra-high performance liquid chromatography-quadrupoletime-of-flight mass spectrometry
- ANCOVA
analysis of covariance
- FDR
false discovery rate
- PC
glycerophosphocholines
- PE
glycerophosphoethanolamines
- LysoPC
lysophosphatidylcholines
- LysoPE
lysophosphatidylethanolamine
Footnotes
Disclosures:
Dr. Shields serves as an expert witness in tobacco company litigation on behalf of plaintiffs. The other authors declare that they have no conflict of interest.
References
- 1.Berman ML, et al. Providing a Science Base for the Evaluation of Tobacco Products. Tobacco Regulatory Science. 2015;1(1):76–93. doi: 10.18001/TRS.1.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patti GJ. Separation strategies for untargeted metabolomics. Journal of separation science. 2011;34(24):3460–3469. doi: 10.1002/jssc.201100532. [DOI] [PubMed] [Google Scholar]
- 3.Hsu PC, et al. Feasibility of identifying the tobacco-related global metabolome in blood by UPLC-QTOF-MS. J Proteome Res. 2013;12(2):679–691. doi: 10.1021/pr3007705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathe EA, et al. Non-invasive urinary metabolomic profiling identifies diagnostic and prognostic markers in lung cancer. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-14-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benowitz NL, Zevin S, Jacob P., 3rd Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther. 1998;287(3):958–962. [PubMed] [Google Scholar]
- 6.Jacob P, 3rd, et al. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2011;879(3–4):267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84(11):5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Stable EJ, Benowitz NL, Marin G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24(2):171–179. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 9.Gelal A, Jacob P, 3rd, Yu L, Benowitz NL. Disposition kinetics and effects of menthol. Clinical pharmacology and therapeutics. 1999;66(2):128–135. doi: 10.1053/cp.1999.v66.100455001. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch Surg. 2006;391(6):603–613. doi: 10.1007/s00423-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence D, Cadman B, Hoffman AC. Sensory properties of menthol and smoking topography. Tob Induc Dis. 2011;9(Suppl 1):S3. doi: 10.1186/1617-9625-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benowitz NL, et al. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochimica et biophysica acta. 1998;1408(2–3):79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 14.Canales L, et al. Developmental cigarette smoke exposure: liver proteome profile alterations in low birth weight pups. Toxicology. 2012;300(1–2):1–11. doi: 10.1016/j.tox.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling YS, Liang HJ, Chung MH, Lin MH, Lin CY. NMR- and MS-based metabolomics: various organ responses following naphthalene intervention. Molecular bioSystems. 2014;10(7):1918–1931. doi: 10.1039/c4mb00090k. [DOI] [PubMed] [Google Scholar]
- 16.Munder PG, Modolell M, Andreesen R, Weltzien HU, Westphal O. Lysophosphatidylcholine (Lysolecithin) and its Synthetic Analogues. Immunemodulating and Other Biologic Effects. Springer Seminars in Immunopathology. 1979;2(2):187–203. [Google Scholar]
- 17.Mehta D. Lysophosphatidylcholine: an enigmatic lysolipid. American journal of physiology. Lung cellular and molecular physiology. 2005;289(2):L174–175. doi: 10.1152/ajplung.00165.2005. [DOI] [PubMed] [Google Scholar]
- 18.Ryborg AK, Johansen C, Iversen L, Kragballe K. Lysophosphatidylcholine induces keratinocyte differentiation and upregulation of AP-1- and NF-kappaB DNA-binding activity. Acta dermato-venereologica. 2004;84(6):433–438. doi: 10.1080/00015550410016930. [DOI] [PubMed] [Google Scholar]
- 19.Gillett MP, Besterman EM. Plasma concentrations of lysolecithin and other phospholipids in the healthy population and in men suffering from atherosclerotic diseases. Atherosclerosis. 1975;22(1):111–124. doi: 10.1016/0021-9150(75)90072-6. [DOI] [PubMed] [Google Scholar]
- 20.Aiyar N, et al. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Molecular and cellular biochemistry. 2007;295(1–2):113–120. doi: 10.1007/s11010-006-9280-x. [DOI] [PubMed] [Google Scholar]
- 21.Rikitake Y, et al. Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(4):1006–1012. doi: 10.1161/01.atv.20.4.1006. [DOI] [PubMed] [Google Scholar]
- 22.Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. Journal of immunology. 2005;174(5):2981–2989. doi: 10.4049/jimmunol.174.5.2981. [DOI] [PubMed] [Google Scholar]
- 23.Ren P, Zhang JG, Xiu L, Yu ZT. Clinical significance of phospholipase A2 group IIA (PLA2G2A) expression in primary resected esophageal squamous cell carcinoma. European review for medical and pharmacological sciences. 2013;17(6):752–757. [PubMed] [Google Scholar]
- 24.Xing XF, et al. Phospholipase A2 group IIA expression correlates with prolonged survival in gastric cancer. Histopathology. 2011;59(2):198–206. doi: 10.1111/j.1365-2559.2011.03913.x. [DOI] [PubMed] [Google Scholar]
- 25.Muller DC, et al. Metabolomics using GC-TOF-MS followed by subsequent GC-FID and HILIC-MS/MS analysis revealed significantly altered fatty acid and phospholipid species profiles in plasma of smokers. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2014;966:117–126. doi: 10.1016/j.jchromb.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Wang-Sattler R, et al. Metabolic profiling reveals distinct variations linked to nicotine consumption in humans--first results from the KORA study. PloS one. 2008;3(12):e3863. doi: 10.1371/journal.pone.0003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu T, et al. Effects of smoking and smoking cessation on human serum metabolite profile: results from the KORA cohort study. BMC medicine. 2013;11:60. doi: 10.1186/1741-7015-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong JC, Zhao L, Xue X, Zou L, Zhang X, Liang X. Lysophosphatidylcholine profiling of plasma: discrimination of isomers and discovery of lung cancer biomarkers. Metabolomics. 2010;6(4):478–488. [Google Scholar]
- 29.Guo Y, et al. Probing gender-specific lipid metabolites and diagnostic biomarkers for lung cancer using Fourier transform ion cyclotron resonance mass spectrometry. Clinica chimica acta; international journal of clinical chemistry. 2012;414:135–141. doi: 10.1016/j.cca.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Bisogno T, et al. Biosynthesis and degradation of bioactive fatty acid amides in human breast cancer and rat pheochromocytoma cells--implications for cell proliferation and differentiation. European journal of biochemistry / FEBS. 1998;254(3):634–642. doi: 10.1046/j.1432-1327.1998.2540634.x. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, et al. Connexin 32 and its derived homotypic gap junctional intercellular communication inhibit the migration and invasion of transfected HeLa cells via enhancement of intercellular adhesion. Molecular medicine reports. 2011;4(5):971–979. doi: 10.3892/mmr.2011.509. [DOI] [PubMed] [Google Scholar]
- 32.MJC, DCJ, GD Identification and analysis of polymer additives using packed-column supercritical fluid chromatography with APCI mass spectrometric detection. The Analyst. 1998;123:1827–1833. [Google Scholar]
- 33.Brosnan JT. Glutamate, at the interface between amino acid and carbohydrate metabolism. The Journal of nutrition. 2000;130(4S Suppl):988S–990S. doi: 10.1093/jn/130.4.988S. [DOI] [PubMed] [Google Scholar]
- 34.Vulimiri SV, Misra M, Hamm JT, Mitchell M, Berger A. Effects of mainstream cigarette smoke on the global metabolome of human lung epithelial cells. Chemical research in toxicology. 2009;22(3):492–503. doi: 10.1021/tx8003246. [DOI] [PubMed] [Google Scholar]
- 35.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. The American journal of physiology. 1999;277(6 Pt 1):L1067–1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 36.Rutten EP, Engelen MP, Wouters EF, Schols AM, Deutz NE. Metabolic effects of glutamine and glutamate ingestion in healthy subjects and in persons with chronic obstructive pulmonary disease. The American journal of clinical nutrition. 2006;83(1):115–123. doi: 10.1093/ajcn/83.1.115. [DOI] [PubMed] [Google Scholar]
- 37.Engelen MP, Schols AM, Does JD, Deutz NE, Wouters EF. Altered glutamate metabolism is associated with reduced muscle glutathione levels in patients with emphysema. American journal of respiratory and critical care medicine. 2000;161(1):98–103. doi: 10.1164/ajrccm.161.1.9901031. [DOI] [PubMed] [Google Scholar]
- 38.Barry B, Halliwell HEP. Cigarette Smoke and Oxidative Stress. Springer; 2007. [Google Scholar]
- 39.Mandal A, Mishra S, Mandal P. Sex Specific Differences in GABA and Glutamate Levels in Response to Cigarette Smoke. Scientific Reports. 2013;2(1):1–2. [Google Scholar]
- 40.Luksch H, et al. Silencing of selected glutamate receptor subunits modulates cancer growth. Anticancer Res. 2011;31(10):3181–3192. [PubMed] [Google Scholar]
- 41.Peters SA, Huxley RR, Woodward M. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ open. 2014;4(12):e005663. doi: 10.1136/bmjopen-2014-005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MH, et al. Gender differences in the association between smoking and dyslipidemia: 2005 Korean National Health and Nutrition Examination Survey. Clinica chimica acta; international journal of clinical chemistry. 2011;412(17–18):1600–1605. doi: 10.1016/j.cca.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239(1):260–267. doi: 10.1016/j.atherosclerosis.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Munir R, et al. Atypical plasma lipid profile in cancer patients: cause or consequence? Biochimie. 2014;102:9–18. doi: 10.1016/j.biochi.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Howlader N, et al. SEER Cancer Statistics Review, 1975–2011. U. S.National Institutes of Health; Bethesda, MD: 2014. [Google Scholar]
- 46.Jagadapillai R, et al. Developmental cigarette smoke exposure: kidney proteome profile alterations in low birth weight pups. Toxicology. 2012;299(2–3):80–89. doi: 10.1016/j.tox.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selige J, Tenor H, Hatzelmann A, Dunkern T. Cytokine-dependent balance of mitogenic effects in primary human lung fibroblasts related to cyclic AMP signaling and phosphodiesterase 4 inhibition. Journal of cellular physiology. 2010;223(2):317–326. doi: 10.1002/jcp.22037. [DOI] [PubMed] [Google Scholar]
- 48.Matera MG, Page C, Cazzola M. PDE inhibitors currently in early clinical trials for the treatment of asthma. Expert opinion on investigational drugs. 2014;23(9):1267–1275. doi: 10.1517/13543784.2014.921157. [DOI] [PubMed] [Google Scholar]
- 49.Vignola AM. PDE4 inhibitors in COPD--a more selective approach to treatment. Respiratory medicine. 2004;98(6):495–503. doi: 10.1016/j.rmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.