Abstract
Background: Body mass index (BMI) and endometriosis have been inversely associated. To address gaps in this research, we examined associations among body composition, endometriosis, and physical activity.
Materials and Methods: Women from 14 clinical sites in the Salt Lake City, Utah and San Francisco, California areas and scheduled for laparoscopy/laparotomy were recruited during 2007–2009. Participants (N = 473) underwent standardized anthropometric assessments to estimate body composition before surgery. Using a cross-sectional design, odds of an endometriosis diagnosis (adjusted odds ratio [aOR]; 95% confidence interval [CI]) were calculated for anthropometric and body composition measures (weight in kg; height in cm; mid upper arm, waist, hip, and chest circumferences in cm; subscapular, suprailiac, and triceps skinfold thicknesses in mm; arm muscle and fat areas in cm2; centripetal fat, chest-to-waist, chest-to-hip, waist-to-hip, and waist-to-height ratios; arm fat index; and BMI in kg/m2). Physical activity (metabolic equivalent of task-minutes/week) and sedentariness (average minutes sitting on a weekday) were assessed using the International Physical Activity Questionnaire-Short Form. Measures were modeled continuously and in quartiles based on sample estimates. Adjusted models were controlled for age (years, continuous), site (Utah/California), smoking history (never, former, or current smoker), and income (below, within 180%, and above of the poverty line). Findings were standardized by dividing variables by their respective standard deviations. We used adjusted models to examine whether odds of an endometriosis diagnosis were moderated by physical activity or sedentariness.
Results: Inverse relationships were observed between endometriosis and standardized: weight (aOR = 0.71, 95% CI 0.57–0.88); subscapular skinfold thickness (aOR = 0.79, 95% CI 0.65–0.98); waist and hip circumferences (aOR = 0.79, 95% CI 0.64–0.98 and aOR = 0.76, 95% CI 0.61–0.94, respectively); total upper arm and upper arm muscle areas (aOR = 0.76, 95% CI 0.61–0.94 and aOR = 0.74, 95% CI 0.59–0.93, respectively); and BMI (aOR = 0.75, 95% CI 0.60–0.93), despite similar heights. Women in the highest versus lowest quartile had lower adjusted odds of an endometriosis diagnosis for: weight; mid-upper arm, hip, and waist circumferences; total upper arm and upper arm muscle areas; BMI; and centripetal fat ratio. There was no evidence of a main effect or moderation of physical activity or sedentariness.
Conclusion: In a surgical cohort, endometriosis was inversely associated with anthropometric measures and body composition indicators.
Keywords: : adiposity, anthropometry, body composition, endometriosis, muscle mass
Introduction
At least 11% of women have endometriosis,1,2 a gynecologic disease characterized by endometrial tissue found outside the uterine cavity.3,4 Recent findings suggest that women with endometriosis compared to women without endometriosis are leaner, as measured by body mass index (BMI), while other findings suggest no difference.5–8 These equivocal findings may be a function of varying study populations, diagnostic approaches for endometriosis, or measurement techniques for body composition.1,9–12
Researchers have questioned whether body composition as indicated by BMI has an etiologic role or is a reflection of other factors that may be associated with the development of endometriosis, such as physical activity, parity, or cigarette smoking.7,11,13–19 Nevertheless, BMI has been associated with gynecological functioning. Underweight young adult women are at increased risk for menstrual dysfunction relative to normal weight women20 while women who are overweight are at increased risk for various gynecological disorders, including menstrual dysfunction20 and impaired fecundity.21
Despite its near universal acceptance as a proxy for body composition (i.e., level of adiposity), BMI does not directly measure body composition, especially the amount and distribution of adipose and muscle tissue. Tissue type in the context of obesity is important to distinguish; the biological activity of adipose and muscle tissue depends on the amount, distribution,22 and type of muscle fiber, which differs based on amount of adipose tissue.23
A more accurate measurement of body composition to distinguish between adipose tissue and muscle mass requires anthropometric or other more specialized assessments (e.g., dual-energy x-ray absorptiometry [DXA], bioelectric impedance, or hydrostatic weighing).24 Anthropometry and some types of specialized assessments, such as DXA, may also capture the location and regional distribution of adipose tissue,25 which may be informative about the pathophysiology of endometriosis.
In addition, much of the research on the relationship between BMI and endometriosis has not considered physical activity, although such activity has been associated with endometriosis in some11,13,14,19 but not all studies.26,27 In addition, physical activity and BMI have associated in a bidirectional relationship.28 However, the relationships among physical activity, BMI, and disease are unclear.29
To address the gaps in current research regarding body habitus and endometriosis, we assessed the relationship between anthropometric body composition indicators and a surgical diagnosis of endometriosis. We used anthropometric measures, body composition indicators, and body fat distribution ratios. In addition, we investigated whether physical activity may moderate the association between body composition and endometriosis.
Material and Methods
Study design and populations
Data were used from the Endometriosis, Natural History, Diagnosis and Outcomes (ENDO) Study; these methods have been described elsewhere.1 Our study sample comprised 475 women scheduled for gynecologic laparoscopy or laparotomy irrespective of surgical indication (e.g., pelvic pain, pelvic mass, and menstrual irregularities) from 14 clinical sites located in the Salt Lake City, Utah and San Francisco, California metropolitan areas between 2007 and 2009. Eligibility criteria were as follows: aged 18–44 years, currently menstruating, no history of cancer except for nonmelanoma skin cancer, no breastfeeding for 6 months or more, and no injectable hormone treatment within the past 2 years. By design, women with previously surgically visualized endometriosis (prevalent disease) were excluded to capture incident diagnoses. Two women cancelled their surgeries resulting in a sample of 473 women.
Data collection and operational definitions
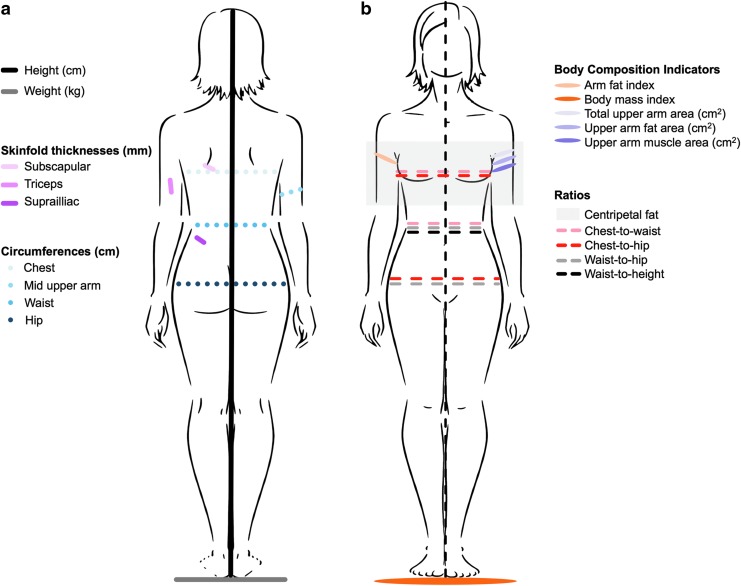
Upon enrollment and ∼2 months before surgery, trained research staff conducted in-person computer-assisted interviews with women to capture sociodemographic, lifestyle, and medical history information. Next, staff performed standardized anthropometric assessments with a protocol,25 as illustrated in Figure 1a. The following measurements were taken using calibrated and certified equipment: height (centimeters; cm) using a fixed stadiometer (214 Road Rod portable stadiometer [seca Corporation, Hamburg, Germany; US office, Hanover, MD] or the wooden Shorr Board [Shorr Productions, Olney, MD]); weight (kilograms; kg) using a balance scale; triceps, subscapular, and suprailiac skinfold thicknesses (millimeters; mm) using a Lange Skinfold Caliper (Beta Technology, Inc., Santa Cruz, CA); and mid-upper arm, waist, and hip body circumferences (cm) using tape measures. Chest circumference (cm) was estimated from self-reported bra size using well-established algorithms.30 Bra size was used instead of directly measuring chest size to (a) decrease women's discomfort that could arise when measuring around the chest and (b) address variability in the possible position of breasts on the chest. Waist circumference measurements were taken at the natural indentation of the waist.25 To maximize reliability, all measurements were taken twice. If the first two measurements differed by ≥0.5 cm for height, ≥0.1 kg for weight, ≥4 mm for any skinfold thickness, or ≥0.5 cm for body circumferences, then a third measurement was taken.
FIG. 1.
(a) Methodology for performing anthropometric assessments and (b) body composition indicators and body fat distribution ratios calculated using anthropometric measures.
Anthropometric measurements were averaged for analysis and then converted to various indicators of muscularity and adiposity (described below), called body composition indicators hereon in this article.31–33 Body circumferences were used to derive ratios indicating body fat distribution. Body composition indicators and body fat distribution ratios are illustrated in Figure 1b. Formulas used to derive body composition indicators are as follows: arm fat index = (upper arm fat area/total upper arm area) × 100; BMI = weight (kg)/height (m)2; total upper arm area = mid upper arm circumference (cm)2/(4 × π); upper arm fat area = total upper arm area − upper arm muscle area; upper arm muscle area = ([mid upper arm circumference(cm)] − [(triceps skinfold thickness[cm]) × π]2)/(4 × π). BMI categories were defined as follows: underweight <18.5 kg/m2; normal weight ≥18.5 and ≤25.0 kg/m2; overweight ≥25.0 and ≤30 kg/m2; and obese ≥30 kg/m2.34 Formulas used to derive body fat distribution ratios were as follows: centripetal fat = subscapular skinfold thickness (mm)/(subscapular skinfold thickness [mm] + triceps skinfold thickness [mm]); chest-to-waist = chest circumference (cm)/waist circumference (cm); chest-to-hip = chest circumference (cm)/hip circumference (cm); waist-to-hip = waist circumference (cm)/hip circumference (cm); and waist-to-height = waist circumference (cm)/height (cm).
Women completed the International Physical Activity Questionnaire-Short Form (IPAQ-SF) to assess degree of physical activity and inactivity.35 The IPAQ-SF captures physical activity as frequency (days), duration (hours and minutes), and intensity of physical activity, which were used to compute metabolic equivalent of task (MET)-minutes of weekly physical activity (continuous). Importantly, the IPAQ enabled us to assess inactivity as frequency (days) and duration (hours and minutes) sitting on a weekday (continuous) to assess sedentariness.28,35 Finally, women provided serum samples to assess serum cotinine (ng/mL) as a biomarker of current nicotine exposure; this measure can differentiate active from either passive or no cigarette smoking. The institutional review boards for all participating sites approved this study. Participants gave written informed consent before enrollment and any data collection.
Endometriosis diagnosis
Endometriosis was defined as disease visualized during surgery, which is the U.S. gold standard.36,37 Surgeons completed standardized operative reports specifically designed to capture postsurgical diagnoses and accompanying morbidity. The report also included the revised American Society for Reproductive Medicine (r-ASRM) scoring system for staging endometriosis, which was categorized as follows: stage I/minimal (scores 1–5); stage II/mild (scores 6–15); stage III/moderate (scores 16–40); or stage IV/severe (scores ≥40).38 The inter-rater agreement for postoperative diagnosed endometriosis among specialized expert surgeons in this study was good (Fleiss kappa = 0.69, 95% confidence interval [CI] 0.64–0.74).39
Statistical analysis
The descriptive phase of the analysis included inspection of data for completeness and distributions. Missing data were minimal for anthropometric data (n ≤ 11) and IPAQ-SF (n = 91) and were missing at random. To minimize potential bias, we addressed missing physical activity data by imputing the mode for the same activity type among women with the same endometriosis diagnosis.40
We examined associations between anthropometric measures and endometriosis status using the Wilcoxon nonparametric rank-sum and Fisher's exact tests for continuous and categorical variables, respectively. We utilized logistic regression analysis to model each anthropometric measurement in relation to the odds of an endometriosis diagnosis adjusting for age (continuous) and site (Utah/California), as well as smoking history (never, former, or current smoker) and income (below, within 180%, and above of the poverty line) based on prior research,41–47 and because the added confounders changed the beta coefficient of the adiposity variables by more than 10% when included in our models.48 To support interpretability of the findings for continuous variables, we standardized the variables by dividing them by their respective standard deviations (the resulting unit for these variables is one standard deviation). We assessed associations between odds of an endometriosis diagnosis and anthropometric and body composition measures continuously and as quartiles based on sample estimates (first quartile as the reference).
We empirically assessed the main effects of physical activity and sedentariness and for potential effect modification between anthropometric and body composition measures and both physical activity (weekly MET-minutes) and sedentariness (average weekday minutes spent sitting). We assessed for significant changes in the adjusted odds ratio (aORs) and accompanying 95% CIs. When we assessed potential moderation, we used hierarchical clustering methods to identify a reduced set of anthropometric measures that were most strongly associated with odds of an endometriosis diagnosis within each cluster to minimize multicollinearity. To investigate if pain could be a confounding factor for physical activity engagement, we assessed for differences in self-reported chronic or cyclic pain by endometriosis diagnosis among different activity levels (high, moderate, and low as indicated by the IPAQ-SF).
We conducted sensitivity analyses to assess the robustness of our findings. This included assessing for significant changes in findings based on choice of comparison group. We compared women with endometriosis to women with a (a) postoperative diagnosis of a normal pelvis (no pathology found) and (b) specific gynecologic disorder, fibroids. Other supporting analyses included using categorized measurements for waist circumference, waist-to-hip ratio, waist-to-height ratio, and BMI,31,33,34,49–51 replacing self-reported smoking with serum cotinine concentrations modeled continuously and categorically (none [9–9.99 ng/mL], passive [10.00–99.99 ng/mL], and active [100.00–595.31 ng/mL] exposure),52 and restricting physical activity analyses among participants for whom data were not imputed. Finally, we assessed model fit and whether the anthropometric measures and body composition measures increased explained variance beyond BMI and weight as measured by Pseudo R2 values, which estimate goodness of fit. All analyses were performed using either Stata (v. 11; College Station, TX) or SAS (v. 9.4; Cary, NC).
Results
Compared with participants without endometriosis, affected women were younger and more likely to be nulligravida, nulliparous, and nonsmokers, have lower cotinine concentrations, and reside in households above the poverty line (Table 1). Overall, women with endometriosis had lower adiposity and lower muscle mass compared with women without endometriosis (Table 2). Preoperative diagnoses for surgery were as follows: pelvic pain (n = 206, 42%), pelvic mass (n = 74, 15%), menstrual irregularities (n = 60, 12%), fibroids (n = 49, 10%), tubal ligation (n = 48, 10%), and infertility (n = 35, 7%). The incidence of endometriosis was 40% (n = 190) and varied from 50% of women with stage 1%–21% with stage 2, 18% with stage 3, and 11% with stage IV. About 31% of women were diagnosed with other gynecologic pathologies (e.g., uterine fibroids, pelvic adhesions, and benign ovarian cysts) while 29% had postoperative diagnoses of a normal pelvis.
Table 1.
Characteristics of Participants by Surgically Visualized Endometriosis (N = 473)
| Characteristic | Endometriosis (n = 190) | No endometriosis (n = 283) |
|---|---|---|
| Demographic | ||
| Age (years), mean ± SD | 32.0 ± 6.8 | 33.6 ± 7.1* |
| Race, n (%) | ||
| Hispanic | 24 (12.6) | 39 (13.8) |
| Non-Hispanic white | 142 (74.7) | 212 (74.9) |
| Non-Hispanic black | 1 (0.5) | 7 (2.5) |
| Asian/Pacific Islander/Native American | 13 (6.8) | 15 (5.3) |
| Other | 4 (2.1) | 7 (2.5) |
| Multiracial | 6 (3.2) | 3 (1.1) |
| Income, n (%) | ||
| Below poverty line | 17 (9.1) | 37 (13.3)* |
| Within 180% of poverty line | 12 (6.4) | 41 (14.7)* |
| Above poverty line | 158 (84.5) | 201 (72.0)* |
| Lifestyle | ||
| Smoking status, n (%) | ||
| Never | 137 (72.1) | 173 (61.6)* |
| Former | 33 (17.4) | 61 (21.7)* |
| Current | 20 (10.5) | 47 (16.7)* |
| Serum cotinine (ng/mL), mean ± SD | 17.0 ± 57.4 | 30.2 ± 78.2* |
| Total MET-minutes/week of physical activity, mean ± SD | 3504.1 ± 3225.4 | 3881.7 ± 3761.2 |
| Mean (±SD) weekday sitting time | 404.6 ± 258.5 | 408.2 ± 444.3 |
| Reproductive history | ||
| Parity conditional on gravidity, n (%) | ||
| No prior pregnancy | 81 (42.6) | 74 (26.3)* |
| Prior pregnancy without birth | 22 (11.6) | 25 (8.9)* |
| Prior pregnancy with birth | 87 (45.8) | 182 (64.8)* |
p < 0.05. Significance for the bivariate analyses was assessed using the Wilcoxon nonparametric rank-sum and Fisher's exact tests for continuous and categorical variables, respectively.
MET, metabolic equivalent of task; SD, standard deviation.
Table 2.
Mean Anthropometric Comparisons of Women by Endometriosis Status (N = 473)
| Endometriosis (n = 190) mean ± SD | No endometriosis (n = 283) mean ± SD | |
|---|---|---|
| Anthropometric measures | ||
| Height (cm) | 165.3 ± 7.1 | 165.4 ± 7.5 |
| Weight (kg) | 72.0 ± 20.2 | 79.9 ± 23.9* |
| Skinfold thicknesses (mm) | ||
| Subscapular | 20.9 ± 11.2 | 24.5 ± 12.8* |
| Suprailiac | 24.2 ± 13.4 | 26.2 ± 14.0 |
| Triceps | 28.2 ± 10.0 | 29.4 ± 9.7 |
| Circumferences (cm) | ||
| Mid-upper arm | 30.7 ± 5.6 | 32.6 ± 6.1* |
| Chest | 85.2 ± 9.6 | 87.9 ± 11.1* |
| Waist | 85.6 ± 16.9 | 91.3 ± 18.4* |
| Hip | 106.8 ± 15.1 | 112.0 ± 17.7* |
| Body composition indicators | ||
| Arm fat indexa | 47.8 ± 11.1 | 47.2 ± 10.1 |
| Body mass index, continuous (kg/m2)b | 26.3 ± 7.2 | 29.1 ± 8.3* |
| Body mass index, categoricalc | ||
| Underweight | 5 (1.81) | 8 (4.23)* |
| Normal weight | 93 (33.57) | 97 (51.32)* |
| Overweight | 70 (25.27) | 39 (20.63)* |
| Obese | 109 (39.35) | 45 (23.81)* |
| Total upper arm area (cm2)d | 77.3 ± 29.4 | 87.3 ± 35.1* |
| Upper arm fat area (cm2)e | 38.0 ± 18.2 | 42.4 ± 19.8* |
| Upper arm muscle area (cm2)f | 38.7 ± 16.0 | 44.6 ± 19.6* |
| Body fat distribution ratios | ||
| Centripetal fat ratiog | 0.41 ± 0.10 | 0.44 ± 0.09* |
| Chest-to-waist ratioh | 1.01 ± 0.11 | 0.98 ± 0.11* |
| Chest-to-hip ratioi | 0.80 ± 0.07 | 0.79 ± 0.08 |
| Waist-to-hip ratioj | 0.80 ± 0.09 | 0.81 ± 0.09* |
| Waist-to-height ratiok | 0.52 ± 0.10 | 0.55 ± 0.11* |
P < 0.05. Significance for bivariate analyses was assessed using the Wilcoxon nonparametric rank-sum and Fisher's exact tests for continuous and categorical variables, respectively.
Arm fat index = (upper arm fat area/total upper arm area) × 100.
Body mass index = [weight (kg)]/[height2 (m2)].
Presented as number (%). Body mass index categories were defined as: underweight <18.5 kg/m2, normal weight 18.5–24.5 kg/m2, overweight 25–29.9 kg/m2, and obese ≥30 kg/m2 (National Health, Lung, and Blood Institute, 1998).
Total upper arm area = [mid upper arm circumference (cm)2]/(4 × π).
Upper arm fat area = [total upper arm area − upper arm muscle area].
Upper arm muscle area = ([mid upper arm circumference (cm)] − [(triceps skinfold thickness [cm]) × π]2)/(4 × π).
Centripetal fat ratio = [subscapular skinfold thickness (mm)]/([subscapular skinfold thickness (mm)] + [triceps skinfold thickness (mm)]).
Chest-to-waist ratio = [chest circumference (cm)]/[waist circumference (cm)].
Chest-to-hip ratio = [chest circumference (cm)]/[hip circumference (cm)].
Waist-to-hip ratio = [waist circumference (cm)]/[hip circumference(cm)].
Waist-to-height ratio = [waist circumference (cm)]/[height (cm)].
The odds of an endometriosis diagnosis were inversely associated with several anthropometric measures and body composition indicators in adjusted models (Table 3). These measures and indicators included standardized: weight (aOR = 0.71, 95% CI 0.57–0.88); subscapular skinfold thickness (aOR = 0.79, 95% CI 0.65–0.98); mid-upper arm, waist, and hip circumferences (aOR = 0.76, 95% CI 0.61–0.93, aOR = 0.79, 95% CI 0.64–0.98, and aOR = 0.76, 95% CI 0.61–0.94, respectively); total upper arm and upper arm muscle areas (aOR = 0.76, 95% CI 0.61–0.94 and aOR = 0.74, 95% CI 0.59–0.93, respectively); and BMI (aOR = 0.75, 95% CI 0.60–0.93).
Table 3.
Anthropometric Measures, Indicators of Body Composition, Fat Distribution, and Odds of a Surgically Visualized Endometriosis Diagnosis (N = 473)
| Unadjusted OR (95% CI) | Adjusted ORa(95% CI) | |
|---|---|---|
| Anthropometry | ||
| Height | 0.98 (0.81–1.18) | 0.89 (0.73–1.09) |
| Weight | 0.67 (0.55–0.83) | 0.71 (0.57–0.88) |
| Skinfold thickness | ||
| Subscapular | 0.73 (0.60–0.89) | 0.79 (0.65–0.98) |
| Suprailiac | 0.86 (0.71–1.04) | 0.95 (0.78–0.16) |
| Triceps | 0.88 (0.73–1.06) | 0.91 (0.75–1.10) |
| Circumferences | ||
| Mid upper arm | 0.71 (0.58–0.87) | 0.76 (0.61–0.93) |
| Chest | 0.76 (0.63–0.93) | 0.84 (0.68–1.03) |
| Waist | 0.71 (0.58–0.87) | 0.79 (0.64–0.98) |
| Hip | 0.72 (0.59–0.88) | 0.76 (0.61–0.94) |
| Body composition indicators | ||
| Arm fat index | 1.06 (0.88–1.28) | 1.08 (0.88–1.31) |
| Body mass index, continuous | 0.68 (0.55–0.84) | 0.75 (0.60–0.93) |
| Body mass index, categoricalb | ||
| Underweight | Reference | Reference |
| Normal weight | 0.65 (0.21–2.06) | 0.76 (0.23–2.54) |
| Overweight | 0.35 (0.11–1.14) | 0.45 (0.13–1.58) |
| Obese | 0.26 (0.08–0.83) | 0.32 (0.10–1.21) |
| Total upper arm area | 0.72 (0.58–0.88) | 0.76 (0.61–0.94) |
| Upper arm fat area | 0.79 (0.65–0.96) | 0.83 (0.67–1.01) |
| Upper arm muscle area | 0.69 (0.55–0.87) | 0.74 (0.59–0.93) |
| Body fat distribution ratios | ||
| Centripetal fat | 0.76 (0.63–0.92) | 0.82 (0.67–1.00) |
| Chest-to-waist | 1.31 (1.08–1.58) | 1.21 (0.98–1.48) |
| Chest-to-hip | 1.12 (0.93–1.35) | 1.17 (0.96–1.43) |
| Waist-to-hip | 0.84 (0.69–1.02) | 0.95 (0.78–1.16) |
| Waist-to-height | 0.72 (0.59–0.88) | 0.81 (0.66–1.01) |
Continuous variables are standardized (variables divided by their respective standard deviation. Original units: weight in kg; height in cm; mid-upper arm, waist, hip, and chest circumferences in cm; subscapular, suprailiac, and triceps skinfold thicknesses in mm; arm muscle and fat areas in cm2; and body mass index in kg/m2. Standardized by dividing variables by their respective standard deviation. Standardized unit = one standard deviation. Bold numbers indicate significant findings.
Odds ratios (ORs) adjusted for age in years (continuous), smoking history (never, former, or current smoker), income (below, within, or above the poverty line), and site (California/Utah).
In original units. Body mass index categories were defined as: underweight <18.5 kg/m2, normal weight 18.5–24.5 kg/m2, overweight 25–29.9 kg/m2, and obese ≥30 kg/m2.34
CI, confidence interval; OR, odds ratio.
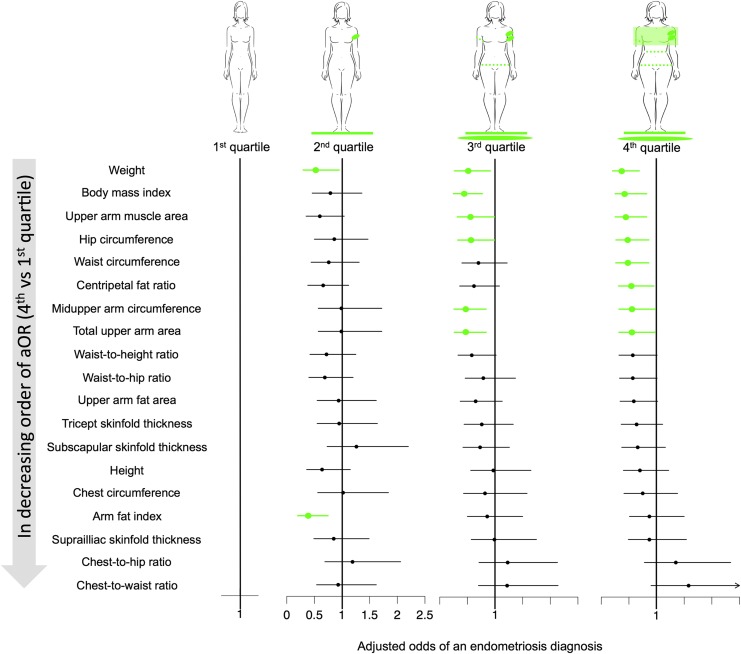
Women in the highest quartiles for several anthropometric measurements, body composition indicators, and body fat distribution ratios had lower adjusted odds of an endometriosis diagnosis than those in the lowest quartile (Fig. 2). These measures, indicators, and ratios included: weight; mid upper arm, hip, and waist circumferences; total upper arm and upper arm muscle areas; BMI; and centripetal fat ratio. Quartile ranges for anthropometric and body composition measures related to first, second, third, and fourth quartiles, respectively, were as follows: Subscapular skinfold thickness ≤13.00, 13.00−20.00, 20.00−30.00, ≥30.00; Suprailiac skinfold thickness ≤13.00, 13.00−22.20, 22.20−33.20, ≥33.20; Triceps skinfold thickness ≤22.00, 22.00−28.60, 28.60−35.00, ≥35.00; Height ≤160.8, 160.8−165.0, 165.0−169.8, ≥169.8; Mid-upper arm circumference ≤27.20, 27.20−30.40, 84.00−98.20, ≥35.00; Chest circumference ≤78.06, 8.06−83.86, 83.86−91.42, ≥91.42; Waist circumference ≤74.60, 74.60−84.00, 84.00−98.20, ≥98.20; Hip circumference ≤98.00, 98.00−106.0, 106.0−117.8, ≥117.8; Waist-to-hip ratio ≤0.74, 0.74−0.79, 0.79−0.85, ≥0.85; Chest-to-waist ratio, ≤0.92, 0.92−1.00, 1.00−1.08, ≥1.08; Chest-to-hip ratio ≤0.75, 0.75−0.79, 0.79−0.83, ≥0.83; Waist-to-height ratio ≤0.45, 0.45−0.51, 0.51−0.59, ≥0.59; BMI ≤22.00, 22.00−26.00, 26.00−31.60, ≥31.60; Total upper arm area ≤58.87, 58.87−73.54, 73.54−97.48, ≥97.48; Upper arm muscle area ≤30.14, 30.14−37.35, 37.35−47.95, ≥47.95; upper arm fat area ≤26.68, 26.68−36.95, 36.95−50.34, ≥50.34; Arm fat index ≤42.17, 42.17−48.19, 48.19−53.64, ≥53.64; and Centripetal fat ratio ≤0.35, 0.35−0.43, 0.43−0.49, ≥0.49.
FIG. 2.
Anthropometric measurements in quartiles and adjusted odd ratios of endometriosis. Green indicates significant findings.
There was no evidence of a main effect of physical activity or sedentariness (data not shown). There was also no evidence of moderation between anthropometric and body composition measures and both physical activity and sedentariness (data not shown). We observed no differences by endometriosis diagnosis status for all but one comparison (data not shown); the sole exception was that among women who reported engaging in “high” physical activity, those with endometriosis were more likely to report cyclic pain compared to women without endometriosis; n = 46 (46.5%) and n = 35 (24.0%), respectively. Significance of the findings was lost when restricting the comparison group to either having fibroids or no pathology, yet general patterns were still observed in that aOR's remained ≤1.0. For the adjusted models, Pseudo R2 values were between 0.0545 and 0.0757. There was no significant improvement in explained variation when accounting for anthropometric and body composition measures beyond weight and BMI (data not shown). All findings were upheld in sensitivity analyses.
Discussion
In the first study known to us to use multiple anthropometric measurements to assess adiposity and muscle mass, we observed inverse relationships between body composition measures and odds of endometriosis without effect modification from physical activity. Findings were similar across standardized continuous measures and measures in quartiles. Although previous researchers have reported an inverse association between BMI and endometriosis, our study is the first to suggest that being lean in adipose tissue and muscle mass are associated with greater odds of an endometriosis diagnosis. Our findings are strengthened by our assessment of body composition with standardized anthropometric measures and our definition of endometriosis using the United States gold standard of surgical visualization.
Using data from a study that had an exposure cohort design, we found that women without endometriosis had greater adiposity and upper arm muscle mass than women with the disease. Our findings corroborate earlier findings of inverse associations between body compositions as measured by BMI7 or self-measured waist-to-hip ratio53 and endometriosis as measured via surgical visualization or self-reported physician diagnosed, respectively. In addition, in adjusted models, we found an absence of a relationship between waist-to-hip ratio and endometriosis diagnosis; these findings are similar to those in a study that included friend controls and an operative sample.54
Our novel research suggests that a more nuanced understanding of body composition–adipose tissue amount and distribution, as well as muscle mass–might help characterize gynecologic disease risk, as opposed to simpler and more global measures of obesity, such as BMI. Many researchers who study obesity use BMI as a measure, including in the context of gynecologic disease. For example, low BMI appears to be protective against ovarian cancer in premenopausal women.55 However, we found that low adiposity was associated with disease: women with endometriosis were more likely to be lean than women without endometriosis. Interestingly, women with endometriosis have the same or greater risk for ovarian cancer than women without endometriosis.56–58 We suggest that future researchers could advance understanding of relationships between obesity and gynecologic disease by looking more precisely at body composition rather than BMI alone. In our study, low BMI was associated with increased odds of an endometriosis diagnosis. When considering estimates for body composition, adiposity measures were not only inversely associated with endometriosis but also with muscle mass. Thus, by looking at estimates of both adipose tissue and muscle mass, we provide a more comprehensive understanding of what tissue types may contribute to the increased odds of an endometriosis diagnosis.
By studying body composition, researchers and healthcare providers may gain understanding of disease mechanisms. Questions for future research include: why is leanness not protective in endometriosis whereas it is in other gynecologic diseases? How can clinicians assess patients for endometriosis risk more effectively? To date, there are no known biomarkers or noninvasive diagnostic methods of incident endometriosis suitable for population research or clinical care.59–62 Body composition provides another means for researchers to investigate potential involvement of an altered adipose tissue milieu in endometriosis characterized by a different immunological profile63–66 or omental fat protease expression.67–70 Studying body composition also could open new research avenues into the role of muscle mass in mechanisms related to the development of endometriosis.
Clinicians could benefit from recognizing that the extremes in body composition may have implications for gynecologic diseases, with a lean body habitus now being associated with endometriosis building upon long-standing recognition that obesity is associated with polycystic ovarian syndrome.71 Healthcare providers could also consider incorporating anthropometric measures of adiposity and muscle mass that could offer a more global understanding of women's disease risk profile. Incorporating body composition into clinical decision-making could be helpful given that no screening tools exist and the only way to definitively diagnose endometriosis is via surgery.
Despite the study's many strengths, careful interpretation of the findings is needed relative to important study limitations, most notably its cross-sectional analysis. The natural history of endometriosis is unknown; it is possible that body habitus, physical activity, and sedentariness in childhood and/or adolescence are important to the development of endometriosis,11 consistent with a possible in utero origin for endometriosis.72,73 In addition, while measurements were taken about 2 months before surgery, women were unaware of their postoperative diagnoses at the time of measurement. This is an important consideration for self-reported data, including bra size, given the potential for reporting errors.74 Another key limitation is reliance on a clinical population comprising women who sought care and underwent surgery. The extent to which the findings are upheld at the population level remain to be established, particularly because 11%, or more, of women may have unrecognized disease.1 As is the case with observational research in general, we recognize the potential for residual confounding. This includes variables related to behaviors, including dietary intake, a factor important for body composition and for which we had little data, and disease related symptoms such as pain, which was likely not a factor in our sample given that we observed no differences in pain and physical activity engagement by endometriosis diagnosis status.
Conclusions
Using established protocols for assessing body composition and endometriosis, we found that a lean body habitus is associated with endometriosis, when controlling for potential confounders and considering physical activity. This study is the first, to our knowledge, to include comprehensive anthropometric assessments to assess adiposity, as well as muscle mass, thereby overcoming limitations from previous research. Our research can support future research into mechanisms of the disease, which could support our understanding of the role of body composition in development of disease and the care of women at risk for developing or diagnosed with endometriosis.
Acknowledgments
The authors gratefully acknowledge Denise Lamb, RN, Study Coordinator and other study personnel for their attention to quality when collecting these data. Sponsorship: The study was funded by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH) (contracts NO1-DK-6-3428; NO1- DK-6-3427; 10001406-02). Dr. Backonja was funded as a doctoral fellow through the NIH/National Institute of Nursing Research (NINR) Graduate Partnership Program and as a postdoctoral fellow through the NIH, National Library of Medicine (NLM) Biomedical and Health Informatics Training Program at the University of Washington (Grant No. T15LM007442).
Author Disclosure Statement
None of the authors have any commercial associations that might create a conflict of interest in connection with submitted article.
References
- 1.Buck Louis GM, Hediger ML, Peterson CM, et al. ; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil Steril 2011;96:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olive DL, Schwartz LB. Endometriosis. N Engl J Med 1993;328:1759–1769 [DOI] [PubMed] [Google Scholar]
- 3.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers PA, D'Hooghe TM, Fazleabas A, et al. Defining future directions for endometriosis research: Workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reprod Sci 2013;20:483–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backonja U, Buck Louis GM, Lauver DR. Overall adiposity, adipose tissue distribution, and endometriosis: A systematic review. Nurs Res 2016;65:151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol 2005;121:94–98 [DOI] [PubMed] [Google Scholar]
- 7.Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril 2005;84:1366–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafay Pillet MC, Schneider A, Borghese B, et al. Deep infiltrating endometriosis is associated with markedly lower body mass index: A 476 case–control study. Hum Reprod 2012;27:265–272 [DOI] [PubMed] [Google Scholar]
- 9.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systematic review. Obes Rev 2007;8:307–326 [DOI] [PubMed] [Google Scholar]
- 10.Engstrom JL, Paterson SA, Doherty A, Trabulsi M, Speer KL. Accuracy of self-reported height and weight in women: An integrative review of the literature. J Midwifery Womens Health 2003;48:338–345 [DOI] [PubMed] [Google Scholar]
- 11.Kvaskoff M, Bijon A, Clavel-Chapelon F, Mesrine S, Boutron-Ruault MC. Childhood and adolescent exposures and the risk of endometriosis. Epidemiology 2013;24:261–269 [DOI] [PubMed] [Google Scholar]
- 12.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004;160:784–796 [DOI] [PubMed] [Google Scholar]
- 13.Cramer DW, Wilson E, Stillman RJ, et al. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986;255:1904–1908 [PubMed] [Google Scholar]
- 14.Dhillon PK, Holt VL. Recreational physical activity and endometrioma risk. Am J Epidemiol 2003;158:156–164 [DOI] [PubMed] [Google Scholar]
- 15.Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: A matched case-control study. Environ Res 2007;103:121–129 [DOI] [PubMed] [Google Scholar]
- 16.Garavaglia E, Ricci E, Chiaffarino F, et al. Leisure and occupational physical activity at different ages and risk of endometriosis. Eur J Obstet Gynecol Reprod Biol 2014;183:104–108 [DOI] [PubMed] [Google Scholar]
- 17.Viganò P, Somigliana E, Panina P, Rabellotti E, Vercellini P, Candiani M. Principles of phenomics in endometriosis. Hum Reprod Update 2012;18:248–259 [DOI] [PubMed] [Google Scholar]
- 18.Vitonis AF, Baer HJ, Hankinson SE, Laufer MR, Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod 2010;25:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitonis AF, Hankinson SE, Hornstein MD, Missmer SA. Adult physical activity and endometriosis risk. Epidemiology 2010;21:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lake JK, Power C, Cole TJ. Women's reproductive health: The role of body mass index in early and adult life. Int J Obes Relat Metab Disord 1997;21:432–438 [DOI] [PubMed] [Google Scholar]
- 21.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril 2004;81:384–392 [DOI] [PubMed] [Google Scholar]
- 22.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93(11 Suppl 1):S57–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 2002;282:E1191–E1196 [DOI] [PubMed] [Google Scholar]
- 24.Shah NR, Braverman ER. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS One 2012;7:e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohman TG, Roche AF, Martorell R, eds. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books, 1988 [Google Scholar]
- 26.Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet Gynecol Scand 1997;76:559–562 [DOI] [PubMed] [Google Scholar]
- 27.Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: A hospital-based case-control study. Ann Epidemiol 1997;7:267–741 [DOI] [PubMed] [Google Scholar]
- 28.Ekelund U, Sepp H, Brage S, et al. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr 2006;9:258–265 [DOI] [PubMed] [Google Scholar]
- 29.Yerrakalva D, Mullis R, Mant J. The associations of “fatness,” “fitness,” and physical activity with all-cause mortality in older adults: A systematic review. Obesity (Silver Spring) 2015;23:1944–1956 [DOI] [PubMed] [Google Scholar]
- 30.Thoma ME, Hediger ML, Sundaram R, et al. ; ENDO Study Working Group. Comparing apples and pears: Women's perceptions of their body size and shape. J Womens Health (Larchmt) 2012;21:1074–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashwell M. Waist to height ratio and the Ashwell® Shape Chart could predict the health risks of obesity in adults and children in all ethnic groups. Nutr Food Sci 2005;35:359–364 [Google Scholar]
- 32.Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor, MI: The University of Michigan Press, 1990 [Google Scholar]
- 33.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 1995, p. 854. [PubMed]
- 34.National Health, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 1998. Report No.: NIH 98–4083. Available at: www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf Accessed May16, 2017 [Google Scholar]
- 35.IPAQ Research Committee. International Physical Activity Questionnaire-Short Form. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)–Short and Long Forms, November 2005. Available at: www.ipaq.ki.se Accessed May16, 2017
- 36.Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil Steril 2012;98:591–598 [DOI] [PubMed] [Google Scholar]
- 37.Kennedy S, Bergqvist A, Chapron C, et al. ; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 2005;20:2698–2704 [DOI] [PubMed] [Google Scholar]
- 38.American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821 [DOI] [PubMed] [Google Scholar]
- 39.Schliep KC, Stanford JB, Chen Z, et al. Interrater and intrarater reliability in the diagnosis and staging of endometriosis. Obstet Gynecol 2012;120:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little RJA, Rubin DB. 2002. Statistical analysis with missing data, 2nd ed. Hoboken, N.J: Wiley [Google Scholar]
- 41.Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: Analysis of NHANES II. Am J Public Health 1987;77:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buck Louis GM, Hediger ML, Pena JB. Intrauterine exposures and risk of endometriosis. Hum Reprod 2007;22:3232–3236 [DOI] [PubMed] [Google Scholar]
- 43.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: A population-based study. Obes Res 2005;13:1466–1475 [DOI] [PubMed] [Google Scholar]
- 44.Kwok S, Canoy D, Soran H, et al. Body fat distribution in relation to smoking and exogenous hormones in British women. Clin Endocrinol (Oxf) 2012;77:828–833 [DOI] [PubMed] [Google Scholar]
- 45.Matalliotakis IM, Cakmak H, Fragouli YG, Goumenou AG, Mahutte NG, Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet 2008;277:389–393 [DOI] [PubMed] [Google Scholar]
- 46.McLaren L. Socioeconomic status and obesity. Epidemiol Rev 2007;29:29–48 [DOI] [PubMed] [Google Scholar]
- 47.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum Reprod 2002;17:2715–2724 [DOI] [PubMed] [Google Scholar]
- 48.Maldonano G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–936 [DOI] [PubMed] [Google Scholar]
- 49.Bray GA, Gray DS. Obesity. Part I—Pathogenesis. West J Med 1988;149:429–441 [PMC free article] [PubMed] [Google Scholar]
- 50.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0 · 5 could be a suitable global boundary value. Nutr Res Rev 2010;23:247–269 [DOI] [PubMed] [Google Scholar]
- 51.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ 1995;311:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health 1988;78:699–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: Results from 20 years of follow-up within the Nurses' Health Study II prospective cohort. Hum Reprod 2013;28:1783–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCann SE, Freudenheim JL, Darrow SL, Batt RE, Zielezny MA. Endometriosis and body fat distribution. Obstet Gynecol 1993;82:545–549 [PubMed] [Google Scholar]
- 55.Wang J, Yang DL, Chen ZZ, Gou BF. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: A systematic review and meta-analysis. Cancer Epidemiol 2016;42:1–8 [DOI] [PubMed] [Google Scholar]
- 56.Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br J Cancer 2014;110:1878–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melin A, Sparén P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod 2006;21:1237–1242 [DOI] [PubMed] [Google Scholar]
- 58.Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case–control studies. Lancet Oncol 2012;13:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borrelli GM, Abrão MS, Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum Reprod 2014;29:253–266 [DOI] [PubMed] [Google Scholar]
- 60.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: A systematic review. Hum Reprod Update 2010;16:651–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: A systematic review of putative biomarkers. Hum Reprod Update 2011;17:637–653 [DOI] [PubMed] [Google Scholar]
- 62.Nisenblat V, Prentice L, Bossuyt PM, et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 2016;13:7:CD012281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adya R, Tan BK, Chen J, Randeva HS. Protective actions of globular and full-length adiponectin on human endothelial cells: Novel insights into adiponectin-induced angiogenesis. J Vasc Res 2012;49:534–543 [DOI] [PubMed] [Google Scholar]
- 64.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A 2001;98:6390–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem 2011;286:13460–13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol 2005;174:6820–6828 [DOI] [PubMed] [Google Scholar]
- 67.Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: A rationale for endometriotic invasiveness. Fertil Steril 2001;75:152–159 [DOI] [PubMed] [Google Scholar]
- 68.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: A proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 2013;139:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Center for Biotechnology Information. MMP9 matrix metallopeptidase 9 [Homo sapiens (human)]. [Last updated 2016. July 9]. Available at: www.ncbi.nlm.nih.gov/gene/4318 Accessed May16, 2017
- 70.Williams KE, Miroshnychenko O, Johansen EB, et al. Urine, peritoneal fluid and omental fat proteomes of reproductive age women: Endometriosis-related changes and associations with endocrine disrupting chemicals. J Proteomics 2015;113:194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmadi A, Akbarzadeh M, Mohammadi F, Akbari M, Jafari B, Tolide-Ie HR. Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian J Endocrinol Metab 2013;17:672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brosens I, Benagiano G. The endometrium from the neonate to the adolescent. J Matern Fetal Neonatal Med 2016;29:1195–1199 [DOI] [PubMed] [Google Scholar]
- 73.Signorile PG, Baldi F, Bussani R, et al. New evidence of the presence of endometriosis in the human fetus. Reprod Biomed Online 2010;21:142–147 [DOI] [PubMed] [Google Scholar]
- 74.Wright MCM. Graphical analysis of bra size calculation procedures. Int J Cloth Sci Technol 2002;14:41–45 [Google Scholar]