Abstract
Listeria monocytogenes is an important cause of foodborne illness hospitalization, fetal loss, and death in the United States. Listeriosis incidence rate varies significantly among population subgroups with pregnant women, older persons, and the Hispanic population having increased relative risks compared with the other subpopulations. Using estimated rates of listeriosis per subpopulation based on FoodNet data from 2004 to 2009, we evaluate the expected number of cases and incidence rates of listeriosis in the US population and the pregnant women subpopulation as the demographic composition changes over time with respect to ethnicity, pregnancy status, and age distribution. If the incidence rate per subpopulation is held constant, the overall US population listeriosis incidence rate would increase from 0.25 per 100,000 (95% confidence interval [CI]: 0.19–0.34) in 2010 to 0.28 (95% CI: 0.22–0.38) in 2020 and 0.32 (95% CI: 0.25–0.43) in 2030, because of the changes in the population structure. Similarly, the pregnancy-associated incidence rate is expected to increase from 4.0 per 100,000 pregnant women (95% CI: 2.5–6.5) in 2010 to 4.1 (95% CI: 2.6–6.7) in 2020 and 4.4 (95% CI: 2.7–7.2) in 2030 as the proportion of Hispanic pregnant women increases. We further estimate that a reduction of 12% in the exposure of the US population to L. monocytogenes would be needed to maintain a constant incidence rate from 2010 to 2020 (current trend), assuming infectivity (strain virulence distribution and individual susceptibility) is unchanged. To reduce the overall US population incidence rate by one-third (Healthy People 2020 goal) would require a reduction in exposure (or infectivity) to L. monocytogenes of 48% over the same time period. Reduction/elimination in exposure of pregnant and Hispanic subpopulations alone could not meet this target. This information may be useful in setting public health targets, developing risk management options, and in interpreting trends in the public health burden of foodborne listeriosis in the United States.
Keywords: : Listeria monocytogenes, demographics, US population, pregnant, elderly, Hispanic
Introduction
Listeria monocytogenes is an important cause of foodborne illness, foodborne illness hospitalization, fetal loss, and death in the United States. Listeriosis incidence rates vary drastically across population subgroups (Rocourt, 1996; Painter and Slutsker, 2007; Mook et al., 2010; Goulet et al., 2012; Pouillot et al., 2012; Silk et al., 2012). For example, in the United States, pregnant women were found to have a relative risk of listeriosis of 115 (95% confidence interval [CI] 69–205) compared to women of the same age, with Hispanic women having an independent relative risk of 4.8 (95% CI: 3.1–7.1) compared to women of other ethnicities (Pouillot et al., 2012). Similarly, individuals aged ≥85 years have a relative risk of 54 (95% CI: 37–79) compared to the 15–44-year-old population, and the Hispanic population has been found to have an independent relative risk of 1.8 (95% CI: 1.3–2.5) (Pouillot et al., 2012). A change in demographics, in which the relative size of one or more of these susceptible populations changes, is thus expected to impact observed incidence and incidence rates of listeriosis at the population level. Currently, groups expected to increase in relative size include older Americans and the Hispanic population (Census Bureau, 2014).
Analyses of data from epidemiological surveillance networks in the United States have not found significant increases (or decreases) in the number of cases of listeriosis (Silk et al., 2013; Crim et al., 2014; Huang et al., 2016; Powell, 2016) since at least 2010. The Healthy People 2020 target for reducing the incidence rate of laboratory confirmed cases of L. monocytogenes is 0.2 cases per 100,000 people, a reduction from an estimated baseline of 0.3 cases per 100,000 people reported in 2006–2008 (HHS, 2014). The objective of this study is to understand the role demographic changes in the US population play in the burden of illness and incidence of listeriosis and to use this information to interpret recent trends in the disease and understand implications for public health targets. We'll show that the number of cases of listeriosis in a subpopulation can be expressed as a function of the subpopulation size, the probability of infection following the ingestion of a L. monocytogenes cell in this subpopulation (which we refer to as “infectivity,” a function of the virulence of the strains, and the susceptibility of the individuals), and the number of L. monocytogenes cells ingested by this subpopulation (exposure). Using this quantitative relationship, three questions will be answered: (i) what is the expected impact of predicted changes in US demographics on the burden of illness and incidence rate of listeriosis for the period 2010 to 2030, assuming exposure and infectivity are constant during this time period? (ii) what does the current observed steady-state incidence rate for listeriosis in the US population imply with regard to changes in exposure (or infectivity), given demographic changes? and (iii) what magnitude of reduction in exposure (or infectivity) to L. monocytogenes would be required to meet the Healthy People 2020 target of a 1/3 reduction in the total listeriosis incidence rate from 2010 to 2020, given predicted demographic changes?
Materials and Methods
Framework
Under the one-hit theory of the L. monocytogenes process of infection (Haas et al., 1999; Teunis and Havelaar, 2000; FAO/WHO, 2004), the expected number of cases of listeriosis C when a population eats m meals can be expressed as
 |
where j = 1, …, m is an index for each meal, dj is the dose (no. of L. monocytogenes) ingested through the meal j, and rj is the average probability that a pathogen from serving j will survive the host-pathogen response and initiate infection (Haas et al., 1999; Schmidt et al., 2013). Assuming that the rj parameter is constant within a given subpopulation i (FAO/WHO, 2004) (e.g., individuals of the same age, ethnicity, or pregnancy status) and equal to ri, this equation leads to
 |
for a given subpopulation, where Ci is the number of cases in the subpopulation I, and mi is the number of meals ingested by this subpopulation. Because r is usually very small for L. monocytogenes (FAO/WHO, 2004),  . Under this limit, the equation simplifies to
. Under this limit, the equation simplifies to
 |
where  is the sum of L. monocytogenes ingested by the subpopulation i. This equation can be expressed as
is the sum of L. monocytogenes ingested by the subpopulation i. This equation can be expressed as
 |
where Ni is the number of individuals in the subpopulation, and  is the mean number of L. monocytogenes ingested by the subpopulation. For each subpopulation i, for a given year y, the expected number of cases Ci,y is then proportional to the number of individuals in this subpopulation Ni,y multiplied by the mean number of L. monocytogenes ingested per meal, that year, for this subpopulation
is the mean number of L. monocytogenes ingested by the subpopulation. For each subpopulation i, for a given year y, the expected number of cases Ci,y is then proportional to the number of individuals in this subpopulation Ni,y multiplied by the mean number of L. monocytogenes ingested per meal, that year, for this subpopulation  , that is,
, that is,
 |
We can estimate the expected change in the incidence rate of listeriosis in the US population from demographic changes alone by assuming that infectivity  and exposure
and exposure  (or its product
(or its product  ) do not change with time. Under the assumption of constant incidence rate Ii in each subpopulation with time (i.e., constant
) do not change with time. Under the assumption of constant incidence rate Ii in each subpopulation with time (i.e., constant  ), the expected number of cases in a given year y2 may be estimated as:
), the expected number of cases in a given year y2 may be estimated as:
 |
where  are the incidence rates for subpopulation i estimated in a given year (e.g., y1). With this assumption, the number of cases and incidence rates in the US population are calculated as
are the incidence rates for subpopulation i estimated in a given year (e.g., y1). With this assumption, the number of cases and incidence rates in the US population are calculated as
 |
respectively. This equation and assumption are used in classical standardization methods (Rothman et al., 2008).
Under the assumption of a constant infectivity ri,y over years (i.e., the same virulence of circulating strains and same susceptibility of individuals within subpopulations) but allowing the average dose (exposure) to vary, the number of cases expected for a subpopulation i in year y2 can be estimated as
 |
where pi is the fractional change in the average L. monocytogenes dose from year y1 to year y2, that is,  . Applying this formula to the subpopulations, one can estimate the change in exposure required to explain trends in listeriosis or to attain public health goals.
. Applying this formula to the subpopulations, one can estimate the change in exposure required to explain trends in listeriosis or to attain public health goals.
Calculation of  , the subpopulation sizes
, the subpopulation sizes
Demographic data for the United States were obtained for 2010, 2020, and 2030 using US Census Bureau population projections (2009 projections for the 2010 data and 2014 projections (the latest available) for the 2020 and 2030 data) by single year of age, sex, race, and ethnicity (Census Bureau, 2009, 2014). For 2010, the 2009 population projection data were used instead of data from the 2010 Census, so that the demographic methods and the population categories match as closely as possible to be able to directly compare results obtained for 2010 to results obtained for 2020 and 2030. Since US Census data vary slightly from US Census Bureau projection data, method consistency was prioritized to obtain unbiased trend information. The number of pregnant women was estimated using 9/12th of the estimated number of children <1 year of age for that year, state, and ethnicity, thus accounting for the 9-month gestational period. The number of pregnancies that did not result in live birth was estimated using published, national ethnicity-specific rates of induced abortion and fetal loss (i.e., 0.57 and 0.47 pregnancies that did not result in live birth per one pregnancy that resulted in live birth for the non-Hispanic and Hispanic populations, respectively) (Ventura et al., 2009). Following the rationale of others (Jamieson et al., 2009; Silk et al., 2012), 2/12th of the resulting estimates were used for these corrections because these pregnancies last an average of 2 months.
Calculation of Ii, incidence rate in the subpopulations
The incidence rates of listeriosis per subpopulation are calculated from FoodNet data from 2007 to 2009. FoodNet is a population-based active surveillance system that tracks trends in laboratory-confirmed cases of pathogens commonly transmitted through food (Jones et al., 2007). A listeriosis case patient was defined, following FoodNet definition, as a case of invasive listeriosis where L. monocytogenes was isolated from a normally sterile site (blood, cerebrospinal fluid, placental tissue, or fetal tissue) (Silk et al., 2012). We did not apply a multiplication factor to our results that would account for underdiagnosis; however, the true incidence of listeriosis may be higher because of underdiagnosis, as Scallan et al. (2011) estimated that one out of two invasive listeriosis cases was not diagnosed in the United States. Outbreak associated cases (<1% of cases) were excluded from the model to reduce correlations between cases.
A mixed negative-binomial generalized-linear model (Henao et al., 2010; Bates et al., 2015) was used to calculate the rate of nonpregnancy associated listeriosis according to considered demographic covariates identified in Pouillot et al. (2012) for the same dataset. The considered covariates are (1) ethnicity (Hispanic vs. non-Hispanic); (2) an interaction between age group (>31 days to 14 years, 15–44 years, 45–59 years, 60–69 years, 70–79 years, 80–84 years, and ≥85 years) and ethnicity; and (3) an interaction between 3-year study periods (2004–2006 vs. 2007–2009) and sex (male vs. female, with incidence rates for women constant from 2004 to 2009 and different for men in 2004–2006 and 2007–2009) [see Table 1 and text in Pouillot et al. (2012)]. The US state (illness location) was considered as a random effect on the intercept. A second mixed generalized-linear model was developed and used specifically for pregnancy associated cases and nonpregnancy associated cases among women of reproductive age (15–44 years). The covariates considered are pregnancy status and an interaction between ethnicity and study period [see Table 2 and text in Pouillot et al. (2012)].
Table 1.
Annual US Population Predictions for Foodborne Invasive Listeriosis: 2010, 2020, and 2030
| Population group | Population size | Predicted no. of cases | 95% CI | Predicted incidence ratea | 95% CI | |
|---|---|---|---|---|---|---|
| 2010 | Nonpregnant | 306,292,754 | 632 | 502–816 | 0.21 | 0.16–0.27 |
| Pregnant | 3,585,019 | 143 | 88–232 | 4.0 | 2.5–6.5 | |
| Total population | 309,877,773 | 775 | 590–1048 | 0.25 | 0.19–0.34 | |
| 2020 | Nonpregnant | 330,686,893 | 802 | 635–1045 | 0.24 | 0.19–0.32 |
| Pregnant | 3,472,497 | 144 | 89–234 | 4.1 | 2.6–6.7 | |
| Total population | 334,159,390 | 946 | 724–1279 | 0.28 | 0.22–0.38 | |
| 2030 | Nonpregnant | 355,516,995 | 996 | 787–1299 | 0.28 | 0.22–0.37 |
| Pregnant | 3,534,758 | 155 | 95–253 | 4.4 | 2.7–7.2 | |
| Total population | 359,051,753 | 1151 | 882–1552 | 0.32 | 0.25–0.43 |
Invasive listeriosis is defined as case of listeriosis where Listeria monocytogenes was isolated from a normally sterile site.
Incidence rate is expressed as per 100,000 people for the nonpregnancy associated cases and the total population and per 100,000 pregnant women for the pregnancy associated cases.
CI, confidence interval.
Table 2.
Fractional Reduction in the Average Number of Listeria monocytogenes Consumed Required to Reach Specific Incidence Rates in 2020
| Target | No change in the overall incidence rate from 2010 to 2020 | 1/3 Reduction of the overall incidence rate from 2010 to 2020 |
|---|---|---|
| Total population | 0.12 | 0.48 |
| >60 Years of age population only | 0.17 | 0.67 |
| >70 Years of age population only | 0.25 | 0.89 |
| Pregnant women only | 0.76 | NR |
| Hispanic population only (including Hispanic pregnant women) | 0.51 | NR |
| Hispanic population and pregnant women | 0.41 | NR |
Example: to observe no change in the overall incidence rate from 2010 to 2020, a reduction of 12% of the average number of L. monocytogenes consumed for all subpopulations would be required, or a reduction of 76% of the average number of L. monocytogenes consumed for the pregnant women (with no change in exposure of all other subpopulations)
NR, non-reachable.
Calculation of listeriosis incidence rates
The demographic data for 2010, 2020, and 2030 derived from the US Census Population Projections were used to calculate 2010 and expected future rates of listeriosis given that the incidence rate of the various population subgroups is held constant, according to Equations (1) and (2) (Census Bureau, 2009, 2014). The incidence rates used for standardization were those calculated for the 2007–2009 study period (Pouillot et al., 2012; Silk et al., 2012). A parametric bootstrap procedure was used to provide CIs around the incidence rates (Bates et al., 2015). In the bootstrap procedure, normally distributed random-effect values are simulated when calculating the 2010, 2020, and 2030 US population projections.
Calculation of the exposure change required to attain a given incidence rate
Using (Eq. 3), we estimated changes in exposure (or infectivity) associated with two scenarios as follows: (1) no change in listeriosis incidence rate for the US population from 2010 to 2020 (current trend) and (2) reduction of the overall US incidence rate by 1/3 from 2010 to 2020 (Healthy People 2020 target).
All statistical analyses were performed in the R statistical software package (R Development Core Team, 2008).
Results
Listeriosis incidence rates
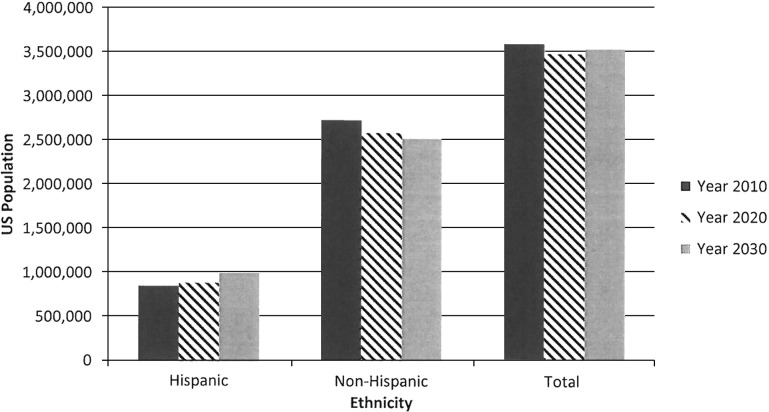
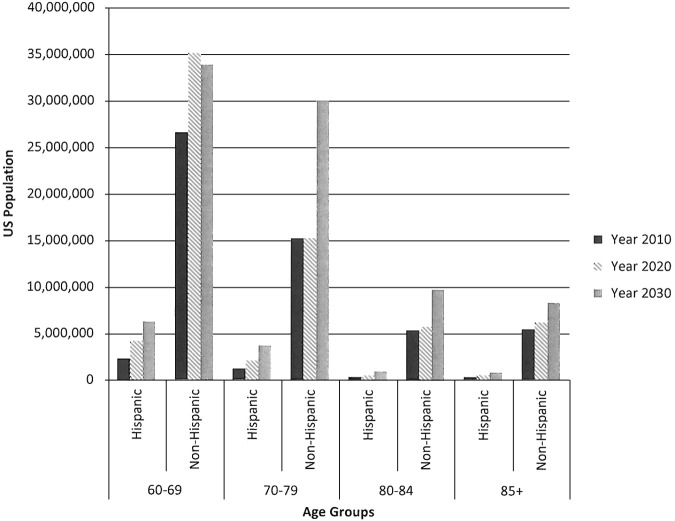
According to the US Census 2009 and 2014 Population Projections, the total US population will increase in size from 2010 to 2030 and population subgroups with increased relative risks for listeriosis will increase as a proportion of the population (Table 1). The US Hispanic population is projected to increase from 16.0% of the total population in 2010 to 19.0% in 2020 and 21.6% in 2030 (Fig. 1). A stable number of pregnant women are projected from 2010 to 2030, with an increasing proportion of women of Hispanic ethnicity. Finally, the US Census Bureau projects an aging population (Fig. 2).
FIG. 1.
Histogram of the pregnant population demographics by ethnicity for the years 2010, 2020, and 2030 (Data: US Census Bureau).
FIG. 2.
Histogram of the four oldest age group population demographics by ethnicity for the years 2010, 2020, and 2030 (Data: US Census Bureau).
The total annual foodborne listeriosis cases is estimated to increase from 775 in 2010 to 946 in 2020 and 1151 in 2030, if exposure and infectivity are constant during this time period. These increases are partly due to overall increases in the population, but are also due to variations in the population structure. Indeed, the projected demographic changes would lead to an increase in the incidence rate per 100,000 individuals from 0.25 in 2010 to 0.28 in 2020 and to 0.32 in 2030, if exposure and infectivity are constant over this time period. The number of pregnancy-associated cases is expected to increase slightly from 143 in 2010 to 144 in 2020 and 155 in 2030, even as the proportion of pregnant women in the overall population is projected to decline (Census Bureau, 2009, 2014). The incidence rate per 100,000 pregnant women would also increase from 4.0 in 2010 to 4.1 in 2020 and 4.4 in 2030 (Fig. 3), if exposure and infectivity are unchanged, as the proportion of Hispanic pregnant women (with a higher relative risk) increases. Overall, the proportion of pregnancy associated cases would decrease from 19% (143/775) in 2010 to 15% (144/946) in 2020 to 13% (155/1151) in 2030.
FIG. 3.
(a) Predicted incidence rate for the pregnant population expressed per 100,000 pregnant women. (b) Predicted incidence rate for the nonpregnant population (square) and the total population (triangle) expressed per 100,000 people, a lag on the x-axis was added for visibility. (c) Number of cases for the pregnant population. (d) Number of cases for the nonpregnant population (square) and the total population (triangle), a lag on the x-axis was added for visibility. Segments represent 95% bootstrap confidence intervals for each figure.
Scenario 1: change in L. monocytogenes exposure associated with a constant listeriosis incidence rate
Given the projected changes in demographic structure described above, in order for the overall incidence rate to remain constant from 2010 to 2020 [as observed to date (Powell, 2016)], the product of exposure and infectivity  must be reduced. Assuming infectivity is unchanged during this time period, we estimate that exposure (the average number of L. monocytogenes consumed per meal) by all subpopulations must be reduced by 12%. This reduction in exposure could arise in a variety of ways, including from larger reductions in L. monocytogenes exposure to subpopulations with higher subpopulation incidence rates. For example, an estimated decrease in exposure of 25% for the >70-year-old population or 76% for the pregnant population over this time period would lead to a constant overall incidence rate from 2010–2020 (Table 2).
must be reduced. Assuming infectivity is unchanged during this time period, we estimate that exposure (the average number of L. monocytogenes consumed per meal) by all subpopulations must be reduced by 12%. This reduction in exposure could arise in a variety of ways, including from larger reductions in L. monocytogenes exposure to subpopulations with higher subpopulation incidence rates. For example, an estimated decrease in exposure of 25% for the >70-year-old population or 76% for the pregnant population over this time period would lead to a constant overall incidence rate from 2010–2020 (Table 2).
Scenario 2: change in L. monocytogenes exposure required to reach the Healthy People 2020 listeriosis incidence rate goal
Given projected changes in US population demographics from 2010 to 2020, a 48% reduction in the average number of L. monocytogenes consumed by all subpopulations (Table 2) would be required to reduce overall incidence rate by 1/3, assuming infectivity is unchanged. If older age groups are exclusively targeted, the declines required are even larger (67% for >60 years and 89% for >70 years). Achieving a reduction by 1/3 in the overall population incidence rate in the United States could not be attained if exposure of pregnant women only, the Hispanic population only, or both of these subpopulations only were reduced or even eliminated (Table 2). Eliminating exposure to Listeria in pregnant women and the Hispanic population in 2020 results in an 18.9% reduction in incidence rate.
Discussion
As the US population ages and the proportion of the population that is of Hispanic ethnicity increases from 2010 to 2020, an increase in listeriosis cases and incidence rates would be expected if exposure and infectivity remain unchanged (more specifically, if no changes in the subpopulation incidence rates occur from those estimated for 2007–2009, the years from which the baseline incidence rates were calculated) (Census Bureau, 2009, 2014). In addition, the proportion of pregnancy associated cases would decrease while the proportion of cases associated with older adults would increase, under these assumptions. While Census projections indicate that the proportion of Hispanic pregnant women in the population will increase through 2030, the projected decline in non-Hispanic pregnant women leads to an estimated decreasing proportion of pregnancy-associated cases overall in 2020 and 2030. In Europe, L. monocytogenes infections have been increasing in number since 2008, and the proportion of pregnancy associated cases is decreasing (Goulet et al., 2008). These trends might be, in part, due to changes in population demographics, including an increased proportion of older adults in the population (Goulet et al., 2008). A Dutch study of the effects of aging population demographics on Listeria incidence also found that incidence rates were expected to increase from 2020 to 2060 in the Netherlands (Bouwknegt et al., 2013).
As explored in Scenario 1, the absence of any change in listeriosis incidence rate may reflect a balance of demographic changes and exposure changes, such as lower contamination rates of foods, which have been recently reported for some ready-to-eat foods (Luchansky et al., 2017). Our results suggest that an overall decrease of 12% in exposure to L. monocytogenes of all subpopulations to L. monocytogenes would lead to a constant incidence rate from 2010 to 2020. It is also possible that an approximately constant incidence rate is being achieved through more significant decreases in exposure of more vulnerable subpopulations. The observed trend could also be due to a combination of an increase in exposure in some subpopulations concurrent with a more important decrease in other subpopulations. A decrease in the infectivity (virulence of the strains and/or susceptibility of the individuals) is also theoretically possible, but has not been observed to date. Whole genome sequencing analyses of food-associated strains combined with human clinical data may provide insights into this possibility (Ronholm et al., 2016).
We examined the possibility of reaching the Healthy People 2020 target (reduction in incidence rate by 1/3) by reducing listeriosis exposure. Our results suggest that elimination of L. monocytogenes exposure to pregnant women and the Hispanic population has the potential to reduce incidence rate by ∼1/5, but a larger reduction in incidence rate would require exposure reductions for additional subpopulations, for example, older adults. Alternatively, a reduction of ∼50% in exposure to all subpopulations would reach the 2020 target.
The distribution of L. monocytogenes levels in foods is known to be exceptionally skewed, with a very large majority of food being noncontaminated, some food being contaminated at low level (few cells per grams), and some food, supporting bacterial growth, being contaminated at an extremely high level (FDA/FSIS, 2003; FAO/WHO, 2004). With such a highly skewed distribution, reductions in the mean level in foods (estimated for two scenarios) could be achieved by an overall reduction of contamination for all food but, much more efficiently, by targeted reductions of the few highly contaminated products.
Conclusions
Our results demonstrate the importance of considering demographic changes when interpreting trends in the public health burden or incidence rate of foodborne listeriosis in the United States. This information and the model developed may further inform risk managers when setting or evaluating progress toward L. monocytogenes public health targets and when developing L. monocytogenes risk management options. Reducing L. monocytogenes contamination of foods and reducing exposure through targeted education campaigns to promote safer food handling or decrease consumption of foods at higher risk for contamination among high risk subpopulations are among possible risk management strategies.
Acknowledgments
The authors thank Mohammad Alam, Beverly Wolpert, and Sherri Dennis for their contribution to this study. The authors thank the local, state, and federal FoodNet partners who collect, report, and manage the FoodNet data. This work was also supported by an appointment to the Research Participation Program at the Center for Food Safety and Applied Nutrition administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Disclosure Statement
No competing financial interests exist.
References
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48 [Google Scholar]
- Bouwknegt M, van Pelt W, Havelaar AH. Scoping the impact of changes in population age-structure on the future burden of foodborne disease in the Netherlands, 2020–2060. Int J Environ Res Public Health 2013;10:2888–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census Bureau. 2009 national population projections (supplemental). Population Projections. 2009. Available at: http://www.census.gov/population/projections/data/national/2009.html Accessed October17, 2016
- Census Bureau. 2014 national population projections. Population Projections. 2014. Available at: http://www.census.gov/population/projections/data/national/2014.html Accessed October17, 2016
- Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, Cartter M, Tobin-D'Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Lance S, Tauxe R, Henao OL. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 us sites, 2006–2013. MMWR Morb Mortal Wkly Rep 2014;63:328–332 [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO. 2004. Risk assessment of Listeria monocytogenes in ready to eat foods—Technical report. Page 269. Microbiological Risk Assessment Series, no 5. Food and Agriculture Organization of the United Nations and World Health Organization, Rome [Google Scholar]
- FDA/FSIS. 2003. Quantitative assessment of relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready-to-eat foods. Page 541. Food and Drug Administration, United States Department of Agriculture, Centers for Disease Control and Prevention [Google Scholar]
- Goulet V, Hebert M, Hedberg C, Laurent E, Vaillant V, De Valk H, Desenclos JC. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clin Infect Dis 2012;54:652–660 [DOI] [PubMed] [Google Scholar]
- Goulet V, Hedberg C, Le Monnier A, de Valk H. Increasing incidence of listeriosis in France and other European countries. Emerg Infect Dis 2008;14:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas CN, Rose JB, Gerba CP. Quantitative Microbial Risk Assessment. New York: Wiley, 1999 [Google Scholar]
- Henao OL, Scallan E, Mahon B, Hoekstra RM. Methods for monitoring trends in the incidence of foodborne diseases: Foodborne diseases active surveillance network 1996–2008. Foodborne Pathog Dis 2010;7:1421–1426 [DOI] [PubMed] [Google Scholar]
- HHS. Reduce infections caused by Listeria monocytogenes transmitted commonly through food. Healthy People 2020, Food Safety: FS-13. 2014. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/food-safety/objectives Accessed December29, 2016
- Huang JY, Henao OL, Griffin PM, Vugia DJ, Cronquist AB, Hurd S, Tobin-D'Angelo M, Ryan P, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Wolpert BJ, Patrick ME. Infection with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance—Foodborne diseases active surveillance network, 10 US sites, 2012–2015. MMWR Morb Mort Wkly Rep 2016;65:368–371 [DOI] [PubMed] [Google Scholar]
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. H1n1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451–458 [DOI] [PubMed] [Google Scholar]
- Jones TF, Scallan E, Angulo FJ. Foodnet: Overview of a decade of achievement. Foodborne Pathog Dis 2007;4:60–66 [DOI] [PubMed] [Google Scholar]
- Luchansky J, Chen Y, Porto-Fett ACS, Pouillot R, Shoyer BA, Johnson-DeRycke R, Eblen DR, Hoelzer K, Shaw WK, Jr, Van Doren JM, Catlin M, Lee J, Tikekar R, Gallagher D, Lindsay JA; The Listeria Market Basket Survery Multi-Institutional Team, Dennis S. Survey for Listeria monocytogenes in/on ready-to-eat foods from retail establishments in the United States (2010–2013): Assessing potential changes of pathogen prevalence and levels in a decade. J Food Prot 2017. DOI: 10.4315/0362-028X.JFP-16-420 [DOI] [PubMed] [Google Scholar]
- Mook P, Grant KA, Little CL, Kafatos G, Gillespie IA. Emergence of pregnancy-related listeriosis amongst ethnic minorities in England and Wales. Euro Surveill 2010;15:17–23 [DOI] [PubMed] [Google Scholar]
- Painter J, Slutsker L. Listeriosis in humans. In: Ryser ET, Marth EH, eds. Listeria, Listeriosis and Food Safety. 3rd ed. Boca Raton: CRC Press, 2007, 85–109 [Google Scholar]
- Pouillot R, Hoelzer K, Jackson KA, Henao OL, Silk BJ. Relative risk of listeriosis in foodborne diseases active surviellance network (FoodNet) sites according to age, pregnancy, and ethnicity. Clin Infect Dis 2012;54:S405–S410 [DOI] [PubMed] [Google Scholar]
- Powell MR. Trends in reported foodborne illness in the United States; 1996–2013. Risk Anal 2016;36:1589–1598 [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2008. https://www.r-project.org/ Accessed June12, 2017 [Google Scholar]
- Rocourt J. Risk factors for listeriosis. Food Control 1996;7:195–202 [Google Scholar]
- Ronholm J, Nasheri N, Petronella N, Pagotto F. Navigating microbiological food safety in the era of whole-genome sequencing. Clin Microbiol Rev 2016;29:837–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia: Wolters Kluwer, 2008 [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis 2011;17:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Pintar KD, Fazil AM, Topp E. Harnessing the theoretical foundations of the exponential and beta-Poisson dose-response models to quantify parameter uncertainty using Markov chain Monte Carlo. Risk Anal 2013;33:1677–1693 [DOI] [PubMed] [Google Scholar]
- Silk BJ, Date KA, Jackson KA, Pouillot R, Holt KG, Graves LM, Ong KL, Hurd S, Meyer R, Marcus R, Shiferaw B, Norton DM, Medus C, Zansky SM, Cronquist AB, Henao OL, Jones TF, Vugia DJ, Farley MM, Mahon BE. Invasive listeriosis in the foodborne diseases active surveillance network (FoodNet), 2004–2009: Further targeted prevention needed for higher-risk groups. Clin Infect Dis 2012;54:S396–S404 [DOI] [PubMed] [Google Scholar]
- Silk BJ, Mahon BE, Griffin PM, Gould H, Tauxe RV, Crim SM, Jackson KA, Gerner-Smidt P, Herman KM, Henao OL. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR Morb Mort Wkly Rep 2013;62:448–452 [PMC free article] [PubMed] [Google Scholar]
- Teunis PF, Havelaar AH. The Beta Poisson dose-response model is not a single-hit model. Risk Anal 2000;20:513–520 [DOI] [PubMed] [Google Scholar]
- Ventura SJ, Abma JC, Mosher WD, Henshaw SK. Estimated pregnancy rates for the United States, 1990–2005: An update. Natl Vital Stat Rep 2009;58. [PubMed] [Google Scholar]