Abstract
Introduction
The potential impact of targeting different components of an adverse lipid profile in populations with multiple cardiovascular risk factors is not completely clear. This study aims to assess the association between different components of the standard lipid profile with all-cause mortality and hospitalization due to cardiovascular events in a high-risk population.
Methods
This prospective registry included high risk adults over 30 years old free of cardiovascular disease (2008–2012). Diagnosis of hypertension, dyslipidemia or diabetes mellitus was inclusion criterion. Lipid biomarkers were evaluated. Primary endpoints were all-cause mortality and hospital admission due to coronary heart disease or stroke. We estimated adjusted rate ratios (aRR), absolute risk differences and population attributable risk associated with adverse lipid profiles.
Results
51,462 subjects were included with a mean age of 62.6 years (47.6% men). During an average follow-up of 3.2 years, 919 deaths, 1666 hospitalizations for coronary heart disease and 1510 hospitalizations for stroke were recorded. The parameters that showed an increased rate for total mortality, coronary heart disease and stroke hospitalization were, respectively, low HDL-Cholesterol: aRR 1.25, 1.29 and 1.23; high Total/HDL-Cholesterol: aRR 1.22, 1.38 and 1.25; and high Triglycerides/HDL-Cholesterol: aRR 1.21, 1.30, 1.09. The parameters that showed highest population attributable risk (%) were, respectively, low HDL-Cholesterol: 7.70, 11.42, 8.40; high Total/HDL-Cholesterol: 6.55, 12.47, 8.73; and high Triglycerides/HDL-Cholesterol: 8.94, 15.09, 6.92.
Conclusions
In a population with cardiovascular risk factors, HDL-cholesterol, Total/HDL-cholesterol and triglycerides/HDL-cholesterol ratios were associated with a higher population attributable risk for cardiovascular disease compared to other common biomarkers.
Introduction
Between 1990 and 2013, age-standardized death rates from cardiovascular and circulatory diseases fell by 22% in Western Europe, while ischemic heart disease and stroke remain the main causes of years of life lost [1]. Therefore, primary prevention strategies based on identification of total cardiovascular risk are essential for cardiovascular disease (CVD) control. Risk assessment tools to estimate the patient's 10-year risk of developing CVD have become the cornerstone to identify high-risk people for primary prevention. Both the Framingham-based equations [2] and the European Systematic COronary Risk Evaluation (SCORE) algorithm [3] (the most widely used for clinical practice guidelines), include total cholesterol and high density lipoprotein cholesterol (HDL-C) as the main lipid parameters. The Framingham risk equations were developed during the peak incidence of CVD in the United States, and they perform well in similar populations but may overestimate risk by up to 50% in contemporary European populations, where the incidence of CVD is lower [4]. On the other hand, the SCORE risk prediction chart assesses risk in people up to 65 years of age, but estimation of absolute risk of coronary heart disease and CVD in the elderly is needed for targeted preventive activities, particularly in low-incidence, low-mortality Southern European countries, where life expectancy continues to increase [5].
Low density lipoprotein cholesterol (LDL-C) is the predominant cholesterol-carrying lipoprotein, and is considered to be the main atherogenic lipoprotein. However other lipoproteins such as (HDL-C or very low density lipoprotein have shown repeatedly to play a role in atherogenesis. Recent epidemiological data suggests that isolated low HDL-C in people with normal LDL-C and triglyceride (TG) levels is equivalent to elevated LDL-C as a coronary risk factor [6–8]. Moreover, low HDL-C levels and the ratio of total serum cholesterol (TC) to HDL-C levels have been introduced in the novel CVD risk scores, such as the QRISK and QRISK2 [9]; the latter being currently recommended by the National Clinical Guideline Centre for Cardiovascular Risk Assessment for primary prevention of CVD [10].
There is limited clinical data however, that prospectively evaluates the association of lipid markers and cardiovascular risk in high-risk populations. In addition, the potential impact on attributable risk of hypothetical interventions targeting novel lipid markers has not been fully explored in contemporary populations with additional cardiovascular risk factors such as hypertension or diabetes. The aim of the present study was to prospectively estimate and compare the attributable risk associated with several lipid markers for all-cause mortality and hospitalization due to CVD in participants with at least one of the three major cardiovascular risk factors: hypertension, diabetes or dyslipidemia, receiving usual care and participating in the ESCARVAL-RISK project [11] “EStudio CARdiometabolico VALenciano” in a Mediterranean population.
Methods
ESCARVAL-RISK is an observational cohort study in individuals with cardiovascular risk factors (hypertension, dyslipidemia, or diabetes mellitus) and free of previous CVD. Treatment for hypertension, diabetes or dyslipidemia, as well as other concomitant diseases, was left to the discretion of primary care physicians and patients were treated according to current clinical guidelines. Therefore, the ESCARVAL-RISK study was specifically designed to investigate associations between three major cardiovascular risk factors and CVD in the real world setting of clinical practice.
Study population
The Valencia Community is a Mediterranean region located on the east coast of Spain, with a total population of 4.980.689 according to the 2015 census. The cohort was recruited from a sample of patients receiving healthcare by the Valencia Health System. Every user of this system has a unique patient identifier, corresponding to a centralized, individual electronic clinical record. The unique patient identifier allows linkage between relevant clinical databases where various variables were collected. Detailed information about the sample size recruitment has been published elsewhere [11].
Briefly, we included 73,302 participants of both sexes, aged 30 years or older with a diagnosis of hypertension, diabetes mellitus, and/or dyslipidemia, with no previous cardiovascular events who attended a primary healthcare center for routine health services. Of the total population, there was missing data on body weight for 12,209 participants, on serum creatinine for 5,175 participants, and on other variables of interest for 4,456 participants. After excluding these participants, our final sample size included 51,462 participants. Information was collected from ABUCASIS, which is the electronic health record (EHR) that registers patient data in the Valencia region.
Baseline data collection
Data on age, sex, smoking and medication for treating hypertension, diabetes, and hypercholesterolemia was collected from the EHR. Blood pressure was measured up to three times on the same day in the sitting position following the European guidelines on CVD prevention in clinical practice [12]. Hypertension was defined as a mean systolic blood pressure ≥140 mm Hg, a mean diastolic blood pressure ≥90 mm Hg, a recorded physician diagnosis, or medication use. Diabetes was defined as a non-fasting glucose level of ≥200 mg/dl, a recorded physician diagnosis, medication use, or an HbA1c ≥ 6.5%. TC was measured enzymatically using the Cholesterol High Performance reagent (Roche Diagnostics). HDL-C was measured using a direct HDL reagent (Roche Diagnostics). LDL-C was calculated using the Friedwald formula [13]. High cholesterol was defined as a serum total cholesterol >200 mg/dL, recorded diagnosis or medication use. Non-HDL cholesterol was measured according to the difference between TC and HDL-C. Triglycerides were measured using Hitachi 704 Analyzer which is serviced by Roche Diagnostics (formerly Boehringer-Mannheim Diagnostics), Indianapolis. Also non-HDL minus LDL-cholesterol was calculated. Body mass index (BMI) was calculated by dividing measured weight in kilograms by height in squared meters and obesity was defined as a BMI ≥30 kg/m2.
Mortality and hospitalization follow-up
The follow up period was from January 2008 to December 2012. Participants were followed up until the first episode of hospitalization for CHD or stroke or for death. Data on all-cause mortality was collected. At the time of inclusion, information about cardiovascular risk factors and their active treatments as well as smoking habit and biochemistry lab values were collected from the EHR. Mortality data were obtained from death certificates registered in the Spanish National Death Index. The cause of hospitalization was determined by the codes assigned according to the International Classification of Diseases, 10th Revision (ICD-10). Cause-specific hospitalization was defined as the first in-hospital admission for CHD (ICD codes 410–414) or stroke (ICD codes 430–438, 444). Cardiovascular hospitalizations or mortality during follow-up were assessed by annual mortality and morbidity surveillance reviews of hospitalization and death records. Follow-up data was available for 99.8% of subjects for mortality and for 99.2% of subjects for morbid events. Time to first event was calculated as the difference between the date of the baseline examination and the date of the hospital admission, date of death or 31 December 2012, whichever occurred first.
The study was conducted according to the standards of the International Guidelines for Ethical Review of Epidemiological Studies (Council for International Organizations of Medical Sciences-CIOMS-Geneva, 1991). The ESCARVAL-RISK study [11] was reviewed and approved by the Valencia Committee for Ethics and Clinical Trials of the Center for Public Health Research (DGSP-CSISP). Patient data collected from the ABUCASIS EHR during the study were anonymized, making it impossible to use the information to identify the patients. The data generated during the study were handled according the Spanish Law 5/1999 and corresponding regulations. All of the researchers with access to study data were required to sign a document guaranteeing confidentiality. No informed consent from patients was required.
Statistical analysis
Age-adjusted rates for mortality and cardiovascular hospitalization end-points were estimated using Poisson regression for individual data with over-dispersion correction. Multi-adjusted rate differences were estimated from semi-parametric Aalen additive hazard models. Statistical models were adjusted for age (continuous-modelled as restricted cubic splines with five knots), sex (male, female), BMI (continuous), hypertension (no, yes), hypertension medication (no, yes), diabetes (no, yes), diabetes medication (no, yes), smoking status (never, former, current), high LDL-C (<130 mg/dL, ≥130 mg/dL), low HDL-C (≤40 mg/dL for men; ≤50 mg/dL for women) and use of cholesterol-lowering medication (no, yes). Adjusted population attributable risks (PARs) for dichotomous lipid biomarkers were calculated by using the standard formula PAR = 1 – ΣjΣi pij / RRi| j [14]. In this formula, the subscript i denotes one of two categories of the lipid biomarkers (with each participant classified according to the presence of the corresponding biomarker being used to calculate the PAR), the subscript j is an index for all strata obtained after cross-classifying the study sample for all adjusted covariates, pij is the proportion of total cases in the study population in each stratum after cross-classifying the dichotomous biomarker category and all adjusted covariates, and RRi|j is the adjusted hazard ratio for the endpoint of interest comparing participants with and without the biomarker in stratum j of covariates, from Cox proportional hazards regression. Adjusted PARs represent the estimated fraction of deaths that would be avoided in the population, had participants above a given cut-off of the biomarker been below it, assuming that the effects are causal and that other risk factors remain unchanged. We created 55,000 bootstrap samples to obtain the standard errors and 95% confidence intervals for PAR.
Results
Participant characteristics
A total of 51,462 patients with at least one cardiovascular risk factor were included in the study. The main characteristics of the study population, grouped by the study endpoints, are shown in Table 1. Hypertension was present in 79% and diabetes in 37% of the participants. Thirty percent were receiving lipid-lowering drug treatment. During an average follow-up of 3.2 years, the EHR recorded 919 deaths (80,705.3 person-years at risk) 1666 hospitalizations for CHD (78,643.85 person-years at risk) and 1510 stroke hospitalizations (79,130.76 person-years at risk). Age-adjusted rates (deaths/10,000 person-years) of CVD and mortality endpoints by CVD risk factors per quartiles of each lipid parameter are described in Table 2.
Table 1. Participant characteristics according to presence or absence of all-cause mortality and CVD hospitalization end-points.
| All-cause mortality | CHD hospitalization | Stroke hospitalization | |||||
|---|---|---|---|---|---|---|---|
| Overall (N = 51,462) | No (N = 50,543) | Yes (N = 919) | No (N = 49,796) | Yes (N = 1666) | No (N = 49,952) | Yes (N = 1510) | |
| Age, mean years (SD) | 62.65 (12.07) | 62.45 (12.02) | 73.37 (10.21) | 62.5 (12.1) | 67.19 (10.36) | 62.41 (12.05) | 70.44 (9.89) |
| Men, % | 47.59 | 47.26 | 65.61 | 47.07 | 63.09 | 47.35 | 55.56 |
| BMI, kg/m2, mean (SD) | 29.53 (4.83) | 29.54 (4.83) | 29.19 (4.89) | 29.52 (4.84) | 29.87 (4.59) | 29.54 (4.84) | 29.29 (4.55) |
| Obesity, % | 42.09 | 42.12 | 40.48 | 41.99 | 44.90 | 42.15 | 40.07 |
| Former smoking, % | 20.78 | 20.66 | 27.53 | 20.39 | 32.59 | 20.67 | 24.57 |
| Current smoking, % | 22.22 | 22.26 | 20.46 | 22.39 | 17.29 | 22.35 | 17.95 |
| Diabetes, % | 37.20 | 36.88 | 54.41 | 36.62 | 54.44 | 36.76 | 51.59 |
| Glucose lowering medication, % | 19.39 | 19.51 | 12.95 | 19.00 | 31.15 | 19.14 | 27.95 |
| Systolic blood pressure, mean mmHg (SD) | 136.24 (18.19) | 136.17 (18.16) | 140.19 (19.64) | 136.11 (18.14) | 140.32 (19.26) | 136.11 (18.14) | 140.75 (19.17) |
| Diastolic blood pressure, mean mmHg (SD) | 79.36 (10.85) | 79.41 (10.84) | 76.71 (10.83) | 79.4 (10.83) | 78.4 (11.37) | 79.39 (10.83) | 78.52 (11.41) |
| Hypertension, % | 78.98 | 78.78 | 90.42 | 78.55 | 92.14 | 78.59 | 92.05 |
| Antihypertensive medication, % | 43.56 | 43.95 | 22.20 | 43.10 | 57.20 | 43.27 | 53.18 |
| Chronic kidney disease, % | 15.01 | 14.69 | 32.43 | 14.69 | 24.31 | 14.66 | 26.36 |
| Total cholesterol, mean mg/dL (SD) | 211.01 (40.97) | 211.26 (40.93) | 197.58 (41.02) | 211.35 (40.84) | 201.02 (43.61) | 211.25 (40.9) | 203.12 (42.49) |
| HDL-cholesterol, mean mg/dL (SD) | 52.7 (13.94) | 52.74 (13.93) | 50.55 (13.98) | 52.83 (13.94) | 48.86 (13.2) | 52.76 (13.95) | 50.8 (13.38) |
| Non-HDL-cholesterol, mean mg/dL (SD) | 158.31 (39.41) | 158.52 (39.39) | 147.04 (38.7) | 158.52 (39.3) | 152.16 (42) | 158.49 (39.36) | 152.32 (40.49) |
| LDL-cholesterol, mean mg/dL (SD) | 126.2 (34.33) | 126.36 (34.31) | 117.62 (34.37) | 126.4 (34.25) | 120.06 (36.16) | 126.34 (34.3) | 121.47 (35.03) |
| Triglycerides, mean mg/dL (SD) | 149.69 (100.01) | 149.89 (100.29) | 138.46 (82.37) | 149.47 (100.13) | 156.38 (96.15) | 149.86 (100.51) | 143.95 (81.65) |
| Ratio total/HDL-cholesterol | 4.22 (1.2) | 4.22 (1.2) | 4.12 (1.17) | 4.22 (1.2) | 4.34 (1.27) | 4.22 (1.2) | 4.2 (1.19) |
| Ratio triglycerides/HDL-cholesterol | 3.26 (3.01) | 3.26 (3.02) | 3.11 (2.69) | 3.25 (3.01) | 3.63 (3.07) | 3.26 (3.03) | 3.18 (2.36) |
| Dyslipidemia, % | 88.23 | 88.39 | 79.22 | 88.04 | 93.76 | 88.20 | 89.01 |
| Lipid lowering medication, % | 30.28 | 30.60 | 12.73 | 29.80 | 44.60 | 30.09 | 36.76 |
CVD: cardiovascular disease; CHD: coronary heart disease; SD: statistical deviation; BMI: body mass index; HDL: High density lipoprotein; LDL: low density lipoprotein
Table 2. Age and sex-adjusted rates of all-cause mortality and CVD hospitalization by quartile of serum lipids.
| Quartile | p-value | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total cholesterol | |||||
| Median (range), mg/dL | 165 (118, 183) | 165 (184, 210) | 223 (211, 237) | 257 (238, 329) | |
| All-cause mortality | |||||
| Cases (person-years) | 341 (43,630.74) | 255 (43,449.59) | 184 (41,295.09) | 139 (41,238.03) | |
| Rate | 60.3 | 55.5 | 51.0 | 49.5 | 0.027 |
| CHD hospitalization | |||||
| Cases (person-years) | 609 (42,385.83) | 429 (42,671.84) | 306 (40,716.51) | 322 (40,637.52) | |
| Rate | 126.0 | 97.7 | 80.6 | 95.2 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 504 (42,683.04) | 388 (42,774.50) | 321 (40,668.79) | 297 (40,714.40) | |
| Rate | 98.3 | 85.0 | 84.7 | 93.9 | 0.361 |
| HDL cholesterol | |||||
| Median (range), mg/dL | 38 (27, 43) | 38 (44, 51) | 56 (52, 61) | 69 (62, 98) | |
| All-cause mortality | |||||
| Cases (person-years) | 315 (45,630.38) | 228 (42,053.36) | 191 (42,115.12) | 185 (39,814.59) | |
| Rate | 66.0 | 52.9 | 46.0 | 50.7 | <0.001 |
| CHD hospitalization | |||||
| Cases (person-years) | 633 (44,381.46) | 435 (41,240.43) | 337 (41,467.21) | 261 (39,322.61) | |
| Rate | 135.8 | 103.5 | 83.7 | 73.6 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 487 (44,749.58) | 378 (41,346.15) | 364 (41,439.38) | 281 (39,305.62) | |
| Rate | 110.8 | 90.4 | 86.3 | 71.9 | <0.001 |
| Non-HDL cholesterol | |||||
| Median (range), mg/dL | 115 (74, 131) | 115 (132, 156) | 169 (157, 183) | 204 (184, 276) | |
| All-cause mortality | |||||
| Cases (person-years) | 325 (43,768.99) | 253 (42,218.93) | 192 (42,588.25) | 149 (41,037.27) | |
| Rate | 58.0 | 55.9 | 50.0 | 52.7 | 0.154 |
| CHD hospitalization | |||||
| Cases (person-years) | 558 (42,614.69) | 420 (41,425.83) | 352 (41,964.77) | 336 (40,406.42) | |
| Rate | 117.6 | 98.0 | 88.0 | 96.4 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 477 (42,867.75) | 383 (41,571.73) | 339 (41,923.08) | 311 (40,478.17) | |
| Rate | 92.1 | 85.2 | 85.2 | 99.8 | 0.492 |
| LDL cholesterol | |||||
| Median (range), mg/dL | 88 (50, 102) | 88 (103, 124) | 135.75 (125, 148) | 166 (149, 224) | |
| All-cause mortality | |||||
| Cases (person-years) | 314 (41,816.10) | 246 (41,215.15) | 203 (43,304.18) | 156 (43,278.03) | |
| Rate | 60.9 | 56.0 | 50.4 | 49.7 | 0.015 |
| CHD hospitalization | |||||
| Cases (person-years) | 577 (40,679.63) | 397 (40,445.12) | 342 (42,666.73) | 350 (42,620.22) | |
| Rate | 128.4 | 95.6 | 83.1 | 94.4 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 460 (40,965.45) | 369 (40,527.55) | 370 (42,657.15) | 311 (42,690.59) | |
| Rate | 95.5 | 85.2 | 90.2 | 91.1 | 0.606 |
| Non-HDL minus LDL cholesterol | |||||
| Median (range), mg/dL | 16.6 (7.6, 21.0) | 16.6 (22.0, 30.0) | 35 (30.6, 40.3) | 48.25 (41.0, 93.0) | |
| All-cause mortality | |||||
| Cases (person-years) | 308 (51,216.52) | 239 (42,451.48) | 206 (38,554.97) | 166 (37,390.47) | |
| Rate | 52.6 | 52.7 | 53.8 | 58.5 | 0.319 |
| CHD hospitalization | |||||
| Cases (person-years) | 449 (50,328.06) | 431 (41,582.03) | 391 (37,851.46) | 395 (36,650.16) | |
| Rate | 85.5 | 99.8 | 102.5 | 118.1 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 448 (50,354.07) | 420 (41,656.99) | 335 (37,963.04) | 307 (36,866.61) | |
| Rate | 81.4 | 94.8 | 87.7 | 101.0 | 0.013 |
| Triglycerides | |||||
| Median (range), mg/dL | 74 (43, 91) | 74 (92, 124) | 147 (126, 176) | 232 (179, 628) | |
| All-cause mortality | |||||
| Cases (person-years) | 256 (43,230.56) | 249 (43,280.85) | 222 (42,904.17) | 192 (40,197.86) | |
| Rate | 52.8 | 53.2 | 52.0 | 59.2 | 0.347 |
| CHD hospitalization | |||||
| Cases (person-years) | 354 (42,575.48) | 400 (42,462.60) | 426 (42,111.74) | 486 (39,261.89) | |
| Rate | 80.9 | 91.6 | 99.6 | 130.7 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 355 (42,606.01) | 400 (42,519.69) | 410 (42,135.18) | 345 (39,579.85) | |
| Rate | 77.5 | 88.2 | 96.0 | 101.2 | <0.001 |
| Ratio total/HDL cholesterol | |||||
| Median (range), mg/dL | 2.96 (2.11, 3.35) | 2.96 (3.38, 4.03) | 4.42 (4.06, 4.86) | 5.58 (4.92, 8.29) | |
| All-cause mortality | |||||
| Cases (person-years) | 261 (42,536.33) | 220 (42,720.99) | 238 (42,608.40) | 200 (41,747.72) | |
| Rate | 53.8 | 49.1 | 55.9 | 58.0 | 0.276 |
| CHD hospitalization | |||||
| Cases (person-years) | 370 (41,798.70) | 392 (41,961.01) | 435 (41,806.98) | 469 (40,845.02) | |
| Rate | 87.2 | 91.8 | 102.1 | 119.8 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 386 (41,761.15) | 366 (42,108.46) | 388 (41,901.78) | 370 (41,069.34) | |
| Rate | 81.3 | 81.3 | 92.4 | 107.3 | <0.001 |
| Ratio TG/HDL cholesterol | |||||
| Median (range), mg/dL | 1.20 (0.58, 1.57) | 1.20 (1.61, 2.44) | 3.05 (2.49, 3.84) | 5.49 (3.95, 17.88) | |
| All-cause mortality | |||||
| Cases (person-years) | 229 (42,638.68) | 232 (43,056.65) | 241 (42,876.82) | 217 (41,041.30) | |
| Rate | 51.5 | 49.4 | 54.6 | 61.6 | 0.041 |
| CHD hospitalization | |||||
| Cases (person-years) | 286 (42,105.98) | 395 (42,282.16) | 466 (42,014.74) | 519 (40,008.84) | |
| Rate | 70.3 | 91.1 | 107.7 | 133.2 | <0.001 |
| Stroke hospitalization | |||||
| Cases (person-years) | 321 (42,083.52) | 409 (42,283.75) | 398 (42,119.24) | 382 (40,354.22) | |
| Rate | 73.2 | 90.2 | 92.0 | 107.3 | <0.001 |
CVD: cardiovascular disease; CHD: coronary heart disease; HDL: High density lipoprotein; LDL: low density lipoprotein
Age and sex-adjusted rates: events/10,000 person-years
Lipid parameters, mortality and hospitalization for CHD or stroke rates
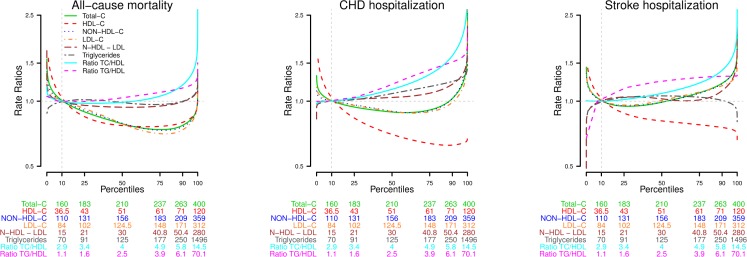
Age-adjusted mortality rates (deaths/10,000 person-years) showed a significant positive association with the TG/HDL-C ratio and an inverse association with TC, HDL-C and LDL-C. Regarding the risk of hospitalization for CHD, a significant positive association was observed in non-HDL-C minus LDL-C, TG, TC/HDL-C ratio and TG/HDL-C ratio, and an inverse relationship with TC, HDL-C and LDL-C. For hospitalization due to stroke, there was a significant positive association with LDL-C, non-HDL-C minus LDL-C, TG, TC/HDL-C ratio and TG/HDL-C ratio, and an inverse association with HDL-C. After further adjustment for other cardiovascular risk factors, however, the association of elevated TC and LDL-C levels and CHD hospitalization was no longer significant, either in the relative or additive scales (Tables 3 and 4). For stroke, however, the association with LDL-C remained significant in fully adjusted models. The rate ratio and rate differences (95% CI) for all–cause mortality and CVD hospitalization after a 3.2-year follow-up comparing the 75th versus 25th percentile of lipid biomarkers concentrations are shown in the S1 Table, with consistent findings. Fig 1 shows the fully adjusted dose-response association between lipid values and risk of mortality and hospitalization due to CHD and stroke. A sensitivity analysis was performed in participants according to current lipid-lowering treatment (S1 Fig), but no relevant differences were found between groups.
Table 3. Rate differences after a 5-year follow-up by altered lipid levels.
| All-cause mortality | CHD hospitalization | Stroke hospitalization | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| High total cholesterol a | -10.70 (-18.08, -3.33) | -16.36 (-24.15, -8.57) | -30.62 (-41.12, -20.12) | -5.76 (-16.65, 5.13) | -2.84 (-12.50, 6.82) | 11.16 (0.95, 21.38) |
| Low HDL-cholesterolb | 11.55 (4.10, 19.00) | 7.79 (0.12, 15.46) | 40.46 (30.20, 50.73) | 26.55 (16.07, 37.03) | 25.61 (15.45, 35.76) | 17.96 (7.45, 28.47) |
| High non-HDL-cholesterolc | -18.16 (-37.71, 1.39) | -23.66 (-43.31, -4.02) | -47.98 (-75.79, -20.16) | -27.67 (-55.59, 0.25) | -26.68 (-51.56, -1.80) | -16.05 (-41.12, 9.03) |
| High LDL-cholesterold | -8.32 (-15.04, -1.60) | -15.26 (-22.30, -8.22) | -22.51 (-31.72, -13.29) | -1.88 (-11.32, 7.57) | -3.56 (-12.62, 5.50) | 6.91 (-2.46, 16.27) |
| High non-HDL minus LDL-cholesterole | 5.47 (-1.48, 12.41) | 1.53 (-5.57, 8.63) | 15.46 (5.53, 25.38) | 5.58 (-4.55, 15.71) | 3.80 (-5.38, 12.98) | -2.43 (-11.85, 6.99) |
| High triglyceridesf | 0.57 (-6.56, 7.70) | -2.02 (-9.69, 5.64) | 25.44 (14.97, 35.91) | 7.94 (-3.50, 19.37) | 10.59 (1.08, 20.09) | -3.61 (-13.74, 6.53) |
| High total/HDL-cholesterolg | 2.17 (-4.97, 9.30) | 2.22 (-5.54, 9.99) | 18.10 (7.37, 28.83) | 23.14 (11.61, 34.67) | 16.04 (6.24, 25.84) | 14.55 (3.80, 25.31) |
| High triglycerides/HDL-cholesterolh | 5.14 (-1.93, 12.21) | 3.77 (-3.45, 10.98) | 35.76 (25.64, 45.87) | 22.13 (11.75, 32.51) | 12.98 (3.90, 22.06) | 4.25 (-5.29, 13.78) |
CVD: cardiovascular disease; CI: confidence interval HDL: High density lipoprotein; LDL: low density lipoprotein
Rate Differences: events/10.000 person-years (95%CI)
Model 1 is adjusted for age and sex. Model 2 is Model 1 further adjusted for smoking status (never, former, current), obesity (no, yes), diabetes (no, yes), hypertension (no, yes), chronic kidney disease (no, yes), anti-hypertensive medication (no, yes), glucose-lowering medication (no, yes), lipid-lowering medication (no, yes). Models for specific lipid biomarkers have been additionally adjusted as follows
Total-cholesterola is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes)
HDL-cholesterolb is further adjusted by LDL ≥ 130 mg/dL (no, yes)
Non-HDL-cholesterolc is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes)
LDL-cholesterold is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes)
Non-HDL minus LDL-cholesterole is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes) and LDL-C ≥ 130 mg/dL (no, yes)
Triglyceridesf further adjusted by total cholesterol > 200 mg/dL (no, yes) and HDL ≤ 40 for men and ≤ 50 for women (no, yes)
Total cholesterol/HDLg is further adjusted by total cholesterol (mg/dL); and
Triglycerides/HDLh is further adjusted by total cholesterol (mg/dL).
Table 4. Population attributable risk (95% CI) for all-cause mortality and CVD hospitalization by altered lipid levels.
| Prevalence | All-cause mortality | CHD hospitalization | Stroke hospitalization | |
|---|---|---|---|---|
| High total cholesterola | 60.19% | |||
| RR | 0.83 (0.73, 0.96) | 0.95 (0.86, 1.06) | 1.14 (1.02, 1.27) | |
| PAR | -9.18 (-16.44, -2.12) | -2.32 (-7.41, 2.69) | 6.60 (1.19, 11.91) | |
| Low HDL-cholesterolb | 31.61% | |||
| RR | 1.19 (1.04, 1.37) | 1.31 (1.18, 1.45) | 1.23 (1.1, 1.37) | |
| PAR | 5.32 (1.02, 9.64) | 8.92 (5.41, 12.44) | 6.64 (3.09, 10.20) | |
| High non-HDL-cholesterolc | 94.51% | |||
| RR | 0.84 (0.68, 1.05) | 0.86 (0.72, 1.02) | 0.9 (0.75, 1.09) | |
| PAR | -16.71 (-41.12, 5.88) | -15.25 (-34.09, 2.50) | -9.85 (-29.16, 8.46) | |
| High LDL-cholesterold | 43.99% | |||
| RR | 0.85 (0.74, 0.98) | 0.98 (0.88, 1.09) | 1.09 (0.98, 1.21) | |
| PAR | -5.93 (-11.12, -0.70) | -0.76 (-4.54, 3.01) | 3.14 (-0.94, 7.20) | |
| High Non-HDL minus LDL-cholesterole | 51.14% | |||
| RR | 1.03 (0.9, 1.17) | 1.08 (0.98, 1.19) | 0.99 (0.89, 1.1) | |
| PAR | 1.08 (-4.68, 6.71) | 3.58 (-1.18, 8.28) | -0.54 (-5.19, 4.15) | |
| High Triglyceridesf | 36.12% | |||
| RR | 1.05 (0.91, 1.23) | 1.11 (0.99, 1.24) | 0.99 (0.88, 1.11) | |
| PAR | 1.65 (-3.18, 6.33) | 3.95 (-0.26, 8.09) | -0.49 (-4.63, 3.62) | |
| High Total/HDL-cholesterolg | 29.46% | |||
| RR | 1.1 (0.94, 1.3) | 1.31 (1.17, 1.47) | 1.2 (1.06, 1.35) | |
| PAR | 2.20 (-1.49, 5.88) | 7.10 (4.05, 10.17) | 4.58 (1.44, 7.70) | |
| High triglycerides/HDL-cholesterolh | 39.42% | |||
| RR | 1.16 (1.01, 1.33) | 1.28 (1.16, 1.42) | 1.08 (0.97, 1.2) | |
| PAR | 4.96 (0.28, 9.61) | 9.91 (5.85, 13.91) | 2.81 (-1.27, 6.84) |
CI: confidence interval; CHD: coronary heart disease; CVD: cardiovascular disease; PAR: population attributable risk; RR: rate ratio; HDL: High density lipoprotein; LDL: low density lipoprotein
Model 1 is adjusted for age and sex. Model 2 is Model 1 further adjusted for smoking status (never, former, current), obesity (no, yes), diabetes (no, yes), hypertension (no, yes), chronic kidney disease (no, yes), anti-hypertensive medication (no, yes), glucose lowering medication (no, yes), lipid-lowering medication (no, yes). Models for specific lipid biomarkers have been additionally adjusted as follows
Total-cholesterola is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes)
HDL-cholesterolb is further adjusted by LDL-C ≥ 130 mg/dL (no, yes)
Non-HDL-cholesterolc is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes)
LDL-cholesterold is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes)
Non-HDL minus LDL-cholesterole is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes) and LDL-C ≥ 130 mg/dL (no, yes)
Triglyceridesf is further adjusted by total cholesterol > 200 mg/dL (no, yes) and HDL ≤ 40 for men and ≤ 50 for women (no, yes)
Total cholesterol/HDLg is further adjusted by total cholesterol (mg/dL); and
Triglycerides/HDLh is further adjusted by total cholesterol (mg/dL).
Fig 1. Age and sex-adjusted rates ratios for all-cause mortality and CVD hospitalization by serum lipid levels.
Models are adjusted for age, sex, smoking status (never, former, current), obesity (no, yes), diabetes (no, yes), hypertension (no, yes), chronic kidney disease (no, yes), anti-hypertensive medication (no, yes), glucose lowering medication (no, yes), lipid-lowering medication (no, yes). Models for specific lipid biomarkers have been additionally adjusted as follows: 1) Total-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 2) HDL-cholesterol is further adjusted by LDL.C ≥ 130 mg/dL (no, yes); 3) Non-HDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 4) LDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 5) Non-HDL minus LDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes) and LDL-C ≥ 130 mg/dL (no, yes); 6) Triglycerides is further adjusted by total cholesterol > 200 mg/dL (no, yes) and HDL ≤ 40 for men and ≤ 50 for women (no, yes); 7) Total cholesterol/HDL is further adjusted by total cholesterol (mg/dL); and 7) Triglycerides/HDL is further adjusted by total cholesterol (mg/dL).
Disease burden associated with lipid parameters
Multi-adjusted differences in rate of events/10,000 person-years of mortality and CVD endpoints (attributable risk) are shown in Table 3. Low HDL-C and a high TC/HDL-C ratio were associated with the absolute risk of hospitalization for CHD and stroke. High TG/HDL-C also increased the absolute risk of hospitalization for CHD. The population attributable risks (PAR) associated with the lipid parameters are shown in Table 4. Low HDL-C, high TC/HDL-C and high TG/HDL-C were associated with a PAR for mortality of 5.3%, 2.2% and 5.0%, respectively. For CHD hospitalization the PAR was 8.9%, 7.1% and 9.9%, respectively. For stroke hospitalization, the attributable risk was low HDL-C (6.6%) and high TC/HDL-C (4.6%).
Discussion
In this Mediterranean population with at least one major cardiovascular risk factor, HDL-C levels and the ratios of TC/HDL-C and TG/HDL-C were associated with all-cause mortality and risk of hospitalization due to CHD and stroke, while LDL-C was associated with stroke but not with CHD. These data were confirmed in a sensitivity analysis carried out in participants not receiving lipid-lowering therapy at baseline. The PARs associated with HDL-C, TC/HDL-C and TG/HDL-C ranged from 4.5% (CI95% 4.4–7.7; PAR of stroke associated to elevated TC/HDL-C) to 9.9% (CI95% 5.8–13.9; PAR of CHD associated with elevated TG/HDL-C).
Characteristics of the study population and the source of data
Participants included in the analysis had a sociodemographic profile comparable to the overall selected population. The age and sex-adjusted rates in our population were lower compared to the United States and other European countries [4, 5], reflecting a country-specific profile of low cardiovascular risk, as supported by the SCORE study [3].
Relationship between lipid parameters and cardiovascular risk in previous studies in Spain
The age-adjusted absolute rates for mortality or hospitalization for CHD or stroke by lipid values and indexes presented in this paper are consistent with previously published studies carried out in Spain and elsewhere. The relationship between lipid parameters and rates of cardiovascular risk has been explored previously in Spain, although these were performed in the 1990s. The ERICE [15] and the FRESCO [5] studies included data from 11 population cohorts in seven Spanish regions. The results of the FRESCO study were consistent with our finding that HDL-C was the lipid factor most strongly associated with cardiovascular risk. In the ERICE study, total cholesterol did not show a statistical association with cardiovascular events, however it was not sufficiently powered to assess the impact of HDL-C. Recent data from a study carried out in patients recently diagnosed with diabetes mellitus [16], identified the ratio of non-HDL-C to HDL-C as a significant predictor of cardiovascular events, while LDL-C and TC did not show significant associations.
Lipid parameters as predictors of vascular events
Studies performed worldwide in different populations have found associations between cardiovascular events and low HDL-C [17–26]. Other studies have found associations with the TC/HDL-C ratio [27–29], yet others have showed conflicting data [30, 31].
It seems clear that low HDL-C is a strong and independent risk factor for CVD. HDL-C particles may act as a protective factor against atherosclerosis via multiple biological mechanisms [32]: effluxing cellular cholesterol, diminishing cellular death, decreasing vascular constriction, reducing inflammatory response, protecting from pathological oxidation, combating bacterial infection, lessening platelet activation, regulating gene expression by virtue of microRNAs, and improving glucose metabolism.
Data from the Jupiter Study [33] has shown that baseline LDL-C was not associated with CVD events. In another recent publication [34] of data from more than 350,000 people from three cohorts (REasons for Geographic And Racial Differences in Stroke [REGARDS], Kaiser Permanente Southern California [KPSC] and Atherosclerosis Risk In Communities [ARIC]) the results suggested that the association between LDL-C and CHD in contemporary studies may be diminished by the preferential use of statins in high risk individuals, while the association with HDL-related markers remains. While we have not found relevant differences between participants based on treatment at baseline (S1 Fig), we cannot rule out the presence of a time-varying residual confounding effect by statin use during the follow-up period in our study population.
It is also important to note that with regard to prediction of vascular events, the most commonly used predictive scales for cardiovascular risk (Framingham [2], SCORE [3]) consider total cholesterol, HDL cholesterol and the ratio of total cholesterol to HDL-C to be the strongest predictors. In the most recent and widely accepted QRISK2 [9], however, the lipid parameter included for cardiovascular risk calculation is the ratio TC/HDL-C. The NICE dyslipidemia guideline [10] recommends using the QRISK2 risk assessment tool to assess cardiovascular risk for the primary prevention of CVD in people aged 84 and younger. So ESCARVAL study results agree with these data in order to conclude that nowadays, lipid parameters as HDL-C or Total Cholesterol / HDL-C are more strongly associated with cardiovascular events than the most used in clinical practice as LDL-C, and are better predictors to estimate the cardiovascular risk, especially in high risk patients.
Prospective studies measuring not only HDL and LDL cholesterol levels, but also the number and size of particles, are needed to further elucidate the association between lipid particles and cardiovascular risk.
Multi-adjusted rate differences in mortality or hospitalizations: PAR
In order to further explore the relationship between lipid parameters and cardiovascular risk, we analyzed the multi-adjusted rate difference in mortality and hospitalizations as well as the PAR by altered lipid levels (Table 3). This value can be interpreted as the average annual increase in mortality and risk of hospitalization due to a cardiovascular event on an absolute scale, attributable to the factor considered and the relative amount of avoidable deaths and hospitalization in the population studied, respectively.
The PAR provides an estimated measure of the public health impact of a potential intervention targeting specific risk factors, in the hypothetical scenario where the association of these markers and CVD risk is causal, and the other risk factors remain unchanged. For most of the evaluated endpoints (mortality and hospitalization for CHD or stroke), low HDL showed a higher PAR than the other lipid markers included in the study. Few studies have analyzed the impact of lipid particles and PAR, and their results are inconsistent. The Framingham Offspring study followed a cohort with a mean baseline age of 51 years for two decades, finding that low levels of HDL-C, high LDL-C and high levels of TG in any combination, were associated with increased CVD risk. In fact, the highest PARs were for the groups including high LDL-C, especially in the presence of concomitantly low HDL-C and/or high TG [35]. Another study, in which the incidence was similar to that of the United States in the 1970s, found that low HDL-C and high TC were associated with a similar PAR to the one found in our study [36]. Discrepancies on the impact of LDL-C or TC on cardiovascular risk may be explained by our selection criteria, which included a higher risk profile and higher use of statins. PAR for cardiovascular risk associated with lipid parameters in other contemporary studies are, however, scarce.
Strengths and limitations
There are some limitations to the current study that should be mentioned. Firstly, the lower CVD risk inherent to the Mediterranean population could limit the generalizability of our results. In addition, the results apply only to individuals with at least one cardiovascular risk factor and cannot be extrapolated to the general population.
The results of this observational study should be considered within the advantages and limitations of registry-based data [37]: the use of EHRs offers a timely alternative and these databases provide a low-cost means of accessing rich longitudinal data on large populations for epidemiologic research. Using EHR for data collection reflects real clinical practice, in contrast to data from clinical trials. Another potential advantage, as in the current study, is the large number of participants and events, which provided enough statistical power and a valuable framework in which to assess the attributable risk of mortality, CHD, and stroke to cardiovascular risk factors in the short term in a real life setting.
The mean follow-up was 3.2 years. Although this is a relatively short time period, we evaluated a high risk study population, which resulted in 919 deaths (80,705.3 person-years at risk), 1666 hospitalizations for CHD (78,643.85 person-years at risk), and 1510 hospitalizations for stroke. Most cardiovascular risk scales predict cardiovascular risk at 10 years, which has been proposed as a limitation for high-risk populations. It has become increasingly clear that from a public health perspective and in clinical practice the need for shorter-term scales in order to intensify interventions, avert clinical inertia and improve therapeutic adherence. The lack of association between LDL-C and cardiovascular events in the current study could be influenced by a shorter time scale used, raising the question of whether LDL-C is a good short-term predictor of cardiovascular events in high-risk patients. In a study with longer follow-up period, the association between LDL-C and events may be prove to be stronger.
Conclusions
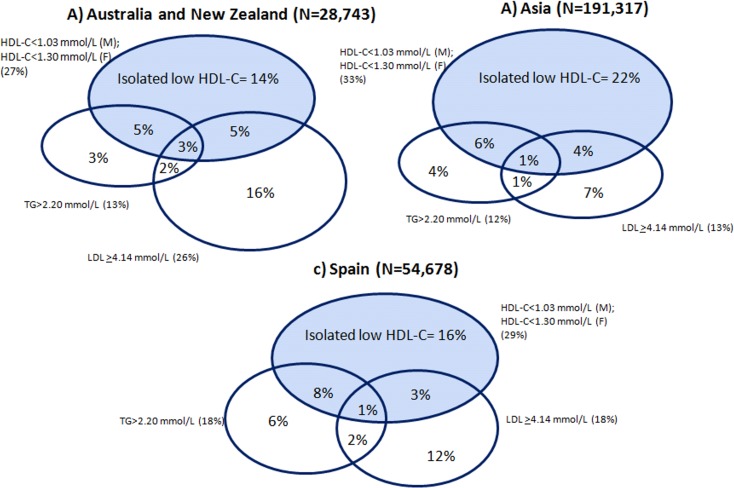
Clinical trials have clearly established that reduced LDL-C levels are associated with fewer cardiovascular events in both high- and low-risk populations. However, despite advances in research for prevention and acute treatment, including new therapeutic agents, cardiovascular disease is still the first cause of death in developed and developing countries. Identification of high risk patients is critical in order to propose effective prevention strategies. There is a significant proportion of patients with LDL-C levels within the normal range, whether in lipid-lowering therapy or not, in whom cardiovascular events do not appear to have been satisfactorily prevented [38]. Many individuals who reach LDL-C targets still possess an atherogenic lipid profile with residual risk. In a recent meta-analysis, around 15% of people from population cohorts in Asia had isolated low HDL-C (patients with normal levels of triglycerides and LDL-C) [39]. We found a similar proportion in our study (Fig 2). In light of these results, one should consider including low HDL, with normal TG and normal LDL as a higher risk category. Individuals exhibiting this form of lipid abnormality are at increased risk of CHD, but at the same time, are not likely to receive lipid-lowering medication according to current clinical guidelines, based on their levels of triglycerides and LDL-C. So, probably we are not identifying high risk patients properly. Nowadays, other lipid parameters in addition to LDL-C should be included for a more accurate evaluation of CV risk and to determine which patients must receive preventive treatment.
Fig 2. Proportion of patients with low HDL-cholesterol, including those with isolated low HDL-cholesterol.
Comparison between Australian, Asian and Spanish cohorts. Completed from Huxley RR et al. Circulation. 2011;124:2056–2064) (M = Male; F = Female). Isolated low HDL-cholesterol: patients with normal levels of triglycerides and LDL-Cholesterol.
For patients with hypertension, diabetes or dyslipidemia, our results suggest that those with low HDL or with high ratios of TC/HDL-C or TG/HDL-C belong to a higher risk category for CVD. According these results, the atherogenic index or HDL-C or TG/HDL-C might be included in new CVD risk equations in order to increase the validity of the risk patient assessment and to improve the therapeutic decision-making. However, further research using these risk markers is needed in order to know which one achieves a better risk adjustment model.
In conclusion, this cohort study in a population with high cardiovascular risk shows that HDL-C and TC/HDL-C and TG/HDL-C ratios may be better predictors for mortality and CVD than other lipid parameters commonly used in clinical practice, with relevant practical implications.
Supporting information
(DOCX)
Models are adjusted for age, sex, smoking status (never, former, current), obesity (no, yes), diabetes (no, yes), hypertension (no, yes), chronic kidney disease (no, yes), anti-hypertensive medication (no, yes), glucose-lowering medication (no, yes), lipid-lowering medication (no, yes). Models for specific lipid biomarkers have been additionally adjusted as follows: 1) Total-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 2) HDL-cholesterol is further adjusted by LDL-C ≥130 mg/dL (no, yes); 3) Non-HDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 4) LDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 5) Non-HDL minus LDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes) and LDL-C ≥ 130 mg/dL (no, yes); 6) Triglycerides is further adjusted by total cholesterol > 200 mg/dL (no, yes) and HDL ≤ 40 for men and ≤ 50 for women (no, yes); 7) Total cholesterol/HDL is further adjusted by total cholesterol (mg/dL); and 7) Triglycerides /HDL is further adjusted by total cholesterol (mg/dL).
(TIF)
Acknowledgments
We thank everyone who contributed significantly to the work. We thank the Conselleria de Sanitat (Generalitat Valenciana) for allowing us to conduct this study. The authors are also grateful to Merck Sharp & Dohme Corp for supporting the Continuing Medical Education course on cardiovascular disease topics addressed to all researchers of ESCARVAL study.
ESCARVAL Study Group: Abad J, Abril A, Abu Omar L, Abu-elbar EF, Acamer F, Aceña P, Aguilar MC, Aguilar MT, Aguilar N, Aguilar M, Aguilar NI, Aguilella L, Aguilera V, Agulles E, Agulló E, Agullo J, Aicart MD, Mora A, Alamar J, Alamo P, Alapont B, Albelda R, Albertos F, Alcalde G, Aldana G, Aldea J, Aldeguer I, Alemañ S, Alfonso P, Aliaga I, Almarcha N, Almela A, Almenar E, Alonso P, Alonso J, Alos N, Alvarez B, Perez A, Amat T, Amongero F, Amoros T, Hernández AB, Andres NT, Andres N, Andres I, Andreu R, Andreu M, Gimenez MA, Antolín B, Antolín MA, Anton A, Antón MA, Anton N, Antonaya MA, Año E, Aparisi JL, Aquila E, Aracil MT, Araez A, Aragón M, Arayo I, Argente MA, Armengol S, Arnau A, Arnau A, Arquiola J, Artero A, Asencio A, Avelino E, Ayala G, Azizieh A, Azorin MT, Badia JA, Balaguer MJ, Ballester J, Baño A, Barbera J, Barcelo C, Barea M, Barrachina A, Barranco M, Barreiro MA, Batalla M, Bataller E, Botella E, Camaro B, Ruiz B, Alvarez B, Beguer JC, Bel MM, Belda MV, Belenguer G, Belenguer E, Belmonte P, Beltran M, Beltran M, Belvis A, Beneito M, Benlloch MJ, Berenguer JL, Berenguer M, Berenguer V, Berna I, Bernard A, Bertolin A, Betancor I, Bijedic S, Blanes D, Blasco M, Blasco MC, Blasco MR, Blasco Z, Boix MI, Boluda MJ, Bonmati T, Bono C, Bori V, Borrás C, Borras C, Bosca J, Botella MJ, Botella A, Botella MS, Box RR, Bretó C, Brines C, Brotons ML, Bueno J, Bueno C, Buigues M, Buitrago A, Burguera MD, Cabrera MA, Cabrera MR, Cabrera AM, Cadenas E, Calafat I, Calatayud MC, Calcedo A, Calpe V, Calvillo A, Camarasa C, Camaro B, Campo J, Canals N, Candau E, Candela V, Canet M, Cano P, Cano M, Cantos F, Cantos C, Cañas E, Cañones I, Capilla N, Carbonell JM, Carbonell F, Carcelen E, Cardona A, Carrasco A, Carrascosa E, Carrascosa LM, Carratala M, Carratala RM, Carreño E, Carrillo MC, Cartagena ME, Casado EJ, Casanova B, Casanova G, Casanova AT, Casanova JJ, Casaut M, Cases I, Cases EP, Casino M, Casorran A, Castañeda C, Castaño A, Castaño C, Castellanos M, Castello M, Castillo R, Castillo C, Castro A, Catala M, Cebrian MD, Celdran MT, Cercos MV, Cerezo J, Cervera I, Cervera A, Cerverón A, Chafer MF, Checa E, Chesa, Chilet I, Chiva MD, Chova S, Cintas JM, Civera M, Clar C, Clave MA, Clemente MR, Clemente A, Climent R, Climent V, Climent C, Coll MJ, Collado I, Collado C, Colomer M, Comes S, Compañ MJ, Belloch C, Contreras JA, Corbacho A, Cornejo F, Cortilla A, Cucala E, Cuenca M, Davo M, De Gregorio C, De Haro J, De La Cruz M, De La Sen C, De La Torre J, De Rafael J, Debon M, Del Moral MD, Del Olmo ML, Delgado J, Diaz M, Diego C, Diez R, Dolz I, Domingo E, Domingo J, Dominguez V, Ducaju M, Dura H, Dura MD, Egea A, Sanz E, Escalante LM, Escorihuela L, Escrig J, Espert S, Espinosa MN, Esplugues R, Espuig J, Esquembre R, Esteban JV, Esteban MA, Estelles C, Esteve F, Estrela T, Falco MD, Faubel MR, Faus E, Feliu M, Fenoll F, Fernandez MT, Fernandez A, Fernandez C, Fernandez MA, Fernandez R, Fernandez AJ, Fernandez F, Fernandez M, Fernandez MC, Fernández T, Fernandez V, Ferrandis MV, Ferrandiz J, Ferrando R, Ferrando J, Ferrer C, Ferrer FL, Ferrer E, Ferrer AM, Flores M, Flores C, Font A, Font J, Fontana E, Fontoba J, Forcada MC, Fornes FJ, Fornes MV, Fortea A, Fraile MB, Frances A, Domingo F, Miralles F, Franco JA, Frau JA, Fuente J, Fuentes R, Fuster T, Fuster M, Fuster R, Fuster M, Gadea R, Galan JV, Galán JF, Galera R, Galiano MT, Gallardo JA, Gallego MC, Galvez AI, Gaona J, Garcia A, Garcia R, Garcia J, Garcia C, Garcia E, Garcia N, Garcia IA, Garcia A, Garcia V, García T, García ME, Garcia MC, Garcia RM, Garcia M, Garcia I, Garcia R, Garcia-calvo MB, Garcia-orad C, Gargallo MI, Garri S, Garrido A, Gascon MO, Gaspar A, Gasull V, Gavalda E, Gea A, Ggenoves AV, Gil MJ, Gil F, Gil A, Gil S, Gil E, Gil MM, Gilabert MA, Gilabert P, Gilabert A, Gimenez E, Giménez M, Gimenez D, Gimenez E, Gimeno J, Gimeno A, Gimeno M, Gimeno M, Giner MA, Giner M, Gisbert C, Gomez MD, Gomez M, Gomez A, Gomez N, Gómez F, Gomez R, Gomez-ferrer R, Gonzalez J, Gonzalez MA, Gonzalez E, Gonzalez R, Gonzalez AI, González MJ, Gonzalez A, González MJ, Gonzalez L, Gonzalez A, Gonzalvez JL, Gonzalvez M, Gracia JJ, Gras S, Grau J, Grau A, Gualde D, Guijo L, Guillard M, Guillen MJ, Guinot E, Gutierrez J, Gutierrez J, Guzman N, Haya A, Hermida E, Hernandez JM, Hernandez A, Hernandez C, Hernandez E, Hernandez I, Hernandis M, Herrero JV, Herrero C, Herrero IM, Herrero V, Hervella I, Hidalgo C, Huertas M, Huertas MC, Ibáñez C, Ibarra M, Ibor E, Ibor JF, Iborra P, Illan E, Pereiro I, Insa C, Marin I, Baldó I, Alfonso I, Ivorra VM, Iznardo V, Izquierdo F, J.Gomez D, Jara B, Javaloyes P, Nadal J, Jimenez T, Jimenez P, Jorda M, Jordá MD, Josa C, Alonso J, Jover JM, Juan V, Montañes JJ, Juan G, Julbe A, Junquero B, Laborda M, Lafuente JL, Laparra E, Sorribes L, Larrauri JM, Larrey D, Latorre MA, Latorre E, Latorre A, Latorre RM, Lavarias M, Leon AMª, Leon A, Lillo E, Llabata C, Llerena AJ, Llinares J, Llinares MJ, Llisterri R, Llopis C, Llopis S, Llopis F, Llopis C, Llopis J, Lloret C, Lluch F, Lluch R, Lluna C, Lusar M, Lope M, Lopez E, Lopez MI, Lopez MJ, Lopez S, Lopez A, Lopez J, Lopez C, Lopez A, Lorena M, Lorente JL, Lorente P, Lorenzo A, Lozano MR, Lozano A, Aliaga MC, Cubedo MD, Avinent MJ, Burgals ML, Mallea MP, Soldado MS, Azpilicueta MT, Suárez MT, Maella P, Maestre L, Magdaleno AM, Mahiques L, Malonda C, Maluenda MT, Manclus C, Manrique MJ, Manzanero MA, Mañas MD, Mañez MA, Mañogil A, López M, March MC, Marco J, Marco MD, Marco JV, Marcos MP, Mures MC, Bru MT, Marin P, Marin M, Marin M, Marmol MI, Mars H, Marti B, Marti A, Marti N, Martin RM, Martín JC, Martin MJ, Martinez M, Martinez MJ, Martinez P, Martinez R, Martinez F, Martinez D, Martinez C, Martinez M, Martinez MC, Martinez J, Martínez S, Martorell V, Mascarell E, Mascaros E, Masegosa C, Masia A, Masjoan M, Masmano C, Masmano R, Masmano JV, Mata RM, Mateo JM, Mateu I, Mayor J, Mecho MD, Medina PA, Medina MA, Medina F, Medina AM, Medina A, Medina B, Medrano A, Mencia G, Menero AC, Meseguer A, Mialaret A, Micó JM, Milián S, Millan M, Minguez J, Miquel L, Miquel C, Mir F, Mir JE, Miralles MC, Miralles S, Miravalls R, Mollá D, Molla E, Molla C, Moller E, Moncholi R, Monfort M, Monrabal JA, Monsonis R, Montagud BM, Montalva P, Montaña JJ, Montes AM, Monton J, Montoro J, Morales M, Morata J, Moreno J, Moreno MJ, Moreno AM, Moreno L, Moreno MC, Morera M, Morera R, Morote A, Mouriño A, Moya M, Mulet MJ, Murillo OM, Narganes D, Navarrete JM, Navarro MA, Navarro G, Navarro C, Navarro RM, Navarro R, Navarro JM, Navarro M, Navarro FJ, Navarro R, Navarro M, Nebot L, Nieto F, Nieto CP, Noguera I, Noguera MD, Noguera JA, Nos MD, Novoa MC, Nuevalos C, Ocampo MF, Ochando I, Ochando RM, Ojeda F, Oliver A, Oliver R, Olmos J, Orellana C, Oriente J, Ortega JV, Ortiz de Salazar A, Ortiz M, Ortiz MT, Ortuño E, Oyarzabal M, Pacheco MJ, Padilla JA, Palacios MA, Palacios DC, Pallares JM, Palomar MA, Pappalardo E, Paradis T, Pardo JL, Pardo C, Paredes R, Paredes ML, Pareja M, Pardo A, Parra D, Parreño MD, Parrondo P, Pascual M, Pastor J, Pastor MA, Pastor RM, Pastor FM, Paya C, Pedro A, Peiró J, Penades M, Peñas P, Perello J, Perez MJ, Perez P, Perez C, Perez MJ, Perez MA, Perez M, Perez CA, Perez I, Perez J, Perez S, Perez D, Perez MC, Perez M, Pérez C, Peris J, Peris JM, Pertusa S, Pérez S, Peydro R, Pico MA, Pico MV, Pilar JM, Lahoz P, Pinto A, Piña V, Pla R, Plana C, Plaza MJ, Ponce F, Poveda A, Poveda B, Prada MP, Pradas AM, Prieto RJ, Pruñosa E, Puchades A, Puchades E, Puerta F, Puigcerver MT, Pujades A, Quiles F, Quiles L, Quiles A, Quintana JV, Quintana P, Raduan A, Raga R, Raga S, Ramirez R, Ramirez J, Ramiro S, Ramon L, Ramos M, Ramos JR, Ramos M, Reig B, Requena E, Revert A, Revert A, Revert MD, Reyes J, Ribera JA, Ribera JP, Ribes J, Ribes AM, González R, Rico M, Ridaura MA, Riera C, Ripoll A, Ripoll M, Ripoll RS, Ripoll J, Roca MT, Roca P, Roca A, Roda J, Rodenas E, Rodrigo A, Rodriguez JJ, Rodriguez N, Rodriguez MD, Rodriguez V, Rodriguez A, Rodriguez T, Rodriguez M, Rodriguez MC, Rodriguez A, Rodriguez I, Rodríguez V, Rodriguez MI, Roig M, Roig A, Rojo M, Roman F, Romero MJ, Romero A, Romero P, Romero MI, Romero MR, Romero PB, Ros C, Ros V, Rosa I, Roselló C, Rosello E, Rubio C, Rubio B, Rubio A, Rubio P, Rubiols C, Rueda JA, Ruiz A, Ruiz N, Ruiz C, Ruíz I, Ruiz R, Ruiz M, Ruso MD, Sabater JM, Saez MR, Saez AB, Saez D, Sáez A, Saiz RM, Sala R, Sala P, Salanova JL, Salanova A, Salas Y, Sales L, San-Nicolas EM, Sanchez MJ, Sanchez M, Sanchez C, Sanchez F, Sanchez E, Sanchez J, Sanchez JI, Sánchez A, Sanchez F, Sanchez MC, Sanchez Y, Sanchis C, Sanchis L, Sanchis D, Sanchis MD, Sanchis T, Sancho S, Sancho N, Saneugenio A, Sanjuan C, Sanmartin A, Sanmartin M, Sanmartin MC, Sansano MR, Santamaria E, Santisteban R, Santonja A, Santos E, Sanz S, Sapiña F, Savall R, Schmuke E, Schwarz G, Segarra G, Segui E, Segura MB, Segura JM, Segura MD, Seijo MA, Selles D, Sempere MA, Serra M, Serra PJ, Serra G, Serrano A, Serrano C, Sierra JA, Silvestre E, Silvestre R, Sisternas MC, Siurana J, Siurana B, Sola AM, Solanas JV, Solanes A, Jerez S, Soler E, Soler JM, Sorli MA, Sosa J, Soto MG, Soto J, Suárez MG, Suberviola V, Martinez S, Talens A, Talens A, Tamarit A, Tarancón V, Tarin MJ, Tellado JL, Ten MT, Tercero A, Crescencio T, Terol A, Calvo T, Tirado JM, Tomas R, Tomas MT, Tomas A, Tormo N, Torralba V, Torralba F, Torres MJ, Torres M, Torres S, Torres I, Torres MT, Torres ML, Tortola D, Trespalacios JL, Trull MJ, Truyols J, Tur MD, Tur A, Ubeda F, Uceda L, Vaello M, Valencia P, Valera F, Valero JM, Valero R, Valladares B, Valles J, Vaquerizo ME, Varas M, Velasco N, Vendrell F, Vera JL, Vercher C, Verdú I, Verdu L, Verdu L, Vergara V, Vicedo I, Vicente ME, Vidal MT, Vidal J, Vidal JJ, Vidiella F, Vieira D, Vilanova I, Vilar MD, Villanueva PA, Villanueva P, Viturro C, Viudes JA, Vivas MA, Vizcaino A, Gómez X, Yañez MR, Zaragoza A, Zaragoza A, Zsigmond C.
Data Availability
The data are not publicly available as the local ministry (Conselleria de Sanitat, Generalitat Valenciana) and steering committee had not included unrestricted data sharing in the protocol at the time of approval of the study by the corresponding ethics committee and unrestricted data sharing was not included in the consent form. There are however opportunities for collaboration. Enquiries can be submitted to Drs. Josep Redon (josep.redon@uv.es) and Maria Tellez-Plaza (maria.tellez@uv.es) at the Institute for Biomedical Research Hospital Clinic of Valencia (INCLIVA). Conselleria de Sanitat is the legal organization which imposes these restrictions. Information can be requested to D. Salvador Peiró Moreno (peiro_bor@gva.es) from Ministry of Health of Valencia/Conselleria de SanitatSanitat.
Funding Statement
The authors received no specific funding for this work.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. www.thelancet.com Published online. December 18, 2014.
- 2.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83: 356–62. [DOI] [PubMed] [Google Scholar]
- 3.Conroy RM, Pyöräla K, Fitzgerald AP, Sans S, Menotti A, De Backer G et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 4.Brindle P, Emberson J, Lampe F, Walker M, Whincup P, Fahey T et al. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. BMJ. 2003;327:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrugat J, Subirana I, Ramos R, Vila J, Marín-Ibañez A, Guembe MJ et al. ; FRESCO Investigators. Derivation and validation of a set of 10-year cardiovascular risk predictive functions in Spain: the FRESCO Study. Prev Med. 2014. April;61:66–74. doi: 10.1016/j.ypmed.2013.12.031 [DOI] [PubMed] [Google Scholar]
- 6.Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality: a 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol. 1997;17:107–113. [DOI] [PubMed] [Google Scholar]
- 7.Assmann G, Cullen P, Schulte H. The Munster Heart Study (PROCAM):results of follow-up at 8 years. Eur Heart J. 1998;19(suppl A):A2–A11. [PubMed] [Google Scholar]
- 8.Lamarche B, Despres JP, Moorjani S, Cantin B, Dagenais GR, Lupien PJ. Prevalence of dyslipidemic phenotypes in ischemic heart disease (prospective results from the Quebec Cardiovascular Study). Am J Cardiol. 1995;75:1189–1195. [DOI] [PubMed] [Google Scholar]
- 9.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007. July 21;335(7611):136 doi: 10.1136/bmj.39261.471806.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Clinical Guideline Centre (UK). NICE clinical guideline CG181. Lipid modification. Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease Clinical guideline. Methods, evidence and recommendations. National Institute for Health and Care Excellence; (UK: ). July 2014. Available at: http://guidance.nice.org.uk/CG67. Accessed August 30,2016. [PubMed] [Google Scholar]
- 11.Gil-Guillen V, Orozco-Beltran D, Redon J, Pita-Fernandez S, Navarro-Pérez J, Pallares V et al. Rationale and methods of the cardiometabolic Valencian study (Escarval-Risk) for validation of risk scales in Mediterranean patients with hypertension, diabetes or dyslipidemia. BMC Public Health. 2010. November 22;10:717 doi: 10.1186/1471-2458-10-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–414.) doi: 10.1093/eurheartj/ehm316 [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. June;18(6):499–502. [PubMed] [Google Scholar]
- 14.Azimi SS, Khalili D, Hadaegh F, Yavari P, Mehrabi Y, Azizi F. Calculating population attributable fraction for cardiovascular risk factors using different methods in a population based cohort study. J Res Health Sci. 2015. Winter;15(1):22–7. [PubMed] [Google Scholar]
- 15.Gabriel R, Brotons C, Tormo MJ, Segura A, Rigo F, Elosua R; on behalf of the ERICE study group. The ERICE-score: the New Native Cardiovascular Score for the Low-risk and Aged Mediterranean Population of Spain. Rev Esp Cardiol (Engl Ed). 2014. August 21 pii: S1885–5857(14)00244-8. [DOI] [PubMed] [Google Scholar]
- 16.Piniés JA, González-Carril F, Arteagoitia JM, Irigoien I, Altzibar JM, Rodriguez-Murua JL et al. ; Sentinel Practice Network of the Basque Country. Development of a prediction model for fatal and non-fatal coronary heart disease and cardiovascular disease in patients with newly diagnosed type 2 diabetes mellitus: the Basque Country Prospective Complications and Mortality Study risk engine (BASCORE). Diabetologia. 2014. November;57(11):2324–33. doi: 10.1007/s00125-014-3370-1 [DOI] [PubMed] [Google Scholar]
- 17.Tikhonoff V, Casiglia E, Mazza A, Scarpa R, Thijs L, Pessina AC et al. Low-density lipoprotein cholesterol and mortality in older people. J Am Geriatr Soc. 2005. December;53(12):2159–64. doi: 10.1111/j.1532-5415.2005.00492.x [DOI] [PubMed] [Google Scholar]
- 18.Sritara P, Cheepudomwit S, Chapman N, Woodward M, Kositchaiwat C, Tunlayadechanont S et al. ; Electricity Generating Authority of Thailand. Twelve-year changes in vascular risk factors and their associations with mortality in a cohort of 3499 Thais: the Electricity Generating Authority of Thailand Study. Int J Epidemiol. 2003. June;32(3):461–8. [DOI] [PubMed] [Google Scholar]
- 19.Sritara P, Patoomanunt P, Woodward M, Narksawat K, Tulyadachanon S, Ratanachaiwong W et al. Associations between serum lipids and causes of mortality in a cohort of 3,499 urban Thais: The Electricity Generating Authority of Thailand (EGAT) study. Angiology. 2007 Dec-2008. January;58(6):757–63. [DOI] [PubMed] [Google Scholar]
- 20.Koller MT, Leening MJ, Wolbers M, Steyerberg EW, Hunink MG, Schoop R et al. Development and validation of a coronary risk prediction model for older U.S. and European persons in the Cardiovascular Health Study and the Rotterdam Study. Ann Intern Med. 2012. September 18;157(6):389–97 doi: 10.7326/0003-4819-157-6-201209180-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettinger WH, Wahl PW, Kuller LH, Bush TL, Tracy RP, Manolio TA et al. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation.1992. September;86(3):858–69. [DOI] [PubMed] [Google Scholar]
- 22.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM et al. ; Treating to New Targets Investigators. HDL cholesterol,very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007. September 27;357(13):1301–10. doi: 10.1056/NEJMoa064278 [DOI] [PubMed] [Google Scholar]
- 23.Reddy VS, Bui QT, Jacobs JR, Begelman SM, Miller DP, French WJ; Investigators of National Registry of Myocardial Infarction (NRMI) 4b–5. Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am J Cardiol. 2015. March 1;115(5):557–62. doi: 10.1016/j.amjcard.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Toth PP, Simko RJ, Palli SR, Koselleck D, Quimbo RA, Cziraky MJ. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2012. September 14;11:109 doi: 10.1186/1475-2840-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation 1989; 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 26.Bruckert E, Hansel B. HDL-c is a powerful lipid predictor of cardiovascular diseases. Int J Clin Pract, November 2007, 61, 11, 1905–1913 doi: 10.1111/j.1742-1241.2007.01509.x [DOI] [PubMed] [Google Scholar]
- 27.Rodondi N, Locatelli I, Aujesky D, Butler J, Vittinghoff E, Simonsick et al. ; Health ABC Study. Framingham risk score and alternatives for prediction of coronary heart disease in older adults. PLoS One. 2012;7(3):e34287 doi: 10.1371/journal.pone.0034287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pikula A, Beiser AS, Wang J, Himali JJ, Kelly-Hayes M, Kase CS et al. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study.Neurology. 2015. February 3;84(5):472–9 doi: 10.1212/WNL.0000000000001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elshazly MB, Quispe R, Michos ED, Sniderman AD, Toth PP, Banach M et al. Patient-Level Discordance in Population Percentiles of the Total Cholesterol to High-Density Lipoprotein Cholesterol Ratio in Comparison With Low-Density Lipoprotein Cholesterol and Non-High-Density Lipoprotein Cholesterol: The Very Large Database of Lipids Study (VLDL-2B). Circulation. 2015. August 25;132(8):667–76 doi: 10.1161/CIRCULATIONAHA.115.016163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pocock SJ, McCormack V, Gueyffier, Boutitie F, Fagard RH, Boissel JP on behalf of the INDANA project steering committee. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ 2001;323:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arsenault BJ, Rana JS, Stroes ES, Després JP, Shah PK, Kastelein JJ et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009. December 29;55 (1):35–41. doi: 10.1016/j.jacc.2009.07.057 [DOI] [PubMed] [Google Scholar]
- 32.Kontush A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovascular Research (2014) 103, 341–349. doi: 10.1093/cvr/cvu147 [DOI] [PubMed] [Google Scholar]
- 33.Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM et al. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial.Circulation. 2015. December 8;132(23):2220–9. doi: 10.1161/CIRCULATIONAHA.115.016857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colantonio LD, Bittner V, Reynolds K, Levitan EB, Rosenson RS, Banach M et al. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation. 2016. January 19;133(3):256–64. doi: 10.1161/CIRCULATIONAHA.115.011646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson C, Lyass A, Vasan RS, Massaro JM, D'Agostino RB Sr, Robins SJ. Long-term risk of cardiovascular events across a spectrum of adverse major plasma lipid combinations in the Framingham Heart Study. Am Heart J. 2014. December;168(6):878–83.e1 doi: 10.1016/j.ahj.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 36.Khalili D, Sheikholeslami FH, Bakhtiyari M, Azizi F, Momenan AA, Hadaegh F. The incidence of coronary heart disease and the population attributable fraction of its risk factors in Tehran: a 10-year population-based cohort study. PLoS One. 2014. August 27;9(8):e105804 doi: 10.1371/journal.pone.0105804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey JA, Schwartz BS, Stewart WF, Adler NE. Using electronic healthrecords for population healthresearch: a review of methodsand applications. Annu. Rev. Public Health 2016. 37:61–81. doi: 10.1146/annurev-publhealth-032315-021353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–234 [DOI] [PubMed] [Google Scholar]
- 39.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T et al. ; Asia Pacific Cohort Studies Collaboration and the Obesity in Asia Collaboration. Isolated Low Levels of High-Density Lipoprotein Cholesterol Are Associated With an Increased Risk of Coronary Heart Disease An Individual Participant Data Meta-Analysis of 23 Studies in the Asia-Pacific Region. Circulation. 2011;124:2056–2064 doi: 10.1161/CIRCULATIONAHA.111.028373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Models are adjusted for age, sex, smoking status (never, former, current), obesity (no, yes), diabetes (no, yes), hypertension (no, yes), chronic kidney disease (no, yes), anti-hypertensive medication (no, yes), glucose-lowering medication (no, yes), lipid-lowering medication (no, yes). Models for specific lipid biomarkers have been additionally adjusted as follows: 1) Total-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 2) HDL-cholesterol is further adjusted by LDL-C ≥130 mg/dL (no, yes); 3) Non-HDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 4) LDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes); 5) Non-HDL minus LDL-cholesterol is further adjusted by HDL ≤ 40 for men and ≤ 50 for women (no, yes) and LDL-C ≥ 130 mg/dL (no, yes); 6) Triglycerides is further adjusted by total cholesterol > 200 mg/dL (no, yes) and HDL ≤ 40 for men and ≤ 50 for women (no, yes); 7) Total cholesterol/HDL is further adjusted by total cholesterol (mg/dL); and 7) Triglycerides /HDL is further adjusted by total cholesterol (mg/dL).
(TIF)
Data Availability Statement
The data are not publicly available as the local ministry (Conselleria de Sanitat, Generalitat Valenciana) and steering committee had not included unrestricted data sharing in the protocol at the time of approval of the study by the corresponding ethics committee and unrestricted data sharing was not included in the consent form. There are however opportunities for collaboration. Enquiries can be submitted to Drs. Josep Redon (josep.redon@uv.es) and Maria Tellez-Plaza (maria.tellez@uv.es) at the Institute for Biomedical Research Hospital Clinic of Valencia (INCLIVA). Conselleria de Sanitat is the legal organization which imposes these restrictions. Information can be requested to D. Salvador Peiró Moreno (peiro_bor@gva.es) from Ministry of Health of Valencia/Conselleria de SanitatSanitat.