Abstract
Background
Transient aphasias are common in the first few days after resective surgery to the language-dominant hemisphere. The specific speech and language deficits that occur are related to the location of the surgical site, and may include impairments in fluency, lexical access, repetition, and comprehension. The impact of these transient aphasias on connected speech production has not previously been investigated.
Aims
The goals of this study were to characterize the nature of connected speech deficits in the immediate post-surgical period, and to determine which deficits resolve completely within 1 month.
Methods & Procedures
Forty-three patients undergoing resective surgery in the left (language-dominant) hemisphere participated in the study. Brief connected speech samples were obtained before surgery, and at 2-3 days post-surgery. In a subset of 24 patients (all of whom presented with aphasia in the immediate post-surgical period), follow-up samples were also obtained at 1 month post-surgery. The samples were transcribed and coded in CHAT format. Ten connected speech measures were derived from each speech sample, and were then compared by time point.
Outcomes & Results
At 2-3 days post surgery, deficits were observed in all 10 connected speech measures in comparison to pre-surgical samples: there were decreases in words per minute, words per utterance, and the use of embedded clauses, and increases in phonological errors, lexical access difficulties, morphosyntactic errors, filled pauses, false starts and retraced sequences. The proportion of closed class words could be perturbed in either direction. At 1 month post-surgery, 8 of the 10 connected speech measures had significantly improved, and all measures reflecting structural features (words per utterance, number of embeddings, morphosyntactic errors, proportion of words that were closed class) were equivalent to the pre-surgical time point. Subtle deficits persisted in some other measures; in particular, there were more phonological errors and lexical access difficulties than at the pre-surgical point.
Conclusions
Transient aphasias after left hemisphere surgery impacted all aspects of connected speech in the immediate post-surgical period. Most of these deficits were largely or completely resolved by 1 month post surgery, but some subtle impairments persisted.
Keywords: connected speech, aphasia, resective surgery
Introduction
Long-term language outcomes after resective surgery to the language-dominant hemisphere are generally excellent (Davies et al., 1994; Duffau, Gatignol, Mandonnet, Capelle, & Taillandier, 2008; Hermann, Wyler, & Somes, 1991; Loring, Meador, & Lee, 1994; Penfield & Roberts, 1959; Sanai, Mirzadeh, & Berger, 2008; Whittle, Pringle, & Taylor, 1998). However, while long-term outcomes are positive, transient aphasias are common in the immediate post-surgical period, within the first few days after surgery (Benzagmout, Gatignol, & Duffau, 2007; Duffau et al., 2003; Falconer & Serafetinides, 1963; Helmstaedter, Gleissner, Zentner, & Elger, 1998; Katz et al., 1989; Loring et al., 1994; Lubrano, Draper, & Roux, 2010; Penfield & Roberts, 1959; Peraud, Ilmberger, & Reulen, 2004; Roberts, 1955; Sanai et al., 2008; Wilson et al., 2015). A recent study in which post-surgical aphasias were characterized using the Western Aphasia Battery (WAB; Kertesz, 1982) showed that 2-3 days after surgery, 71% of patients met criteria for aphasia according to the WAB (Wilson et al., 2015). Transient deficits can occur in many language domains, including fluency, naming, repetition, comprehension, reading and writing (Loring et al., 1994; Wilson et al., 2015), and the specific impairments that occur are systematically related to the location of the surgical site (Wilson et al., 2015). The majority of transient aphasias resolve within a month (Falconer & Serafetinides, 1963; Loring et al., 1994; Penfield & Roberts, 1959; Sanai et al., 2008; Wilson et al., 2015), and the only language function in which persistent deficits have been documented is naming; these persistent lexical access deficits, if they occur at all, are generally relatively mild (Davies et al., 1998; Krauss et al., 1996; Langfitt & Rausch, 1996; Saykin et al., 1995; Sherman et al., 2011; Wilson et al., 2015).
Acquired aphasia invariably affects the production of connected speech, and quantitative analysis of connected speech can provide rich information about the function of multiple language domains (MacWhinney, Fromm, Forbes, & Holland, 2011; Saffran, Berndt, & Schwartz, 1989; Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997; Wilson et al., 2010). Sentence construction is reflected in features such as the mean length of utterances, the use of embedded clauses, the presence of morphosyntactic errors, and the balance between open and closed class items. Lexical access can be assessed by tallying phenomena such as semantic errors, circumlocution, empty speech, and abandoned utterances, while phonological errors and neologisms can indicate lexical or phonemic encoding deficits. Other phenomena that contribute to an overall impression of fluency can be readily quantified, such as words per minute, and different kinds of pauses and self-corrections. To our knowledge, the impact of transient post-surgical aphasias on connected speech has not previously been investigated. There are at least two reasons why such an investigation could be informative.
First, quantitative analysis of connected speech is capable of characterizing some aspects of language function that have not been well captured by the WAB or by the other less comprehensive language assessments that have been reported to date in the immediate post-operative period. In particular, the WAB uses a single 10-point scale to quantify fluency, whereas previous studies have shown that fluency is a multidimensional construct incorporating features such as speech rate, phrase length, articulatory agility and syntactic structure, which are not necessarily affected similarly (Goodglass, Quadfasel, & Timberlake, 1964; Thompson et al., 2012; Wilson et al., 2010). Moreover, the WAB does not provide any summary measure that quantifies the presence of phonemic paraphasias or neologisms, and although lexical access is quantified by means of the naming subscore, the items used are rather high in frequency, and confrontation naming is less ecologically valid than naturally occurring lexical access difficulties in language use.
A second reason to use quantitative analysis of connected speech is that it may be capable of detecting subtle deficits (Thompson et al., 1997) that may not be detected on aphasia batteries such as the WAB, or when using less comprehensive language assessments. Sensitivity to subtle deficits is important in order to document how long it takes for transient post-surgical language deficits to resolve completely.
Therefore in this study, we obtained and analyzed brief connected speech samples from patients before and after left hemisphere resective surgery in order to address two goals: first, to characterize the nature of connected speech deficits in the immediate post-surgical period, and second, to determine which deficits resolve completely within 1 month.
Methods
Patients
Forty-three patients undergoing left hemisphere resective surgery were included in this study. The inclusion criteria were: (1) left hemisphere resection in perisylvian language regions or the anterior temporal lobe; (2) left hemisphere dominance for language confirmed with Wada test, pre-surgical language deficits, or magnetoencephalography lateralization; (3) native speaker of English; (4) availability of recorded pre-surgical connected speech sample from the WAB (description of picnic scene); recorded post-surgical picnic description (2-3 days post-surgery); complete post-surgical WAB (2-3 days post-surgery).
The study was approved by the institutional review boards of UCSF and the University of Arizona, and all participants gave written informed consent. Demographic information and the distribution of etiologies are shown in Table 1. Surgical procedures have been described previously (Wilson et al., 2015).
Table 1.
Demographic and etiological information
| Age | 43.4 ± 15.0 years (range: 19 to 74) | |
| Sex | 20 male, 23 female | |
| Handedness | 36 right-handed, 6 left-handed, 1 ambidextrous | |
| Education | 15.4 ± 2.8 years (range 12 to 20) | |
| Time since first symptoms | 1510 ± 3281 days (range 4 days to 40 years) | |
| Etiology | Low grade glioma | 11 |
| High grade glioma | 25 | |
| Epileptogenic focus | 6 | |
| Vascular malformation | 1 | |
Acquisition of connected speech samples and other language measures
The connected speech samples comprised the description of a picnic scene from the WAB. The WAB was generally administered by MB, a speech-language pathologist, except in a few cases in which it was administered by a trained research assistant. Audio recordings were made for later analysis.
A consecutive series of 79 patients met the first 3 inclusion criteria described above. However, in 36 of these patients, the required data were not all obtained. With the exception of 2 patients (one of whom was untestable 2-3 days post-surgery due to global aphasia, and one of whom was suspected to be malingering), the reasons for not acquiring data in the remaining 34 patients were situational (e.g., patients who did not live in the San Francisco bay area and so were not available for pre-surgical assessment), so the 43 included patients comprise a largely representative sample of a consecutive series.
When patients met WAB criteria for aphasia at the 2-3 days post-surgery time point, follow-up language testing at 1 month post-surgery was attempted. Twenty-nine of the 43 patients met criteria for aphasia at 2-3 days post-surgery, and of those, follow-up speech samples were obtained for 24 of the 29. The remaining 5 were not obtained due to situational factors, mostly because patients did not live in the bay area.
The mean length of the speech samples was 139.2 ± 81.1 words (not including filled pauses, false starts, or retraced sequences) at the pre-surgical time point, 139.9 ± 95.9 words at the 2-3 days post-surgical time point, and 171.6 ± 50.3 words at the 1 month post-surgical time point. These lengths did not differ significantly from one another (all p ≥ 0.33).
While the WAB at 2-3 days post-surgery was required for inclusion in this study, the WAB was also administered at the pre-surgical and 1 month time points in most patients (pre: 39/43; 1 month: 21/24). A 15-item version of the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983) was also administered in most patients (pre: 39/43, 2-3 days: 41/43; 1 month: 22/24).
Neuroimaging
Thirty-four of the 43 patients received post-surgical MRI scans, on which the extent of resection and any adjacent infarction was manually delineated as described previously (Wilson et al., 2015). To illustrate the distribution of surgical sites in the patient group, a lesion overlay was computed using VLSM version 2.56 (Bates et al., 2003). The lesion overlay showed that most patients’ resections were located in left anterior temporal or left frontal regions (Figure 1).
Figure 1.
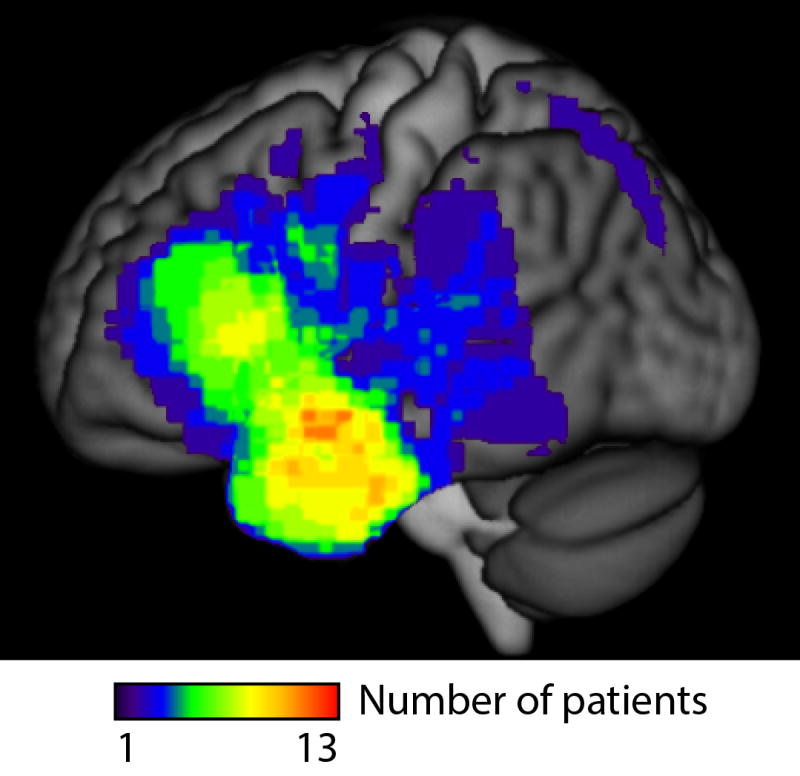
Lesion overlay. MRI was available for 34 of the 43 patients. Most patients’ resections were located in left anterior temporal or left frontal regions, with a maximal overlap of 13 patients in the left anterior temporal lobe.
Transcription, coding and analysis of connected speech samples
Transcription and coding of the connected speech samples followed the CHAT system (MacWhinney, 2000, 2012), including newer codes that were introduced for quantifying aphasic speech (MacWhinney et al., 2011). CHAT provides a standardized way to represent naturalistic speech samples in aphasia with the means to capture phonological, lexical and morphosyntactic disturbances. Speech samples were transcribed using EUDICO Linguistic Annotator (ELAN; Wittenburg, Brugman, Russel, Klassmann, & Sloetjes, 2006) in order to encode precise timing information. All speech samples were transcribed in standard English orthography except for the use of International Phonetic Alphabet (IPA) to transcribe phonemic paraphasias and neologisms. After transcription and coding, each morpheme was tagged for part of speech using the program mor (MacWhinney, 2000), then manually disambiguated. The speech samples were transcribed and coded by AM and AC, undergraduates in speech, language, and hearing sciences, who were each trained by SMW. All samples were reviewed and revised as needed by AM and SMW to maximize consistency.
An in-house MATLAB program was used to derive 10 connected speech measures from the coded and tagged transcriptions in the ELAN files, and to perform statistical analyses. The set of language measures are outlined in Table 2. All transcription, coding and analysis procedures have been described in detail previously (Yagata et al., 2017). Two refinements were made for the present study. First, non-sentence utterances were not automatically counted toward the measure of morphosyntactic errors. Such utterances were only counted when they were judged to be agrammatic rather than conversationally appropriate isolated phrases (e.g. an isolated noun phrase that serves as a self-contained utterance). Second, all of the measures which involved counts per hundred words of features indicative of impairment were transformed by adding 1 and taking the natural logarithm; there were 6 such measures (see Table 2). This was done to reduce the impact of extreme values, which arose quite frequently given that some of the speech samples were brief.
Table 2.
Connected speech measures
| Measure | Definition |
|---|---|
| Words per minute | the total number of words produced divided by the time spent speaking; neologisms and other errors were included, while filled pauses, false starts, and retraced sequences were not included. |
| Phonological errors | phonemic paraphasias or neologisms (per hundred words, log-transformed); these may reflect retrieval of word forms but more often indicate phonemic encoding problems. Examples: |
| and he's playing with a [kaɪp]. (phonemic paraphasia, nonword) | |
| and they're maybe out paying in <the> uh the water. (phonemic paraphasia, real word) | |
| listening to the radio eating a [pɪtnɪs]. (neologism with known target) | |
| Impaired lexical access | word-level semantic errors, neologisms with unknown targets, incomplete utterances, empty speech, circumlocution, semantically anomalous utterances (per hundred words, log-transformed). Examples: |
| and here's a house with a chair. (word-level semantic error: chair for car) | |
| an(d) uh a car with <a v-> a [vaɪg]. (neologism with unknown target) | |
| um and then there's two people sitting on I think on like… (incomplete utterance) | |
| that are just all kinds of things. (empty speech) | |
| and further out there is an individual that is sitting on some sort of floating device that um he can protrude himself to the water on. (circumlocution) | |
| and then you go down <to> towards the water you'll find a couple pieces t- ˄- to the water. (semantically anomalous utterance) | |
| Words per utterance | the total number of words divided by the number of utterances |
| Embedded clauses | embedded clauses were required to contain a subject or a finite verb form; multiple embeddings per utterance could be counted (per hundred words). Example: |
| I assume it's the man who's reading his book. | |
| Morphosyntactic errors | agrammatic utterances, paragrammatic utterances, word-level morphological errors, omitted words, errors in formal lexical devices (e.g. incorrect determiners) (per hundred words, log-transformed). Examples: |
| reading. glasses. um car parked in driveway. (agrammatic) | |
| the rest of <the front> the front available for ð- ð- cutting of the you know to fill the <flower> flower bed. (paragrammatic) | |
| the man and the woman is sailing the sailboat. (morphological) | |
| there's kid flying a kite. (omitted word) | |
| Proportion closed class | the number of closed class items divided by the total number of open and closed class items. Examples: |
| an(d) I think this and this and this. (increased proportion of closed class words) | |
| child. kite. dog. (decreased proportion of closed class words) | |
| Filled pauses | um, uh, and er (per hundred words, log-transformed) |
| False starts | words that were abandoned after only one or two phonemes had been produced (per hundred words, log-transformed). Example: |
| and there is a deck raɪ- right here on the ocean or on the bay. | |
| Retraced sequences | sequences of one or more complete words that were made redundant by subsequent repetitions, amendments, elaborations or alternative expressions (per hundred words, log-transformed). Example: |
| um <there are> there are trees <to the> <the> um uh adjacent to the lake. |
Conventions in examples: angled brackets indicate retraced material; hyphens indicate false starts
Statistical analysis
For each of the 10 connected speech measures, as well as the WAB Aphasia Quotient (AQ) and the BNT, the following statistical comparisons were carried out with paired t-tests. First, measures at 2-3 days post-surgery were compared to pre-surgical values. The purpose of this comparison was to identify language deficits induced by surgery in the immediate post-operative period.
Next, this same analysis was carried out for a subset of 14 patients who did not meet WAB criteria for aphasia at 2-3 days post-surgery, and separately for the remaining 29 patients who did present with aphasia. These analyses were aimed at determining whether there were subtle connected speech deficits in patients who did not meet WAB criteria for aphasia.
Then, in the 24 of 29 patients with acute aphasia for whom 1 month follow-up data were acquired, the 1 month time point was compared to the 2-3 days post-surgical time point to identify measures that significantly improved between these time points, and the 1 month time point was compared to the pre-surgical time point to identify any changes in connected speech measures that were not yet resolved by 1 month post-surgery.
Paired t-tests were one-tailed, assuming that deficits would be maximal at 2-3 days post-surgery, and minimal at the pre-surgical time point (Falconer & Serafetinides, 1963; Loring et al., 1994; Penfield & Roberts, 1959; Sanai et al., 2008; Wilson et al., 2015). Since the proportion of closed class words can be perturbed in either direction in aphasia (reflecting either agrammatism or empty speech), tests were carried out on the absolute value of the deviation from 0.60, which is the mean proportion of closed class items in healthy control participants describing the picnic scene (Wilson et al., 2010).
Each of the five sets of comparisons was separately corrected for multiple comparisons using the false discovery rate procedure (Benjamini & Hochberg, 1995; Yekutieli & Benjamini, 1999). There were 12 comparisons per set: 10 connected speech measures and 2 standard language measures (AQ and BNT). Tests were considered significant at q < 0.05 (false discovery rate).
Results
Excepts from speech samples at each time point from a single representative patient are shown in Appendix 1.
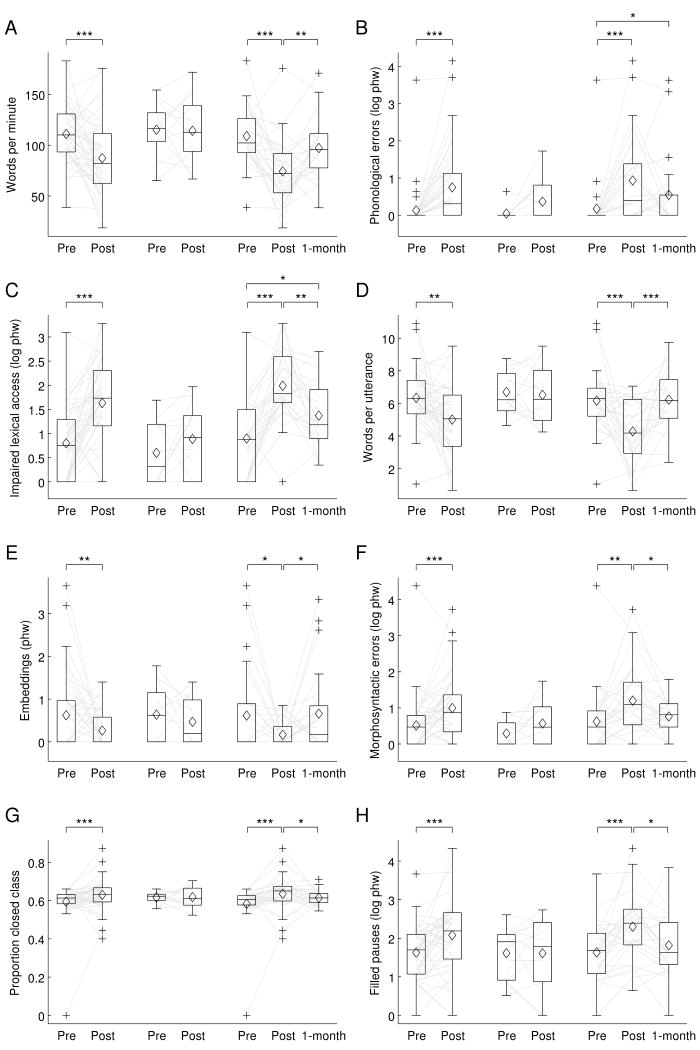
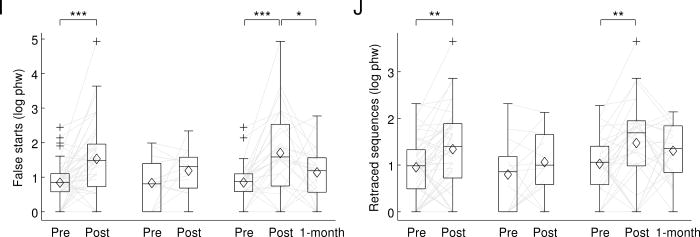
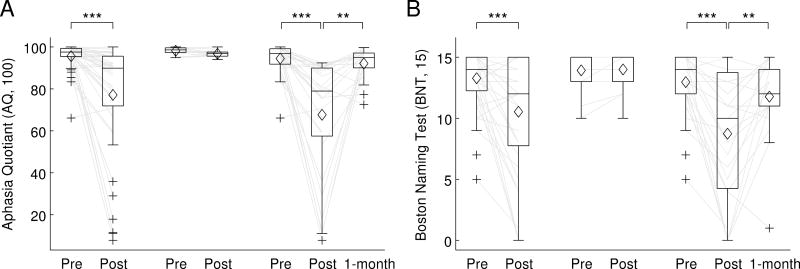
At 2-3 days post-surgery, the patient cohort as a whole showed statistically significant changes indicative of impairment on all 10 connected speech measures (Table 3; Figure 2). On average, there were fewer words per minute, more phonological errors, more occurrences of impaired lexical access, fewer words per utterance, fewer embedded clauses, more morphosyntactic errors, greater deviations of the proportion of closed class items from typical, and more filled pauses, false starts, and retraced sequences. There were also significant declines in the AQ and BNT (Table 3; Figure 3).
Table 3.
Statistical comparisons of connected speech measures and standard language measures
| Patients Time points | All 43 patients Pre vs post | 14 non-aphasic post Pre vs post | 29 aphasic post Pre vs post | 24 aphasic post Post vs 1-month | 24 aphasic post Pre vs 1-month | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | q | p | q | p | q | p | q | p | q | |
| Words per minute | < 0.001 | < 0.001 *** | 0.455 | 0.546 | < 0.001 | < 0.001 *** | 0.002 | 0.004 ** | 0.039 | 0.077 |
| Phonological errors (log phw) | < 0.001 | < 0.001 *** | 0.027 | 0.165 | < 0.001 | < 0.001 *** | 0.130 | 0.142 | 0.007 | 0.044 * |
| Impaired lexical access (log phw) | < 0.001 | < 0.001 *** | 0.101 | 0.172 | < 0.001 | < 0.001 *** | 0.001 | 0.003 ** | 0.007 | 0.044 * |
| Words per utterance | 0.001 | 0.001 ** | 0.364 | 0.485 | < 0.001 | < 0.001 *** | < 0.001 | < 0.001 *** | 1.000 | 1.000 |
| Embeddings (phw) | < 0.001 | 0.009 ** | 0.198 | 0.297 | 0.015 | 0.015 * | 0.022 | 0.030 * | 1.000 | 1.000 |
| Morphosyntactic errors (log phw) | < 0.001 | < 0.001 *** | 0.062 | 0.172 | < 0.001 | 0.001 ** | 0.016 | 0.024 * | 0.150 | 0.180 |
| Proportion closed class | < 0.001 | < 0.001 *** | 0.094 | 0.172 | < 0.001 | < 0.001 *** | 0.006 | 0.012 * | 0.110 | 0.147 |
| Filled pauses (log phw) | < 0.001 | < 0.001 *** | 1.000 | 1.000 | < 0.001 | < 0.001 *** | 0.013 | 0.022 * | 0.022 | 0.054 |
| False starts (log phw) | < 0.001 | < 0.001 *** | 0.059 | 0.172 | < 0.001 | < 0.001 *** | 0.040 | 0.048 * | 0.045 | 0.077 |
| Retraced sequences (log phw) | 0.002 | 0.002 ** | 0.093 | 0.172 | 0.005 | 0.006 ** | 1.000 | 1.000 | 0.018 | 0.054 |
| Aphasia Quotient (AQ, 100) | < 0.001 | < 0.001 *** | 0.022 | 0.165 | < 0.001 | < 0.001 *** | 0.001 | 0.003 ** | 0.081 | 0.121 |
| Boston Naming Test (BNT, 15) | < 0.001 | < 0.001 *** | 1.000 | 1.000 | < 0.001 | < 0.001 *** | 0.001 | 0.003 ** | 0.014 | 0.054 |
Significance after correction for false discovery rate:
q ≤ 0.05
q ≤ 0.01
q ≤ 0.001.
Figure 2.
Connected speech measures. In each plot, the first pair of boxes show pre-surgical and 2-3 days post-surgical measures for all 43 patients. The next pair of boxes show pre- and post-surgical measures for only the 14 patients who did not meet WAB criteria for aphasia at 2-3 days post-surgery. The final three boxes show pre- and post-surgical measures for the 29 patients who presented with aphasia at 2-3 days post surgery, and 1-month follow-up measures for 24 of these patients. (A) Words per minute. (B) Phonological errors (log phw). (C) Impaired lexical access (log phw). (D) Words per utterance. (E) Embedded clauses (phw). (F) Morphosyntactic errors (log phw). (G) Proportion closed class. (H) Filled pauses (log phw). (I) False starts (log phw). (J) Retraced sequences (log phw). phw = per hundred words; boxes = inter-quartile range; whiskers = the range not including outliers; pluses = outliers; horizontal lines = medians; diamonds = means; light gray lines = trajectories of each patient. * = q ≤ 0.05 (corrected for false discovery rate); ** = q ≤ 0.01 (corrected for false discovery rate); *** = q ≤ 0.001 (corrected for false discovery rate).
Figure 3.
Standard language measures. (A) Aphasia Quotient (AQ) from the Western Aphasia Battery. (B) Boston Naming Test. See figure 2 caption for definitions.
Fourteen out of 43 patients (33%) did not meet WAB criteria for aphasia at 2-3 days post-surgery. In these 14 patients, none of the connected speech measures or standard language measures changed significantly after correction for multiple comparisons (Table 3; Figures 2 and 3).
The remaining 29 patients (67%) met WAB criteria for aphasia at 2-3 days post-surgery. In these patients, there were statistically significant changes consistent with impairment on all 10 connected speech measures, and the AQ and BNT measures also declined significantly (Table 3; Figures 2 and 3).
At 1 month post-surgery, follow-up testing was carried out on 24 out of these 29 patients. Eight of the 10 connected speech measures, as well as the AQ and BNT, showed statistically significant changes indicative of recovery (Table 3; Figure 2). The only measures that did not improve significantly were the rates of phonological errors and retraced sequences.
When connected speech measures at 1 month post-surgery were compared to pre-surgical measures, 2 of the 10 measures showed statistically significant changes indicating that deficits had not yet fully resolved: patients produced more phonological errors, and experienced more lexical access difficulties (Table 3; Figure 2). It is noteworthy that an additional 4 connected speech measures showed marginally significant changes (0.05 ≤ q ≤ 0.08): patients tended to produce fewer words per minute, and more filled pauses, false starts and retraced sequences (Table 3; Figure 2). There was no change in AQ, but there was a marginally significant decline in BNT scores (Table 3; Figure 3).
An ancillary analysis was carried out in which the 14 patients who did not meet WAB criteria for aphasia at 2-3 days post were also included, with 1 month follow-up measures imputed to be identical to their pre-surgical values. The same set of measures showed significant or marginal changes in this analysis, suggesting that these findings can be generalized to the whole cohort, not just the patients who presented with aphasia at 2-3 days post-surgery.
Discussion
The first goal of this study was to characterize the nature of connected speech deficits in the immediate post-surgical period after dominant hemisphere resective surgery. We found that at 2-3 days post-surgery, all 10 connected speech measures showed statistically significant changes at the group level, in directions associated with impairment, relative to pre-surgical values. These deficits were largely confined to the 29 out of 43 patients who met WAB criteria for aphasia; in the subset of 14 patients who did not present with aphasia, no connected speech measures changed significantly after correction for multiple comparisons.
The observation that multiple connected speech measures changed in directions consistent with impairment is consistent with our recent finding that fluency is reduced in transient post-surgical aphasias (Wilson et al., 2015). The present study extends this previous finding by specifying precisely how fluency is impacted. Specifically, we documented deficits in lexical access (impaired lexical access), phonological retrieval and/or encoding (phonological errors), and sentence construction (words per utterance, embedded clauses, morphosyntactic errors, changes in the proportion of closed class words). Lexical access impairments in connected speech are not surprising, given that confrontation naming has been shown to be impaired previously (Loring et al., 1994; Wilson et al., 2015) and in the present study. However, the phonological and sentence construction deficits that we demonstrated are novel findings, since these language domains are not well captured by the WAB or by other approaches that have been used to investigate post-surgical aphasias.
At 1 month post-surgery, most patients who had presented with aphasia in the immediate post-operative period were dramatically improved; there were statistically significant improvements in 8 out of 10 connected speech measures, and both standard language measures. However despite this substantial recovery, some subtle language deficits remained that were most evident in the connected speech analysis. In particular, the rates of phonological errors and lexical access difficulties were higher at 1 month post-surgery than they had been at the pre-surgical time point; these changes were statistically significant after correction for multiple comparisons. In previous studies of longer term language outcomes after resective surgery, confrontation naming has been the only language measure in which persistent deficits have been reported (Davies et al., 1998; Krauss et al., 1996; Langfitt & Rausch, 1996; Saykin et al., 1995; Sherman et al., 2011; Wilson et al., 2015). It is possible that phonemic encoding deficits might have contributed to reduced scores on some of these confrontation naming assessments.
An additional 4 connected speech measures—words per minute, filled pauses, false starts, and retraced sequences—showed trends relative to the pre-surgical time point that were marginally significant (0.05 ≤ q ≤ 0.08). These are all features of normal speech: all people speak slowly sometimes, insert um and uh, and correct themselves. Yet taken together, these features can give rise to an overall impression of dysfluency.
The remaining 4 connected speech measures—words per utterance, numbers of embedded clauses, morphosyntactic errors, and proportion of closed class words—were essentially equivalent to pre-surgical levels at 1 month post-surgery. These 4 measures all relate to sentence construction, and suggest that syntactic production mechanisms are especially resilient to damage from resective surgery in language regions, possibly because brain regions important for syntactic processing are quite widely distributed (Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Caplan et al., 2007; Blank, Balewski, Mahowald, & Fedorenko, 2016; Wilson et al., 2016).
The standard language measures (WAB and BNT) did not show significant changes from the pre-surgical time point to the 1 month post-surgical time point after correction for multiple comparisons, although there was a marginal trend for BNT scores to be reduced. This suggests that quantitative analysis of connected speech may be more sensitive to the subtle deficits that remain at this time point. It should be noted that 1 month post-surgery does not represent a stable end point, and it is likely that remaining language deficits, which were already mild in most cases, would further resolve over time.
Our study had several notable limitations. First, the speech samples were descriptions of single pictures, and were relatively short. The means of elicitation of connected speech samples (e.g. describing a single picture, describing a sequence of pictures, retelling of a familiar story, sharing a past experience, describing how to do something) can impact the language measures that are derived from them (Armstrong, 2000; Fergadiotis & Wright, 2011; Nicholas & Brookshire, 1993). It is generally recommended that quantitative analyses of connected speech be based on samples of at least 150 words (Saffran et al., 1989), and while the mean length of our samples was in this vicinity, many individual samples were shorter. Shorter samples will inevitably lead to less reliable estimates of connected speech measures, as has been demonstrated empirically (Brookshire & Nicholas, 1994; Boyle, 2015). Although previous studies have shown robust brain-behavior relationships based on picnic description speech samples (e.g. Wilson et al., 2010), it would have been preferable to acquire longer samples, or even better, to have obtained multiple samples during multiple sessions per time point (Salthouse, 2007). Longer speech samples, or multiple samples per time point, would enable us to estimate test-retest reliability and would increase our sensitivity to detect changes. Due to the brevity of our samples, all null results need to be interpreted with caution. In particular, it is plausible that the 4 connected speech measures that were marginally changed at 1 month post-surgery relative to pre-surgery would show statistically significantly residual changes if longer samples or more samples were obtained.
Second, there are some respects in which the CHAT coding scheme does not precisely capture important dimensions of aphasic speech. For instance, agrammatism and paragrammatism are not distinguished at the utterance level. Moreover, in the derived scores that constituted our connected speech measures, in several instances we collapsed across related phenomena, for example the measure of impaired lexical access included word-level semantic errors, neologisms with unknown targets, incomplete utterances, empty speech, circumlocution, and semantically anomalous utterances. A more fine-grained analysis with longer speech samples should be able to able to examine these features of connected speech independently of one another. Related to these issues, even though our analyses were quantitative, there are always subjective decisions to be made when transcribing and coding connected speech samples. For instance, it is not always obvious whether atypical expressions are colloquialisms or errors, or how to assess criteria for utterance boundaries, or whether an error is syntactic or semantic. We attempted to maximize consistency by having two authors review and revise all transcriptions.
Third, in this study we did not attempt to distinguish between patterns with damage to different brain regions, or different etiologies (e.g. high grade gliomas, low grade gliomas, epileptogenic foci). We have previously shown that the nature of transient post-surgical aphasias depends on which specific brain regions are resected (Wilson et al., 2015). With a larger number of patients, and longer speech samples, it would be informative in future studies to investigate how different connected speech measures are impacted based on surgical site and/or etiology.
Fourth, we did not acquire 1 month follow-up data for individuals who did not meet WAB criteria for aphasia at the 2-3 days post-surgical time point. This prevented us from precisely quantifying the extent of residual deficits at 1 month post-surgery in the whole cohort. However, a conservative analysis in which these patients without aphasia were imputed to have 1 month measures identical to pre-surgical measures showed that even in this optimistic scenario, there would still be subtle residual deficits at the group level.
These limitations do not diminish the two main conclusions of our study. First, at 2-3 days post surgery, all 10 connected speech measures changed, reflecting impairments in multiple language domains including fluency, lexical access, phonological encoding, and sentence construction. Second, while most aphasic symptoms were largely resolved by 1 month post surgery, at least some connected speech measures still reflected subtle persisting deficits; specifically, there were more phonological errors and lexical access difficulties than there had been before surgery.
Acknowledgments
We thank Leah Swanson, Alexa Bautista, Daniel Lam, and Jessica DeLeon for their assistance with this project, and all of the patients for their participation in our research.
Funding: This research was supported in part by the National Institutes of Health (National Institute on Deafness and Other Communication Disorders) under grants DC013270, DC012379, NS065120, and OD00862.
Appendix 1
Below are excerpts from speech samples at each of the three time points from one patient, a 29-year old female. This patient was quite representative, in that she showed many of the typical deficits that were commonly observed at 2-3 days post-surgery, and in that her language returned to baseline by 1 month post-surgery. Conventions: angled brackets indicate retraced material; hyphens indicate false starts; pauses are indicated by (.) = 1 second, (..) = 2 seconds, (…) = 3 or more seconds.
Pre-surgical sample excerpt
um we got a tree here.
a little house.
they're having like a picnic.
out in their front yard, I guess, <out in the> kinda out in the country.
flying a kite.
um they got a lake here.
someone's out (.) uh on a sailboat out on a lake.
they got the dog.
having a picnic.
the radio.
and (…) um the boat dock there.
2-3 days post-surgical sample excerpt
well they're having a picnic (.) um and drinking. (..)
they're having a kite. (…)
um (..) oh they're um (.) on (.) a (..) s- (..) <on a [snoʊbαɹt]> on a board.
they're trying to… (…)
h- uh di- (..) ð- this guy is um (.) on the bike.
he's riding… (..)
um I mean a picnic.
and they got the dog here. (…)
and he's um watching music. (..)
he's l- watching a (.)[pɪηpαn]. (.)
<I mean I> I mean a um magazine. (.)
and she's having a drink.
1 month post-surgical sample excerpt
there's people having a picnic outside.
um they're having…
uh <he's> well he reading a book.
having um (.) drinks outside.
enjoying flying the kite.
enjoying time on (.) uh the boat.
and (..) uh playing with the dog.
someone's even going fishing. (.)
just a lot of people doing a lot of stuff outside, enjoying nice weather.
References
- Armstrong E. Aphasic discourse analysis: The story so far. Aphasiology. 2000;14:875–892. doi: 10.1080/02687030050127685. [DOI] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- Benzagmout M, Gatignol P, Duffau H. Resection of World Health Organization grade II gliomas involving Broca's area: Methodological and functional considerations. Neurosurgery. 2007;61:741–753. doi: 10.1227/01.neu.0000298902.69473.77. [DOI] [PubMed] [Google Scholar]
- Blank I, Balewski Z, Mahowald K, Fedorenko E. Syntactic processing is distributed across the language system. NeuroImage. 2016;127:307–323. doi: 10.1016/j.neuroimage.2015.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. Stability of word-retrieval errors with the AphasiaBank stimuli. American Journal of Speech-Language Pathology. 2015;24:S953–S960. doi: 10.1044/2015_ajslp-14-0152. [DOI] [PubMed] [Google Scholar]
- Brookshire RH, Nicholas LE. Speech sample size and test-retest stability of connected speech measures for adults with aphasia. Journal of Speech and Hearing Research. 1994;37:399–407. doi: 10.1044/jshr.3702.399. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters G, Kennedy D, Alpert N, Makris N, Dede G, Reddy A. A study of syntactic processing in aphasia II: Neurological aspects. Brain and Language. 2007;101:151–177. doi: 10.1016/j.bandl.2006.06.226. [DOI] [PubMed] [Google Scholar]
- Davies KG, Bell BD, Bush AJ, Hermann BP, Dohan FC, Jr, Jaap AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–419. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Davies KG, Maxwell RE, Jennum P, Dhuna A, Beniak TE, Destafney E, Fiol ME. Language function following subdural grid-directed temporal lobectomy. Acta Neurologica Scandinavica. 1994;90:201–206. doi: 10.1111/j.1600-0404.1994.tb02706.x. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Lopes M, Van Effenterre R. Functional recovery after surgical resection of low grade gliomas in eloquent brain: Hypothesis of brain compensation. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery. 2008;109:461–471. doi: 10.3171/jns/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Falconer MA, Serafetinides EA. A follow-up study of surgery in temporal lobe epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1963;26:154–165. doi: 10.1136/jnnp.26.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergadiotis G, Wright HH. Lexical diversity for adults with and without aphasia across discourse elicitation tasks. Aphasiology. 2011;25:1414–1430. doi: 10.1080/02687038.2011.603898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Quadfasel FA, Timberlake WH. Phrase length and the type of severity of aphasia. Cortex. 1964;1:133–153. [Google Scholar]
- Helmstaedter C, Gleissner U, Zentner J, Elger CE. Neuropsychological consequences of epilepsy surgery in frontal lobe epilepsy. Neuropsychologia. 1998;36:681–689. doi: 10.1016/S0028-3932(97)00134-6. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. Journal of Neurosurgery. 1991;74:560–566. doi: 10.3171/jns.1991.74.4.0560. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Katz A, Awad IA, Kong AK, Chelune GJ, Naugle RI, Wyllie E, Lüders H. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30:763–771. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, Lesser RP. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Archives of Neurology. 1996;53:72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP. Effects of temporal lobectomy on generative fluency and other language functions. Archives of Clinical Neuropsychology. 1994;9:229–238. doi: 10.1093/arclin/9.3.229. [DOI] [PubMed] [Google Scholar]
- Lubrano V, Draper L, Roux FE. What makes surgical tumor resection feasible in Broca's area? Insights into intraoperative brain mapping. Neurosurgery. 2010;66:868–875. doi: 10.1227/01.neu.0000368442.92290.04. [DOI] [PubMed] [Google Scholar]
- MacWhinney B. The CHILDES Project: Tools for Analyzing Talk. Mahwah, NJ: Lawrence Erlbaum; 2000. [Google Scholar]
- MacWhinney B. The CHILDES Project: Tools for Analyzing Talk—Electronic Edition. MS: Carnegie Mellon University; Aug 6, 2012. 2012. [Google Scholar]
- MacWhinney B, Fromm D, Forbes M, Holland A. AphasiaBank: Methods for studying discourse. Aphasiology. 2011;25:1286–1307. doi: 10.1080/02687038.2011.589893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas LE, Brookshire RH. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. Journal of Speech and Hearing Research. 1993;36:338–350. doi: 10.1044/jshr.3602.338. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Speech and brain-mechanisms. Princeton, NJ: Princeton University Press; 1959. [Google Scholar]
- Peraud A, Ilmberger J, Reulen HJ. Surgical resection of gliomas WHO grade II and III located in the opercular region. Acta Neurochirurgica. 2004;146:9–17. doi: 10.1007/s00701-003-0165-4. [DOI] [PubMed] [Google Scholar]
- Roberts L. Handedness and cerebral dominance. Transactions of the American Neurological Association, (80th Meeting) 1955:143–148. [PubMed] [Google Scholar]
- Saffran EM, Berndt RS, Schwartz MF. The quantitative analysis of agrammatic production: procedure and data. Brain and Language. 1989;37:440–479. doi: 10.1016/0093-934X(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Implications of within-person variability in cognitive and neuropsychological functioning for the interpretation of change. Neuropsychology. 2007;21:401–411. doi: 10.1037/0894-4105.21.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. New England Journal of Medicine. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Stafiniak P, Robinson LJ, Flannery KA, Gur RC, O’Connor MJ, Sperling MR. Language before and after temporal lobectomy: Specificity of acute changes and relation to early risk factors. Epilepsia. 1995;36:1071–1077. doi: 10.1111/j.1528-1157.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Jette N. Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia. 2011;52:857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. doi: 10.1080/02687039708248473. [DOI] [Google Scholar]
- Thompson CK, Cho S, Hsu CJ, Wieneke C, Rademaker A, Weitner BB, Weintraub S. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26:20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle IR, Pringle AM, Taylor R. Effects of resective surgery for left-sided intracranial tumours on language function: a prospective study. Lancet. 1998;351:1014–1018. doi: 10.1016/S0140-6736(97)08295-0. [DOI] [PubMed] [Google Scholar]
- Wilson SM, DeMarco AT, Henry ML, Gesierich B, Babiak M, Miller BL, Gorno-Tempini ML. Variable disruption of a syntactic processing network in primary progressive aphasia. Brain. 2016 doi: 10.1093/brain/aww218. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Lam D, Babiak MC, Perry DW, Shih T, Hess CP, Chang EF. Transient aphasias after left hemisphere resective surgery. Journal of Neurosurgery. 2015;123:581–593. doi: 10.3171/2015.4.JNS141962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenburg P, Brugman H, Russel A, Klassmann A, Sloetjes H. ELAN: A professional framework for multimodality research. Proceedings of LREC 2006, Fifth International Conference on Language Resources and Evaluation.2006. [Google Scholar]
- Yagata SA, Yen M, McCarron A, Bautista A, Lamair-Orosco G, Wilson SM. Rapid recovery from aphasia after infarction of Wernicke's area. Aphasiology. 2017 doi: 10.1080/02687038.2016.1225276. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekutieli D, Benjamini Y. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. Journal of Statistical Planning and Inference. 1999;82:171–196. doi: 10.1016/S0378-3758(99)00041-5. [DOI] [Google Scholar]