Abstract
For clinicians, Pseudomonas aeruginosa is a nightmare pathogen that is one of the top three causes of opportunistic human infections. Therapy of P. aeruginosa infections is complicated due to its natural high intrinsic resistance to antibiotics. Active efflux and decreased uptake of drugs due to cell wall/membrane permeability appear to be important issues in the acquired antibiotic tolerance mechanisms. Bacterial cell wall biosynthesis enzymes have been shown to be essential for pathogenicity of Gram-negative bacteria. However, the role of these targets in virulence has not been identified in P. aeruginosa. Here, we report knockout (k.o) mutants of six cell wall biosynthesis targets (murA, PA4450; murD, PA4414; murF, PA4416; ppiB, PA1793; rmlA, PA5163; waaA, PA4988) in P. aeruginosa PAO1, and characterized these in order to find out whether these genes and their products contribute to pathogenicity and virulence of P. aeruginosa. Except waaA k.o, deletion of cell wall biosynthesis targets significantly reduced growth rate in minimal medium compared to the parent strain. The k.o mutants showed exciting changes in cell morphology and colonial architectures. Remarkably, ΔmurF cells became grossly enlarged. Moreover, the mutants were also attenuated in vivo in a mouse infection model except ΔmurF and ΔwaaA and proved to be more sensitive to macrophage-mediated killing than the wild-type strain. Interestingly, the deletion of the murA gene resulted in loss of virulence activity in mice, and the virulence was restored in a plant model by unknown mechanism. This study demonstrates that cell wall targets contribute significantly to intracellular survival, in vivo growth, and pathogenesis of P. aeruginosa. In conclusion, these findings establish a link between cell wall targets and virulence of P. aeruginosa and thus may lead to development of novel drugs for the treatment of P. aeruginosa infection.
Introduction
Pseudomonas aeruginosa is a wide-spread Gram-negative bacterium. It is reported as the third leading cause of nosocomial infections responsible for life-threatening infections of immunocompromised and cystic fibrosis patients and is main cause of hospital-acquired infections, particularly for burns victims [1, 2].
Biofilm forming bacteria like P. aeruginosa are armed with success-arsenals such as environmental adaption to distinct environments with low nutriments showing a characteristic regulation in gene expression profile [3]. Biofilm formation also leads to a higher antibiotic tolerance and gives the necessary time to acquire antibiotic resistance [3, 4]. Antibiotic resistance mechanisms have been studied extensively in P. aeruginosa. Common mechanisms/factors are active efflux and/or decreased uptake of drugs (membrane permeability), modification of the drug, oxidative phosphorylation, lipopolysaccharide (LPS) composition, cyclic di-guanosine monophosphate (c-di-GMP) levels and quorum sensing [3–8].
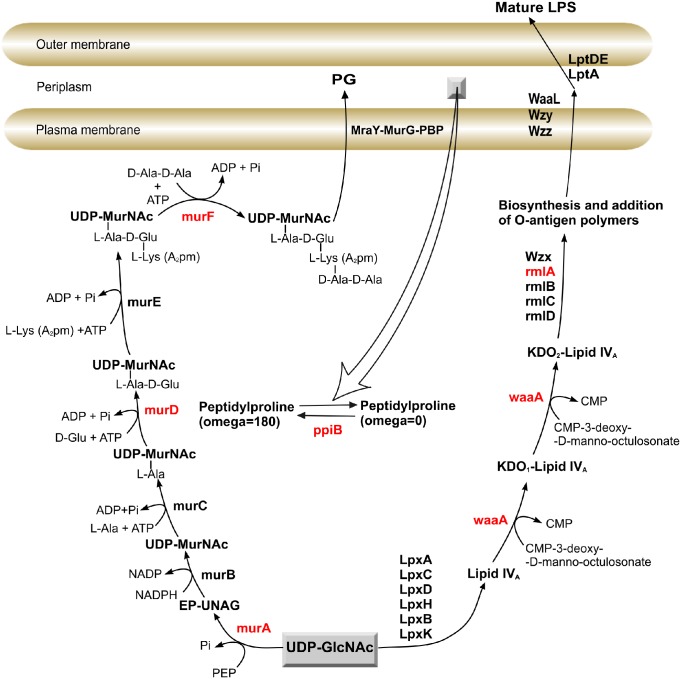
Most of P. aeruginosa strains develop high intrinsic resistance as a result of inefficient antibiotics uptake across the outer membrane [9]. A prominent constituent of the outer membrane is cell wall polymer, peptidoglycan, which is vital for bacterial survival. As in many other gram negative bacteria, the biosynthetic pathway of peptidoglycan consists of two-stage process. The first process takes place in the cytoplasm catalysed by murA. This is followed by reduction reactions of the enol-pyruvate moiety to D-lactate, yielding UDP-N-acetylmuramate catalysed by murB. The later product enters series of pentapeptide side chain additions on the newly reduced D-lactyl group which is processed by murC, murD, murE and murF (Fig 1) [10, 11].
Fig 1. Schematic representation of peptidoglycan and LPS biosynthesis pathway in P. aeruginosa.
The targeted genes are colored in red.
The second process deals with the transfer of the precursor across the inner membrane and addition to the growing cell wall polymers [11]. Every antibiotic that is introduced into clinical use has limited or no effect on mur enzymes, except murA, which is inhibited by phosphonomycin [12]. Another promising target in P. aeruginosa cell-wall is the LPS biosynthesis pathway. The sugar 3-deoxy-D-manno-octulosonic acid (Kdo) is an essential component of the lipopolysaccharide (LPS) of Escherichia coli and other Gram-negative bacteria [13–15]. The Kdo sugar synthesis mediated by waaA (3-deoxy-D-manno-octulosonic-acid (Kdo) transferase) which makes sequential addition of two Kdo sugars onto a molecule of lipid IVA, a key precursor of lipid A (Fig 1). Belunis et al. [16] demonstrated that the 3-deoxy-D-manno-octulosonic-acid (Kdo) transferase gene is essential for growth of E. coli. Another LPS decoration is the O-specific polysaccharide, made of replicated oligosaccharide units. Most of the enzymes involved in the biosynthesis of O polysaccharides are encoded by genes clustered in the rfb locus (rml locus in P. aeruginosa) [17]. Mutations among rfb gene cluster in E. coli K-12 lead to loss of O antigen and inability to survive in its natural environment [18]. Moreover, previous studies have noted that the organism’s components residing in the periplasm are essential for P. aeruginosa pathogenesis and virulence [19]. Peptidyl-prolyl cis-trans isomerase B (ppiB), one of the most important enzymes in periplasmic area, which catalyses the cis-trans isomerization of proline peptide bonds, have been detected and isolated from both the periplasm of the E. coli and P. aeruginosa [19–21].
The lack of novel antibiotics against P. aeruginosa and related drug resistant Gram-negative bacteria is mainly responsible for the failure to control drug resistance [22, 23]. P. aeruginosa strain PAO1 has one of the largest bacterial genomes sequenced with over 5.500 predicted genes [24]. A multidisciplinary approach has been used to assess and validate drug targets, genetically and biochemically, characterise these targets and generate new hit [25, 26]. Based on bioinformatics and biological data assessment of PAO1 and other Gram-negative organisms, a target list of genes encoding proteins for which there are no structures and little biochemical data have been identified and predicted to have an essential function in this pathogen [25, 27].
The pathogenesis of P. aeruginosa infections is multifaceted, as illustrated by numerous virulence features. Previous studies have shown that the majority of bacterial outer leaflet murein, and LPS biosynthesis enzymes are crucial for the survival of most Gram-negative bacteria [11, 13–16, 18, 28]. However, no direct relationship between these genes and virulence of P. aeruginosa has been established. In order to fill the innovation gap and to search new targets for novel antibiotics development against P. aeruginosa infections, we used “evaluate and design” strategy. In this study, a sub-set of the PAO1 cell wall, murein and LPS biosynthesis targets were subjected to in-vivo gene deletion by constructing single-gene knockout strains of PAO1 followed by survival experiments of these mutants in vitro and in vivo. We report here the results obtained with a number of k.o mutants in murA, PA4450; murD, PA4414; murF, PA4416; ppiB, PA1793; rmlA, PA5163; waaA, PA4988. By using this genetic approach, we could assess directly the role of selected targets in pathogenesis and virulence in mice.
Material and methods
Ethics statement
All animal experiments were carried out according to recommendations of the European Commission and the law for the Care and Use of Laboratory Animals of the Government of the Federal Republic of Germany. The protocol and experiments were approved by the responsible German authorities (the Ethics Committee of Animal Experiments, The Landesuntersuchungsamt-the government of Rheinland-Pfalz, Federal Republic of Germany, Permit Number: 23 177-07/G09-15-001.
Construction of suicide vectors for replacement of target genes
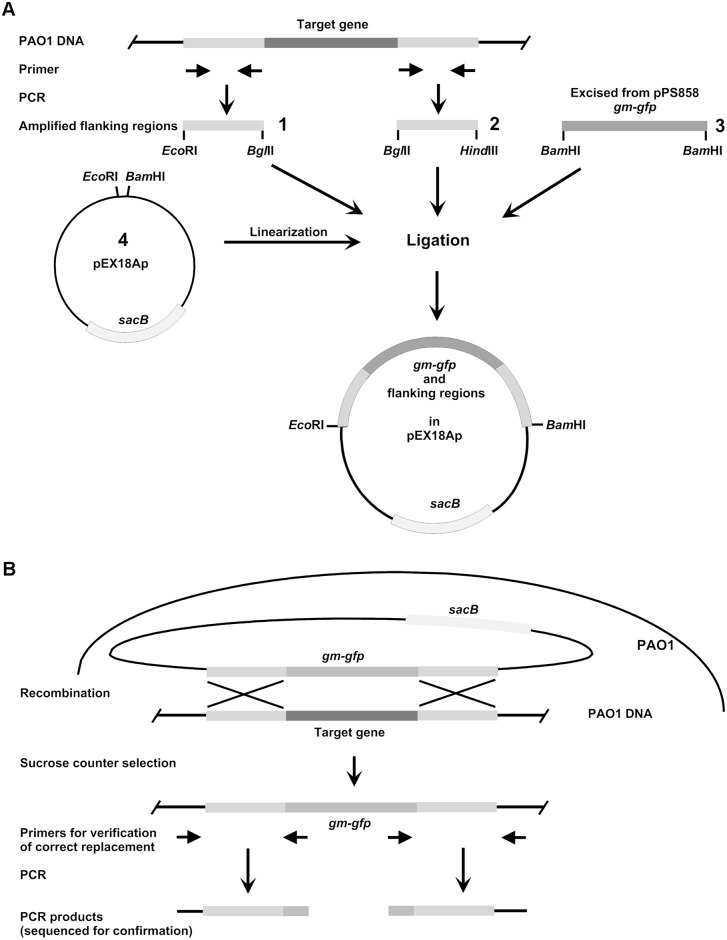
For gene replacement the sacB-based strategy based on the pEX18Ap suicide vector [29, 30] was used. The suicide vectors for deletion of target genes were constructed by amplification of approximately 400 bp flanking regions using primers with integrated restriction sites (Table 1) allowing directed insertion into mobilisable vector pEX18Ap. PCR-products of upstream regions were cleaved with EcoRI and BglII and downstream regions with HindIII and BglII (only in case of PA1793 (ppiB) BamHI instead of BglII). The gentamycin-GFP cassette of pPS858 was excised using BamHI and vector pEX18Ap was digested with EcoRI and HindIII. Cleaved fragments and vector were then combined in ligation mixture (Fig 2). The constructs were confirmed by sequencing and then transformed into the E. coli donor strain ST18 which was used for conjugational transfer of the plasmids into P. aeruginosa PAO1 wild-type (WT) as described previously [29]. Briefly, E. coli ST18 containing the plasmid were streaked and transferred to LB plate containing aminolevulinic acid, carbenicillin 100 μg/mL and gentamicin 10 μg/mL. After controls and main cultures with and without antibiotics, in 1.5 mL reaction tube 100 μL PAO1 and 1 mL ST18 culture were mixed. The mixture was centrifuged at 11.000 g for 1 min, the pellet was suspended in 100 μL LB medium and plated on LB agar plate overnight at 37°C. Using an inoculation needle, the colonies from the conjugation plate transferred to LB-antibiotic plate and incubated overnight at 37°C. Single colonies obtained on the conjugation plate restreaked on LB-antibiotic agar plates. After counter-selection on LB-agar plates containing 5% sucrose and 80 μg/ml gentamycin, the obtained clones were tested for carbenicillin sensitivity by replica plating. In order to confirm the loss of plasmid borne DNA due to recombination events, genomic DNA was isolated from the potential k.o mutants and used in PCR together with primers specific for internal sequences of backbone of the plasmid. The correct replacement of the target gene by the gentamycin cassette was confirmed by site specific PCR and sequencing. Primers located in approximately 800 and 1400 base pairs distance from the ends of the cassette and directed outwards were used in combination with primers located minimum 500 base pairs up-and downstream from the target gene. The resulting PCR products were isolated and sequenced.
Table 1. Sequences of primers used for amplification of flanking regions of target genes, pEX18Ap vector backbone and Gm-gfp cassette.
| Target gene | Flanking region | Primer sequence 5´-3´ |
|---|---|---|
| PA1793 (ppiB) | upstream | GGCAATCGCCAGCGAATTC |
| GCGACGAGATCTGTGGGTAATCCGCTTTGTC | ||
| downstream | GCGACGGGATCCAGCGATGAGCGTCCTGTTC | |
| GCGACGAAGCTTCCAGCGGCGCAGGCGCATG | ||
| PA4414 (murD) | upstream | GGAGGAGAATTCGTACCTGCTGATTCCCAAC |
| GGAGGAAGATCTGCTCTCTTCGTCCTCAACG | ||
| downstream | GGAGGAAGATCTGATGCTGTCGGTGTTGCG | |
| GGAGGGAAGCTTCACGCAGACCTTGGCGATC | ||
| PA4416 (murF) | upstream | GGAGGAGAATTCCTGGAAAAAGTCCTGGAGG |
| GGAGGAAGATCTGCGGCACCTCCCAGGCGGC | ||
| downstream | GGAGGAAGATCTCTAATGCTCCTGCTGCTGG | |
| GGAGGGAAGCTTGACTGCCAGAAGTACTTCC | ||
| PA4450 (murA) | upstream | GGAGGAGAATTCGAGATCCTGCCTTTGCAGG |
| GGAGGAAGATCTTGCAATGATCCCCCGTGGG | ||
| downstream | GGAGGAAGATCTCGGAGGCTGTCGCGCAAATG | |
| GGAGGGAAGCTTCTTGATCACGTCGACCTGG | ||
| PA4988 (waaA) | upstream | GGAGGAGAATTCGAGATGGTCACGGTGTGCG |
| GGAGGAAGATCTCATGGGCGCGCAGCTTAGC | ||
| downstream | GGAGGAAGATCTGTAGCGCTCGCCCCGTCGG | |
| GGAGGGAAGCTTCGTGACCATCCAGTGGTTC | ||
| PA5163 (rmlA) | upstream | GGAGGAGAATTCCGACGAACTCAAGGTCGTG |
| GGAGGAAGATCTGTGGTCCCTGCTCGCTCAG | ||
| downstream | GGAGGAAGATCTGAAAGCGACCCGCCTGGC | |
| GGAGGGAAGCTTCCAGAAGTCGGTGGTCTTG | ||
| pEX18Ap vector | Fw | TATGTACTGTGTTAGCGG |
| Rev | AAACTCTGGCTCACCG | |
| Gm-gfp cassette | Fw | AACTTTGTATAGAGAGCCACTGCG |
| Rev | TTAGGTGGCTCAAGTATGGGC |
Fig 2. General scheme of construction of suicide vectors (A) and replacement of target genes (B).
A: For construction of suicide vectors for replacement of target genes, regions of approximately 400 bp flanking the target up- and downstream, respectively, were amplified using primers with integrated restriction sites allowing directed insertion into mobilisable vector pEX18Ap. PCR-products of upstream regions (1) were cleaved with EcoRI and BglII and downstream regions (2) with HindIII and BglII (only in case of PA1793 BamHI instead of BglII). The gentamycin-GFP cassette (3) of pPS858 was excised using BamHI and vector pEX18Ap was linearised with EcoRI and HindIII. Cleaved fragments 1, 2, and 3 and linearised vector pEX18Ap (4) were then combined in ligation mixture. The gentamycin-GFP cassette ligation to up and downstream fragments aided by the compatible cohesive ends between BamHI and BglII. Then E. coli donor strain ST18 was transformed with this mixture. Afterwards the suicide constructs were transferred from E. coli ST18 into P.aeruginosa PAO1 by conjugation. B: After conjugational transfer of suicide constructs recombinational replacement of the target genes and loss of vector backbone was forced by sucrose counter selection and gentamycin selection, respectively. Total DNA was isolated from knock out mutants and correct gene replacement was confirmed by sequencing extended PCR-products of the flanking regions.
Agarose bead preparation with P. aeruginosa PAO1 WT and P. aeruginosa PAO1 mutants
Agarose beads were prepared as described previously [31] with some modifications. P. aeruginosa PAO1 (ATCC 47085) wild-type (WT) was cultured overnight at 37°C in LB and cell wall targets mutants were cultivated in LB supplemented with gentamycin (80 μg/ml). After centrifugation at 1500 g for 10 min sedimented bacteria were resuspended in 1 ml sterile PBS (pH 7.4) and added to 5 ml 2% Agarose prewarmed to 50°C. The bacteria-agarose mixture was transferred rapidly to 5 ml 50°C prewarmed heavy white mineral oil. After intensive vortexing the mixture was cooled on ice for 5 minutes followed by centrifugation at 1500 g for 10 min. The resulting agarose beads were washed three times in sterile PBS (pH 7.4). The load of bacteria in the agarose beads was quantified by plating 10-fold serial dilutions on Columbia blood agar plates. The inoculums for infection in mice were prepared by diluting the bead suspension with PBS (pH 7.4) to 2x108 CFU/ml.
Lung infection of mice and quantification of bacteria in the lung
For the infection studies we used female NMRI outbred mice at the age of min. 6 weeks. The animals were anesthetized using a Ketamin-Xylazin mixture applied by subcutaneous injection. Mice were intratracheally infected with 50 μl of the bead suspension at a concentration of 2x108 CFU/ml resulting in 1x107 CFU. After 72 hours of infection, the mice were sacrificed by CO2 inhalation. Lungs were isolated and homogenized through cell strainer. Mouse lung homogenates were diluted 1:1, 1:10 and 1:100 with sterile PBS (pH 7.4). The resulted dilutions were plated on Columbia blood agar plates in case of P. aeruginosa PAO1 wild-type, and with addition of gentamycin (80 μg/ml) for Knockout mutants. The colony counts were determined for each dilution after overnight incubation at 37°C.
Macrophage-mediated bactericidal assay
Macrophage-mediated bactericidal assays were carried out as described previously with few modifications [32]. The murine macrophage cell line J774A.1 (Leibniz-Institut DSMZ—Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH) was used to examine the survival in vitro. Macrophages were grown in DMEM supplemented with 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37°C. Mid-log-phase P. aeruginosa PAO1 wild-type grown in LB broth at 37°C and for mutants with addition of gentamycin were collected by centrifugation at 6.000 g and suspended to an OD600 of 0.4 in DMEM without FBS. Macrophages (5 × 107) were incubated in 1 ml DMEM with the wild-type and mutants of P. aeruginosa (5 ×107 CFU) for 30 min at 37°C. In order to eliminate extracellular P. aeruginosa, three washes by centrifugation at 163 g for 5 min at room temperature in PBS for wild-type and with PBS containing 400 μg/ml gentamycin were carried out. After the final wash, macrophages were allowed to adhere to tissue culture flasks in DMEM medium supplemented with gentamicin (400 μg/ml). After two hours of incubation, macrophages with internalized bacteria were harvested and lysed with 0.25% SDS and 0.025% SDS and the live intracellular bacteria were counted by plating serial dilutions of the lysates on LB plates for wild-type and LB containing gentamycin (80 μg/ml) for the mutants. The bacterial counts were determined with respect to the CFU/ml.
Plant virulence assays
The plant virulence assay was performed in lettuce leafs as described previously [33]. 10 μl of stationary-phase cultures were diluted in LB medium (107 CFU/ml; OD600 = 0.01), washed twice and resuspended in 10 mM MgSO4. Bacterial suspensions were inoculated into the midribs of Romaine lettuce leaves. Alu-dishes containing Whatman filter papers soaked with 10 mM MgSO4 and inoculated leaves were kept at room temperature for seven days. Symptoms were monitored daily for seven days.
Culture conditions and growth curve
P. aeruginosa PAO1 was grown in LB medium or minimal M9 medium, if not otherwise stated. M9 medium contained 2% glucose as a carbon source in all cases. For growth of all PAO1 mutants both medium types were supplemented with gentamycin. For growth characteristics comparison, glycerol (2%), instead of glucose as a carbon source, was used.
Determination of extracellular DNA
For the determination of the release of DNA by P. aeruginosa PAO1 cultures, the bacteria were grown in LB medium for up to 31 h. The cultures were centrifuged at 15.700 g for 10 min and the supernatant was mixed with loading buffer. The samples were subjected to 1% agarose gel stained with GelStar Nucleic Acid Gel Stain (Lonza, USA) and photographed under UV-light. Quantitative analysis of DNA on gel images was performed by ImageLab software (Bio-Rad Laboratories).
Preparation for field emission scanning electron microscopy(FESEM)
P. aeruginosa PAO1 wild-type and the mutant strains were grown in LB and M9 medium. The media for k.o mutant were supplemented with gentamycin (80 μg/ml). 5 ml of midlog-phase cultures (OD600 of 0.7–0.9) were centrifuged and washed twice in PBS (pH 7.4). Samples were fixed with a solution containing 2.5% glutaraldehyde for 2 hours at room temperature and washed twice with PBS. Samples were further washed with TE buffer (20 mM TRIS, 2 mM EDTA, pH 6.9). After washing with TE buffer samples placed onto poly-l-lysine-coated slides and then dehydrated with 10%, 30%, 50%, 70%, 90%, 100% acetone, each step for 10 min on ice. The last 100% acetone step was performed at room temperature. Samples were then critical-point-dried with liquid CO2 (CPD 030, Bal-Tec, Liechtenstein) mounted onto aluminium stubs and sputter coated with gold (SCD 500, Bal-Tec, Liechtenstein) before examination in a Zeiss field emission scanning electron microscope Gemini DSM852 (Zeiss, Oberkochen, Germany) at an acceleration voltage of 5 kV using the Everhart-Thornley SE detector and the inlens SE detector in a 50:50 ratio. Images were stored onto a 230 MB MO disk. GIMP (version 2.0) software (http://www.gimp.org/) was used for images measurement.
Results
Construction of k.o mutants in P. aeruginosa PAO1
For validation of cell wall biosynthesis genes as potential new drug targets in P. aeruginosa, we created single gene knockout strains of P. aeruginosa PAO1 using sacB-based strategy on the pEX18Ap suicide vector (Fig 2). After the putative k.o mutants were identified by their carbenicillin-sensitive, gentamycin-resistant, and sucrose-tolerant phenotype, correct insertion of Gm-gfp cassette at the target site and excision of pEX18Ap vector backbone was confirmed. The latter event was examined by PCR using total DNA isolated from k.o-candidates together with primers specific to pEX18Ap vector backbone and Gm-gfp cassette (S1 Fig). In case where no fragment could be amplified, clones were further tested for replacement of target gene by Gm-gfp cassette. For this, primer combinations located minimum 500 bp from the flanking regions and those located in the cassette directed outwards were used. This means that the four primers combined in four pairs of which two were expected to result in a fragment in case of correct insertion. Amplified fragments were purified and sequenced for final confirmation.
Growth characteristics of the k.o mutants: ΔrmlA failed to grow
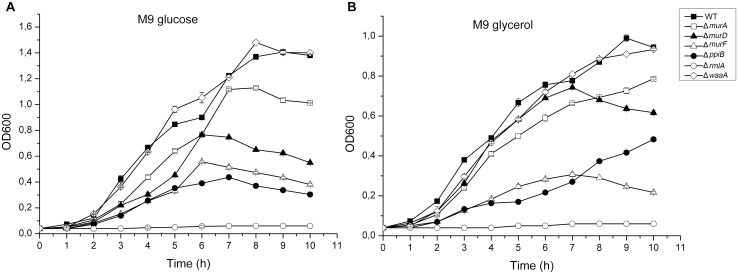
Prior to studying the effect of the k.o mutations on the mice model, we first examined the growth characteristics of the wild-type and the mutant strains in both minimal and complex media. In liquid minimal medium M9 with glucose as the sole carbon source at 37°C, ΔmurA, ΔmurD, ΔmurF and ΔppiB mutant strains showed significantly reduced growth rate compared to that of the wild-type (Fig 3A). In minimal M9 medium with glycerol as carbon source, ΔmurA, ΔmurD, ΔmurF and ΔppiB mutant strains also showed significantly reduced growth rate compared to the parent strain (Fig 3B). Unexpectedly, ΔrmlA mutant strain failed totally to grow in M9 neither when supplemented with glucose nor with glycerol. The doubling time of all mutants except ΔwaaA grown in the M9 medium either with glucose or glycerol was significantly longer compared to the wild-type strain. Unexpectedly the wild-type culture displayed short stationary-phase between 5 and 6 hours in M9 plus glucose and began to grow exponentially again after 6 hours. In glycerol supplemented media this short delayed growth appeared also between 6 and 7 hours. In addition, cultures of the mutant strains did not achieve the same final optical density as the parent strain in the M9 medium. In LB medium all mutants exhibited similar growth rate to wild-type except delayed growth in initial mid-log of ΔrmlA cultures (data not shown).
Fig 3. Growth characteristics of P. aeruginosa PAO1 wild-type and cell wall targets mutants.
(A) Growth curves in M9 minimal medium with glucose as the sole carbon source. (B) Growth curves in M9 minimal medium with glycerol as sole carbon source. The standard errors of the mean for three independent experiments are shown.
Morphological and colony architectural changes
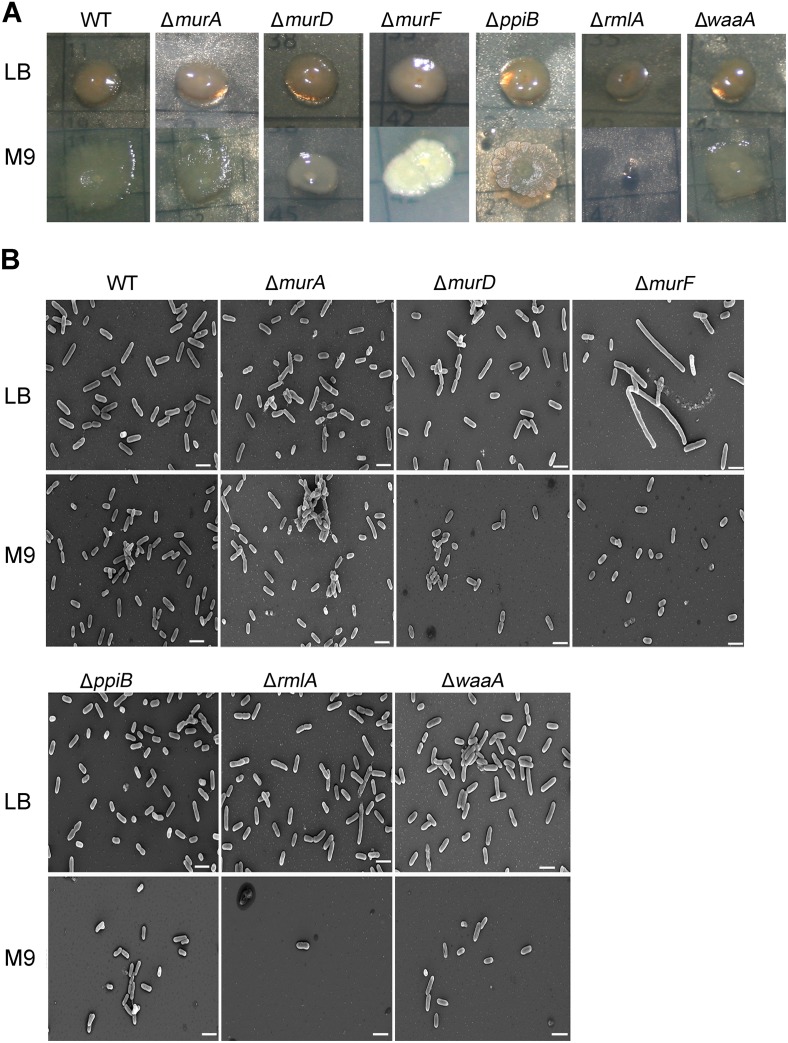
Using PAO1 k.o mutant cultures grown in minimal and complex media, we investigated colony morphology of PAO1 wild-type, ΔmurA, ΔmurD, ΔmurF, ΔppiB, ΔrmlA and ΔwaaA grown in vitro. For colony morphology studies, single colonies were inoculated onto fresh medium plates (LB or M9 supplemented with glucose). All strains used in this study formed smooth colonies when cultured on LB. We noted that the ΔmurA and ΔmurF colonies in LB had different color with slightly shining and smoother textured surfaces (Fig 4A). On the other hand, dramatic changes in colonies morphology were observed when the wild-type and mutant strains were grown on M9 agar. All mutants colonies were smaller compared to wild-type except ΔrmlA strain which lost the ability to grow in minimal media (Fig 4A). Such effect in rmlA mutant (Fig 3) has also been reported for E. coli [18]. ΔmurA strain showed similar undulated colonies like wild-type but were smaller in size. ΔmurD formed flat smooth colonies with less undulations while ΔwaaA formed lobed and smooth colonies. Moreover, ΔmurF and ΔppiB colonies had irregular and highly textured surfaces with marked wrinkles (Fig 4A). Taken together, the deletion of cell wall biosynthesis genes influenced the morphological appearance especially in minimal conditions. Scanning electron microscopy showed that whereas PAO1 wild-type grown in LB exhibited normal rod shape morphology with a cell length of 1.2–2.5 μm (Fig 4B), ΔmurA, ΔmurD, ΔppiB, ΔrmlA and ΔwaaA mutants grown in LB exhibited no alteration in cell morphology. Markedly ΔmurF cells were highly elongated (Fig 4B) up to 6.8 μm compared to the wild-type and other mutants. This abnormal cell elongation is likely caused by defective cell division because most of elongated cells didn’t show any cell dividing pattern which may indicate that the formation of septa is suppressed in ΔmurF mutants [34]. Thus, ΔmurF may have an important role in septal formation and late cell division events [34, 35]. In M9 medium, length measurements demonstrated clearly that ΔmurD and ΔmurF cells are shorter when compared to wild-type and other mutants. For ΔrmlA strain in M9 very few cell found to be documented, because ΔrmlA strain lost growth ability in M9 medium and most probably these few cells were from the initial inoculum. These prominent and different changes in cell size, different colony morphologies and surface structure demonstrated that each Knockout gene affects the cellular phenotype differently.
Fig 4. Morphological and colonial architectures changes in the knockout strains.
(A) Colony morphology on LB (higher panel) and on M9 supplemented with glucose (Lower panel). (B) FESEM analysis of P. aeruginosa wild-type and cell wall mutants, all strains cultivated on LB (higher panel) and on M9 supplemented with glucose (Lower panel). Scale bar is indicated at the bottom of each image. The scale bars always represent 2 μm.
Extracellular DNA in P. aeruginosa cultures
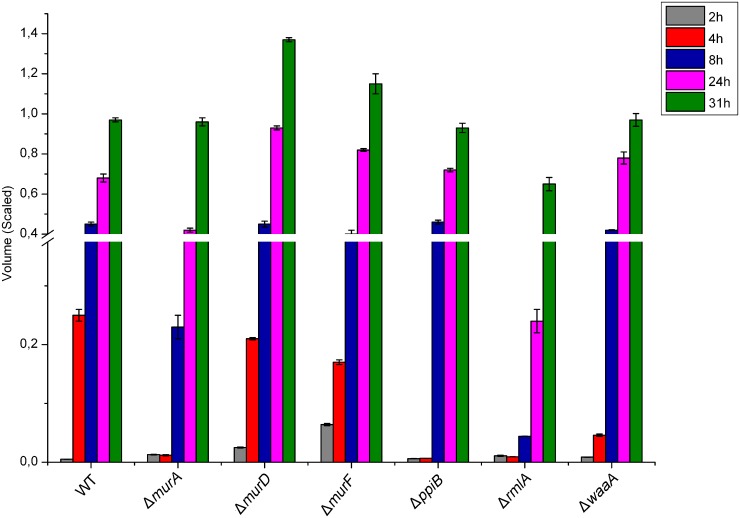
P. aeruginosa PAO1 as well as clinical P. aeruginosa isolates are known to release extracellular DNA [4, 36]. Accumulated evidence confirms that the extracellular DNA functions as a cell-to-cell interconnecting matrix component in biofilms [4, 36–38]. Moreover, extracellular DNA induces antibiotic resistance in biofilms [4]. The extracellular DNA in the medium of growing P. aeruginosa PAO1 strains was assessed by stained agarose gel electrophoresis. The LB medium was used because different growth rates were observed in minimal medium. Software-aided DNA density volume measurements, showed that the P. aeruginosa cultures except culture of ΔmurF, contained a low basal level of extracellular DNA in the initial-log phase of growth (2 hours), and that a large amount of DNA was released in the late-log phase of growth (Fig 5). After 4 hours, sudden increase of extracellular DNA in wild-type, ΔmurD and ΔmurF cultures was observed, while the DNA amount remained low in the other mutants. This increase was followed by substantial increases of extracellular DNA after 8 hours in all except in ΔrmlA cultures. Obviously ΔrmlA cultures contained the lowest extracellular DNA among the whole time course. Fig 5 shows that a large amount of extracellular DNA was released specifically in the late-log phase of ΔmurD growth. The low level of DNA release in ΔrmlA cultures correlated well with the delayed initial mid-log in LB and suggested that quorum sensing and biofilms might be affected seriously in this mutant [36–38].
Fig 5. Cell wall genes dependent release of extracellular DNA.
PAO1wild-type and cell wall targets mutants were grown in LB medium. Supernatant samples were collected after the respected time. DNA release was delayed and was much lower in the PAO1 rmlA mutants. Standard errors represent the mean of three independent experiments.
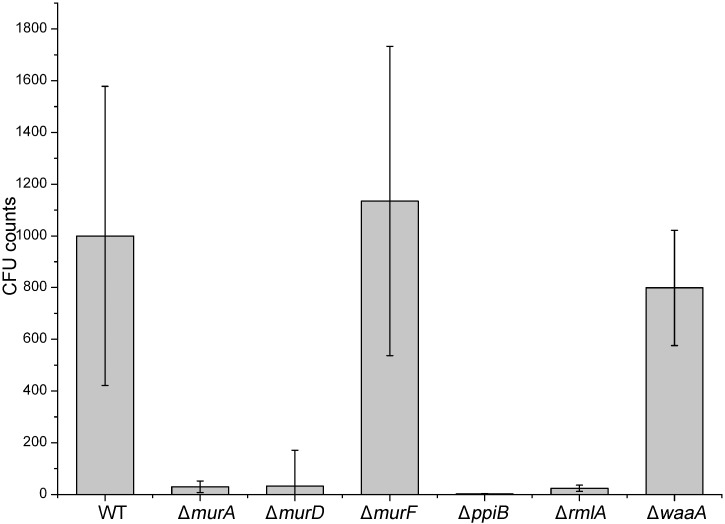
Cell wall mutants are sensitive to macrophage-mediated killing
On the basis of the association of outer leaflet murein, and LPS biosynthesis genes with virulence of most Gram-negative bacteria, we hypothesized that deletion of the these genes would lead to attenuation of intracellular macrophage growth [11, 13–16, 18, 28]. Encouraged by the growth rate decrease of most of the mutants in defined media, we measured the sensitivity of bacterial cells to macrophage-mediated killing. We performed macrophage cell culture invasion assay for both the wild-type and mutants using the murine J774 macrophage cell line in vitro. Macrophage cultures were infected with bacterial suspensions and intracellular bacteria were recovered in order to determine the number of surviving bacterial cells. As shown in Fig 6, all cell wall mutants were much more sensitive to macrophage-mediated killing than wild-type PAO1. These results suggested that cell wall biosynthesis genes are necessary for survival inside macrophages.
Fig 6. Macrophage-mediated bactericidal assay.
Deletion of cell wall biosynthesis genes resulted in a significantly decreased survival of the bacteria. Standard errors of mean of 3 experimental points are shown.
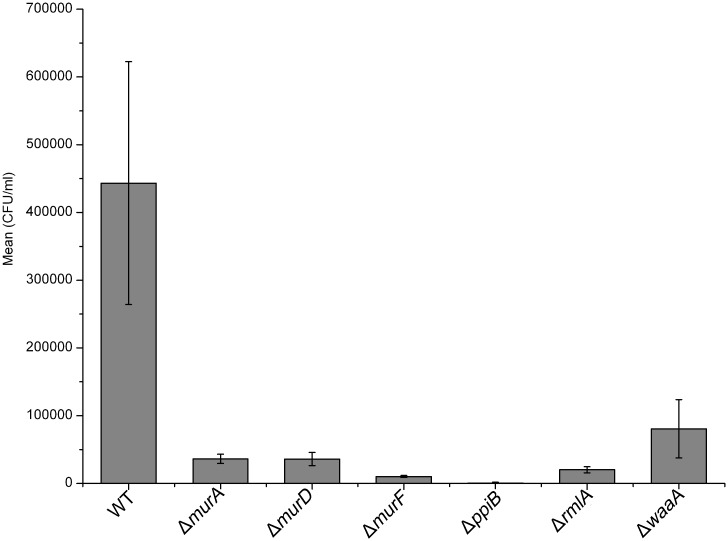
Intratracheal lung infection of NMRI mice
For further validation of outer cell wall biosynthesis genes as potential targets for novel drugs against P. aeruginosa infections, NMRI mice were intratracheally infected with agarose beads loaded with P. aeruginosa PAO1 and mutants as described in the method section. In our preliminary studies, incubation duration of 72 hours was found to yield the most significant results. Mice were separated in different groups of 10 animals each. 72 hours post infection the lungs were dissected and their homogenates were plated in different dilutions for quantification of the bacterial infectivity. In this study, P. aeruginosa PAO1 ΔmurF and ΔwaaA did not show any decrease in total bacterial counts compared to the wild-type (WT) strain (Fig 7). In contrast, ΔmurA, ΔmurD, ΔppiB and ΔrmlA show reduced bacterial burden in mice. These results demonstrated that the murA, murD, ppiB and rmlA genes are required for infectivity and growth of P. aeruginosa in an in vivo model of infection while murF and waaA genes not.
Fig 7. Assessment of the effect of cell wall targets gene deletion in vivo.
Quantification of P. aeruginosa colonies grown in the lung of mice intratracheally infected with agarose beads loaded with the P. aeruginosa mutants in comparison to PAO1 wild-type (WT).
The k.o mutations in cell wall targets compromise P. aeruginosa in a plant virulence model
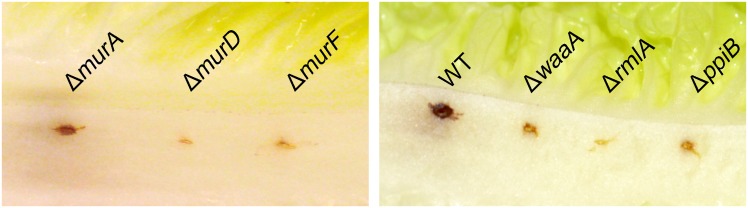
Apparently, the deletion of cell wall biosynthesis genes substantially decreased the in vitro intracellular survival in the murine macrophages and the in vivo survival in NMRI mice model of infection. For further assessment of the missing genes on the virulence of P. aeruginosa, we employed the lettuce leaf model of infection. Earlier studies used the plant as an in vivo model for identification of unknown P. aeruginosa virulence factors (genes) in mammalian pathogenesis [33, 39–41]. All these studies clearly demonstrated that the P. aeruginosa virulence mechanisms and factors are conserved between plant and animal models [40, 41]. Interestingly, the plant model disclosed a significant difference between the wild-type and mutants in infection symptoms. We found that lettuce was not susceptible to infection by ΔmurD, ΔmurF and ΔrmlA (Fig 8). These mutants strain did not cause any infection symptoms to the leaves even after a prolonged incubation period. In contrast, the wild-type, caused necrotic lesions just after three days post-infection. ΔwaaA and ΔppiB elicited delayed weak rotting symptoms on lettuce stems. Surprisingly, ΔmurA proliferated in lettuce leaves and elicited disease symptoms similar to those elicited by wild-type (Fig 8). At day 7 of infection, ΔmurA P. aeruginosa strain invaded the entire midrib of a lettuce leaf resulting in severe maceration (S2 Fig); the infection severity is even more than what was observed for wild-type.
Fig 8. Plant (lettuce) virulence assay with the P. aeruginosa PAO1 and cell wall biosynthesis genes mutants.
The figure represents lettuce midribs after 5 days of infection. Infection by PAO1 wild-type and murA mutant shows necrosis and tissue maceration. Three independent experiments gave similar results.
Discussion
Drug development against P. aeruginosa infections requires detailed information on the putative drug targets which are validated. Construction and characterisation of specific gene deletions of targets in the host strain is an excellent way for such validations. We constructed knockout mutants of six cell wall targets to investigate the essentiality of candidate genes. We employed a verified knockout system using pEX18Ap suicide vector as our successful tool for knockout [29, 30]. We were able to inactivate the cell wall targets at the bacterial chromosomal locus. In support of a role for cell wall biosynthesis and involvement in environmental versatility of P. aeruginosa [11–14, 16, 18–21, 24], the construction of the P. aeruginosa murA, murD, murF, ppiB, rmlA and waaA mutants resulted in dramatic changes in the growth properties in minimal media compared with the wild-type (Figs 3 and 4). Major changes in the size and morphology of the colonies were also observed when cultures were grown on solid media. Previously, cell elongation in P. aeruginosa was reported to be caused by nutrient deprivation and anaerobic respiration [34, 42]. It was postulated that bacteria elongate to increase their nutrient uptake and to respond to nitric oxide, a spontaneous byproduct of the anaerobic respiration. Remarkably, here we observed that ΔmurF cells were highly elongated (Fig 4B) compared to the wild-type and other mutants. Unlike the previous reports, our results suggest that cell elongation could be a consequence of gene function loss and has significant effects on the physiological process, cell division and on formation of septal peptidoglycan [34, 35]. The issues underlying the short cell size exhibited by wild-type and mutants cells and their subsequent adaptation to growth on minimal medium have not been further clarified. In this study we have clearly demonstrated that knockout of cell wall targets strongly remodels the extracellular DNA release in LB media. Significantly, ΔrmlA released very low DNA amount which suggests that rmlA contributes to interbacterial signaling and multi-cellular development processes in Pseudomonas biofilms [4, 36–38].
Furthermore, our results also demonstrated the impaired ability of these mutants to replicate in murine macrophages. In this study, we tested the infectivity of PAO1 mutants in the lung infection model. Using this method we are able also to examine directly whether individual gene knockout in PAO1 has lethality effect in the PAO1 strain in the lung. With two major exceptions, the growth of these k.o mutants was severely diminished in vivo in mice compared to that of wild-type (Fig 7). This indicates that P. aeruginosa PAO1 ΔmurF and ΔwaaA do not constitute a regulator controlling P. aeruginosa growth-associated virulence, but the other targets do so. Interestingly, the P. aeruginosa PAO1 ΔmurF and ΔwaaA mutants were attenuated for intracellular macrophage survival.
Discernibly, several studies have shown that the P. aeruginosa virulence determinants are conserved within animal and plant models [33, 40, 41]. The mutant of murA proliferated in lettuce leaves and severely caused disease symptoms even more than the wild-type especially at late infection phase (S2 Fig). It is interesting to speculate that murA mutation was spontaneously complemented in the plant model. The peptidoglycan building stone, UDP-N-MurAc, forms from UDP-N-acetyl-D-glucosamine (UNAG) and phosphoenolpyruvic acid via murA reactions. murA catalyzes unusual reaction where the transfer of the enolpyruvyl moiety of phosphoenolpyruvic acid to the 3′-hydroxyl group of UNAG mediated through cleavage of C-O bond of PEP and not via addition-elimination process utilizing the high energy P-O bond [43, 44]. The shikimate pathway in plants contains AroA (5-enolpyruvylshikimate-3-phosphate synthase), the only enzyme known to catalyze the identical unusual reaction as murA [44, 45]. This finding indicates that the AroA enzyme from the plant might replace the function of murA. Other possible explanation is the abolition of the synthesis of UDP-N-MurAc, could be replaced by another similar sugar from the plant.
Understanding the details of aftereffect of the functional loss of targeted gene would lay the framework for a focused strategy of drug design. Future work could now be directed towards whole-genome effects of these mutants. Microarray-based comparative techniques could be applied to learn more about the interplay between these mutations and ecological versatility of this pathogen. The current report provides initial insights into the relationship between the targets studied and the cell and colony characteristics of P. aeruginosa. This study also provides definitive evidence that cell wall targets activity contributes to intramacrophage/mice survival and pathogenesis. Thus, these results identify peptidoglycan/LPS assembly in the P. aeruginosa cell wall as essential targets which are expected to significantly increase the probability of finding novel anti-pseudomonas therapeutic drugs.
Supporting information
Knockout of the native genes in the PAO1 strain was verified by PCR analysis. Chromosomal DNA of mutant strains was used as templates in PCR reactions. Upper gel part): using internal specific primers for Gm-gfp cassette, the expected 425 bp. Which confirm the insertion of the Gm-gfp cassette at the chromosomal locus. Lower gel part): For these mutants the absence of pEX18Ap vector backbone was confirmed by using backbone specific primers (538 bp PCR Product). The PCR reactions were analyzed by gel-electrophoresis on 1% agarose gel. The first lane contains molecular size markers (GeneRuler 1 kb DNA Ladder, Thermo Scientific).
(PDF)
The figure represents lettuce midribs after 6 and 7 days of infection. Infection by murA mutant shows severe necrosis/maceration the infection symptoms more than wild-type. Three independent experiments gave similar results.
(PDF)
Acknowledgments
We thank Dr. Max Schobert for the kind donation of plasmids. We would like to thank Stefanie Heß and Dr. Fatima Jonas, LIONEX GmbH for their help with Macrophage-mediated bactericidal assays.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work received funding from the Seventh Framework Program of the European Commission under grant agreement No. 223461(Aeropath). The funder provided support in the form of salaries for authors [AAE, SS, WO, YB, CH, MM and MS], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. AAE, SS, WO and MS are employed by LIONEX Diagnostics and Therapeutics GmbH. YB was employed during Aeropath project by LIONEX Diagnostics and Therapeutics GmbH. HW was supported by a doctoral scholarship from Deutscher Akademischer Austauschdienst (DAAD). CH and MM are employed by mfd Diagnostics GmbH. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4(4):551–60. Epub 1998/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodey GP, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983;5(2):279–313. Epub 1983/03/01. . [DOI] [PubMed] [Google Scholar]
- 3.Amini S, Hottes AK, Smith LE, Tavazoie S. Fitness landscape of antibiotic tolerance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2011;7(10):e1002298 Epub 2011/10/27. doi: 10.1371/journal.ppat.1002298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4(11):e1000213 Epub 2008/11/22. doi: 10.1371/journal.ppat.1000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34(5):634–40. Epub 2002/02/02. doi: 10.1086/338782 . [DOI] [PubMed] [Google Scholar]
- 6.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19(8):419–26. Epub 2011/06/15. doi: 10.1016/j.tim.2011.04.005 . [DOI] [PubMed] [Google Scholar]
- 7.Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436(7054):1171–5. Epub 2005/08/27. doi: 10.1038/nature03912 . [DOI] [PubMed] [Google Scholar]
- 8.Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151(Pt 2):373–83. Epub 2005/02/09. doi: 10.1099/mic.0.27463-0 . [DOI] [PubMed] [Google Scholar]
- 9.Giamarellou H. Prescribing guidelines for severe Pseudomonas infections. J Antimicrob Chemother. 2002;49(2):229–33. Epub 2002/01/30. 11815561. [DOI] [PubMed] [Google Scholar]
- 10.Rogers HJ, Perkins H.R. and Ward J.B.. Biosynthesis of peptidoglycan In: Rogers HJ, editor. Microbial Cell Walls and Membranes. London: Chapman and Hall; 1980. p. 239–97. [Google Scholar]
- 11.van Heijenoort J. Biosynthesis of the bacterial peptidoglycan unit. In: Ghuysen J-MaH R., editor. Bacterial Cell Wall Amsterdam: Elsevier; 1994. p. 39–54. [Google Scholar]
- 12.Christensen BG, Leanza WJ, Beattie TR, Patchett AA, Arison BH, Ormond RE, et al. Phosphonomycin: structure and synthesis. Science. 1969;166(3901):123–5. Epub 1969/10/03. . [DOI] [PubMed] [Google Scholar]
- 13.Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–70. Epub 1990/01/01. doi: 10.1146/annurev.bi.59.070190.001021 . [DOI] [PubMed] [Google Scholar]
- 14.Raetz CR. Bacterial endotoxins: extraordinary lipids that activate eucaryotic signal transduction. J Bacteriol. 1993;175(18):5745–53. Epub 1993/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267(2):54–61. Epub 1992/08/01. . [DOI] [PubMed] [Google Scholar]
- 16.Belunis CJ, Clementz T, Carty SM, Raetz CR. Inhibition of lipopolysaccharide biosynthesis and cell growth following inactivation of the kdtA gene in Escherichia coli. J Biol Chem. 1995;270(46):27646–52. Epub 1995/11/17. . [DOI] [PubMed] [Google Scholar]
- 17.Schnaitman CA, Klena JD. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57(3):655–82. Epub 1993/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson G, Neal B, Liu D, Hobbs M, Packer NH, Batley M, et al. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol. 1994;176(13):4144–56. Epub 1994/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imperi F, Ciccosanti F, Perdomo AB, Tiburzi F, Mancone C, Alonzi T, et al. Analysis of the periplasmic proteome of Pseudomonas aeruginosa, a metabolically versatile opportunistic pathogen. Proteomics. 2009;9(7):1901–15. Epub 2009/04/01. doi: 10.1002/pmic.200800618 . [DOI] [PubMed] [Google Scholar]
- 20.Compton LA, Davis JM, Macdonald JR, Bachinger HP. Structural and functional characterization of Escherichia coli peptidyl-prolyl cis-trans isomerases. Eur J Biochem. 1992;206(3):927–34. Epub 1992/06/15. . [DOI] [PubMed] [Google Scholar]
- 21.Edwards KJ, Ollis DL, Dixon NE. Crystal structure of cytoplasmic Escherichia coli peptidyl-prolyl isomerase: evidence for decreased mobility of loops upon complexation. J Mol Biol. 1997;271(2):258–65. Epub 1997/08/15. doi: 10.1006/jmbi.1997.1151 . [DOI] [PubMed] [Google Scholar]
- 22.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6(1):29–40. Epub 2006/12/13. doi: 10.1038/nrd2201 . [DOI] [PubMed] [Google Scholar]
- 23.Walsh C. Where will new antibiotics come from? Nat Rev Microbiol. 2003;1(1):65–70. Epub 2004/03/26. doi: 10.1038/nrmicro727 . [DOI] [PubMed] [Google Scholar]
- 24.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–64. Epub 2000/09/13. doi: 10.1038/35023079 . [DOI] [PubMed] [Google Scholar]
- 25.Moynie L, Schnell R, McMahon SA, Sandalova T, Boulkerou WA, Schmidberger JW, et al. The AEROPATH project targeting Pseudomonas aeruginosa: crystallographic studies for assessment of potential targets in early-stage drug discovery. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013;69(Pt 1):25–34. Epub 2013/01/09. doi: 10.1107/S1744309112044739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moynie L, Leckie SM, McMahon SA, Duthie FG, Koehnke A, Taylor JW, et al. Structural insights into the mechanism and inhibition of the beta-hydroxydecanoyl-acyl carrier protein dehydratase from Pseudomonas aeruginosa. J Mol Biol. 2013;425(2):365–77. Epub 2012/11/24. doi: 10.1016/j.jmb.2012.11.017 . [DOI] [PubMed] [Google Scholar]
- 27.Krasowski A, Muthas D, Sarkar A, Schmitt S, Brenk R. DrugPred: a structure-based approach to predict protein druggability developed using an extensive nonredundant data set. J Chem Inf Model. 2011;51(11):2829–42. Epub 2011/10/15. doi: 10.1021/ci200266d . [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Quinn PJ. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog Lipid Res. 2010;49(2):97–107. Epub 2009/10/10. doi: 10.1016/j.plipres.2009.06.002 . [DOI] [PubMed] [Google Scholar]
- 29.Thoma S, Schobert M. An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol Lett. 2009;294(2):127–32. Epub 2009/05/12. . [DOI] [PubMed] [Google Scholar]
- 30.Schweizer HP, Hoang TT. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158(1):15–22. Epub 1995/05/26. . [DOI] [PubMed] [Google Scholar]
- 31.Starke JR, Edwards MS, Langston C, Baker CJ. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22(6):698–702. Epub 1987/12/01. doi: 10.1203/00006450-198712000-00017 . [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Boucher JC, Hibler NS, Deretic V. Virulence properties of Pseudomonas aeruginosa lacking the extreme-stress sigma factor AlgU (sigmaE). Infect Immun. 1996;64(7):2774–81. Epub 1996/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahme LG, Tan MW, Le L, Wong SM, Tompkins RG, Calderwood SB, et al. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci U S A. 1997;94(24):13245–50. Epub 1997/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goehring NW, Beckwith J. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr Biol. 2005;15(13):R514–26. Epub 2005/07/12. doi: 10.1016/j.cub.2005.06.038 . [DOI] [PubMed] [Google Scholar]
- 35.Marshall WF, Young KD, Swaffer M, Wood E, Nurse P, Kimura A, et al. What determines cell size? BMC Biol. 2012;10:101 Epub 2012/12/18. doi: 10.1186/1741-7007-10-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, et al. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol. 2006;59(4):1114–28. Epub 2006/01/25. doi: 10.1111/j.1365-2958.2005.05008.x . [DOI] [PubMed] [Google Scholar]
- 37.Nemoto K, Hirota K, Murakami K, Taniguti K, Murata H, Viducic D, et al. Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy. 2003;49(3):121–5. Epub 2003/06/20. 70617. . [DOI] [PubMed] [Google Scholar]
- 38.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487 Epub 2002/02/23. doi: 10.1126/science.295.5559.1487 . [DOI] [PubMed] [Google Scholar]
- 39.Goldova J, Ulrych A, Hercik K, Branny P. A eukaryotic-type signalling system of Pseudomonas aeruginosa contributes to oxidative stress resistance, intracellular survival and virulence. BMC Genomics. 2011;12:437 Epub 2011/09/02. doi: 10.1186/1471-2164-12-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268(5219):1899–902. Epub 1995/06/30. . [DOI] [PubMed] [Google Scholar]
- 41.Rahme LG, Ausubel FM, Cao H, Drenkard E, Goumnerov BC, Lau GW, et al. Plants and animals share functionally common bacterial virulence factors. Proc Natl Acad Sci U S A. 2000;97(16):8815–21. Epub 2000/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberger RE, Allen AR, Hansa HG, Holden PA. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa-unsaturates biofilms. Microb Ecol. 2002;43(4):416–23. Epub 2002/06/04. doi: 10.1007/s00248-001-1063-z . [DOI] [PubMed] [Google Scholar]
- 43.Yoon HJ, Lee SJ, Mikami B, Park HJ, Yoo J, Suh SW. Crystal structure of UDP-N-acetylglucosamine enolpyruvyl transferase from Haemophilus influenzae in complex with UDP-N-acetylglucosamine and fosfomycin. Proteins. 2008;71(2):1032–7. Epub 2008/02/06. doi: 10.1002/prot.21959 . [DOI] [PubMed] [Google Scholar]
- 44.Eschenburg S, Priestman M, Schonbrunn E. Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is essential for product release. J Biol Chem. 2005;280(5):3757–63. Epub 2004/11/09. doi: 10.1074/jbc.M411325200 . [DOI] [PubMed] [Google Scholar]
- 45.Bentley R. The shikimate pathway—a metabolic tree with many branches. Crit Rev Biochem Mol Biol. 1990;25(5):307–84. Epub 1990/01/01. doi: 10.3109/10409239009090615 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Knockout of the native genes in the PAO1 strain was verified by PCR analysis. Chromosomal DNA of mutant strains was used as templates in PCR reactions. Upper gel part): using internal specific primers for Gm-gfp cassette, the expected 425 bp. Which confirm the insertion of the Gm-gfp cassette at the chromosomal locus. Lower gel part): For these mutants the absence of pEX18Ap vector backbone was confirmed by using backbone specific primers (538 bp PCR Product). The PCR reactions were analyzed by gel-electrophoresis on 1% agarose gel. The first lane contains molecular size markers (GeneRuler 1 kb DNA Ladder, Thermo Scientific).
(PDF)
The figure represents lettuce midribs after 6 and 7 days of infection. Infection by murA mutant shows severe necrosis/maceration the infection symptoms more than wild-type. Three independent experiments gave similar results.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.