Abstract
Odour-baited technologies are increasingly considered for effective monitoring of mosquito populations and for the evaluation of vector control interventions. The BG-Malaria trap (BGM), which is an upside-down variant of the widely used BG-Sentinel trap (BGS), has been demonstrated to be effective to sample the Brazilian malaria vector, Anopheles darlingi. We evaluated the BGM as an improved method for sampling the African malaria vectors, Anopheles arabiensis. Experiments were conducted inside a large semi-field cage to compare trapping efficiencies of BGM and BGS traps, both baited with the synthetic attractant, Ifakara blend, supplemented with CO2. We then compared BGMs baited with either of four synthetic mosquito lures, Ifakara blend, Mbita blend, BG-lure or CO2, and an unbaited BGM. Lastly, we compared BGMs baited with the Ifakara blend dispensed via either nylon strips, BG cartridges (attractant-infused microcapsules encased in cylindrical plastic cartridge) or BG sachets (attractant-infused microcapsules encased in plastic sachets). All tests were conducted between 6P.M. and 7A.M., with 200–600 laboratory-reared An. arabiensis released nightly in the test chamber. The median number of An. arabiensis caught by the BGM per night was 83, IQR:(73.5–97.75), demonstrating clear superiority over BGS (median catch = 32.5 (25.25–37.5)). Compared to unbaited controls, BGMs baited with Mbita blend caught most mosquitoes (45 (29.5–70.25)), followed by BGMs baited with CO2 (42.5 (27.5–64)), Ifakara blend (31 (9.25–41.25)) and BG lure (16 (4–22)). BGM caught 51 (29.5–72.25) mosquitoes/night, when the attractants were dispensed using BG-Cartridges, compared to BG-Sachet (29.5 (24.75–40.5)), and nylon strips (27 (19.25–38.25)), in all cases being significantly superior to unbaited controls (p < 000.1). The findings demonstrate potential of the BGM as a sampling tool for African malaria vectors over the standard BGS trap. Its efficacy can be optimized by selecting appropriate odour baits and odour-dispensing systems.
Introduction
Large-scale implementation of the two front-line vector control interventions against African malaria vectors, i.e., long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), have led to major reductions in malaria cases contributing just over three quarters of all gains since 2000 [1]. Despite these gains, there appears to be persistent transmission, a significant proportion of which may be occurring outdoors and is not targeted effectively by LLINs and IRS [2, 3]. Other challenges include the growing physiological insecticide resistance in vector populations [4–6] and behavioural responses of the residual malaria vectors, which may also lower their responsiveness to control [7–9]. These challenges, combined with poor user-compliance and human behaviours, such as spending most of the time outside dwellings in the evenings, heavily compromise the likelihood of malaria elimination in many settings [10–12].
An important and closely related problem is the need for new tools to monitor this persistent transmission, especially in areas where a significant proportion of biting occurs outdoors [7, 13, 14], but also in areas where specific interventions need to be matched to certain vector species behaviours and responsiveness. Odour-baited devices have been proposed as potential complementary tools to sample malaria mosquitoes outdoors [15–21], but also to disrupt transmission [22]. Examples of odour-baited traps or human-baited traps previously used for malaria mosquitoes include, the Suna Trap [23], Odour-Baited Mosquito Entry Trap [20, 24], Ifakara-Tent Trap [20], Ifakara Odour-baited stations [25], the MMX trap [26, 27], the Mosquito Landing Box [18] and BG-Sentinel Trap [28].
The BG-Malaria trap (BGM) [29, 30] is a modified version of the original BG-Sentinel (BGS) trap created by Biogents Company, Germany. The BGM trap was adapted to collect Anopheles species. BGM has already been demonstrated as a sensitive method for monitoring Brazilian malaria vectors [29, 30], but it has not previously been tested for African malaria vectors. The main difference between the two traps is the airflow orientation. The BGM is installed upside down, 40 cm above the ground, making it a simple adaptation of BGS. This adaptation was considerably more effective than other commercial traps tested, including the original BGS and CDC-Light Traps, and performed almost as well as human landing catches (HLC) in Brazilian field tests [29].
In the initial trials, the bait used in BGM trap was CO2 obtained from dry ice [29, 30]. However, to improve trap efficacy, other baits such as synthetic human odours should also be explored. The synthetic odour blend developed at Ifakara Health Institute (i.e. Ifakara Blend) is one candidate lure, already demonstrated in long-range village tests to be more attractive to malaria and non-malaria mosquitoes, than real humans [31]. There are several other synthetic attractant variants, potentially equal or perhaps more effective.
Other than the actual mosquito attractants, studies also have demonstrated that performance of these attractants is influenced by the medium from which they are dispensed [32–34]. For example, nylon strips have been used effectively for the release of attractants for host-seeking malaria mosquitoes [31, 33–38]. Other examples of odour dispensing materials have included low density polyethylene (LDPE) sachets [39–41] and glass vials [31, 33]. These have shown that an appropriate selection of the dispensing medium to liberate the odorants should be considered at least as important as the actual attractants or traps.
The main aim of this study was to evaluate the efficiency of an odour-baited BGM trap for sampling host-seeking laboratory-reared An. arabiensis. We examined the efficacy of the BGM relative to the original BGS trap and compared four different attractant types for baiting the BGM. We also tested three different odour-dispensing methods used in the BGM.
Materials and methods
Study site
The work was conducted at Ifakara Health Institute, in a semi-field system facility [42] located at Ifakara branch, in Kilombero district, southeastern Tanzania. The semi-field system consists of three-chambered large screened-enclosures, measuring 28.8m by 21m, with walls made of UV-resistant shade netting, and a polyethylene roof mounted on a raised concrete platform [42]. We used two chambers of this facility, each measuring 28.8m by 7m.
Mosquitoes
Laboratory-reared An. arabiensis mosquitoes were used. The colony (with mosquitoes originally from Lupiro village, Ulanga district, Tanzania) has been maintained in the laboratory at Ifakara Health Institute since 2009. The mosquitoe larvae are reared under standard insectary conditions (29±1°C, 80±5% RH and 12:12h photoperiod) and fed with Tetramin® fish food. Adult mosquitoes were kept in a separate room, where temperatures were maintained at an average temperature of ~27°C and relative humidity at 70–90%. Female and male adults were housed together in a 30 x 30 x 30 cm mating cage and 10% sucrose solution was placed in the cage as a food source. To propagate the colony, the adult female mosquitoes were fed also on human blood (by way of volunteer arm-feeding from five to ten mins every two days). An. arabiensis females aged three to eight days that had not previously taken any blood meals were used for this study. Before beginning of the experiments each night, mosquitoes were selected early and starved by withdrawing the glucose solution, 6 h in advance, to encourage host-seeking behaviour.
BG-Sentinel trap (BGS) and BG-Malaria trap (BGM)
The BGS (BioGents HmGb, Regensburg, Germany) has a cylindrical shape, 35cm in diameter and 40cm in height. Centered inside the trap is a black collecting tube (12cm in diameter x 30cm in length), with a bag for collecting mosquitoes. An electric fan (12V, 14 cm diameter, powered by a 12 V battery) produces a cycle downward flow of air that exits through a gauze cover on the top of the trap and draws in mosquitoes that approach the collecting tube [28]. The interior of the trap can be fitted with different types of mosquito attractants, from which the odours exit through the gauze cover to lure mosquitoes [28].
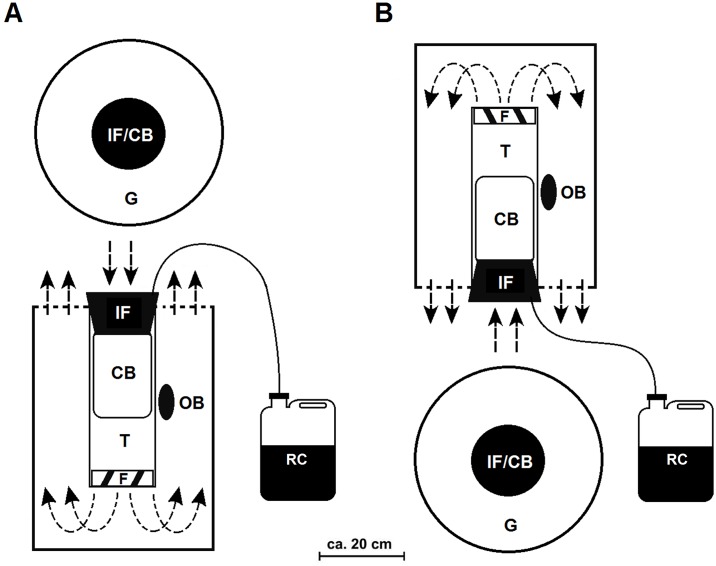
The BGM is an upside down variant of the BGS [29]. The BGM is hanged upside down, 40cm above the ground, with an electrical fan (12V, 14cm diameter, powered by a 12V battery), which produces an upward suction to capture the mosquitoes that approach the trap. The two traps, BGS and BGM, are illustrated in Fig 1.
Fig 1. Illustration of design and functionality of: (A) BG-Sentinel and (B) BG-Malaria.
IF = Intake funnel; CB = Catch Bag; F = Fan; G = Gauze Cover; T = Tube; RC = Recipient of CO2; OB = Odour Bait. Arrows indicate the direction of the airflow. Adapted from Kröckel et al., (2006) and Gama et al., (2013).
Synthetic attractants
Three synthetic mosquito attractants were used: (i) the BG-Lure (BioGents HmGb, Regensburg, Germany), which consists of a mixture of ammonia, L-lactic acid, and caproic acid, in undeclared proprietary concentrations; (ii) the Mbita-5 lure (MB5), which consists of ammonia (2.5%), lactic acid (85%), tetradecanoic acid (0.00025%) and 3-methyl-1-butanol (0.000001%) [38]; (iii) the Ifakara blend (IB), which consists of a mixture of ammonia (2.5%), L-lactic acid (85%), propionic acid (0.1%), butanoic acid (1%), pentanoic acid (0.01%), 3-methylbutanoic acid (0.001%), heptanoic acid (0.01%), octanoic acid (0.01%) and tetradecanoic acid (0.01%) [31]. The IB was used in all the experiments in this work, unlike the BG-Lure and MB5, which were used only in the second experiment.
Attractant dispensing systems
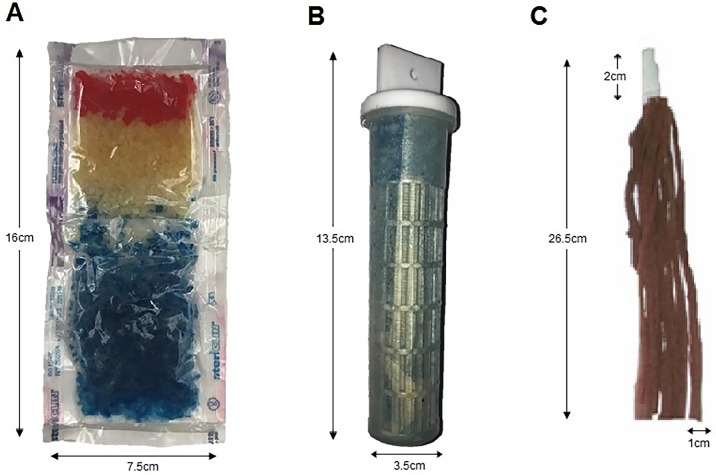
To dispense the synthetic attractants in all the experiments, microcapsules incased in a plastic cartridge (here referred to as BG-Cartridge) supplied by Biogents Company was used. For the experiment where odour-dispensing systems were tested, two others odour-release devices were added. The first was nylon strips, initially tested for dispensing odours attractive to An. gambiae in Tanzania [33]. The strips are small pieces of nylon, each measuring 26.5 x 1.0 cm, and infused with different attractant chemicals that make up the attractant blend. To make up the IB, nine of these strips are batched together (each strip carrying a different blend constituent) and then suspended inside the candidate mosquito trap. The second dispensing system added was the BG-Sachet (also supplied by Biogents Company), which consisted of microcapsules of the IB odorant constituents encased inside a thin plastic sachet. The individual dispensing methods are illustrated in Fig 2.
Fig 2. Devices used for dispensing mosquito attractants.
Panels A and B show attractants infused inside microcapsules supplied by Biogents Company encased in a plastic sachet (BG-Sachet) and plastic cartridge (BG-Cartridge), respectively. Panel C shows a batch of nylon strips, each soaked in solution of a different constituent of the synthetic attractant [33].
Study procedures
The study consisted of three experiments, conducted nightly between 06.00 P.M. and 07.00 A.M.
Experiment 1
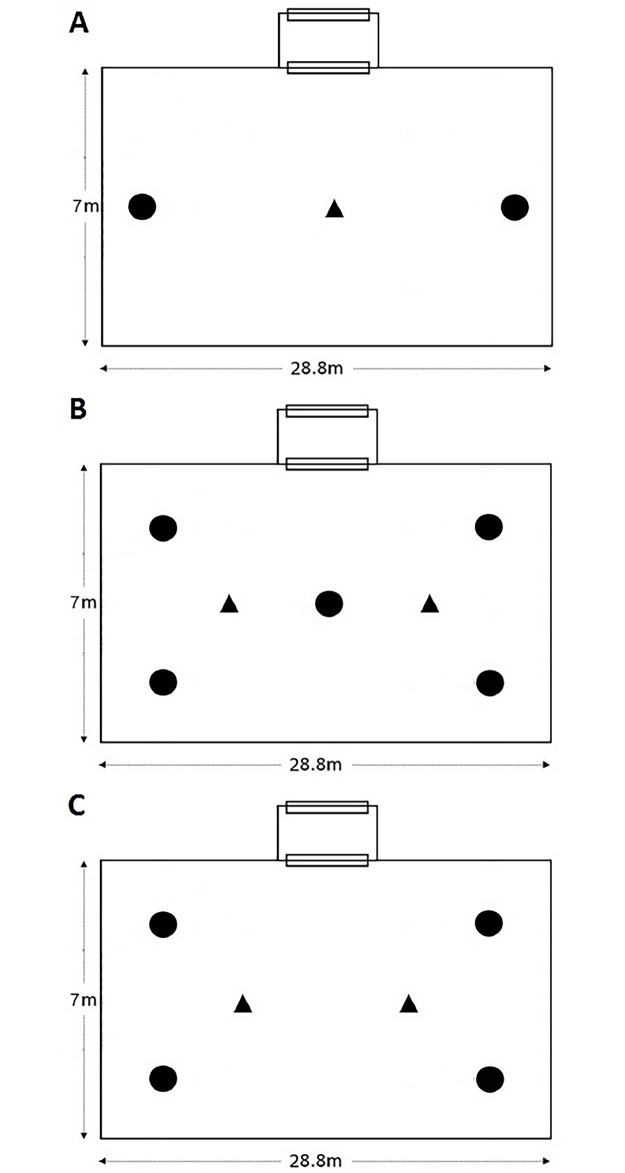
A binary choice experiment (Fig 3A) was conducted to compare BGM against BGS for catching host-seeking An. arabiensis mosquitoes. The traps were baited with the synthetic human odour developed at Ifakara Health Institute [31], supplemented with CO2 gas obtained from yeast-molasses fermentation. Each night, the BGM or BGS were placed at opposite ends of the semi-field testing chamber. This experiment was replicated 10 times, each time releasing 200 adult female An. arabiensis mosquitoes at the center of the test chamber (Fig 3A). Each night the trap locations were interchanged to annul any position-related biases. The traps were both powered by 12V rechargeable batteries. The experiment was left to run overnight. The traps were emptied each morning, and all mosquitoes caught in each trap counted and recorded.
Fig 3. Schematic diagram of the trap positions and mosquito release points within the semi-field system.
Set ups for experiments 1, 2 and 3, are shown in figure panels A, B and C, respectively. Trap positions are shown in circles, and mosquito release points in triangles. In all experiments, the treatment being tested was rotated between the test locations nightly.
Experiment 2
A comparative evaluation was conducted to compare different candidate lures and identify the most effective one for use with the BGM trap for capturing host-seeking An. arabiensis. A 5 x 5 Latin square experiment with four replicates was carried out. Four BGM traps were baited with either of the four candidate lures (Fig 3B), and a fifth BGM was un-baited. In many field and laboratory studies, synthetic mosquito attractants are commonly augmented with CO2, to activate the mosquitoes and synergize attractive effects of the lures. We maintained this practice but also had a separate configuration where CO2 was the only bait, to improve comparison of the lures and separate effects of the CO2. The five different treatments compared in this experiment were (i) Ifakara blend + CO2 (referred to simply as IB); (ii) BG-Lure® + CO2; (iii) Mbita-5 Blend + CO2 (referred to as MB5); (iv) CO2 alone; and (v) a control consisting of non-baited BGM trap. The IB, MB5 and BG-Lure were dispensed using BG-Cartridges supplied by Biogents Company. The CO2 was obtained from a mix of 2L water with 500g molasses plus 35g yeast, delivered from a 5L plastic container, through a 60 cm long plastic tubing measuring 0.5 cm in diameter [43].
For each night’s experiment, 600 host-seeking female An. arabiensis mosquitoes were released in the semi-field chamber. In this experiment, we used two different release points as illustrated in Fig 3B, with 300 mosquitoes released from each point. The locations of the baits were rotated nightly such that after five nights of experimentation, each bait had been to each location at least once. The experiments ran the entire night, and the traps were emptied in the morning, after which number of mosquitoes collected in each trap was recorded for each treatment. The traps were then thoroughly cleaned using a solution of 70% alcohol and dried outdoors before they could be re-used. The experiment was replicated four times over a 20 nights experimental period.
Experiment 3
In the third experiment, we aimed to assess whether the attractiveness of the Ifakara Blend [31] would be affected by the medium from which it is released when used in the BGM supplemented with CO2 from yeast-molasses fermentation. The dispensing devices tested are shown in Fig 2 and were as follows: (i) Nylon strips [33]; (ii) BG-Sachet (iii) BG-Cartridge; and (iv) control (no baited trap). The nylon strips were freshly prepared for this experiment, while both the sachets and cartridges were provided by the manufacturer, Biogents Company.
The dispensing devices were rotated in four positions during four nights in a 4 x 4 Latin square design, such that after a complete set, each dispensing method had been to each of the four selected locations at least once. The locations of the traps are illustrated in Fig 3C. We marked and fixed the individual trap locations, but locations of the lures were rotated nightly in a random fashion. The experiment was replicated 5 times, over a 20 nights experimental period. Each night, a total of 400 host-seeking An. arabiensis mosquitoes were released from two different points in the semi-field test chamber (200 mosquitoes per release point). The number of mosquitoes collected in each trap was recorded each morning.
Data analysis
The analysis was done using R software version 3.3.2 [44]. In all experiments, Generalized Linear Mixed Effects Statistical Models (GLMMs) were used to estimate the number of mosquitoes captured as a function of the different trap types, dispensing devices or lures. To account for the over-dispersion, number of mosquitoes captured (i.e., the mosquito count data) were modeled following a negative binomial distribution with log link function [45]. In the first experiment the main effects were trap type. In the second experiment (testing the effect of different lures on the number of mosquito captured), the main effect was type of lure. In the last experiment (testing the effect of different odour dispensing mechanism), the main effect was type of dispensing devices. To account for the variation in temperatures, winds and any other confounding factors during the study period, the experiment date, replication number and trap locations were treated as random factors for each analysis in respective experiments. Relative risks (RR), and 95% Confidence Intervals (CI) were used to estimate the strength of influence of each main factor. The estimates were considered statistically significant different if p < 0.05. Additionally, pairwise comparison tests were done using Tukey’s honest significance difference post-hoc test (Tukey’s HSD) to assess differences between individual groups.
Ethical considerations
A written and signed informed consent was obtained from the volunteers working in the mosquito-rearing facility, and arm-feeding of mosquitoes in the insectary was done only by volunteering adult males. This study was approved by both Ifakara Health Institute IRB (IHI/IRB/No: 34–2014) and the Medical Research Coordinating Council at the Tanzania National Institute of Medical Research (Certificate No. NIMR/HQ/R.8a/Vol.IX/1903).
Results
Experiment 1: Test to compare BG Malaria and BG Sentinel traps
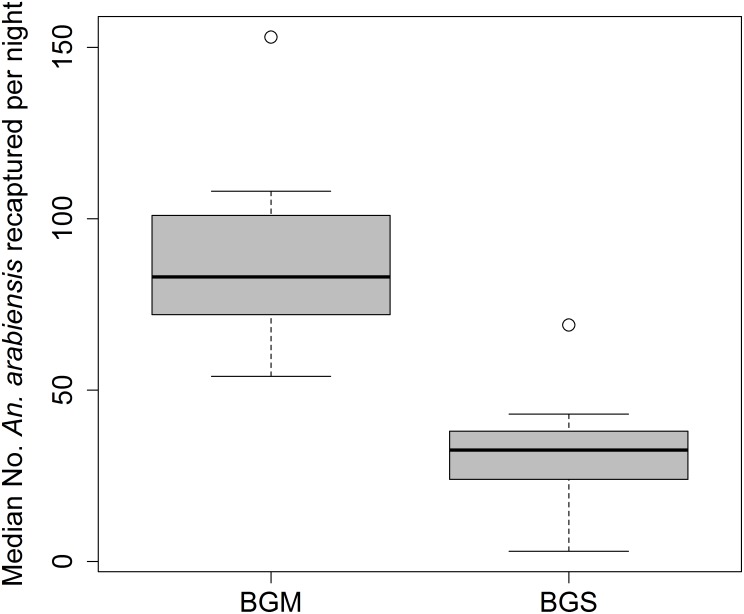
Of the 2000 mosquitoes released in the semi-field system chambers throughout the study period, 60.8% were recaptured by the traps. In this experiment, the BGM trap was tested against the BGS trap in a binary choice assay and both traps baited with IB and CO2. The median nightly catches are shown in Fig 4. The BGM trap was the more effective trap in capturing mosquitoes [RR = 2.76, 95%CI: (1.95–3.89), p < 0.001] as compared to BGS trap. There was no effect of location on the overall mosquito catches between chambers. Location was not found to have any significant impact on the number of mosquito collected per trap (p = 0.773).
Fig 4. Distribution and median number of Anopheles arabiensis recaptured per night using different trapping methods in the semi-field system.
BGM = BG-Malaria trap; BGS = BG-Sentinel trap.
Experiment 2: Test to compare the different mosquito attractants
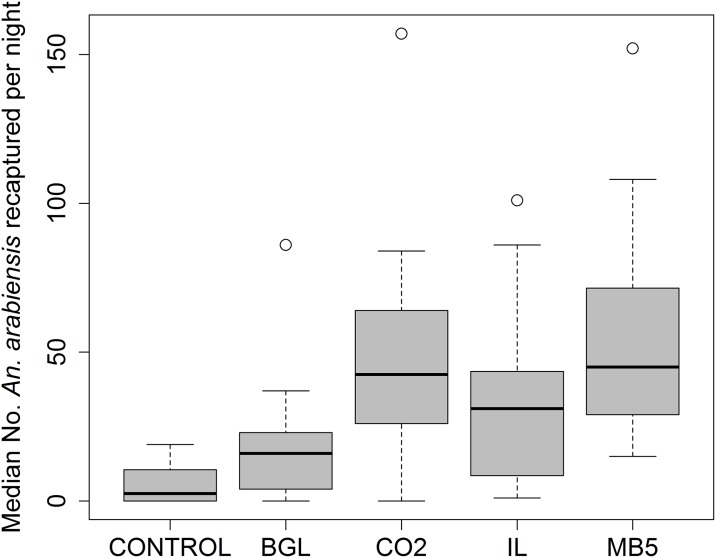
A total of 15000 mosquitoes were used in this experiment, for which 20.8% of the total were re-captured in the traps. The number of mosquitoes caught was influenced significantly by type of attractant used (p < 0.001; DF = 4). While traps baited with MB5, IB and CO2 alone caught statistically similar numbers of mosquitoes, these were all significantly higher than the number caught in traps baited with the BG-Lure (p < 0.001) or the unbaited traps (p < 0.001). The median numbers of mosquitoes caught in traps baited by different lures is shown in Fig 5.
Fig 5. Distribution and median number of Anopheles arabiensis recaptured per night by BGM traps baited with different lures in the semi-field system.
CO2 = Trap baited with only CO2; MB5 = Trap baited with Mbita lure + CO2; IB = Trap baited with Ifakara blend + CO2; BG = Trap baited with BG-Lure + CO2; Control = unbaited trap.
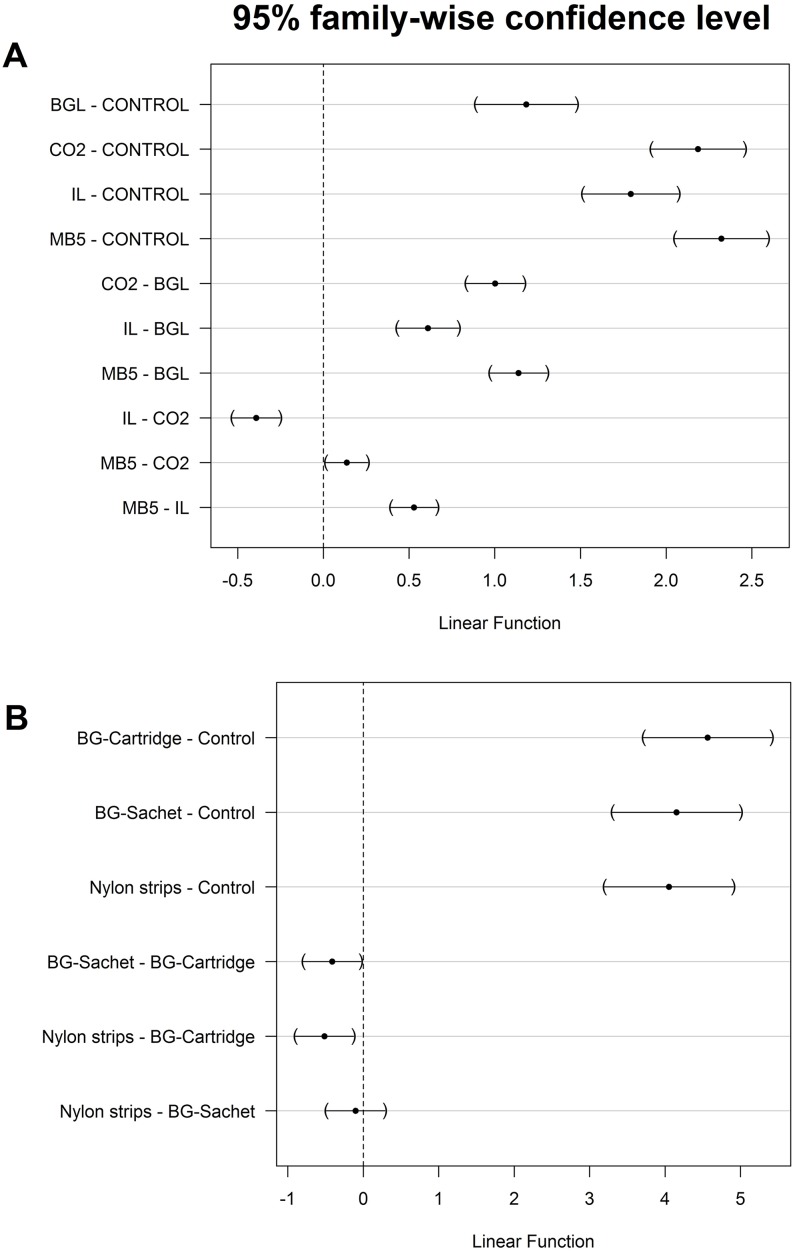
As compared to the un-baited BGM, traps baited with MB5 caught the highest number of mosquitoes [RR = 10.50, 95%CI: (5.80–18.99), p < 0.001], followed by traps baited with CO2 [RR = 8.70, 95%CI: (4.81–15.75), p < 0.001], IB [RR = 6.34, 95%CI: (3.48–11.58), p < 0.001], and BG-Lure [RR = 3.36, 95%CI: (1.84–8.38), p < 0.001]. Pair-wise comparison test using Tukey’s HSD showed that, there is significant difference between CO2 and BG-Lure (z = 0.29, p < 0.05), MB5 and BG-Lure (z = 0.29, p < 0.001) while the rest of the pairs were not significantly different from one another (Fig 6A).
Fig 6. Results of pair-wise post hoc comparison using Tukey’s honestly significance tests (Tukey’s HSD).
Howing similarities and differences between number of mosquitoes caught in traps baited with different lures (Panel A) and number of mosquitoes caught in traps baited with different lures dispensed from different media (Panel B).
Experiment 3: Test to compare different odour-dispensing methods
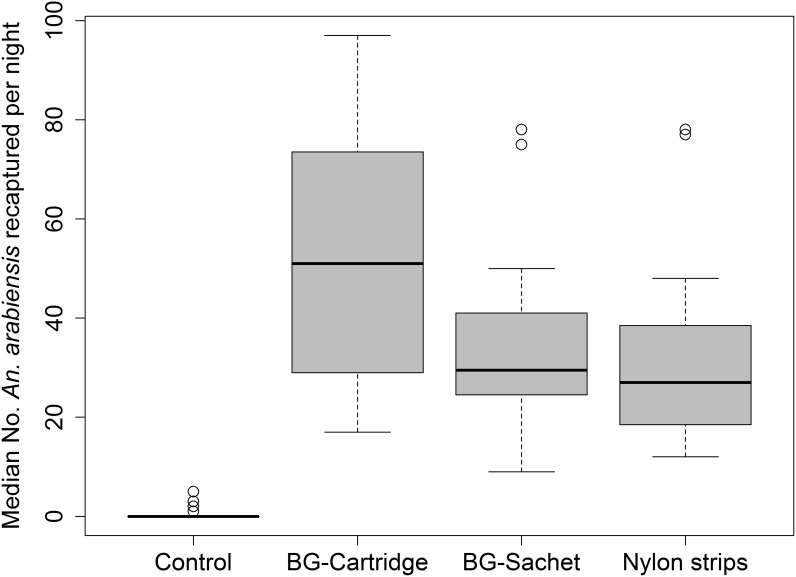
A total of 8000 mosquitoes were used in this experiment, of which 29.9% of the total were re-captured in the traps. The effect of the odour-dispensing mechanism was assessed using BGM trap baited with Ifakara lure dispensed from nylon strips, BG cartridge and BG sachets. Results of this experiment showed significantly higher mosquito catches when using any of the three dispensers, than the control (p < 0.001). The nightly median mosquito catches and the interquartile ranges are shown in Fig 7. The Tukey’s pair-wise comparison showed no significant difference between nylon strips and BG-Sachet (z = 0.15, p = 0.906), but a slight difference between BG-Sachet and BG-Cartridge (z = 0.15, p = 0.027) (Fig 6B). The model results shows that of the lure dispensers tested, BG-Cartridge attracted the most mosquitoes [RR = 96.03 (49.79–185.20), p<0.001)], followed by BG-Sachet [RR = 63.57 (32.92–122.76), p<0.001] and Nylon strips [RR = 57.46 (29.71–111.11), p<0.001] compared to the control.
Fig 7. Distribution and median number of Anopheles arabiensis recaptured per night by BGM traps baited with Ifakara blend released by different odour-release devices in the semi-field system.
BG-Cartridge and BG-Sachet refer to attractants infused inside microcapsules supplied by Biogents Company incased in a cylindrical plastic cartridge and plastic sachet, respectively; Nylon strips refer to batch of nylon strips where each strip is soaked in solution of a different constituent of the synthetic attractant; Control refers to an unbaited trap.
Discussion
The search for effective mosquito sampling tools remains a key priority for vector control practitioners and researchers. While the most appropriate trap may be different from place to place, and from vector species to species, it is important that all traps for large-scale field use are robust, easy to use, low-cost and have minimal need for replacement parts. The chemical nature of the attractants and attractant dispensing systems for use in these traps should also have similar attributes, but also be scalable, safe for human handling and readily available. This study describes the effectiveness of BG-Malaria (BGM) trap, an adaptation of BG-Sentinel (BGS) trap, as a sampling device for host-seeking An. arabiensis mosquitoes under semi-field conditions. Twice as many mosquitoes were caught by the BGM trap than by the BGS trap, which has previously been demonstrated as an effective trap for different mosquito species, including the malaria vector, An. gambiae s.s [46]. The BGS trap was originally designed for sampling Aedes aegypti mosquitoes [28], but because of its ease of use and demonstrated efficacy in a variety of field settings, it has become a good candidate for sampling malaria vectors [16, 46, 47] and is now widely used in research and surveillance settings.
In a field study conducted in Brazil [29], the BGM trap was demonstrated as being highly effective and nearly comparable to human landing catches, the most representative sampling strategy for human-biting mosquitoes [48]. By changing the BGS trap orientation and installing it upside down, 40 cm above the ground, it was observed that collections of the Brazilian malaria vector, An. darlingi were extremely increased [29]. In this current study, our results have demonstrated that similar modifications also work for laboratory-reared African malaria vector, An. arabiensis.
As with the BGS, the spacious interior of the BGM trap allows addition of a wide variety of attractants. Although it was originally proposed to be used with CO2 as bait and the BG-Lure, it has in different occasions been tested when baited with different attractants. In this study, we tested four synthetic attractants and three attractant dispensing methods. We observed that the BGM would work with a variety of attractants. Of all candidate lures tested, the Mbita blend (MB5) tended to be the most attractive, though the differences in attractiveness between lures was not always significant. To evaluate synthetic blends, CO2 was used as an effective mosquito attractant that also synergises other lures and activates mosquitoes [49, 50]. CO2 is routinely added to enhance mosquito responses in laboratory and field studies with odour blends [15, 16, 31, 47, 51]. The same procedure was adopted in this study and a trap baited with CO2 only was tested alongside an un-baited trap control to enable elucidation of any synergistic effects of CO2 with other lures.
Our findings reveal that traps baited with the synthetic blend, MB5, caught the highest number of mosquitoes, compared to the BG-Lure. However, the other attractants also performed well but there were no differences in the caches in traps baited with MB5, IB and CO2. Post hoc analyses suggest minor differences between the lure types, but a clear differences between any of the lures and the controls. Since we found only a minor synergy between the CO2 and the MB5 and IB attractants. This finding suggests that, for purposes of sampling the east African malaria vectors, BGM can be used with a variety of mosquito attractants and that even CO2 alone, in this case derived from yeast-molasses fermentation, may by itself be appropriate. Therefore, where the intention is purely surveillance, rather than vector control, BGM offers significant improvements over BG sentinel traps and can be fitted with any of the multiple synthetic odour lures available commercially or readily in local communities, such as CO2 derived from yeast-molasses fermentation.
The formulation of the MB5 contains ammonia, lactic acid, tetradecanoic acid, 3-methyl-1-butanol and butan-1-amine, and according to semi-field and field studies done in Kenya, it was shown to attract nearly as many mosquitoes as human subjects [38]. In addition, the MB5 was more attractive than IB, consistent with the findings of the present study. Even though the MB5 and IB share some candidate odorant constituents, the concentration of these chemical constituents may affect the responses of mosquitoes. A recent study demonstrated that attraction of mosquitoes to MB5 was concentration-dependent and that addition of 1-dodecanol to MB5 increased catches of female An. gambiae s.l. [36]. What is particularly important in this new study is that the MB5, being a far simpler blend of attractive constituents that IB, also tended to be the more attractive version. It is possible therefore, as concluded also by Okumu [31], that new odour mixtures can be developed with far superior attractiveness than any of the current blends.
Since their original tests, nylon strips are increasingly used as a matrix for dispensing attractant compounds [31, 33–36, 38]. In a comparison between LDPE and nylon strips, Mweresa et al. showed that releasing the IB from nylon strips caused a significantly higher attraction of An. gambiae [37]. Okumu et al., [33] also reported this effect. However, the current study indicated that BG-Cartridge attracted consistently higher proportions of laboratory An. arabiensis. Although, the BG-Cartridge presented the best results in An. arabiensis attraction in our study, these findings were obtained in tests done for short periods, unlike the nylon strips that have been tested previously for long-term dispensation [34, 37]. A previous semi-field study done in western Kenya showed that nylon strips treated with IB remained attractive to host-seeking mosquitoes for up to 40 consecutive nights post-treatment [34]. These findings were extended up to one year in a western Kenya study [37], but apparently this last result was likely affected by bacteria that establish on strips over time producing additional compounds that attract host-seeking mosquitoes. Nonetheless, further studies about alternative and low-cost odour release devices considering the difference in release ratios of semio-chemicals are needed. Thus, the possible matrix should also be tested for their constant release and long-lasting residual activity on target mosquitoes.
One limitation of this study was that we did not compare the BGM to several other trap types, especially the human landing catches, currently considered the gold standard. However, we selected to compare the BGM against an already established outdoor mosquito sampling tool such as the BGS and did not consider it essential to compare it to HLC. Such studies could be done in the future. Our study instead was considered an initial assessment of the potential of BGM for sampling Afro-tropical malaria mosquitoes.
Conclusion
Earlier studies had already demonstrated that the BGM trap was highly effective for trapping the Brazilian malaria vector, An. darlingi [29, 30]. Here, we conclude that this trap is also effective for capturing the African malaria vector, An. arabiensis. To ascertain whether BGM could be an effective representative field sampling tool for malaria mosquitoes, and possibly a substitute the human landing catch, additional tests in field settings in malaria endemic communities are recommended. Overall, we conclude that BGM has potential as an improved sampling tool for malaria vectors in Africa, and that its efficacy can be optimized by selecting appropriate odour baits and appropriate odour-dispensing systems.
Acknowledgments
We thank Mr. Joseph Mgando, who helped to take care of the laboratory-reared mosquitoes during the entire study period. We thank all our Ifakara Health Institute colleagues for their support during the work. EPAB was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Grant 88881.133584/2016-01) and AEE funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico of the Ministério da Ciência, Tecnologia e Inovação (CNPq/MCTI) (Grant 310205/2014-0) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Grant PPM-00502-15). FOO was funded by a Wellcome Trust Intermediate Research Fellowship (Grant WT102350/Z/13/Z) and a Visiting Researcher Fellowship from CNPq/MCTI, (Grant 42070/2013-2).
Data Availability
All relevant data are within the paper.
Funding Statement
EPAB was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Grant 88881.133584/2016-01) and AEE funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico of the Ministério da Ciência, Tecnologia e Inovação (CNPq/MCTI) (Grant 310205/2014-0) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Grant PPM-00502-15). FOO was funded by a Visiting Researcher Fellowship from CNPq/MCTI, (Grant 42070/2013-2) and a Wellcome Trust Intermediate Research Fellowship (Grant WT102350/Z/13/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11. doi: 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malaria Journal. 2011;10(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. Journal of Infectious Diseases. 2012;206(10):1622–9. doi: 10.1093/infdis/jis565 [DOI] [PubMed] [Google Scholar]
- 4.Chandre F, Darriet F, Manguin S, Brengues C, Carnevale P, Guillet P. Pyrethroid cross resistance spectrum among populations of Anopheles gambiae ss from Cote d'Ivoire. Journal of the American Mosquito Control Association. 1999;15(1):53–9. [PubMed] [Google Scholar]
- 5.Chandre F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, et al. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bulletin of the World Health Organization. 1999;77(3):230–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends in Parasitology. 2011;27(2):91–8. doi: 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malaria Journal. 2011;10(80):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HCJ, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67(4):1218–30. doi: 10.1111/evo.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govella NJ, Chaki PP, Killeen GF. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malaria Journal. 2013;12(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissbühler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, et al. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malaria Journal. 2007;6(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn CE, Le Mare A, Makungu C. Malaria risk behaviours, socio-cultural practices and rural livelihoods in southern Tanzania: implications for bednet usage. Social Science & Medicine. 2011;72(3):408–17. [DOI] [PubMed] [Google Scholar]
- 12.Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M, et al. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malaria Journal. 2015;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasites & vectors. 2013;6(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durnez L, Coosemans M. Residual transmission of malaria: An old issue for new approaches Anopheles mosquitoes—new insights into malaria vectors. 2013:671–704. [Google Scholar]
- 15.Mmbando AS, Okumu FO, Mgando JP, Sumaye RD, Matowo NS, Madumla E, et al. Effects of a new outdoor mosquito control device, the mosquito landing box, on densities and survival of the malaria vector, Anopheles arabiensis, inside controlled semi-field settings. Malaria Journal. 2015;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoel DF, Marika JA, Dunford JC, Irish SR, Geier M, Obermayr U, et al. Optimizing Collection of Anopheles gambiae ss (Diptera: Culicidae) in Biogents Sentinel Traps. Journal of Medical Entomology. 2014;51(6):1268–75. doi: 10.1603/ME14065 [DOI] [PubMed] [Google Scholar]
- 17.Matowo NS, Koekemoer LL, Moore SJ, Mmbando AS, Mapua SA, Coetzee M, et al. Combining Synthetic Human Odours and Low-Cost Electrocuting Grids to Attract and Kill Outdoor-Biting Mosquitoes: Field and Semi-Field Evaluation of an Improved Mosquito Landing Box. PloS one. 2016;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matowo NS, Moore J, Mapua S, Madumla EP, Moshi IR, Kaindoa EW, et al. Using a new odour-baited device to explore options for luring and killing outdoor-biting malaria vectors: A report on design and field evaluation of the Mosquito Landing Box. Parasites & Vectors. 2013;6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oketch F, Kotas M, Kihonda J, Killeen G, Moore S. Comparative evaluation of methods used for sampling malaria vectors in the Kilombero Valley, South Eastern Tanzania. The Open Tropical Medicine Journal. 2008;1:51–5. [Google Scholar]
- 20.Dia I, Diallo D, Duchemin J-b, Ba Y, Konate L, Costantini C, et al. Comparisons of human-landing catches and odour-baited entry traps for sampling malaria vectors in Senegal. Journal of Medical Entomology. 2005;42(2):104–9. [DOI] [PubMed] [Google Scholar]
- 21.Jawara M, Smallegange RC, Jeffries D, Nwakanma DC, Awolola TS, Knols BG, et al. Optimizing odour-baited trap methods for collecting mosquitoes during the malaria season in The Gambia. PLoS One. 2009;4(12):e8167 doi: 10.1371/journal.pone.0008167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumu FO, Govella NJ, Moore SJ, Chitnis N, Killeen GF. Potential benefits, limitations and target product-profiles of odor-baited mosquito traps for malaria control in Africa. PLoS One. 2010;5(7):e11573 doi: 10.1371/journal.pone.0011573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiscox A, Otieno B, Kibet A, Mweresa CK, Omusula P, Geier M, et al. Development and optimization of the Suna trap as a tool for mosquito monitoring and control. Malaria Journal. 2014;13(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costantini C, Gibson G, Brady J, Merzagora L, Coluzzi M. A new odour-baited trap to collect host-seeking mosquitoes. Parassitologia. 1993;35(1–3):5 [PubMed] [Google Scholar]
- 25.Okumu FO, Madumla EP, John AN, Lwetoijera DW, Sumaye RD. Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations-The Ifakara Odor-Baited Stations. Parasit Vectors. 2010;3:12 doi: 10.1186/1756-3305-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline DL. Comparison of two American biophysics mosquito traps: The professional and a new counterflow geometry trap. Journal of the American Mosquito Control Association-Mosquito News. 1999;15(3):276–82. [PubMed] [Google Scholar]
- 27.Kline DL. Evaluation of various models of propane-powered mosquito traps. Journal of Vector Ecology. 2002;27:1–7. [PubMed] [Google Scholar]
- 28.Kroeckel U, Rose A, Eiras ÁE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. Journal of the American Mosquito Control Association. 2006;22(2):229–38. doi: 10.2987/8756-971X(2006)22[229:NTFSOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 29.Gama RA, Silva IMd, Geier M, Eiras ÁE. Development of the BG-Malaria trap as an alternative to human-landing catches for the capture of Anopheles darlingi. Memórias do Instituto Oswaldo Cruz. 2013;108(6):763–71. doi: 10.1590/0074-0276108062013013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues MS, Silva IM, Leal LB, Dos Santos CA Jr, Eiras ÁE. Development of a New Mosquito Retention System for the BG-Malaria Trap To Reduce The Damage To Mosquitoes. Journal of the American Mosquito Control Association. 2014;30(3):184–90. doi: 10.2987/14-6423R.1 [DOI] [PubMed] [Google Scholar]
- 31.Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, Mbeyela E, et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PloS One. 2010;5(1):e8951 doi: 10.1371/journal.pone.0008951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torr S, Hall D, Phelps R, Vale G. Methods for dispensing odour attractants for tsetse flies (Diptera: Glossinidae). Bulletin of Entomological Research. 1997;87(03):299–311. [Google Scholar]
- 33.Okumu F, Biswaro L, Mbeleyela E, Killeen G, Mukabana R, Moore S. Using nylon strips to dispense mosquito attractants for sampling the malaria vector Anopheles gambiae ss. Journal of Medical Entomology. 2010;47(2):274–82. [DOI] [PubMed] [Google Scholar]
- 34.Mukabana WR, Mweresa CK, Omusula P, Orindi BO, Smallegange RC, van Loon JJ, et al. Evaluation of low density polyethylene and nylon for delivery of synthetic mosquito attractants. ParasitES & Vectors. 2012;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mweresa CK, Mukabana WR, Omusula P, Otieno B, Gheysens T, Takken W, et al. Evaluation of textile substrates for dispensing synthetic attractants for malaria mosquitoes. Parasite Vectors. 2014;7:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mweresa CK, Mukabana WR, Omusula P, Otieno B, Van Loon JJA, Takken W. Enhancing attraction of African malaria vectors to a synthetic odor blend. Journal of Chemical Ecology. 2016;42(6):508–16. doi: 10.1007/s10886-016-0711-1 [DOI] [PubMed] [Google Scholar]
- 37.Mweresa CK, Otieno B, Omusula P, Weldegergis BT, Verhulst NO, Dicke M, et al. Understanding the long-lasting attraction of malaria mosquitoes to odor baits. PloS one. 2015;10(3):e0121533 doi: 10.1371/journal.pone.0121533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, van Loon JJA, et al. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. Journal of Chemical Ecology. 2012;38(3):235–44. doi: 10.1007/s10886-012-0088-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smallegange RC, Takken W. Host-seeking behaviour of mosquitoes: responses to olfactory stimuli in the laboratory. Olfaction in vector-host interactions. 2010;2:143–80. [Google Scholar]
- 40.Jawara M, Awolola TS, Pinder M, Jeffries D, Smallegange RC, Takken W, et al. Field testing of different chemical combinations as odour baits for trapping wild mosquitoes in The Gambia. PLoS One. 2011;6(5):e19676 doi: 10.1371/journal.pone.0019676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhulst NO, Mbadi PA, Kiss GB, Mukabana WR, van Loon JJ, Takken W, et al. Improvement of a synthetic lure for Anopheles gambiae using compounds produced by human skin microbiota. Malaria Journal. 2011;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferguson HM, Ng'habi KR, Walder T, Kadungula D, Moore SJ, Lyimo I, et al. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malaria Journal. 2008;7(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mweresa CK, Omusula P, Otieno B, Van Loon JJ, Takken W, Mukabana WR. Molasses as a source of carbon dioxide for attracting the malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malaria Journal. 2014;13:160 doi: 10.1186/1475-2875-13-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2015, URL http://wwwR-projectorg. 2016. [Google Scholar]
- 45.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823. 2014. [Google Scholar]
- 46.Schmied WH, Takken W, Killeen GF, Knols BG, Smallegange RC. Evaluation of two counterflow traps for testing behaviour-mediating compounds for the malaria vector Anopheles gambiae ss under semi-field conditions in Tanzania. Malaria Journal. 2008;7(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busula AO, Takken W, Loy DE, Hahn BH, Mukabana WR, Verhulst NO. Mosquito host preferences affect their response to synthetic and natural odour blends. Malaria Journal. 2015;14(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver JB. Mosquito ecology: Field sampling methods: Springer Science & Business Media; 2007. [Google Scholar]
- 49.Gillies M. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): A review. Bulletin of Entomological Research. 1980;70(04):525–32. [Google Scholar]
- 50.Dekker T, Geier M, Cardé RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. Journal of Experimental Biology. 2005;208(15):2963–72. [DOI] [PubMed] [Google Scholar]
- 51.Menger D, Van Loon J, Takken W. Assessing the efficacy of candidate mosquito repellents against the background of an attractive source that mimics a human host. Medical and Veterinary Entomology. 2014;28(4):407–13. doi: 10.1111/mve.12061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.