Abstract
Purpose
Pediatric Hodgkin lymphoma (HL) is a highly curable malignancy. Outcomes for pediatric HL may vary between developed and developing countries for multiple reasons. This study was conducted to ascertain the outcomes of children with HL at our center and to identify risk factors for recurrent disease.
Methods
We analyzed the outcomes of 172 consecutive, previously untreated patients with pediatric HL presenting at our center from 2001 to 2010. Patients were treated with either adriamycin, bleomycin, vinblastine, and dacarbazine or adriamycin, bleomycin, vinblastine, cyclophosphamide, vincristine, prednisone, and procarbazine chemotherapy initially, and radiation to bulky sites or a single site of residual disease when appropriate.
Results
The median duration of follow-up was 77 months. The median age of the patients was 10 years; 127 (74%) of the 172 patients were male. The extent of disease was stage I and II in 59% of the patients. B symptoms were present in 32% of the patients, and 27% had bulky disease. The most common histologic subtype was mixed cellularity (45%). The 5-year overall survival (OS) and progression-free survival (PFS) of the entire cohort were 92.9% and 83.1%, respectively. The 5-year OS rates for patients with stage I, II, III, and IV were 96%, 94.7%, 84%, and 69.8%, respectively. On univariate analysis, advanced stage, response on interim radiologic assessment, and presence of B symptoms significantly predicted inferior PFS and OS. On multivariate analysis, only interim radiologic response significantly predicted PFS (P < .001) and OS (P < .001).
Conclusion
Overall, the outcomes of patients treated at our center are comparable to those observed in other centers in India and globally.
INTRODUCTION
The evolution of treatment over the past 4 decades has altered the outcome of pediatric Hodgkin lymphoma (HL) such that the majority of patients are cured.1 The changes in the management of pediatric HL include better assessment of the extent of disease through improvements in imaging and better understanding of the long-term complications of treatment. Currently, the goal is to minimize exposure and choose appropriate chemotherapy regimens so that long-term effects are minimal. Although developing countries, like India, have kept pace with changes in management of HL globally, there are significant variables that might affect outcome. The biologic and demographic differences that have been noticed in pediatric HL between the West and India include a higher male preponderance, poor nutritional status, younger age at presentation, and increased incidence of a mixed cellularity pathologic subtype in India.2-10 Although variations of mechlorethamine, vincrinstine, procarbazine, and prednisone were used in India initially, adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), which is used to treat adult HL, is used more commonly now.2,4 This is in contrast to Europe and North America where the use of the ABVD regimen is less common because of the increase in long-term cardiac issues and the potential for lung toxicity.11 We, like other centers in India, use ABVD or its hybrid variations because it can be administered in the outpatient clinic, it is economical, and it does not require frequent monitoring of blood counts, all of which are important in a resource-challenged setting.12 This study was conducted to ascertain the outcomes of children with HL treated with ABVD or adriamycin, bleomycin, vinblastine, cyclophosphamide, vincristine, prednisone, and procarbazine (ABV/COPP) chemotherapy at our center and to identify risk factors for recurrence of disease.
METHODS
We analyzed the data of all consecutive, previously untreated pediatric patients with HL, younger than 18 years of age, who were treated at our center from January 1, 2001, to December 31, 2010. This study was approved by the institutional ethical committee. Patients who were diagnosed but refused treatment were not included in the analysis because these patients were not indexed in the hospital registry. All the data were obtained from the patients’ hospital case records for analysis. Durations of symptoms were recorded from the time of onset to the time of presentation to the hospital. Diagnosis of HL was confirmed by histopathologic examination of lymph node biopsy and immunohistochemistry. The WHO system was used for pathologic classification.13 The Cotswold modification of Ann Arbor staging was used to stage the patients.14 The patients underwent contrast-enhanced computed tomography scan of the neck, chest, abdomen, and pelvis for staging and assessment for response. All patients underwent unilateral iliac crest bone marrow aspiration and bone marrow biopsy for diagnosis of bone marrow involvement. Bulky disease was defined as size of lymph nodal mass > 6 cm or mediastinal mass size more than one third of maximal thoracic diameter on chest x-ray.15 The WHO weight-for-age chart was used for nutritional assessment. Patients with a weight for age less than the third centile were considered malnourished.16
The chemotherapy regimens used to treat the patients during the study period were ABVD or ABV/COPP.3,17 In treatment of patients with ABVD or ABV/COPP, the number of cycles of chemotherapy, the addition of involved field radiotherapy (IFRT), and the IFRT dose was individualized on the basis of the decision of the hospital multidisciplinary tumor board. Patients with early-stage disease (stages I and II) and advanced-stage disease (stages III and IV) were scheduled for a minimum of four and six cycles of chemotherapy, respectively. Response was assessed clinically after each cycle and radiologically after completion of four cycles of chemotherapy. A few patients had radiologic assessment of response after completing six cycles. It was the practice during the period of study in our center to give two additional cycles of chemotherapy after documentation of radiologic complete response (CR) for a maximum of eight cycles. In patients who initially had bulky disease, IFRT was given, with a total dose of between 20 and 30 Gy, and in patients with residual disease, IFRT was administered to the site at a dose that was between 30 and 36 Gy. IFRT was administered in a daily fraction of 1.8 to 2 Gy and was given 5 days of the week. Toxicities caused by chemotherapy were captured according to the case records. Patients usually underwent follow-up clinical examinations at 3-month intervals for the first 3 years, then at 6-month intervals for 5 years, and then yearly. Investigations on follow-up were performed only if there were clinical signs or symptoms.
CR was defined as complete disappearance of all clinical and radiologic evidence of disease. Partial response (PR) was a reduction of > 50% of the tumor area (the product of the two greatest diameters), but less than a CR. Appearance of a new lesion or a > 25% increase in an existing lesion was considered progressive disease (PD). All other responses were considered as stable disease (SD).18
Progression-free survival (PFS) was calculated from the initiation of treatment to the date of recurrence or documented progression. Overall survival (OS) was calculated from the date of initiation of treatment to the date of death or date of last follow-up. All patients were censored at the date of last follow-up, or the date of telephonic contact if lost to follow-up, or March 6, 2015, whichever was earliest. PFS and OS were estimated using the Kaplan-Meier method, and variables were compared using the log-rank test. P values < .05 were considered significant. Cox regression analysis was used for multivariate analysis of variables significant on univariate analysis, and results are reported as hazard ratio (HR) with 95% CI. Statistical analysis was performed using SPSS software (IBM SPSS Statistics Version 17; SPSS, Chicago, IL).
RESULTS
Overall
One hundred seventy-two patients with HL were treated at our institute during the 10-year period, and 92 of 172 patients (53%) and 116 of 172 patients (67%) attended the clinic within 1 and 2 years of closure of study, respectively. The median age of the patients was 10 years (range, 2 to 18 years), and 127 of 172 patients (74%) were male (Table 1). Thirty-two patients (19%) had empirically received treatment for tuberculous lymphadenitis before presentation at our hospital and diagnosis of HL. However, none of the 32 patients were found to have active tuberculosis at presentation with HL. Bone marrow involvement was detected in six of 172 patients (3%). Weight-for-age data were available for 166 of 172 patients, and 80 of 166 patients (48%) were malnourished at presentation. The most common histologic subtype was mixed cellularity (45%), followed by nodular sclerosis (35%). All patients received chemotherapy at presentation. ABVD was administered to 120 of 172 patients (70%) and ABV/COPP to 52 of 172 patients (30%). IFRT was given to 32 of 172 patients (19%). Radiologic assessment at four to six cycles of planned treatment showed that 148 of 172 (86%) were in CR, 13 of 172 (8%) were in PR, two of 172 (1%) had SD, and two of 172 (1%) had PD. Both the patients with SD had stage 3 disease, whereas the two patients with PD had stage 2 and stage 4 disease, respectively. Response was not assessed in seven patients because of treatment abandonment (n = 5), death (n = 1), and no available record (n = 1). Eleven patients discontinued treatment (6.3%) and eight of them later presented to the hospital with recurrence.
Table 1.
Baseline Characteristics and Treatment Details
Early-Stage Disease
At presentation, 102 of 172 patients (60%) had stage I and II disease. A median of six cycles of chemotherapy was administered (range, three to eight cycles). Six cycles of chemotherapy were given to 71 of 102 patients (70%), whereas 17 of 102 (17%) received fewer (three to five), and 14 of 102 (14%) received eight. Among the 17 patients who received fewer than six cycles of chemotherapy, one did not attend hospital after three cycles (and later relapsed), six received four cycles with IFRT, nine received four cycles without IFRT (one patient later relapsed), and one received five cycles without IFRT. IFRT was given to 25 of 102 patients with early-stage disease; the indication for IFRT was bulky disease (n = 16; two patients in PR) or PR (n = 1), and it was also administered as part of consolidation after four cycles of chemotherapy (n = 4). In addition, four patients received IFRT despite receiving six cycles of chemotherapy and being in CR. IFRT was not given to 11 of 27 patients (40%) with bulky disease and six of nine patients (66%) in PR.
Advanced-Stage Disease
At presentation, 70 of 172 patients (40%) had stage III and IV disease. A median of eight cycles of chemotherapy were administered (range, two to eight cycles). Of the 11 patients who received fewer than six cycles of chemotherapy, 10 did not attend for treatment regularly, and the remaining patient died as a result of bleomycin toxicity. Among the 10 patients who did not receive proper treatment, seven had a recurrence or progression of disease and one died in an accident; only two are alive and in CR, and these two patients had received four and five cycles of chemotherapy, respectively. Six and eight cycles of chemotherapy were administered to 21 of 70 patients (30%) and 37 of 70 patients (53%), respectively. IFRT was given to seven of 102 patients (7%). The indication for IFRT was bulky disease (n = 5; two with PR). In addition, two patients received IFRT despite having received eight cycles of chemotherapy and being in CR. IFRT was not given to 14 of 19 patients (74%) with bulky disease and four of six patients (66%) with PR or SD.
Survival
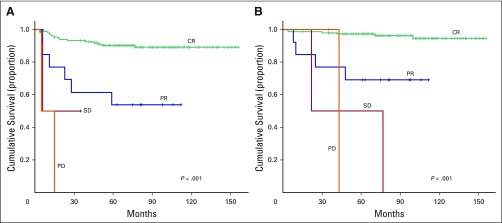
The median duration of follow-up of all patients was 77 months. The actuarial 5-year OS and PFS rates of the entire cohort were 92.9% and 83.1%, respectively. The 5-year PFS rates for stages I, II, III, and IV were 91.7%, 86.6%, 78.3%, and 57.1%, respectively (P = .004) (Fig 1A). The 5-year OS rates for stage I, II, III, and IV were 96%, 94.7%, 84%, and 69.8%, respectively (P = .01; Fig 1B). On univariate analysis, advanced stage, presence of B symptoms, and interim radiologic response significantly predicted inferior PFS and OS (Table 2; Figs 2 and 3). On multivariate analysis, only interim radiologic response significantly predicted PFS (HR, 1.74; 95% CI, 1.35 to 2.24; P < .001) and OS (HR, 2.02; 95% CI, 1.46 to 2.8; P < .001). Sex, serum albumin, elevated lactate dehydrogenase > 400 IU, erythrocyte sedimentation rate > 40 mm/h, hemoglobin < 10.5 g/dL, histology, bulky disease, nutritional status, addition of radiotherapy, delay in presentation of > 1 month, and splenomegaly were not significant factors. The omission of radiotherapy in early-stage patients who had received fewer than six cycles of chemotherapy, in patients in PR in interim radiologic assessment, and in patients with bulky disease was not significant.
Fig 1.
(A) Progression-free survival and (B) overall survival according to stage.
Table 2.
Univariate Analysis for PFS and OS
Fig 2.
(A) Progression-free survival and (B) overall survival according to B symptoms.
Fig 3.
(A) Progression-free survival and (B) overall survival according to interim radiologic response. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Relapse and Mortality
Overall, recurrence of disease was observed in 27 of 172 patients (16%) and two of 172 (1.7%) had primary progression. There were 17 documented deaths, among which 14 were a result of relapse or progression, two were a result of bleomycin toxicity, and one was a result of an accident. Among the 14 deaths caused by relapse of disease or progression, 10 occurred because patients refused further treatment.
Second-Line Treatment
The median duration to relapse of disease from presentation was 15.17 months (range, 1.97 to 79.03 months). Among the 29 patients who had a recurrence or were refractory, 41% had early-stage and 59% had advanced-stage disease at initial diagnosis. Chemotherapy at recurrence of disease was given to 17 of 29 patients (59%; Table 3), and radiotherapy alone was given to two of 29 (7%). On completion of second-line chemotherapy, the CR, PR, and PD rates of response were 11 of 17 (65%), three of 17 (17%), and two of 17 (12%), respectively, and the remaining patients (6%) failed to attend to evaluate response. All 11 patients who achieved CR are alive and well on follow-up, and three of six patients with less than CR have died. Regarding the two patients who received IFRT alone, one is alive and well and the other has died as a result of progression of disease. Ten patients who did not receive further treatment at relapse of disease because of nonattendance at the hospital were found on telephonic/postal inquiry to have died. Consolidation with high-dose chemotherapy supported by peripheral blood stem cell rescue was performed in four of 29 patients (14%) with recurrent disease after second-line treatment and achievement of CR, all of whom remained in CR at last follow-up.
Table 3.
Second-Line Chemotherapy
Toxicity
The most common acute toxicity was febrile neutropenia (grade 4), which was observed in 11 patients. Two patients had bleomycin lung toxicity while on treatment, which was fatal, and both deaths occurred during the fourth cycle of ABVD. We documented three long-term toxicities in our study: infertility in one patient and cardiomyopathy in two patients. There were no cases of second malignancy.
DISCUSSION
Our study provides insight into the long-term outcomes of patients with pediatric HL treated at a tertiary cancer center. We previously published data from our center on outcomes in 134 pediatric patients with HL treated with ABV/COPP chemotherapy during the period 1989 to 1998.3 The 5-year PFS and OS in our previous study were 86.7% and 92.5%, respectively. Unlike this study, the staging was performed using ultrasound and chest x-ray, none of the patients received ABVD, there were more advanced-stage patients (64%), and only 5% received IFRT. The recommendation for diagnosing bone marrow involvement in HL is to perform bilateral iliac crest bone marrow aspiration and biopsy. At our center, unilateral bone marrow testing is preferred because it is associated with less pain when performed under local anesthesia. Bone marrow involvement in HL is rare, and it probably does not have a major impact on the treatment and outcome of patients.19 Mixed cellularity was the most common histologic subtype (45%) in this cohort and this has not changed substantially compared with the previous study and as observed in other studies from India.3-10 Nodular sclerosis is a more common histologic subtype in developed countries.20 The increased mixed cellularity pathology in pediatric HL from developing countries has been attributed to a higher prevalence of Epstein-Barr virus infection.21,22 Children younger than 5 years of age constituted 15% of the current cohort of patients, which is similar to the proportion at other Indian centers.5 This is in contrast to Western data, which show that less than 5% of patients are under 5 years of age.20,23-25 Two thirds of our patients were male, and a similar trend has been reported from other centers in India.4,5,7-10 This phenomenon could be attributed partly to a bias against seeking treatment for female children in Indian society; however, accounting for the trends in other pediatric cancers, such a major difference cannot be explained.26 Malnutrition is a significant problem in developing countries, and it has been associated with poor outcomes in pediatric malignancies, but it was not a significant prognostic factor in this study.27
The treatment offered to our patients was heterogenous over the duration of the report because of the evolution of treatment and imaging. It is therefore difficult to draw any conclusions regarding the preference for a chemotherapy regimen, schedule, and addition of IFRT. However, our study indicates that, with the use of appropriate chemotherapy regimens, children with HL from resource-challenged settings can have similar outcomes to those of the Western population.28 At centers where access to radiation is not available, it can be replaced by six cycles of ABVD or ABV/COPP. Our results show that patients with advanced-stage disease who receive fewer than six cycles of ABVD or ABV/COPP have poor outcomes. The most common reason for patients receiving less than optimal chemotherapy is noncompliance, rather than toxicity. Approximately 10% of early-stage patients who were in CR after six cycles received a further two cycles of chemotherapy, which, in hindsight, is excessive and is not recommended. The reason for this was that these patients were documented to be in CR only after six cycles and it was our policy to give a further two cycles of chemotherapy after CR. Because of improvements in imaging over the duration of this study, we are not treating patients beyond six cycles. Only 19% of our patients received radiation. This was tempered by the fact that it was necessary to avoid radiation unless absolutely necessary because of its impact on growth. The omission of IFRT did not affect outcomes in patients with bulky disease, with PR on interim scans, or with early-stage disease who had received fewer than six cycles of chemotherapy (Table 2). However, this result is not based on randomized data, because the majority of patients had received six cycles of chemotherapy, which could have negated any beneficial effect of IFRT. Many centers from the developing world have reported excellent outcomes when radiotherapy has been omitted from the treatment protocol.5,7,8 A treatment abandonment rate of 6.2% was seen in our study, and this was similar to that which has been reported in other regional cancer centers in India.5 We did not find any significant differences between the ABV/COPP regimen and ABVD. However, these regimens were not compared prospectively in a randomized trial manner.
We did not investigate for subclinical organ dysfunction in our cohort of patients. Our patients received higher doses of anthracyclines (because of the six to eight cycles of treatment) compared with contemporary protocols, and only further long-term follow-up will clearly show the effects of treatment. Treatment-related toxicities could not be captured comprehensively because of the retrospective nature of the study.
It is challenging to offer second-line treatment in developing countries because of financial constraints and the reluctance of parents to accept treatment for their child after failure of the initial therapy. Our results suggest that sustained complete remission can be achieved in 70% of patients if treated, and not all may require consolidation with high-dose chemotherapy. Patients who discontinue treatment with either ABVD or ABV/COPP and have progression of disease subsequently can be treated again effectively with either of these two regimens (Table 3).
This report has its limitations because it is retrospective. However, it is encouraging to note that OS is excellent in this cohort and that it reflects the real-world scenario in a developing country.
Our report illustrates that outcomes of pediatric patients with HL are excellent in developing countries despite the challenges in delivering optimal care. The results of second-line chemotherapy in patients with recurrence have been encouraging. In the future, a risk-adapted approach for treating pediatric HL in developing countries will be necessary, thereby reducing the long-term toxicities of the treatment without compromising cure.
ACKNOWLEDGMENT
We thank all the patients who took part in this study. We also acknowledge the numerous residents, nurses, and faculty who managed these patients over the duration of this study.
AUTHOR CONTRIBUTIONS
Conception and design: Venkatraman Radhakrishnan, Manikanadan Dhanushkodi, Trivadi S. Ganesan, Prasanth Ganesan, Tenali Gnana Sagar
Collection and assembly of data: Venkatraman Radhakrishnan, Manikanadan Dhanushkodi, Shirley Sundersingh, Ganesarajah Selvaluxmy, Rajaraman Swaminathan, Ranganathan Rama
Data analysis and interpretation: Venkatraman Radhakrishnan, Manikanadan Dhanushkodi, Trivadi S. Ganesan, Prasanth Ganesan, Shirley Sundersingh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Venkatraman Radhakrishnan
No relationship to disclose
Manikanadan Dhanushkodi
No relationship to disclose
Trivadi S. Ganesan
No relationship to disclose
Prasanth Ganesan
No relationship to disclose
Shirley Sundersingh
No relationship to disclose
Ganesarajah Selvaluxmy
No relationship to disclose
Rajaraman Swaminathan
No relationship to disclose
Ranganathan Rama
No relationship to disclose
Tenali Gnana Sagar
No relationship to disclose
REFERENCES
- 1. Pizzo PA, Poplack DG: Hodgkin lymphoma, in Pizzo PA and Poplack DG (eds): Principles and Practice of Pediatric Oncology (ed 6). Philadelphia, PA, WultersKlower/Lippincott, Williams & Wilkins, 2011, pp 639-662. [Google Scholar]
- 2.Shanta V, Sastri DV, Sagar TG, et al. A review of Hodgkin’s disease at the Cancer Institute, Madras. Clin Oncol. 1982;8:5–15. [PubMed] [Google Scholar]
- 3.Sagar TG, Chandra A, Raman SG. Childhood Hodgkin disease treated with COPP/ABV hybrid chemotherapy: A progress report. Med Pediatr Oncol. 2003;40:66–69. doi: 10.1002/mpo.10017. [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Kapoor G, Bajpai R. ABVD-based therapy for Hodgkin lymphoma in children and adolescents: Lessons learnt in a tertiary care oncology center in a developing country. Pediatr Blood Cancer. 2016;63:1024–1030. doi: 10.1002/pbc.25935. [DOI] [PubMed] [Google Scholar]
- 5.Trehan A, Singla S, Marwaha RK, et al. Hodgkin lymphoma in children: Experience in a tertiary care centre in India. J Pediatr Hematol Oncol. 2013;35:174–179. doi: 10.1097/MPH.0b013e318271f587. [DOI] [PubMed] [Google Scholar]
- 6.Dinand V, Arya LS. Epidemiology of childhood Hodgkins disease: Is it different in developing countries? Indian Pediatr. 2006;43:141–147. [PubMed] [Google Scholar]
- 7.Arya LS, Dinand V, Thavaraj V, et al. Hodgkin’s disease in Indian children: Outcome with chemotherapy alone. Pediatr Blood Cancer. 2006;46:26–34. doi: 10.1002/pbc.20157. [DOI] [PubMed] [Google Scholar]
- 8.Chandra J, Naithani R, Singh V, et al. Developing anticancer chemotherapy services in a developing country: Hodgkin lymphoma experience. Pediatr Blood Cancer. 2008;51:485–488. doi: 10.1002/pbc.21609. [DOI] [PubMed] [Google Scholar]
- 9.Laskar S, Gupta T, Vimal S, et al. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: Is there a need? J Clin Oncol. 2004;22:62–68. doi: 10.1200/JCO.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor G, Advani SH, Dinshaw KA, et al. Treatment results of Hodgkin’s disease in Indian children. Pediatr Hematol Oncol. 1995;12:559–569. doi: 10.3109/08880019509030770. [DOI] [PubMed] [Google Scholar]
- 11.Hodgson DC. Long-term toxicity of chemotherapy and radiotherapy in lymphoma survivors: optimizing treatment for individual patients. Clin Adv Hematol Oncol. 2015;13:103–112. [PubMed] [Google Scholar]
- 12.Mauz-Körholz C, Metzger ML, Kelly KM, et al. Pediatric Hodgkin lymphoma. J Clin Oncol. 2015;33:2975–2985. doi: 10.1200/JCO.2014.59.4853. [DOI] [PubMed] [Google Scholar]
- 13.Pileri SA, Ascani S, Leoncini L, et al. Hodgkin’s lymphoma: The pathologist’s viewpoint. J Clin Pathol. 2002;55:162–176. doi: 10.1136/jcp.55.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 15.Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 16. WHO Multicentre Growth Reference Study Group: Who Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for- Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland, WHO, 2006. http://www.who.int/childgrowth/standards/en/
- 17.Bonadonna G, Santoro A. ABVD chemotherapy in the treatment of Hodgkin’s disease. Cancer Treat Rev. 1982;9:21–35. doi: 10.1016/s0305-7372(82)80003-0. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 19.Hines-Thomas MR, Howard SC, Hudson MM, et al. Utility of bone marrow biopsy at diagnosis in pediatric Hodgkin’s lymphoma. Haematologica. 2010;95:1691–1696. doi: 10.3324/haematol.2010.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiller CA, Parkin DM. Geographic and ethnic variations in the incidence of childhood cancer. Br Med Bull. 1996;52:682–703. doi: 10.1093/oxfordjournals.bmb.a011577. [DOI] [PubMed] [Google Scholar]
- 21.Dinand V, Dawar R, Arya LS, et al. Hodgkin’s lymphoma in Indian children: Prevalence and significance of Epstein-Barr virus detection in Hodgkin’s and Reed-Sternberg cells. Eur J Cancer. 2007;43:161–168. doi: 10.1016/j.ejca.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Glaser SL, Lin RJ, Stewart SL, et al. Epstein-Barr virus-associated Hodgkin’s disease: Epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Hsu SC, Metzger ML, Hudson MM, et al. Comparison of treatment outcomes of childhood Hodgkin lymphoma in two US centers and a center in Recife, Brazil. Pediatr Blood Cancer. 2007;49:139–144. doi: 10.1002/pbc.20883. [DOI] [PubMed] [Google Scholar]
- 24.Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved-field radiotherapy in pediatric Hodgkin’s disease: The Stanford experience. J Clin Oncol. 1994;12:2160–2166. doi: 10.1200/JCO.1994.12.10.2160. [DOI] [PubMed] [Google Scholar]
- 25.Schellong G, Pötter R, Brämswig J, et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin’s disease: The German-Austrian multicenter trial DAL-HD-90. J Clin Oncol. 1999;17:3736–3744. doi: 10.1200/JCO.1999.17.12.3736. [DOI] [PubMed] [Google Scholar]
- 26.Swaminathan R, Rama R, Shanta V. Childhood cancers in Chennai, India, 1990-2001: Incidence and survival. Int J Cancer. 2008;122:2607–2611. doi: 10.1002/ijc.23428. [DOI] [PubMed] [Google Scholar]
- 27.Radhakrishnan V, Ganesan P, Rajendranath R, et al. Nutritional profile of pediatric cancer patients at Cancer Institute, Chennai. Indian J Cancer. 2015;52:207–209. doi: 10.4103/0019-509X.175841. [DOI] [PubMed] [Google Scholar]
- 28.Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: Final results of the multinational trial GPOH-HD95. J Clin Oncol. 2013;31:1562–1568. doi: 10.1200/JCO.2012.45.3266. [DOI] [PubMed] [Google Scholar]