Abstract
Purpose
Cervical cancer is a major cause of mortality in low- and middle-income countries (LMICs) and the most common cancer diagnosed in women in Botswana. Most women present with locally advanced disease, requiring chemotherapy and radiation. Care co-ordination requires input from a multidisciplinary team (MDT) to deliver appropriate, timely treatment. However, there are limited published examples of MDT implementation in LMICs.
Methods
In May 2015, a weekly MDT clinic for gynecologic cancer care was initiated at Botswana’s national referral facility. The MDT clinic served as a forum for discussion and coordination of patients with gynecologic cancer and consisted of a gynecologist, pathologist, medical oncologist, radiation oncologist, palliative care specialist, and nurse coordinator.
Results
Between May 2015 and December 2015, 135 patients were seen in the MDT clinic. The mean age of the patients was 49 years. Most (60%) of the patients were HIV positive. The most common diagnosis was cervical cancer (60%), followed by high-grade cervical intraepithelial neoplastic lesions (12%) and vulvar cancer (11%). Only data up to September 2015 were assessed for treatment delays. It was found that only 38% of patients needed more than one visit for care coordination before treatment initiation. Among patients with cervical cancer, the median delay from date of biopsy to start of radiation treatment was 39 days (interquartile range, 34 to 57 days) for patients treated after MDT initiation, compared with 108 days (interquartile range, 71 to 147 days) for patients treated before MDT initiation (P < .001).
Conclusion
Implementation of MDT clinics in LMICs is feasible and can help reduce delays in treatment initiation, as demonstrated by a gynecologic MDT clinic in Botswana. Streamlining care through MDT clinics can enhance care coordination and improve clinical outcomes. This model can apply to cancer care in other LMICs.
INTRODUCTION
Cervical cancer is the fourth most common cancer affecting women worldwide, with an estimated 528,000 new cases and 266,000 deaths annually.1 Approximately 85% of these new cases and 87% of deaths occur in low- and middle-income countries (LMICs).1 Because of limited screening programs and high HIV prevalence, cervical cancer is the leading cause of cancer death in Botswana.2
More than 75% of patients with cervical cancer in Botswana have locally advanced disease.3 In Botswana, current treatment includes radiotherapy and cisplatin-based chemotherapy. Radiotherapy, however, is not available in the public sector; hence, patients are referred to the private sector for radiation. On the basis of our pilot data, the median time from diagnosis to treatment is 108 days.3 To decrease cervical cancer morbidity and mortality, it is imperative to identify and address factors contributing to delays.
Cancer management requires action from a range of expert providers over a prolonged time.4 Difficulties in communication and coordination among these various providers can cause cancer care to become fragmented, ultimately contributing to treatment delays and worse outcomes,5,6 which are a potential cause of delay in cervical cancer care in Botswana. Studies in developed countries in which surgeons, pathologists, oncologists, radiologists, social workers or psychologists, and nurses are involved in discussing each case7 suggest that multidisciplinary teams (MDTs) can decrease time to diagnosis, time to treatment, and duplication of investigations, as well as improve accuracy of diagnosis.8 Thus, the establishment of an MDT is warranted.
Actionable knowledge about scaling MDTs into LMICs remains sparse. Results from an international survey showed that breast cancer MDT clinics across the world used different models and lacked standard guidelines.9 In several developing countries, cancer centers do not have formal MDTs.10 Another survey in Arab countries revealed that only 49% of respondents reported having MDTs.11 This could be because establishing MDT clinics in LMICs is difficult because of competing health-care demands, limited health-care personnel, and poor infrastructure.12,13 Although there are established oncology MDT clinics in LMICs in Africa, such as in Uganda and Egypt, there have been no published studies on the direct benefits of these MDT clinics.14,15
The purpose of this paper is to describe the implementation and early outcomes of a gynecologic MDT clinic in Botswana, which, to our knowledge, is the first gynecologic MDT clinic in an LMIC. We also describe the impact of the MDT clinic on reducing treatment delay.
METHODS
Organization Before Weekly Meeting
Through a collaborative effort across providers, we established a weekly gynecologic MDT clinic at Botswana’s national referral facility in Gaborone. A nurse coordinator was assigned to manage patient flow. All new referrals with basic information, including stage, date of biopsy, results of biopsy specimen assessment, and HIV status, were sent to the nurse coordinator, who then procures patient records and ensures that biopsy results are available. All patients to be discussed were contacted by the coordinator the day before the clinic meeting.
Workflow During Weekly Meeting
At the beginning of each clinic meeting, a team composed of a radiation oncologist, clinical oncologist, gynecologist, pathologist, nurse coordinator, and palliative care specialist discuss all patients referred to the MDT clinic. Together, an oncologist and gynecologist examine patients and determine an appropriate care plan. If the patient requires radiation, paperwork to document sponsorship of treatment by the government of Botswana is completed and submitted to the referral office. Investigations for staging or treatment planning are ordered if they have not been done previously. If patients are HIV positive or untested, they are referred to an HIV clinic for antiretroviral treatment evaluation or retesting for HIV. Patients are then counseled on the treatment plan by an oncologist, gynecologist, and palliative care doctor, and given a follow-up date in 2 weeks to make sure all the investigations are completed and all submitted documents are processed.
We evaluated the patient characteristics and radiation treatment delays in patients seen in this clinic. The Wilcoxon rank-sum test was used to compare the delay in treatment before and after MDT clinic initiation. Only data up to September 2015 were assessed for treatment delays, because the radiation department in Botswana closed in September 2015 for an upgrade and patients were referred to South Africa.
RESULTS
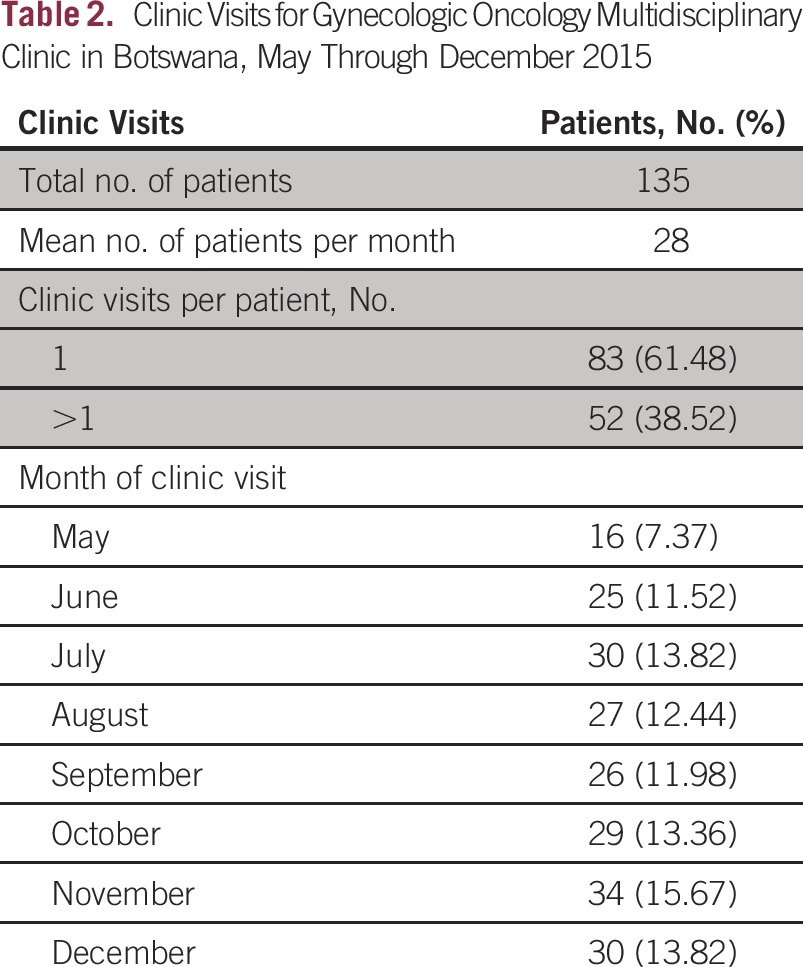
A gynecologic MDT clinic was initiated at Princess Marina Hospital in May 2015. Between May and December 2015, 135 patients were seen in this clinic, with an average of 28 patients per month. Most patients (n = 82; 60%) were HIV positive, 31% (n = 42) were HIV negative, and the HIV status of 11 patients was unknown. The most common diagnosis of patients who came to the MDT clinic was cervical cancer (60%), followed by high-grade preinvasion, or cervical intraepithelial neoplasia 3, lesions (12%) and vulvar cancer (11%). Forty-two percent of patients (n = 57) had locally advanced cancer and needed chemoradiation. Further details of patients seen in the clinic are listed in Table 1. Sixty-two percent of patients (n = 83) needed only one visit for care coordination. Treatment delays were evaluated for radiation treatment as well as surgery. The median delay from date of biopsy to start of radiation treatment was 39 days (interquartile range [IQR], 34 to 57 days) after MDT initiation, compared with 108 days (IQR, 71 to 147 days; P < .001) before MDT initiation. The median delay from clinic visit to the start of radiation treatment was 24 days (IQR, 19 to 31 days) and median delay from clinic visit to surgery was 31 days (IQR, 13 to 43 days) after MDT initiation (Table 2).
Table 1.
Patient, Clinical, and Treatment Characteristics of Patients Seen in a Multidisciplinary Gynecologic Oncology Clinic in Botswana, May 2015 Through December 2015
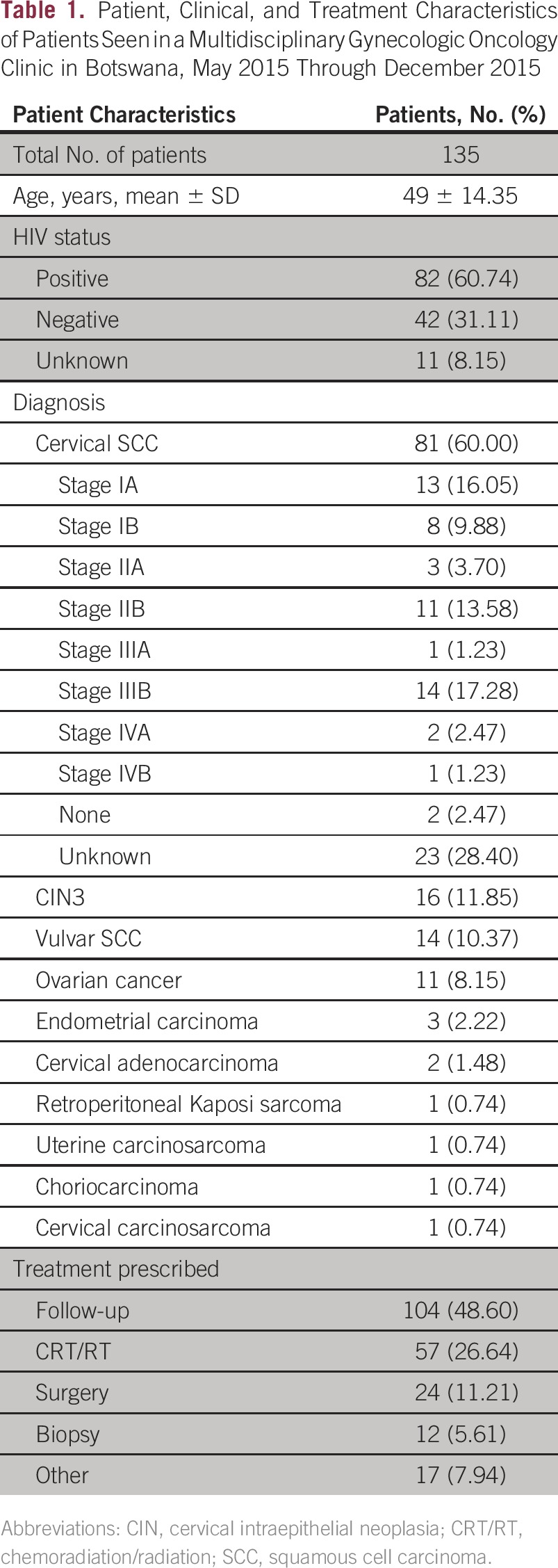
Table 2.
Clinic Visits for Gynecologic Oncology Multidisciplinary Clinic in Botswana, May Through December 2015
DISCUSSION
The complexity of cancer care makes it vulnerable to delay and suboptimal outcomes. By enhancing communication and streamlining care, MDTs are important in reducing treatment delays and improving patient outcomes. The results from the establishment of a gynecologic MDT clinic in Botswana are promising. The time from diagnosis to the start of radiation treatment was reduced by greater than 50% following initiation of the MDT clinic. Furthermore, only 38.5% of patients needed more than one clinic visit, suggesting that even one visit to the MDT clinic was sufficient to facilitate patient navigation through the system.
Similar MDT models are also being piloted for head and neck, palliative care, and breast cancer in Botswana. A follow-up clinic is being piloted where all patients treated with gynecologic cancer are seen after treatment or followed up until signs of toxicities or recurrence appear. All the patients seen in the MDT clinic will be linked to the follow-up clinic and will receive regular reminders for a visit or a telephone call for follow-up.
In summary, establishment of an MDT clinic in LMIC settings is feasible. It can help with care coordination and reducing delays, and can be used more broadly as a model of cancer care for all cancers in Botswana and other LMICs.
ACKNOWLEDGMENT
We thank Nametsegang Babutsi Barati Monare Tebogo Othusitse, Shekinah Elmore, Patricia Eifel, Ann Klopp, Kathleen Schmeler, Memory Nsingo, Lilie Lin, Lawrence Shulman, and Yehoda M. Martei for their contributions to this study.
Footnotes
Supported by the Center for AIDS Research (Grant No. 5-P30-AI-045008-17) and a Conquer Cancer Foundation Young Investigator Award.
Presented at Union for International Cancer Control World Cancer Congress, Paris, France, October 31 to November 3, 2016.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Surbhi Grover
No relationship to disclose
Sebathu Philip Chiyapo
No relationship to disclose
Priya Puri
No relationship to disclose
Mohan Narasimhamurthy
No relationship to disclose
Babe Eunice Gaolebale
No relationship to disclose
Neo Tapela
No relationship to disclose
Doreen Ramogola-Masire
No relationship to disclose
Mukendi K.A. Kayembe
No relationship to disclose
Thabo Moloi
No relationship to disclose
Ponatshego Andrew Gaolebale
No relationship to disclose
REFERENCES
- 1. International Agency for Research on Cancer: Cervical cancer. Estimated incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
- 2.Grover S, Raesima M, Bvochora-Nsingo M, et al. Cervical cancer in Botswana: Current state and future steps for screening and treatment programs. Front Oncol. 2015;5:239. doi: 10.3389/fonc.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grover S. Prospective Cohort of Cervical Cancer Patients in Botswana Treated with Definitive (Chemo) Radiation. Melbourne, Victoria, Australia: IGCS; 2014. [Google Scholar]
- 4.Hong NJ, Wright FC, Gagliardi AR, et al. Examining the potential relationship between multidisciplinary cancer care and patient survival: An international literature review. J Surg Oncol. 2010;102:125–134. doi: 10.1002/jso.21589. [DOI] [PubMed] [Google Scholar]
- 5.Borras JM, Albreht T, Audisio R, et al. Policy statement on multidisciplinary cancer care. Eur J Cancer. 2014;50:475–480. doi: 10.1016/j.ejca.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Fleissig A, Jenkins V, Catt S, et al. Multidisciplinary teams in cancer care: Are they effective in the UK? Lancet Oncol. 2006;7:935–943. doi: 10.1016/S1470-2045(06)70940-8. [DOI] [PubMed] [Google Scholar]
- 7.Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: Retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath LE, Yordan E, Malhotra D, et al. Multidisciplinary care in the oncology setting: Historical perspective and data from lung and gynecology multidisciplinary clinics. J Oncol Pract. 2010;6:e21–e26. doi: 10.1200/JOP.2010.000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini KS, Taylor C, Ramirez AJ, et al. Role of the multidisciplinary team in breast cancer management: Results from a large international survey involving 39 countries. Ann Oncol. 2012;23:853–859. doi: 10.1093/annonc/mdr352. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113:2221–2243. doi: 10.1002/cncr.23844. (suppl 8) [DOI] [PubMed] [Google Scholar]
- 11.El Saghir N, El-Asmar N, Hajj C, et al. Survey of utilization of multidisciplinary management tumor boards in Arab countries. Breast. 2011;20:S70–S74. doi: 10.1016/j.breast.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: Executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12:387–398. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 13.Harford JB, Otero IV, Anderson BO, et al. Problem solving for breast health care delivery in low and middle resource countries (LMCs): Consensus statement from Breast Health Global Initiative. Breast. 2011;20:S20–29. doi: 10.1016/j.breast.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Gakwaya A, Kigula-Mugambe JB, Kavuma A, et al. Cancer of the breast: 5-year survival in a tertiary hospital in Uganda. Br J Cancer. 2008;99:63–67. doi: 10.1038/sj.bjc.6604435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelaziz AO, Elbaz TM, Shousha HI, et al. Survival and prognostic factors for hepatocellular carcinoma: An Egyptian multidisciplinary clinic experience. Asian Pac J Cancer Prev. 2014;15:3915–3920. doi: 10.7314/apjcp.2014.15.9.3915. [DOI] [PubMed] [Google Scholar]


