Abstract
Purpose
To provide resource-stratified (four tiers), evidence-based recommendations on the primary prevention of cervical cancer globally.
Methods
The American Society of Clinical Oncology convened a multidisciplinary, multinational panel of oncology, obstetrics/gynecology, public health, cancer control, epidemiology/biostatistics, health economics, behavioral/implementation science, and patient advocacy experts. The Expert Panel reviewed existing guidelines and conducted a modified ADAPTE process and a formal consensus-based process with additional experts (consensus ratings group) for one round of formal ratings.
Results
Existing sets of guidelines from five guideline developers were identified and reviewed; adapted recommendations formed the evidence base. Five systematic reviews, along with cost-effectiveness analyses, provided evidence to inform the formal consensus process, which resulted in agreement of ≥ 75%.
Recommendations
In all resource settings, two doses of human papillomavirus vaccine are recommended for girls age 9 to 14 years, with an interval of at least 6 months and possibly up to 12 to 15 months. Individuals with HIV positivity should receive three doses. Maximal and enhanced settings: if girls are age ≥ 15 years and received their first dose before age 15 years, they may complete the series; if no doses were received before age 15 years, three doses should be administered; in both scenarios, vaccination may be through age 26 years. Limited and basic settings: if sufficient resources remain after vaccinating girls age 9 to 14 years, girls who received one dose may receive additional doses between age 15 and 26 years. Maximal, enhanced, and limited settings: if ≥ 50% coverage in the priority female target population, sufficient resources, and cost effectiveness, boys may be vaccinated to prevent other noncervical human papillomavirus–related cancers and diseases. Basic settings: vaccinating boys is not recommended.
It is the view of the American Society of Clinical Oncology that health care providers and health care system decision makers should be guided by the recommendations for the highest stratum of resources available. The guideline is intended to complement but not replace local guidelines.
INTRODUCTION
The purpose of this guideline is to provide expert guidance on primary prevention of cervical cancer, via the reduction in human papillomavirus (HPV) infection by HPV vaccine administration, to clinicians, public health leaders, and policymakers in all resource settings. The target population is people at risk for HPV infection and related diseases. Cervical cancer is the most common of the severe outcomes of HPV infection. Other disease outcomes from HPV infection include genital warts, several other anogenital cancers, and oropharyngeal cancers, particularly at the base of the tongue and tonsil.1,2 This guideline focuses on the role of HPV infection in cervical cancer.
Approximately 85% of incident cervical cancers occur in less developed regions, often overlapping with low- and middle-income countries (LMICs) around the world, and represent 12% of cancers among women in those regions. Eighty-seven percent of deaths resulting from cervical cancer occur in these less developed regions.3 Different regions of the world, both among and within countries, differ with respect to access to both primary and secondary prevention. As a result of these disparities, the American Society of Clinical Oncology (ASCO) Resource-Stratified Guidelines Advisory Group chose cervical cancer as a priority topic for guideline development.4,5
HPV causes virtually all cervical cancers and their immediate precursors everywhere in the world. The HPV 16 and HPV 18 subtypes are most associated with cervical cancer. It is estimated that complete coverage with HPV vaccines in the female population could reduce up to 90% of cervical cancer incidence worldwide with the existing vaccines, on the basis of reported worldwide HPV genotype distribution.6-8 There are three prophylactic HPV vaccines approved and recommended in the United States, Europe, and many regions and countries: the bivalent (2vHPV; against HPV 16 and 18),9 quadrivalent (4vHPV; against HPV 6, 11, 16, and 18),10-12 and nine valent (9vHPV; against HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58).13-15 These vaccines prevent (for those who are HPV naïve) and reduce the burden of infection of HPV types that are included in the vaccines (HPV vaccine types) overall. Although there is some cross-protection inferred by the bivalent and quadrivalent vaccines for HPVs that are phylogenetically related to the vaccine HPV types (eg, 45 for 18, 31 and 33 for 1616,17), the duration of this cross-protection remains unclear. As a partial result of failures within different health care systems at levels of prevention (eg, vaccination and screening) and disease treatment and management, there are large regional and global disparities in cervical cancer incidence and mortality.
THE BOTTOM LINE
Primary Prevention of Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Guideline
Guideline Question
What is the optimal method for the primary prevention of cervical cancer?
Target Population
General population
Target Audience
Public health authorities, cancer control professionals, policymakers, obstetricians and gynecologists, pediatricians, other primary care providers, and lay public
Recommendations
Vaccination is the optimal strategy for primary prevention of infection by some types of human papillomavirus (HPV) that cause cervical cancer in the target population. There is no other preventive strategy for this cancer that can substitute for vaccination.
In maximal and enhanced resource settings:
- For which cohorts is routine vaccination recommended in maximal and enhanced resource settings?
- •Recommendation A1a
- Public health authorities, ministries of health, and primary care providers should routinely vaccinate girls, with the target age range being as early as possible, starting at 9 through 14 years of age (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
- •Recommendation A1b
- Public health authorities may set the upper end of the target population higher than 14 years of age, depending on local policies and resources (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
- What numbers of doses and intervals are recommended in maximal and enhanced resource settings?
- •Recommendation A2a
- For girls 9 to 14 years of age who are immune competent, a two-dose regimen is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- •Recommendation A2b
- The interval between two doses should be at least 6 months and may be up to 12 to 15 months (6 months: Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong. 12 to 15 months: Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: weak).
- •Recommendation A2c
- Girls age ≥ 15 years at the time of the first dose or initiation (outside of target population) who receive vaccine should receive three doses (Type: informal consensus-based; Evidence quality: intermediate; Strength of recommendation: moderate).
- Should catch-up for those outside the priority age groups for vaccination be offered for prevention of HPV infection in maximal and enhanced resource settings?
- •Recommendation A3
- For females who have received one dose and are age > 14 years, public health authorities may provide additional doses or complete the series up to 26 years of age (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- Should HPV vaccination of boys be recommended to reduce HPV infection in maximal and enhanced resource settings?*
- •Recommendation A4
- For prevention of cervical cancer, if there is low vaccine coverage of the priority female target population (< 50%) in maximal or enhanced resource settings, vaccination may be extended to boys (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- For prevention of cervical cancer in maximal or enhanced resource settings where vaccine coverage of girls is ≥ 50%, vaccination of boys is not recommended (Type of recommendation: evidence based; Evidence quality: insufficient; Strength of recommendation: weak).
In limited resource settings:
- For which cohorts is routine vaccination recommended in limited resource settings?
- •Recommendation B1a
- Public health authorities, ministries of health, and primary care providers should vaccinate girls as early as possible, starting at 9 through 14 years of age (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
- What numbers of doses and intervals are recommended in limited resource settings?
- •Recommendation B2a
- For girls starting at 9 years of age who are immune competent, a two-dose regimen is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- •Recommendation B2b
- The interval between the doses should be at least 6 months and may be up to 12 to 15 months (6 months: Type of recommendation: evidence based; Evidence for quality: high; Strength of recommendation: strong. 12 to 15 months: Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
- Should catch-up for those outside the priority age groups for vaccination be offered for prevention of HPV infection in limited resource settings?
- •Recommendation B3
- If there are sufficient resources remaining after vaccinating high-priority populations with an adequate target (minimum recommended coverage is ≥ 50% with two doses, with a target of 80%),53 for females who have received one dose and are age > 14 years, public health authorities may provide additional doses or complete the series up to 26 years of age (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- Should HPV vaccination of boys be recommended to reduce HPV infection in limited resource settings?*
- •Recommendation B4
- For prevention of cervical cancer in limited resource settings where vaccine coverage of girls is ≥ 50%, vaccination of boys is not recommended.
- For prevention of cervical cancer, if there is low vaccine coverage of the priority female target population (< 50%) in limited resource settings, vaccination may be extended to boys (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- *Qualifying statement for A4 and B4. Extending vaccination to boys to prevent cervical cancer is not cost effective, unless there is low vaccine coverage of the priority female target population (< 50%). Vaccination may be extended to boys for other reasons, such to prevent other noncervical HPV-related cancers and diseases (eg, genital warts) and/or to reduce more rapidly circulating HPVs.
In basic resource settings:
- For which cohorts is routine vaccination recommended in basic resource settings?
- •Recommendation C1
- Public health authorities, ministries of health, and primary care providers should vaccinate girls in the priority target age group, starting as early as possible through 14 years of age (Type of recommendation: evidence based; Evidence quality: high. Strength of recommendation: strong).
- What numbers of doses and intervals are recommended in basic resource settings?
- •Recommendation C2a
- For girls starting at 9 years of age who are immune competent, a two-dose regimen is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- •Recommendation C2b
- The interval between the doses should be at least 6 months and may be up to 12 to15 (6 months: Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong. 12 to 15 months: Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
- Should catch-up for those outside the priority age groups for vaccination be offered for prevention of HPV infection in basic resource settings?
- •Recommendation C3
- High coverage of priority populations should be emphasized. Where coverage of the primary targeted group of females is high (≥ 50%) and resources allow, the age group may be expanded upward in catch-up efforts (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
- Should HPV vaccination of boys be recommended to reduce HPV infection in basic resource settings?†
- •Recommendation C4
- For prevention of cervical cancer in basic resource settings where vaccine coverage of girls is ≥ 50%, vaccination of boys is not recommended.
- For prevention of cervical cancer, if there is low vaccine coverage of the priority female target population (< 50%) in basic resource settings, vaccination may be extended to boys (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
- †Qualifying statement for C4. Extending vaccination to boys to prevent cervical cancer is not cost effective, unless there is low vaccine coverage of the priority female target population (< 50%). However, if resources allow for efforts to reduce noncervical cancers and diseases and/or reduce more rapidly circulating HPVs, vaccination may be extended to boys.
In all resource settings:
- What vaccination strategy is recommended for women who are HIV positive or immunosuppressed for other reasons (all resource settings)?
- •Recommendation D
- Females who are HIV positive or immunosuppressed for other reasons should follow the same age recommendations but should receive three doses (Type of recommendation: evidence based; Evidence quality: insufficient; Strength of recommendation: weak).
- What vaccination strategy is recommended for women who are pregnant (all resource settings)?
- •Recommendation E
- HPV vaccination is not recommended for pregnant women (Type of recommendation: evidence based; Evidence quality: insufficient; Strength of recommendation: weak).
- What vaccination strategy is recommended for women receiving treatment of cervical cancer precursor lesions (cervical intraepithelial neoplasia grade ≥ 2; eg, conization, loop electrosurgical excision process, or cryotherapy; all resource settings)?
- •Recommendation F
- No recommendation (insufficient data).
Qualifying Statements
Additional qualifying statements: If boys are vaccinated, use the same age-related recommendations as for girls, according to resource settings. Recommendations regarding boys do not apply to men who have sex with men, and readers are referred to Centers for Disease Control and Prevention, Australian, and other guidelines.
Additional Resources
More information, including a Data Supplement, a Methodology Supplement, slide sets, and clinical tools and resources, is available at www.asco.org/rs-cervical-cancer-primary-prev-guideline and www.asco.org/guidelineswiki. Patient information is available at www.cancer.net.
The American Society of Clinical Oncology believes that cancer and cancer prevention clinical trials are vital to inform medical decisions and improve cancer care and that all patients should have the opportunity to participate.
ASCO has established a process for resource-stratified guidelines, which includes mixed methods of guideline development, adaptation of the clinical practice guidelines of other organizations, and formal expert consensus. This article summarizes the results of that process and presents the practice resource-stratified recommendations, which are based in part on expert consensus and adaptation from existing guidelines (described in Results and Appendix Table A1, online only).
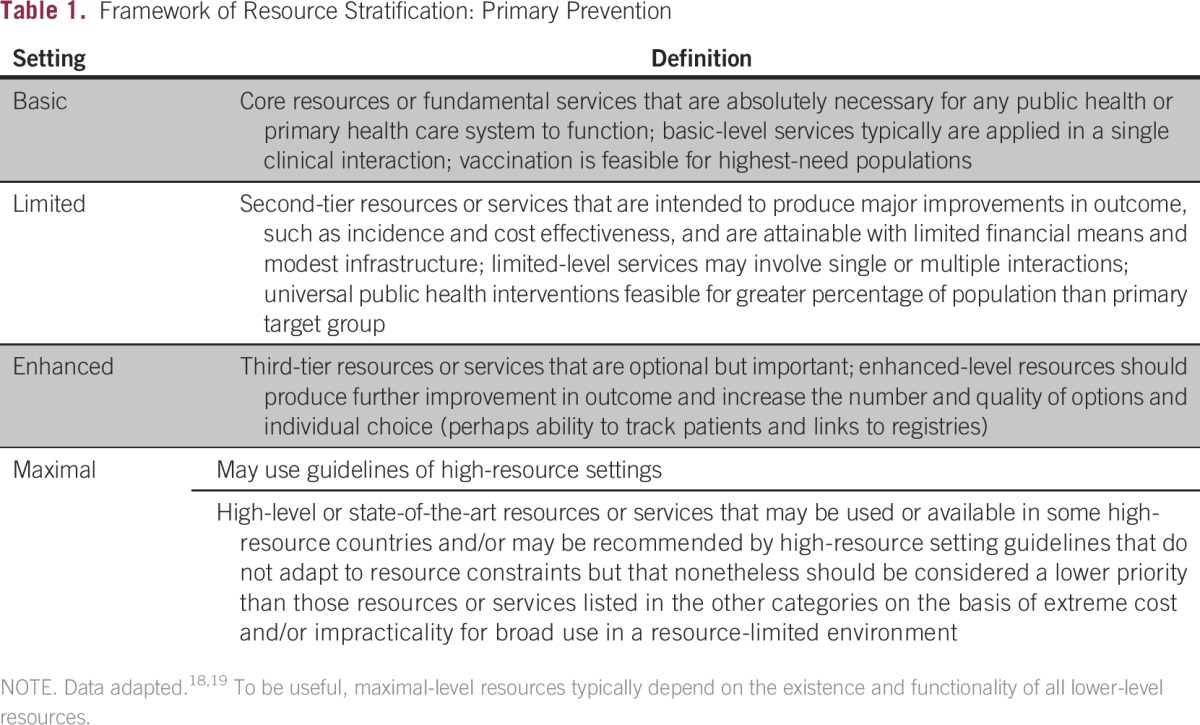
In developing resource-stratified guidelines, ASCO has adopted its framework from the four-tier resource setting approach (basic, limited, enhanced, maximal; Table 1) developed by the Breast Health Global Initiative and modifications to that framework based on the Disease Control Priorities 3.18,19 ASCO uses an evidence-based approach to inform guideline recommendations.
Table 1.
Framework of Resource Stratification: Primary Prevention
GUIDELINE QUESTION
This clinical practice guideline addresses the overarching clinical question: What is the optimal method for primary prevention of cervical cancer in each resource stratum?
METHODS
These recommendations were developed by an Expert Panel with multinational and multidisciplinary representation (Appendix Table A2, online only). The Expert Panel met via teleconference and in person and corresponded through e-mail. On the basis of consideration of the evidence, the authors were asked to contribute to the development of the guideline, provide critical review, and finalize the guideline recommendations. Members of the Expert Panel were responsible for reviewing and approving the penultimate version of the guideline, which was then circulated for external review and submitted to a peer-reviewed journal for editorial review and consideration for publication. This guideline was partially informed by the ASCO-modified Delphi Formal Expert Consensus methodology, according to which the Expert Panel was supplemented by additional experts recruited to rate their agreement with the drafted recommendations. The entire membership of experts is referred to as the consensus panel (the Data Supplement provides a list of members). All ASCO guidelines are ultimately reviewed and approved by the Expert Panel and the ASCO Clinical Practice Guideline Committee before publication. This guideline adaptation was also informed by the ADAPTE methodology20 and consensus processes used together as an alternative to de novo guideline development. Adaptation of guidelines is considered by ASCO in selected circumstances, when one or more quality guidelines from other organizations already exist on the same topic. The objective of the ADAPTE process is to take advantage of existing guidelines to enhance the efficient production, reduce duplication, and promote the local uptake of quality guideline recommendations.
The ASCO adaptation and formal consensus processes begin with a literature search to identify candidate guidelines for adaptation. The Panel used literature searches (1966 to 2015, with additional searches for literature published in specific areas [date parameters, 2005 to 2015]), existing guidelines and expert consensus publications, some literature suggested by the Panel, and clinical experience as guides. Adapted guideline manuscripts are reviewed and approved by the ASCO Clinical Practice Guideline Committee. The review includes two parts: methodologic review and content review.21 The methodologic review was completed by ASCO senior guideline staff (Methodology Supplement). The content review was completed by the Expert Panel. In addition, staff reviewed the methodologies of systematic reviews with the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) instrument.22
The guideline recommendations were crafted, in part, using the GLIDES (Guidelines Into Decision Support) methodology and accompanying BRIDGE-Wiz software.23 Detailed information about the methods used to develop this guideline is available in the Methodology and Data Supplements at www.asco.org/rs-cervical-cancer-primary-prev-guideline.
The ASCO Panel and guidelines staff will work with co-chairs to keep abreast of any substantive updates to the guideline. On the basis of formal review of the emerging literature, ASCO will determine the need to update.
This is the most recent information as of the publication date. For updates and the most recent information and to submit new evidence, please visit www.asco.org/rs-cervical-cancer-primary-prev-guideline and the ASCO Guidelines Wiki (www.asco.org/guidelineswiki).
Guideline Disclaimer
The clinical practice guidelines and other guidance published herein are provided by ASCO to assist providers in clinical decision making. The information herein should not be relied upon as being complete or accurate, nor should it be considered as inclusive of all proper treatments or methods of care or as a statement of the standard of care. With the rapid development of scientific knowledge, new evidence may emerge between the time information is developed and when it is published or read. The information is not continually updated and may not reflect the most recent evidence. The information addresses only the topics specifically identified therein and is not applicable to other interventions, diseases, or stages of diseases. This information does not mandate any particular course of medical care. Furthermore, the information is not intended to substitute for the independent professional judgment of the treating provider, because the information does not account for individual variation among patients. Each recommendation indicates high, moderate, or low confidence that the recommendation reflects the net effect of a given course of action. The use of words like “must,” “must not,” “should,” and “should not” indicates that a course of action is recommended or not recommended for either most or many patients, but there is latitude for the treating physician to select other courses of action in individual cases. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. ASCO provides this information on an as-is basis and makes no warranty, express or implied, regarding the information. ASCO specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. ASCO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information or for any errors or omissions.
Guideline and Conflict of Interest
The Expert Panel was assembled in accordance with ASCO’s Conflict of Interest Policy Implementation for Clinical Practice Guidelines (“Policy,” found at http://www.asco.org/rwc). All members of the Expert Panel completed ASCO’s disclosure form, which requires disclosure of financial and other interests, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as a result of promulgation of the guideline. Categories for disclosure include employment; leadership; stock or other ownership; honoraria, consulting or advisory role; speaker’s bureau; research funding; patents, royalties, other intellectual property; expert testimony; travel, accommodations, expenses; and other relationships. In accordance with the Policy, a majority of the members of the Expert Panel did not disclose any relationships constituting a conflict under the Policy.
RESULTS
As part of the systematic literature review, PubMed, Standards and Guidelines Evidence directory, Cochrane Systematic Review, and National Guideline Clearinghouse databases were searched for guidelines, systematic reviews, and meta-analyses published between 1966 and January 2015. Inclusion criteria identified publications that were (1) on the primary prevention of cervical cancer, (2) developed by multidisciplinary content experts as part of a recognized organizational effort, and (3) published between 1966 and 2015. Searches for cost-effectiveness analyses (CEAs) were also conducted. Articles were excluded from the systematic review if they were (1) meeting abstracts or (2) books, editorials, commentaries, letters, news articles, case reports, or narrative reviews.
A total of nine guidelines and seven systematic reviews were found in the literature search, and their currency, content, and methodology were reviewed. On the basis of content and methodology reviews, the Expert Panel chose guidelines from five public health authorities or guideline developers (the WHO,24 the US Advisory Committee on Immunization Practices [as adopted by the Centers for Disease Control and Prevention (CDC)],7-9,25 the National Advisory Committee on Immunization [NACI; Canada],26 German guidelines,27 and Immunize Australia28), four systematic reviews, and one quantitative review25,29-32 on the primary prevention of HPV infection as the evidentiary basis for the guideline recommendations, along with CEAs. Appendix Table A1 lists links to the guidelines. While this ASCO guideline was nearing publication, the CDC announced a forthcoming change in recommendations regarding doses.33
This ASCO guideline reinforces selected recommendations offered in the WHO, CDC, NACI, German, and Immunize Australia guidelines and acknowledges the effort put forth by the authors and aforementioned societies to produce evidence-based and/or consensus-based guidelines informing practitioners and institutions providing primary prevention of HPV infection. The identified guidelines were published between 2014 and 2015. The Data Supplement includes an overview of these guidelines, including information on the clinical questions, target populations, development methodologies, and key evidence.
GUIDELINES ON PRIMARY PREVENTION OF HPV INFECTION
Clinical Questions and Target Populations of Guidelines Adapted by ASCO
The guidelines adapted in part by ASCO are listed in Appendix Table A1. The WHO guideline, based on the WHO Strategic Advisory Group of Experts (SAGE) systematic review, pertained to the 4vHPV and 2vHPV vaccines, with a target population of preadolescent and adolescent girls age 9 to 13 years (primary population), including those who were immunocompromised (ie, HIV positive), as well as men who have sex with men (MSM). The primary clinical question was the appropriate number of doses.24,32,34 The 2015 CDC guidelines focused on the 9vHPV vaccine, with a target population of US girls and boys age 11 or 12 years, including catch-up (ie, extending the target age range) for females age 13 to 26 years and males age 13 to 21 years who have not received or completed a vaccine series. The CDC stated that men age 22 to 26 years may also receive the vaccine. In the United States, all three vaccines are available.15 This was the highest-quality guideline found on the 9vHPV vaccine (based on AGREE II [Appraisal of Guidelines for Research and Evaluation II] assessment; Methodology Supplement). The clinical questions concerned the age of initial target populations and ages for older populations who had not previously received vaccinations. A previous CDC guideline (2014) examined the 4vHPV vaccine for males and females and included populations (including ages) similar to those in the more recent CDC guideline; its clinical question concerned the routine use of the 4vHPV vaccine for boys.11 This 2014 guideline was preceded by 2010 and 2011 recommendations for boys.12,35 Another previous CDC guideline was on the 2vHPV vaccine, for US females only, age 11 to 26 years, including primary and catch-up populations.9
The German guideline concerned the 4vHPV and 2vHPV vaccines, with a target population of girls (both 4vHPV and 2vHPV) and boys (4vHPV) starting at age 9 years. The summary was in English, and the full guideline was in German; the clinical questions were not explicitly stated in the English-language summary.27 The 2015 Canadian guideline, which was largely based on the WHO SAGE systematic review, had a target population of females and males age 9 to 26 years, as well as immunocompromised persons and MSM, and covered the 4vHPV and 2vHPV vaccines.26 The clinical questions regarded schedule, number of doses, and boosters. The 2016 Australian guideline population included males, females, MSM, and immunocompromised persons. The clinical questions were not available; however, the guideline target population was girls and boys age 12 to 13 years.28
Summary of Guidelines Adapted by ASCO: Development Methodologies and Key Evidence
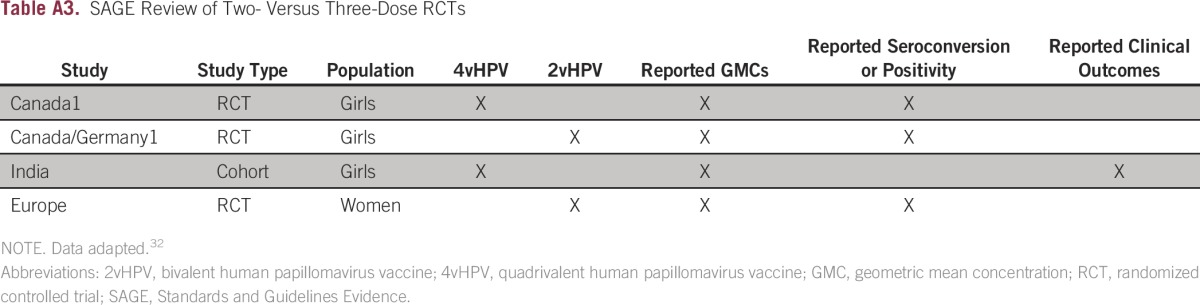
The WHO SAGE methods included systematic and nonsystematic reviews of published and gray literature and critical appraisal with GRADE (Grading of Recommendations Assessment, Development and Evaluation). This guideline received a rating of 82% on the AGREE II (Methodology Supplement). The most important evidence underlying recommendations on the number of doses came from the WHO SAGE systematic review, which included not only randomized clinical trials (RCTs) but also observational studies and publications from the gray literature (defined as preserved and collected but non–peer-reviewed unpublished literature36).32 The primary systematic review examined studies that compared two versus three doses of 2vHPV and 4vHPV vaccines and found randomized evidence from four RCTs (two on 2vHPV and two on 4vHPV; one was later reclassified as a cohort study; Appendix Table A3, online only)37; participants were girls age 9 to 18 years (the trials were referred to as Canada1 BCGov01,38-41 Canada/German1 HPV-048,42-45 Europe [ClinicalTrials.gov identifier: NCT00552279; Esposito et al46], and India [ClinicalTrials.gov identifier: NCT00923702]; since the SAGE review, the India study was published in a peer-reviewed journal that stated the authors reanalyzed the results as an observational cohort study).37,47 There were also four nonrandomized comparisons within RCTs and nonrandomized comparative studies (Canada1,38-41 Canada.Germany1,42-45 Mexico,48 and multinational44,45,49; Table 2 in WHO SAGE Appendix 132), plus other noncomparative, nonrandomized data. The primary outcomes were immunologic, although SAGE also looked at clinical outcomes if studies reported them. All guidelines except the WHO and NACI guidelines presumed the target intervention included three doses of the vaccines.
The Canadian NACI conducted a literature search to update the WHO SAGE literature search and found three observational studies. Otherwise, the NACI guideline used the WHO SAGE evidence base to support its clinical practice guideline, which received 64% on the AGREE II (Methodology Supplement). Its methodology included a committee vote on the NACI recommendations.
CDC guidelines are based on systematic reviews. The CDC adopted the GRADE methodology for critical appraisal of evidence in 2011 and first used it for its guidelines on HPV vaccination for males. Key evidence included clinical trial data (prelicensure, including RCTs, immunogenicity, and immunobridging studies) and cost-effectiveness modeling data. The 2014 recommendations on 4vHPV were based on four RCTs on efficacy and safety guidance from seven clinical trials. The AGREE scores for the CDC 2010 to 2015 guidelines ranged from 42% to 52% (Methodology Supplement).
The German guideline used mixed methods, including evidence based, clinical (informal) consensus, clinical experience, and formal consensus in a nominal group process. This guideline received a rating of 52% on AGREE II. The evidence base primarily came from 28 studies. The Immunize Australia guideline recommendations were based on methods involving an evidence base, expert review, and public comment. The guideline refers to using the highest-quality evidence available and other guidelines. The AGREE score was 54% (Methodology Supplement).
Outcomes
The outcomes or end points in most studies reviewed by the guidelines included immunogenicity, HPV infection, cervical intraepithelial neoplasia (CIN; cervical cancer precursor lesions), and safety.
RESULTS OF ASCO METHODOLOGIC REVIEW
The methodologic review of the guidelines was completed by two ASCO guideline staff members using the Rigour of Development subscale of the AGREE II instrument. The score for the Rigour of Development domain is calculated by summing the scores across individual items in the domain and standardizing the total score as a proportion of the maximum possible score. Detailed results of the scoring and the AGREE II assessment process for this guideline are available in the Methodology Supplement.
FINAL RECOMMENDATIONS
The recommendations were developed by a multinational, multidisciplinary group of experts using evidence from existing guidelines and clinical experience as a guide. The ASCO Expert Panel underscores that health care practitioners who implement the recommendations presented in this guideline should first identify the available resources in their local and referral facilities and endeavor to provide the highest level of care possible with those resources.
Maximal and Enhanced Resource Settings
These recommendations were modified from the following guidelines: WHO, CDC, and Canadian guidelines.
For which cohorts is routine vaccination recommended in maximal and enhanced resource settings?
Recommendation A1a.
Public health authorities, ministries of health, and primary care providers should routinely vaccinate girls, with the target age range being as early as possible, starting at 9 through 14 years of age (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Recommendation A1b.
Public health authorities may set the upper end of the target population higher than 14 years of age, depending on local policies and resources (Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
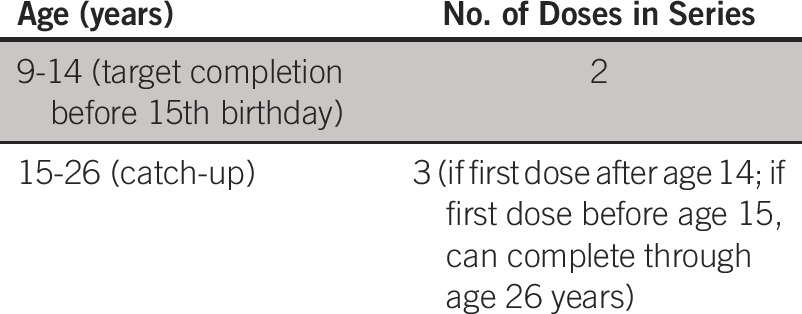
What numbers of doses and intervals are recommended in maximal and enhanced resource settings?
Recommendation A2a.
For girls 9 to 14 years of age who are immune competent, a two-dose regimen is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation A2b.
The interval between two doses should be at least 6 months and may be up to 12 to 15 months (6 months: Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong. 12 to 15 months: Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: weak).
Recommendation A2c.
Girls age ≥ 15 years at the time of the first dose or initiation (outside of target population) who receive vaccine should receive three doses (Type: informal consensus based; Evidence quality: intermediate; Strength of recommendation: moderate).
Source guidelines and discussion.
Vaccination is the optimal strategy for primary prevention for the majority of HPV genotypes that cause cervical cancer in the target population. There is no other preventive strategy that can substitute for vaccination. Vaccination does not protect against all oncogenic HPV types. In trial conditions, the 9vHPV vaccine showed ≥ 96% efficacy in the reduction of persistent infection and cervical, vaginal, and vulvar precursor or preneoplastic lesions for the five additional types included in the vaccine (ie, 31, 33, 45, 52, and 58).15 Protection from infection is improved with higher vaccination coverage.
The lower end of the age range (9 years) is supported by the WHO, CDC (on 9vHPV), German, and Canadian guidelines and by licensure by regulatory authorities (eg, European Medicines Agency [EMA]); this is based on immunogenicity data for girls and boys from age 9 years onward. The upper end of the age range recommendation is based on the WHO position paper34 and the NACI Canadian guidelines.26 In some countries, the upper end ranges from 15 to 16 years.
The RCTs establishing the benefit of vaccination were conducted with three doses. Subsequently, research has investigated the use of two doses with immunogenicity end points. The most important evidence underlying recommendations on the number of doses came from the WHO SAGE systematic review, as described in Summary of Guidelines Adapted by ASCO. The NACI guideline agrees with the WHO recommendations. The data should be evaluated in the future when there is > 4 years of follow-up. There are now data with end points of immunogenicity for 9vHPV (unpublished data). The EMA has stated that 9vHPV can be administered on a two-dose schedule for boys and girls age 9 to 14 years.13
In most clinical trials and guidelines, the interval between the first and second vaccine doses was 6 to 12 months.24,34 The maximum interval between two doses that is still effective is not known. Research comparing the upper end of the interval of 12 months with other intervals has not been conclusive and is ongoing (eg, ClinicalTrials.gov identifier: NCT02568566). A WHO position paper suggested an interval of no greater than 12 to 15 months so that girls complete all doses before they are sexually active, noting that they do not recommend a maximum interval.34(p489)
Although this guideline specifically regards cervical cancer, there is also an additional benefit of HPV vaccination in preventing other HPV-related cancers, such as other anogenital and potentially oropharyngeal cancers.9 This guideline does not make recommendations or review evidence regarding these other cancers.
The source guidelines reviewed safety data, and this subject is discussed in detail in Special Topic C. The ASCO Expert Panel also endorses the recently published International Papillomavirus Society statement on the safety of the vaccines.50
Should catch-up for those outside the priority age groups for vaccination be offered for prevention of HPV infection in maximal and enhanced resource settings?
Recommendation A3.
For females who have received one dose and are age > 14 years, public health authorities may provide additional doses or complete the series up to 26 years of age (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Source guidelines and discussion.
The purpose of catch-up strategies is to address the temporary situation in which some persons are older than the priority target populations. Vaccination up to age 26 years is supported by all three CDC guidelines reviewed,11,12,15 the 2015 Canadian guideline, and evidence reviewed by the WHO. In addition to evidence discussed in these guidelines, Couto et al25 conducted a high-quality meta-analysis of trials published before October 2012, including 13 RCTs, on catch-up vaccination for women age ≥ 16 years, without language restrictions. Included studies were conducted in the United States, Canada, South America, Europe, and Asia and compared the vaccine with placebo or no vaccine. Although the systematic review looked for studies with a variety of outcomes, there were limited data of the effect of vaccination on mortality. For HPV-associated CIN grade ≥ 2 (CIN2+), the pooled risk ratio (RR) was 0.80 (95% CI, 0.62 to 1.02). For HPV-related CIN, the intention-to-treat pooled RR was 0.54 (95% CI, 0.44 to 0.67). All RRs are based on 4 years of follow-up. The RR for pooled outcomes of adverse events was 0.99 (95% CI, 0.91 to 1.08). (This systematic review received a 9.5 AMSTAR rating.)
Increasing the upper age limit of the cohort in which catch-up vaccination is implemented should be based on relative cost effectiveness from high-quality CEAs for each setting or region. CEAs should include (1) an incremental cost-effectiveness ratio (ICER) analysis that is practicable to apply according to the resource setting of a country and (2) at least a two-way sensitivity analysis to include paramount parameters that may act as cost drivers in the model used in references. The CHEERS checklist provides parameters for such criteria.51
Should HPV vaccination of boys be recommended to reduce HPV infection in maximal and enhanced resource settings?
Recommendation A4.
For prevention of cervical cancer, if there is low vaccine coverage of the priority female target population (< 50%) in maximal or enhanced resource settings, vaccination may be extended to boys (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
For prevention of cervical cancer in maximal or enhanced resource settings where vaccine coverage of girls is ≥ 50%, there are insufficient data to recommend for or against vaccination of boys (Type of recommendation: evidence based; Evidence quality: insufficient; Strength of recommendation: weak).
Qualifying statement.
Extending vaccination to boys to prevent cervical cancer is not cost effective, unless there is low vaccine coverage of the priority female target population (< 50%). Vaccination may be extended to boys for other reasons, such as to prevent other noncervical HPV-related cancers and diseases (eg, genital warts) and/or to reduce more rapidly circulating HPVs.
Source guidelines and discussion.
The scope of this guideline extends only to the prevention of cervical cancer; it does not review literature on the prevention of other cancers (eg, oropharyngeal cancer and/or other HPV-related cancers in males). In current practice in the United States, the CDC recommends vaccinating boys starting at 11 or 12 years of age with 4vHPV or 9vHPV. If boys are vaccinated, the age could be as young as 9 years; guidelines vary with regard to the earliest starting age, because they consider noncervical cancers as well as cervical cancer.26,35 In addition, the German, Canadian, and Australian guidelines also support vaccinating boys in maximal settings, with the 4vHPV vaccine (the CDC recommends 9vHPv).
There can be direct benefit in vaccinating male recipients with regard to prevention of male cancer and benefits to female populations by lowering the incidence of HPV-related cervical cancer via herd protection, depending on the coverage level for girls, although it would be less cost effective than increasing vaccine coverage of girls. Predictive models suggest that when coverage in girls is low, including boys might add some benefit to cervical cancer prevention. However, this benefit will be lower than that achieved with increasing girls’ coverage to 80%. A recent meta-analysis of model-predicted outputs from 16 independent transmission models, all representing developed countries, reaffirmed previous findings that there is greater impact by increasing coverage in girls than extending coverage to boys and that the health benefit and cost effectiveness of including boys are maximized when vaccination coverage in girls is low.52 If the coverage in girls has reached 50%, the benefit of adding boys is marginal for cervical cancer prevention (based on CEAs), and the benefit may only apply to reducing the risk of noncervical cancers. Of note, CEAs have been based on theoretic or market prices of the vaccine and not in real government-paid prices. Thus, the benefit of vaccinating boys may be larger than previously estimated. For the goal of reducing cervical cancer, the priority should be providing vaccination to the maximum portion of the target population of girls.
In the opinion of the ASCO Expert Panel, if public health authorities have sufficient resources to devote to the prevention of less common HPV-related cancers other than cervical cancer, the HPV vaccine may be offered to boys. The number of doses would follow the age-related recommendations for females in this guideline.
Limited Resource Settings
The recommendations for the limited resource setting concerning age cohort and number of doses are the same as those for the higher-resourced settings.
For which cohorts is routine vaccination recommended in limited resource settings?
Recommendation B1a.
Public health authorities, ministries of health, and primary care providers should vaccinate girls as early as possible, starting at 9 through 14 years of age (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
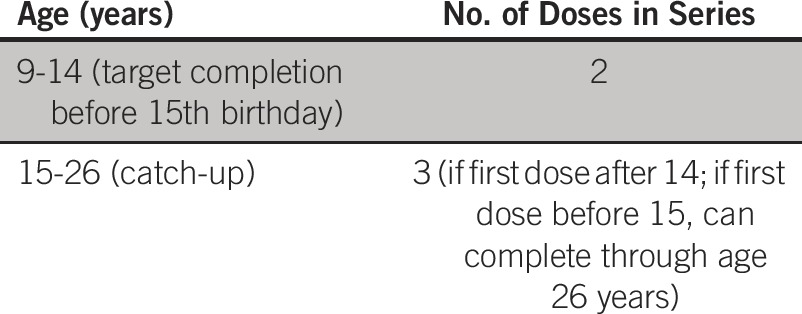
What numbers of doses and intervals are recommended in limited resource settings?
Recommendation B2a.
For girls starting at 9 years of age who are immune competent, a two-dose regimen is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation B2b.
The interval between the doses should be at least 6 months and may be up to 12 to 15 months (6 months: Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong. 12 to 15 months: Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Should catch-up for those outside the priority age groups for vaccination be offered for the prevention of HPV infection in limited resource settings?
Recommendation B3.
If there are sufficient resources remaining after vaccinating high-priority populations with an adequate target (minimum recommended coverage is ≥ 50% with two doses, with a target of 80%),53 for females who have received one dose and are age > 14 years, public health authorities may provide additional doses or complete the series up to 26 years of age (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Should HPV vaccination of boys be recommended to reduce HPV infection in limited resource settings?
Recommendation B4.
For prevention of cervical cancer in limited resource settings where vaccine coverage of girls is ≥ 50%, vaccination of boys is not recommended. For prevention of cervical cancer, if there is low vaccine coverage of the priority female target population (< 50%) in limited resource settings, vaccination may be extended to boys (Type of recommendation: evidence based; Evidence quality: intermediate. Strength of recommendation: moderate).
Qualifying statement.
Extending vaccination to boys to prevent cervical cancer is not cost effective, unless there is low vaccine coverage of the priority female target population (< 50%). Vaccination may be extended to boys for other reasons, such as to prevent other noncervical HPV-related cancers and diseases (eg, genital warts) and/or to reduce more rapidly circulating HPVs.
Source guidelines and discussion.
These recommendations follow the WHO guideline. The ages for boys in limited resource settings should be the same as those for girls in limited resource settings. The exceptions to these recommendations and contraindications are listed in the product specifications and may be affected by lack of resources to deliver the vaccine appropriately (eg, equipment, cold chain, and so on). High coverage of priority target populations should be emphasized, taking into account any relevant sociocultural factors. If there are more resources than are typically found in limited resource settings, the age group of females offered vaccines may be expanded. There is some evidence on the efficacy of vaccination in boys to prevent cervical cancer; however, CEAs are contradictory, and most CEAs conducted in LMICs have found vaccinating boys has only a marginal benefit over vaccinating girls with regard to reducing the risk of cervical cancer, and therefore, reaching female populations should be the priority. Specific CEA publications related to the issue of vaccination of males are discussed in this guideline, in Further Discussion and in Cost Implications.
Basic Resource Settings
Recommendations for the basic resource setting are modified from the WHO guideline.
For which cohorts is routine vaccination recommended in basic resource settings?
Recommendation C1.
Public health authorities, ministries of health, and primary care providers should vaccinate girls in the priority target age group, starting as early as possible through 14 years of age (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
What numbers of doses and intervals are recommended in basic resource settings?
Recommendation C2a.
For girls starting at 9 years of age who are immune competent, a two-dose regimen is recommended (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Recommendation C2b.
The interval between the doses should be at least 6 months and may be up to 12 to 15 months (6 months: Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong. 12 to 15 months: Type of recommendation: evidence based; Evidence quality: low; Strength of recommendation: moderate).
Should catch-up for those outside the priority age groups for vaccination be offered for prevention of HPV infection in basic resource settings?
Recommendation C3.
High coverage of priority populations should be emphasized. Where coverage of the primary targeted group of females is high (≥ 50%) and resources allow, the age group may be expanded upward in catch-up efforts (Type of recommendation: evidence based; Evidence quality: high; Strength of recommendation: strong).
Should HPV vaccination of boys be recommended to reduce HPV infection in basic resource settings?
Recommendation C4.
For prevention of cervical cancer in basic resource settings where vaccine coverage of girls is ≥ 50%, vaccination of boys is not recommended.
For prevention of cervical cancer, if there is low vaccine coverage of the priority female target population (< 50%) in basic resource settings, vaccination may be extended to boys (Type of recommendation: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate).
Qualifying statement.
Extending vaccination to boys to prevent cervical cancer is not cost effective, unless there is low vaccine coverage of the priority female target population (< 50%). However, if resources allow for efforts to reduce noncervical cancers and diseases and/or reduce more rapidly circulating HPVs, vaccination may be extended to boys.
Source guidelines and discussion.
Recommendations for basic resource settings are based on the WHO guideline. In these settings, girls’ HIV status may be unknown at the age of vaccination. Therefore, authorities providing vaccine to girls with unknown HIV status should follow the age-related recommendation. The highest priority is to have high coverage of young girls. If the country or region has a certain amount of resources, these should be devoted first to increasing coverage of girls. Decisions regarding boys depend on prevalence, coverage, resources, and CEAs. In addition, sociocultural issues in some settings may affect policy decision making. Providing catch-up vaccination should not be performed at the expense of achieving high coverage in the recommended priority cohort. Therefore, only if priority populations are vaccinated with sufficient coverage (ie, > 50% with a target of 80%) and additional resources remain should authorities offer catch-up.
Further Discussion on Vaccinating Boys (all settings)
Source guidelines and discussion.
Vaccine coverage is essential to reducing HPV prevalence (circulating HPVs) in the target populations. In basic and limited settings, the highest priority is to have high coverage of girls while still promoting safe sex to reduce transmission of the virus to nonvaccinated girls. Resources should be devoted to reach > 80% coverage of girls. In higher-resource settings, the extension and purposes of vaccinating boys should depend on the coverage level of the primary target population. If there is low coverage of girls (< 50%), vaccination may be extended to boys. If there is high coverage of girls, the recommendation is not to vaccinate boys, except in maximal and enhanced settings, where male vaccination may be offered to prevent other noncervical HPV-related cancers and diseases.
Vaccinating boys can also reduce the viral pool and may contribute to reducing the spread of HPV infections in the population. Thus, vaccination of boys may consequently reduce the overall burden of cervical cancer, as well as other HPV-related diseases, in the female population. However, modeling suggests this benefit is quite limited once a moderate level of coverage among girls has been achieved (C. Wheeler and V. Tsu, personal communication, August 2016). In addition, boys and young men can themselves benefit from the prevention of HPV-related infection and disease (eg, reduction of genital warts and male anogenital cancers). As mentioned, reviewing the benefits of HPV vaccines for outcomes in diseases other than cervical cancer was outside the scope of this guideline.
Performing a CEA is advisable to determine whether a particular resource setting may be able to extend HPV vaccination to boys. CEAs need to take into account the comments presented in this guideline; that is, duration of protection becomes less important as vaccinated cohorts move into the initial decade of sexual activity, because transmission is blocked if enough individuals are immune, and there is not a reservoir of infectious virus in the population, providing males and females are vaccinated. For example, a recent systematic review of CEAs (building on three other systematic reviews of CEAs) found vaccinating males was not cost effective for the prevention of cervical cancer in higher-income countries.29 It was only cost effective if other HPV-related diseases were included in analyses, which might still be the case in many developing countries. The overall societal impact may occur not only in cervical cancer, because many other diseases and cancers are also highly attributable to HPV. This systematic review included studies that reported ICERs in developed countries,29 all with three-dose series. The comparators were primarily female-only vaccination strategies. Outcomes included cervical cancer only, cervical cancer and genital warts, all HPV-related diseases, and anal cancer and/or genital warts for MSM. End points used for comparison were ICERs representing quality-adjusted life-years (QALYs). Seventeen studies were reviewed, including one on MSM, which found a value of $17,970 per QALY gained (for anal and genital outcomes) as a result of vaccinating males at 12 years of age. For cervical cancer only, vaccinating both sexes resulted in $28,713 to $554,317 per QALY gained.29 Higher ICERs obtained for both sexes might still be acceptable if HPV-related diseases are prevalent in a country, whereby the burden of HPV-related disease management would definitely be higher than the cost of primary prevention achieved by vaccinating both sexes.
Special Populations
These recommendations are modified from the WHO and Canadian guidelines.
What vaccination strategy is recommended for women who are HIV positive or immunosuppressed for other reasons (all resource settings)?
Recommendation D.
Females who are HIV positive or immunosuppressed for other reasons should follow the same age recommendations but should receive three doses (Type of recommendation: evidence based; Evidence quality: insufficient; Strength of recommendation: weak).
Source guidelines and discussion.
This recommendation is based on the WHO guideline and also agrees with the Canadian and Australian guidelines. A two-dose scheme is not recommended in this population, because of insufficient data on immunogenicity. If girls’ HIV status is unknown, authorities should provide vaccine to girls following the age-related recommendation for the basic setting. Data on the safety of a three-dose schedule in HIV-positive females and males and in HIV-infected children age 7 to 12 years showed no evidence of harm.34 Most importantly, girls in this population should receive antiretroviral treatment of HIV.
What vaccination strategy is recommended for women who are pregnant (all resource settings)?
Recommendation E.
HPV vaccination is not recommended for pregnant women (Type of recommendation: evidence based; Evidence quality: insufficient; Strength of recommendation: weak).
Source guidelines and discussion.
This recommendation is based on the WHO and Immunize Australia guidelines. HPV vaccination is not recommended during pregnancy, because of lack of sufficient evidence of safety; however, there is no evidence of harm.54,55 It is not necessary to perform a pregnancy test before vaccination or to terminate a pregnancy subsequent to vaccination. Women who have received one or two doses should receive the second and/or third dose at the completion of the pregnancy. There is no need to restart the complete vaccination program.
What vaccination strategy is recommended for women receiving treatment for cervical cancer precursor lesions (CIN2+; eg, conization, loop electrosurgical excision procedure, or cryotherapy; all resource settings)?
Recommendation F.
No recommendation (insufficient data).
Discussion.
There are insufficient data to recommend that women in this population be offered vaccination or not based on their history of HPV infection and/or treatment of cervical cancer precursor lesions. Reports in women who had received HPV vaccines before or after excisional treatment of high-grade cervical disease have shown mixed results, with some studies demonstrating no effect.56-58 In the absence of consistent and persuasive evidence that women with a history of HPV-related abnormalities have any risk for future new infection that is different from women of a similar age, HPV vaccination should be offered according to the age- and resource-related recommendations as given in this guideline. HPV status, including HPV testing or history of HPV-related abnormalities (eg, abnormal cytology results or cervical biopsies), is not part of the decision making for offering HPV vaccine. The likelihood of infection with HPV 16 or 18 increases with the severity of cervical abnormality, and the overall benefit of vaccination would decrease. Women should be advised that results from clinical trials do not indicate the vaccine will have any therapeutic effect on existing HPV infection or cervical lesions.10 Women who receive treatment for precursor lesions and their physicians should follow routine post-treatment follow-up recommendations.4,5,59,60
An article published after the guidelines were adapted presented results from a randomized trial of the bivalent vaccine; some participants had HPV infections, but there were no differences in infection outcomes or efficacy.61,62 Other articles that did not meet the inclusion criteria included a retrospective case-control study of a patient who had undergone loop electrosurgical excision procedure.63 A greater percentage of nonvaccinated women had recurrences of CIN grade 2 to 3, and a multivariate analysis showed a lack of vaccination was prognostic for recurrence. Finally, a nonprespecified and retrospective analysis of results from the double-blind, placebo-controlled RCTs FUTURE I and II64 included women who had been enrolled in the trials without screening and regardless of HPV infection status. There was a statistically significant decreased risk of cervical disease (after previous treatment for cervical disease). Overall, the results of studies of women treated for precursor lesions were negative, were retrospective, included small numbers of patient cases, and/or showed mixed results. Larger, prospective studies would be needed before the Panel discusses making a recommendation for this population.
SPECIAL COMMENTARY
Topic A
In vaccinated cohorts, what is recommended for secondary prevention in terms of cost-effectiveness ratios for the combined strategies?
Vaccination does not replace screening. Until additional data are gathered, vaccinated cohorts will need to be screened. The testing algorithm and interval between screening tests are still under evaluation in many countries. It is likely that the initial change for screening of vaccinated women will be to increase the age at which screening is initiated. Screening after vaccination is discussed in detail in the ASCO Screening Resource-Stratified Guideline.5
Topic B
Is there a need to have a registration system (ie, enrollment, refusal, or surveillance of potential adverse effects) to evaluate the impact and coverage of the strategies?
There is a need for monitoring the implementation of vaccines in terms of coverage and outcomes detected by screening and cancer registries. Strengthened systems for monitoring immunization adverse events are essential for tracking potential adverse effects, especially rare or late-occurring events. The rationale for screening and cancer registries is the need for data over time to track longer-term outcomes, especially cervical cancer outcomes, and the duration of immunity and protection. Surveillance with linkage of screening and vaccination information should occur to inform the safe, effective, and rational integration of these two complementary prevention strategies. In basic and limited resource settings, public health providers need to document the percentage of eligible girls and boys vaccinated. All countries or regions should have basic coverage data documenting the percentage of eligible girls and boys vaccinated. As countries and regions introduce HPV vaccination, they need to update the WHO Expanded Programme on Immunization, with recording of doses administered and collection of reported adverse events. In limited resource settings, policymakers and public health authorities should move toward population-based cancer registries for at least one region in the country. In enhanced resource settings, policymakers and public health authorities should implement a surveillance system to monitor HPV infections and HPV-related precancers. In countries with more resources, policymakers and public health authorities should implement countrywide, regional, and state surveillance systems. Surveillance systems can rule out false associations and identify rare adverse events in the postvaccine licensure period.57 Maximal resource settings should also establish surveillance linking vaccination, screening, and cancer registries through collection of continuous longitudinal data, but achieving this requires strong linking variables.
Topic C: Safety
The safety profile of HPV vaccines has been assessed extensively in RCTs and by robust pharmacovigilance in the postlicensure setting using both passive and active vaccine surveillance. Passive surveillance is the voluntary reporting in daily practice by vaccinated persons (or others) and medical professionals to manufacturers and national surveillance systems, such as the US Vaccine Adverse Event Reporting System (VAERS) and the Australian Therapeutic Goods Administration databases or multinational databases, such as the WHO Global Individual Case Safety Reports Database System and the Scientific and Technical Evaluation of Vaccinational Programs in the European Union. Active surveillance is the implementation of systematic procedures to actively seek and identify clinically significant events that occur within a defined period and/or population and include large postlicensure studies sponsored by the manufacturer or national regulatory authorities. As with all serious vaccine adverse events, it is important that appropriate investigations be carried out promptly to determine whether the event is caused by the vaccine and whether any remedial action is needed. The key challenge faced in pharmacovigilance is to distinguish real adverse events from background conditions that would occur regardless of vaccination. Population-based data on incidence of potential adverse events before vaccination allow analysis of observed and expected rates in vaccinated populations.56,57
Several entities conduct routine adverse event reporting, including VAERS, the EMA, Japan, and others.
After monitoring reports to VAERS, the CDC and US Food and Drug Administration analyzed reports of serious adverse events and deaths, as well as postmarketing data, and found no causal link to HPV4 vaccination.11 The Morbidity and Mortality Weekly Report also refers to other analyses of adverse event reporting, including those from Denmark, Sweden, and France, and reports there have been no findings of any causal link between 4vHPV vaccination and autoimmune, venous thromboembolic, neurologic, or other conditions.
The EMA reviewed publications, clinical trial data, postmarketing data, and reports and found no evidence that HPV vaccines may cause complex regional pain syndrome or postural orthostatic tachychardia syndrome. There is no evidence of higher incidence of these syndromes among vaccinated or unvaccinated girls.65,66
The WHO Global Advisory Committee for Vaccine Safety reviewed safety data, most recently in December 2015, and found no safety signals warranting changes in WHO recommendations.58
The International Papillomavirus Society assessed reviews by the WHO, US Food and Drug Administration, CDC, EMA, International Federation of Gynecology and Obstetrics, UK Medicines & Healthcare Products Regulatory Agency, and Australian Therapeutic Goods Administration and other publications and concluded that there is no evidence that neurologic disease, autoimmune diseases, or deaths are vaccine attributable and emphasized there have been no deaths associated with HPV vaccines.50
This guideline agrees with the International Papillomavirus Society policy statement on the safety of HPV vaccines.
Topic D: Children and Adolescents With History of Sexual Abuse
Offering HPV vaccine in an age-appropriate manner to children and adolescents with a history of sexual abuse is recommended by the CDC, and this population may receive vaccines according to the age- and resource-stratified recommendations in this guideline. There has been a special concern about vaccinating children and adolescents with a history of sexual abuse, given that they may be at higher risk for HPV infection as a result of the cervical, vaginal, or anal trauma associated with forced penetration. The CDC includes this population in its 2016 Immunization Schedules for three doses starting at 9 years of age: “administer HPV vaccine beginning at age 9 years to children and youth with any history of sexual abuse or assault who have not initiated or completed the 3-dose series.”67(p1407) This subject is also discussed in a review by Garland et al.68 Given the strong evidence in support of vaccinating girls as young as age 9 years across all resource settings (basic to maximal and enhanced), girls with a history of sexual abuse would be covered without the need to directly associate vaccination with history of abuse. With regard to vaccinating boys with a history of sexual abuse, the evidence is less clear but is consistent with the overall recommendation that if resources allow, boys with a history of sexual abuse should be vaccinated as young as age 9 years.
UPTAKE
ASCO published “American Society of Clinical Oncology Statement: Human Papillomavirus Vaccination for Cancer Prevention” in April 2016,69 which includes specific literature-informed recommendations to promote HPV vaccination. It has been well established that health care provider recommendation is the strongest predictor of HPV vaccination.70-73 Primary care providers and pediatricians are in a unique position to promote HPV vaccination, given their longstanding relationship with their child and adolescent patients and their parents. Once informed and educated about the importance of HPV vaccination by a trusted source (usually their children’s health care provider), parents are more likely to vaccinate their children. Therefore, at all levels (basic through maximal), education of primary care physicians and pediatricians about the cancer-preventive properties of HPV vaccination and its safety could provide the highest return on investment in cervical cancer primary prevention. Secondary strategies to promote uptake, particularly in settings where cost is not the primary barrier, include reminders (for providers and parents); promotion of HPV vaccination with other vaccines (eg, Tdap); and dissemination of consistent evidence-based, culturally relevant messages among parents, agents of change (eg, teachers or pastors), and providers, particularly with regard to the effectiveness and safety of the vaccine in preventing HPV-related cancers.69,74,75 Furthermore, it has been shown that active vaccination policies at the country level are an important policy-level strategy. Mortensen et al76 found that in countries with active vaccination policies (United Kingdom and Italy), parents tended to trust the national vaccination programs, whereas in countries with passive vaccination strategies (Germany and France), parents needed greater assurance from health care providers and public health workers.
COST IMPLICATIONS
In low-resource settings, cost remains the primary barrier to HPV vaccination. Currently, the lowest pricing ($4.50) is available to countries receiving support from the Global Alliance for Vaccines and Immunization, with 54 countries eligible as of early 2016.77 There are many published CEAs on HPV vaccines. An ASCO literature search focusing on high-quality systematic reviews of published CEAs was conducted. Among systematic reviews found was a 2013 review by Fesenfeld et al30 of CEAs specifically on vaccination and focusing on LMICs. Twenty-five studies were found. The authors comment that delivery and program costs are an important part of total cost, and one group of CEAs found these costs formed an estimated 40% of the cost per girl (assuming the vaccine cost per dose was the international dollar 10 to 25). All but one study of girls found vaccination would be cost effective in most cases. Vaccination is usually second in line of cost effectiveness after routine screening, but this needs high coverage of the female population. Many countries are not able to implement an effective call–recall system for screening as a result of limited resources and logistic barriers. Findings of studies in boys were contradictory. The authors state that if results are pooled, the price relative to the income of a country spent on health is an important factor, unless regions are able to obtain support from donors (usually through a successful public–private partnership) to implement mass vaccination.
Kiatpongsan et al78 published a CEA after Fesenfeld et al30 on two countries in east Africa. It was specific to 9vHPV and used a static natural history disease simulation model. It compared the cost effectiveness of 9vHPV with 2vHPV or 4vHPV for a population of females starting at 9 years of age and included some societal costs. In one country, the ICER for 9vHPV was below per-capita gross domestic product compared with existing vaccines. This showed that the strategy is cost effective.
For maximal resource settings, Armstrong31 published a review of CEAs with US-based models published before February 22, 2010. Eleven studies were included. All the studies included screening as a comparator, unlike in the report by Fesenfeld et al.30 Three of the studies included boys. Model types and assumptions varied, but all found HPV vaccination of girls versus screening alone is cost effective (ICER ≤ $100,000 per QALY gained), especially if the interval was > 1 year.
CEAs support the recommendations in this guideline for, at minimum, vaccination of girls age 9 to 14 years. In the near future, screening will have to accompany vaccination.
LIMITATIONS OF RESEARCH AND FUTURE DIRECTIONS
There were limitations to the evidence informing some of the recommendations, resulting in part from the relatively recent introduction of the vaccine. There were limited published data on
The impact on invasive cervical cancer outcomes
The upper age range for the priority target population of girls starting at 9 years of age
The optimal upper end of the interval (which starts at 6 months)
Two versus three doses of 9vHPV
CEAs of vaccinating boys in limited and basic settings
Pregnant women
Women who have or are receiving treatment for CIN2+
Vaccination of women age > 26 years
Effectiveness studies on two doses for women who are HIV positive or immunosuppressed
Therefore, the Expert Panel suggests research be conducted on these topics. ASCO believes that cancer and cancer prevention clinical trials are vital to inform medical decisions and improve cancer care. All patients should have the opportunity to participate.
ADDITIONAL RESOURCES
Additional information, including data supplements, evidence tables, and clinical tools and resources, can be found at www.asco.org/rs-cervical-cancer-primary-prev-guideline and www.asco.org/guidelineswiki. Patient information is available there and at www.cancer.net. Visit www.asco.org/guidelineswiki to provide comments on the guideline or to submit new evidence.
ACKNOWLEDGMENT
We thank Jean Rene Clemenceau, MD, Noelle LoConte, MD, William Tew, MD, Muhieddine Seoud, MD, the Consensus Ratings Panel, and the American Society of Clinical Oncology (ASCO) Clinical Practice Guidelines Committee for their thoughtful reviews of and insightful comments on this guideline document and Shannon McKernin for her assistance on the manuscript and derivatives. The Expert Panel and ASCO staff dedicate this guideline to the memory of Xavier Castellsagué, our dearly departed colleague and Expert Panel member.
Appendix
Table A1.
Adapted Guidelines and Links
Table A2.
Expert Panel Membership
Table A3.
SAGE Review of Two- Versus Three-Dose RCTs
Footnotes
Deceased.
Clinical Practice Guideline Committee approved: January 9, 2017.
Reprint requests: 2318 Mill Rd, Suite 800, Alexandria, VA 22314; e-mail: guidelines@asco.org.
Editor’s note: This American Society of Clinical Oncology clinical practice guideline provides recommendations, with review and analyses of the relevant literature for each recommendation. Additional information, which may include data supplements, slide sets, patient versions, frequently asked questions, and clinical tools and resources, are available at www.asco.org/rs-cervical-cancer-primary-prev-guideline and www.asco.org/guidelineswiki.
AUTHOR CONTRIBUTIONS
Administrative support: Sarah Temin
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Silvina Arrossi
No relationship to disclose
Sarah Temin
No relationship to disclose
Suzanne Garland
Leadership: Merck Sharp & Dohme, CSL, GlaxoSmithKline
Honoraria: Sanofi Pasteur, HPV standalone scientific symposium Barcelona 2014, lectures and media interviews on HPV best practices in Australia and Japan 2014, International Gynecological Cancer Society, Merck Sharp & Dohme, Merck
Consulting or Advisory Role: Merck Sharp & Dohme
Research Funding: Merck Sharp & Dohme (Inst), CSL (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Pan American Health Organization/WHO, GlaxoSmithKline
Linda O’Neal Eckert
No relationship to disclose
Neerja Bhatla
Research Funding: Merck Sharp & Dohme
Xavier Castellsagué
No relationship to disclose
Sharifa Ezat Alkaff
No relationship to disclose
Tamika Felder
Honoraria: Quest Diagnostics, Genentech, Merck, Hologic
Doudja Hammouda
Travel, Accommodations, Expenses: Merck
Ryo Konno
Honoraria: GlaxoSmithKline, Merck Sharp & Dohme, Qiagen, Roche, Chugai Pharmaceutical
Consulting or Advisory Role: GlaxoSmithKline, Merck Sharp & Dohme
Research Funding: Chugai Pharmaceutical (Inst)
Gilberto Lopes
Honoraria: AstraZeneca, Roche, Merck Serono, Merck Sharp & Dohme, Fresenius Kabi, Novartis, Bristol-Myers Squibb, Janssen-Cilag, Boehringer Ingelheim, Pfizer, Cipla, Sanofi, Eisai, Eli Lilly
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Eli Lilly
Research Funding: Eli Lilly, Pfizer, AstraZeneca, Merck Sharp & Dohme, Eisai, Bristol-Myers Squibb
Expert Testimony: Sanofi
Emmanuel Mugisha
No relationship to disclose
Raul Murillo
No relationship to disclose
Isabel C. Scarinci
No relationship to disclose
Margaret Stanley
Honoraria: Merck Sharp & Dohme
Consulting or Advisory Role: GlaxoSmithKline
Vivien Tsu
No relationship to disclose
Cosette M. Wheeler
Research Funding: GlaxoSmithKline (Inst), Roche (Inst)
Isaac Folorunso Adewole
Honoraria: GlaxoSmithKline
Silvia de Sanjose
Research Funding: Merck (Inst), GlaxoSmithKline (Inst)
REFERENCES
- 1.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: Comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 3. International Agency for Research on Cancer: GLOBOCAN 2012 cervical cancer: Estimated incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
- 4.Chuang LT, Temin S, Berek JS. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline summary. J Oncol Pract. 2016;12:693–696. doi: 10.1200/JCO.2016.68.3789. [DOI] [PubMed] [Google Scholar]
- 5.Jeronimo J, Castle PE, Temin S, et al. Secondary prevention of cervical cancer: ASCO resource-stratified clinical practice guideline. J Glob Oncol. 2017;3:635–657. doi: 10.1200/JGO.2016.006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Sanjosé S, Serrano B, Castellsagué X, et al. Human papillomavirus (HPV) and related cancers in the Global Alliance for Vaccines and Immunization (GAVI) countries: A WHO/ICO HPV Information Centre report. Vaccine. 30 doi: 10.1016/S0264-410X(12)01435-1. D1-D83, vi, 2012 (suppl 4) [DOI] [PubMed] [Google Scholar]
- 7.de Sanjose S, Wheeler CM, Quint WG, et al. Age-specific occurrence of HPV16- and HPV18-related cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1313–1318. doi: 10.1158/1055-9965.EPI-13-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 10.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 11.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males: Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–1708. [PubMed] [Google Scholar]
- 13. European Medicines Agency: Gardasil 9: EPAR—Summary for the public. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003852/human_med_001863.jsp&mid=WC0b01ac058001d124.
- 14. Centers for Disease Control and Prevention: Provider information: Gardasil 9 vaccine information statements. http://www.cdc.gov/vaccines/hcp/vis/vis-statements/hpv-gardasil-9.html.
- 15.Petrosky E, Bocchini JA, Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler CM, Castellsagué X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 17.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson BO, Shyyan R, Eniu A, et al. Breast cancer in limited-resource countries: An overview of the Breast Health Global Initiative 2005 guidelines. Breast J. 2006;12(suppl 1):S3–S15. doi: 10.1111/j.1075-122X.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 19.Horton S, Gauvreau CL. Cancer in low- and middle-income countries: An economic overview. In: Gelband H, Jha P, Sankaranarayanan R, et al., editors. Cancer Disease Control Priorities (ed 3) Washington, DC: International Bank for Reconstruction and Development/World Bank; 2015. [PubMed] [Google Scholar]
- 20. ADAPTE Collaboration: The ADAPTE process: Guideline adaptation—A resource toolkit (version 2.0). http://www.g-i-n.net/document-store/working-groups-documents/adaptation/adapte-resource-toolkit-guideline-adaptation-2-0.pdf.
- 21.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiffman RN, Michel G, Rosenfeld RM, et al. Building better guidelines with BRIDGE-Wiz: Development and evaluation of a software assistant to promote clarity, transparency, and implementability. J Am Med Inform Assoc. 2012;19:94–101. doi: 10.1136/amiajnl-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization Meeting of the strategic advisory group of experts on immunization, April 2014: Conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:221–236. [PubMed] [Google Scholar]
- 25.Couto E, Sæterdal I, Juvet LK, et al. HPV catch-up vaccination of young women: A systematic review and meta-analysis. BMC Public Health. 2014;14:867. doi: 10.1186/1471-2458-14-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Public Health Agency of Canada National Advisory Committee on Immunization: Update on the recommended human papillomavirus (HPV) vaccine immunization schedule. https://www.canada.ca/en/public-health/services/publications/healthy-living/update-recommended-human-papillomavirus-vaccine-immunization-schedule.html. [DOI] [PMC free article] [PubMed]
- 27.Gross G, Becker N, Brockmeyer NH, et al. Vaccination against HPV-associated neoplasias: Recommendations from the current S3 guideline of the HPV management forum of the Paul-Ehrlich Society—AWMF guidelines, Registry No. 082-002 (short version), valid until Dec. 31st, 2018. Geburtshilfe Frauenheilkd. 2014;74:233–241. doi: 10.1055/s-0033-1360170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Australian Government Department of Health: The Australian Immunisation Handbook (ed 10): 4.6 Human papillomavirus. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home∼handbook10part4∼handbook10-4-6#4-6-7.
- 29.Ben Hadj Yahia MB, Jouin-Bortolotti A, Dervaux B. Extending the human papillomavirus vaccination programme to include males in high-income countries: A systematic review of the cost-effectiveness studies. Clin Drug Investig. 2015;35:471–485. doi: 10.1007/s40261-015-0308-4. [DOI] [PubMed] [Google Scholar]
- 30.Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: A systematic review. Vaccine. 2013;31:3786–3804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong EP. Prophylaxis of cervical cancer and related cervical disease: A review of the cost-effectiveness of vaccination against oncogenic HPV types. J Manag Care Pharm. 2010;16:217–230. doi: 10.18553/jmcp.2010.16.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evidence based recommendations on human papillomavirus (HPV) vaccines schedules: Background paper for SAGE discussions. http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf?ua=1.
- 33.Centers for Disease Control and Prevention CDC recommends only two HPV shots for younger adolescents. www.cancerview.ca/TreatmentAndSupport/GRCMain/GRCSAGE/GRCSAGESearch/
- 34.World Health Organization Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89:465–491. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 36. US National Library of Medicine: National Information Center on Health Services Research and Health Care Technology (NICHSR). https://www.nlm.nih.gov/nichsr/hta101/ta10109.html.
- 37.Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA. 2013;309:1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 39.Krajden M, Cook D, Yu A, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clin Vaccine Immunol. 2011;18:418–423. doi: 10.1128/CVI.00489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sankaranarayanan R. 2 vs 3 doses HPV vaccine schedule: Low- and middle-income countries. Presented at the Eurogin 2013 International Multidisciplinary Congress, Florence, Italy, November 3-6, 2013. [Google Scholar]
- 41. Institut National de Santé Publique du Québec: La vaccination des pré-adolescents contre les virus du papillome humain (VPH) au Québec: Deux ou trois doses? http://www.inspq.qc.ca/pdf/publications/1683_VaccinPreAdoVPHQc_2ou3Doses.pdf.
- 42.Romanowski B, Schwarz TF, Ferguson LM, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: Results from a randomized study. Hum Vaccin Immunother. 2014;10:1155–1165. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romanowski B, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: Results from a randomized study. Hum Vaccin. 2011;7:1374–1386. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. GlaxoSmithKline: Evaluation of the safety and immunogenicity of GlaxoSmithKline Biologicals’ HPV vaccine 580299 when administered in healthy females aged 9 – 25 years using an alternative schedule and an alternative dosing as compared to the standard schedule and dosing. http://gsk-clinicalstudyregister.com/files2/ebe3f40a-ef27-469c-8874-35053b5a80d7.
- 45. European Medicines Agency Committee for Medicinal Products for Human Use (CHMP): Assessment report EMA/789820/2013: Cervarix. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000721/WC500160885.pdf.
- 46.Esposito S, Birlutiu V, Jarcuska P, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: Results from a randomized study. Pediatr Infect Dis J. 2011;30:e49–e55. doi: 10.1097/INF.0b013e318206c26e. [DOI] [PubMed] [Google Scholar]
- 47.Sankaranarayanan R. Evaluation of fewer than three doses of HPV vaccination in India. Presented at the WHO Consultation Meeting; Geneva, Switzerland: 2013. [Google Scholar]
- 48.Lazcano-Ponce E, Stanley M, Muñoz N, et al. Overcoming barriers to HPV vaccination: Non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months. Vaccine. 2014;32:725–732. doi: 10.1016/j.vaccine.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 49. Puthanakit T, Huang LM, Chiu CH, et al: Randomized open trial comparing 2-dose regimens of the human papillomavirus 16/18 AS04-adjuvanted vaccine in girls aged 9-14 years versus a 3-dose regimen in women aged 15-25 years. J Infect Dis 214:525-536, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garland SM, Stanley M, Brotherton J, et al. IPVS policy statement on safety of HPV vaccines. Papillomavirus Res. 2016;2:9–10. doi: 10.1016/j.pvr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Reporting Standards (CHEERS): Explanation and elaboration—A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Brisson M, Benard E, Drolet M. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: A systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Pub Health. 2016;1:e8–e17. doi: 10.1016/S2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015. Pediatr Infect Dis J. 2015;34:983–991. doi: 10.1097/INF.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 55.Garland SM, Ault KA, Gall SA, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: A combined analysis of five randomized controlled trials. Obstet Gynecol. 2009;114:1179–1188. doi: 10.1097/AOG.0b013e3181c2ca21. [DOI] [PubMed] [Google Scholar]
- 56.Brotherton JM. Safety of the quadrivalent human papillomavirus vaccine. BMJ. 2013;347:f5631. doi: 10.1136/bmj.f5631. [DOI] [PubMed] [Google Scholar]
- 57.Arnheim-Dahlström L, Pasternak B, Svanström H, et al. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: Cohort study. BMJ. 2013;347:f5906. doi: 10.1136/bmj.f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Global Advisory Committee on Vaccine Safety, 2-3 December 2015. Wkly Epidemiol Rec. 2016;91:21–31. [PubMed] [Google Scholar]
- 59.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829–846. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization . WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention: WHO Guidelines Approved by the Guidelines Review Committee. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 61.Hildesheim A, Gonzalez P, Kreimer AR, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016;215:212.e1–212.e15. doi: 10.1016/j.ajog.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hildesheim A, Wacholder S, Catteau G, et al. Efficacy of the HPV-16/18 vaccine: Final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32:5087–5097. doi: 10.1016/j.vaccine.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013;130:264–268. doi: 10.1016/j.ygyno.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 64.Joura EA, Garland SM, Paavonen J, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. European Medicines Agency: HPV vaccines: EMA confirms evidence does not support that they cause CRPS or POTS. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Human_papillomavirus_vaccines/human_referral_prac_000053.jsp&mid=WC0b01ac05805c516f.
- 66. Centers for Disease Control and Prevention, Food and Drug Administration: Vaccine Adverse Event Reporting System (VAERS). https://vaers.hhs.gov/index.
- 67. doi: 10.15585/mmwr.mm6549a5. Meites E, Kempe A, and Markowitz LE: Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 65:1405-1408, 2016. [DOI] [PubMed]
- 68.Garland SM, Subasinghe AK, Jayasinghe YL, et al. HPV vaccination for victims of childhood sexual abuse. Lancet. 2015;386:1919–1920. doi: 10.1016/S0140-6736(15)00757-6. [DOI] [PubMed] [Google Scholar]
- 69.Bailey HH, Chuang LT, duPont NC, et al. American Society of Clinical Oncology Statement: Human papillomavirus vaccination for cancer prevention. J Clin Oncol. 2016;34:1803–1812. doi: 10.1200/JCO.2016.67.2014. [DOI] [PubMed] [Google Scholar]
- 70.Dorell C, Yankey D, Kennedy A, et al. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: National Immunization Survey-Teen, 2010. Clin Pediatr (Phila) 2013;52:162–170. doi: 10.1177/0009922812468208. [DOI] [PubMed] [Google Scholar]
- 71.Gerend MA, Madkins K, Phillips G, II, et al. Predictors of human papillomavirus vaccination among young men who have sex with men. Sex Transm Dis. 2016;43:185–191. doi: 10.1097/OLQ.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerend MA, Shepherd MA, Lustria ML, et al. Predictors of provider recommendation for HPV vaccine among young adult men and women: Findings from a cross-sectional survey. Sex Transm Infect. 2016;92:104–107. doi: 10.1136/sextrans-2015-052088. [DOI] [PubMed] [Google Scholar]
- 73.Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19-26: Importance of a physician’s recommendation. Vaccine. 2011;29:890–895. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 74.Lehmann CE, Brady RC, Battley RO, et al. Adolescent vaccination strategies: Interventions to increase coverage. Paediatr Drugs. 2016;18:273–285. doi: 10.1007/s40272-016-0177-1. [DOI] [PubMed] [Google Scholar]
- 75.Bratic JS, Seyferth ER, Bocchini JA., Jr Update on barriers to human papillomavirus vaccination and effective strategies to promote vaccine acceptance. Curr Opin Pediatr. 2016;28:407–412. doi: 10.1097/MOP.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 76.Lee Mortensen G, Adam M, Idtaleb L. Parental attitudes towards male human papillomavirus vaccination: A pan-European cross-sectional survey. BMC Public Health. 2015;15:624. doi: 10.1186/s12889-015-1863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavi The Vaccine Alliance Countries eligible for support. http://www.gavi.org/support/apply/countries-eligible-for-support
- 78.Kiatpongsan S, Kim JJ. Costs and cost-effectiveness of 9-valent human papillomavirus (HPV) vaccination in two East African countries. PLoS One. 2014;9:e106836. doi: 10.1371/journal.pone.0106836. [DOI] [PMC free article] [PubMed] [Google Scholar]


