Abstract
In chronic hepatitis B virus (HBV)-infected patients, T helper 17 (Th17) cells are significantly elevated. Th17 cells initiate immune-mediated pathogenesis and have a critical role in the process of HBV-related liver cirrhosis (HBV-LC). The mechanisms underlying this process are attributed to Th17-secreted cytokines, which include interleukin (IL)-17, IL-21 and IL-22; however, a systemic analysis regarding these mechanisms has yet to be conducted. Therefore, the present study aimed to investigate the role of Th17 cells in the pathogenesis of HBV-LC. All randomized clinical trials, case series, case reports and meta-analyses that contained the aforementioned keywords were included in the review process. In addition, unpublished information from the Food and Drug Administration was included. The findings indicated that Th17-secreted cytokines, including IL-17, IL-21 and IL-22, function by activating or silencing hepatic stellate cells, modulating proinflammatory and pro- or antifibrogenic effectors, regulating extracellular matrix formation, upregulating chemokine expression, and inducing hepatocellular damage or hepatoprotection during the HBV-LC process. In addition, Th17 cells and Th17-secreted cytokines may be considered a potential tool in the diagnosis or treatment of HBV-LC. The present review summarized the role of Th17 cells in the pathogenesis of HBV-LC in order to deepen the clinical understanding of the role of Th17 cells and also to support the development of effective therapies for patients with HBV-LC.
Keywords: Th17 cells, interleukin-17, interleukin-21, interleukin-22, chronic hepatitis B virus, fibrosis
1. Introduction
Despite the availability of effective hepatitis B virus (HBV) vaccines, 350–400 million patients worldwide still suffer from chronic HBV infection. In addition, approximately half of the total liver cancer mortalities are attributable to the HBV infection and the worldwide mortality rates associated with liver cancer has increased by 62% in the last 12 years (1). HBV-related liver cirrhosis (HBV-LC) is the primary cause of liver disease-associated morbidity or mortality worldwide (2,3). Excessive accumulation of extracellular matrix components can eventually progress to liver cirrhosis. In addition, liver inflammation or hepatic injury can result in liver cirrhosis. A key step in the development of liver fibrogenesis is the activation of hematopoietic stem cells (HSCs) and the production of a large amount of α-smooth muscle action (α-SMA) and collagen (4,5). Previous studies have suggested that host immunological (6,7) and virological factors (8) may influence the progression of liver fibrosis. It is well known that fibrosis is suppressed by T helper (Th)1 cytokines, whereas Th2 cytokines promote fibrosis (9). Animal models of cell inactivation or depletion have indicated a role for natural killer T (NKT) and natural killer (NK) cells in the regulation of HSC activation and development of liver fibrosis (10–13). In addition, Th17 cells have been reported to be important in the development of liver cirrhosis, particularly in patients with chronic HBV infection (14,15).
Th17 cells are a subset of cluster of differentiation (CD)4+ Th cells, which are characterized by the production of the following cytokines: Interleukin (IL)-17, IL-21, IL-22, IL-26 and tumor necrosis factor (TNF)-α. Th17 cells represent a proinflammatory cell subtype. The formation, development and function of Th17 cells differ from Th1 and Th2 cells (16,17). Transforming growth factor (TGF)-β and proinflammatory cytokines, such as IL-6 and IL-1, serve a crucial, initial role in the process of Th17 cell differentiation (18,19). In addition, the autocrine growth factor IL-21 further accelerates amplification of the process via signal transducer and activator of transcription 3 (STAT3) signal transduction (20,21). IL-23 is able to stabilize and further amplify these cells due to its actions on its receptor complex, after which sequential activation of Janus kinase 2 and STAT3 in Th17 cells occurs (16,22–25). The cytokines secreted by Th17 cells are associated with various functions, including inflammation, regulation and epithelial restitution (26). The present review aimed to discuss the detailed mechanisms of Th17 cells and Th17-associated cytokines, which function by activating or silencing HSCs, modulating proinflammatory and pro- or antifibrogenic effectors, regulating extracellular matrix formation, upregulating chemokine expression, and inducing hepatocellular damage or hepatoprotective effects. An indexed Medline search (https://www.nlm.nih.gov/databases/download/pubmed_medline.html) was conducted in June 2016, using the following keywords: ‘Th-17’, ‘IL-17’, ‘IL-21’, ‘IL-22’, ‘cirrhosis’, ‘chronic hepatitis B viral infection’ and ‘fibrosis’; the search included studies published between 1995 and 2016. This review integrated the most up-to-date evidence, in order to present a comprehensive overview of the progression of HBV-LC.
2. Characteristics of Th17-secreted cytokines
IL-17 is an emerging cytokine family that consists of six family members (termed IL-17A through IL-17F), which are encoded by separate genes. IL-17A has been characterized as a major effector cytokine that is secreted by Th17 cells when the RAR-related orphan receptor transcription factor is activated (27). This process is critical for T cell priming in viral infections (28). Almost all liver cell types have been reported to express IL-17 receptor (IL-17R), including HSCs, biliary epithelial cells, hepatocytes, Kuppfer cells (KCs), biliary epithelial cells and liver sinusoidal endothelial cells (29). When active, IL-17 binds to either IL-17RA or IL-17RC, which are two isoforms of its cognate receptor; subsequently, IL-17 exerts its function (30,31). IL-17 activates various signaling pathways, including the microRNA-23b and nuclear factor-κB, and mitogen-activated protein kinases (MAPKs) pathways (32,33). In vitro treatment with IL-17 has previously been demonstrated to induce expression of C-reactive protein in hepatocytes (34). Furthermore, numerous chemokines and proinflammatory cytokines have been reported to be expressed in HSCs, KCs and biliary epithelial cells (35,36). Therefore, the complex roles of IL-17, which have been observed in murine models of liver disease (35), may be attributed to its effects on various liver cell types.
Th17 cells produce IL-21, which activates CD4+ T cells, NKT cells and follicular helper T cells. IL-21 is a type I cytokine and its receptor is the common cytokine receptor chain (37,38). IL-21 exerts pleiotropic effects due to the broad cellular distribution of IL-21 receptor, which includes CD8+ and CD4+ T cells, dendritic cells, NK cells, B cells, keratinocytes and macrophages (37,39). IL-21 initiates B cell terminal differentiation into plasma cells, regulates immunoglobulin production, induces Th17 differentiation and cooperatively expands CD8+ T cells (40,41). These findings indicated that IL-21 may limit viral persistence and trigger inflammatory pathways, which may promote tissue damage in various organs (42,43).
Th17, Th22 and NK cells produce IL-22, which is an IL-10 cytokine family member (44). IL-22 binds to a cell-surface complex comprising IL-22 receptor 1 (IL-22R1) and IL-10 receptor 2 (IL-10R2) chains; this activity is regulated by interactions with IL-22 binding protein, which is a soluble binding protein. IL-22R1 and IL-10R2 share sequence similarity with an extracellular region of IL-22R1 (45,46). Only cells of epithelial origin express IL-22R1, including liver progenitor cells and hepatocytes (47). Activation of the STAT3 signaling cascade, and the AKT and MAPK pathways, results from targeting of the IL-22R complex, which is composed of IL-22R1 and IL-10R2 (48–50). Acute inflammatory proteins are expressed in response to the IL-22-mediated induction of these signaling pathways (48). In addition, proliferative and/or anti-apoptotic programs are activated and various tissue-specific genes are induced, including serum amyloid A, mucins and antimicrobial proteins (50).
3. The function of Th17-secreted cytokines in HBV-LC
The main factors associated with HBV-LC are considered to be HBV-initiated liver injury and chronic inflammation (49). In addition, the host immune response has a critical role in HBV-LC progression. Previous studies have indicated that Th17 cell-mediated inflammation is closely associated with the progression of chronic HBV infection to fibrosis, and consequently to cirrhosis. In previous studies, patients with HBV-LC exhibited an increase in the percentage of Th17 cells (51,52), and it has been suggested that peripheral Th17 cell frequency and serum IL-17 levels may assist in predicting the severity of liver damage and fibrosis (51–53). An imbalance between Th17 and regulatory T cells (Tregs) may also serve a critical role in the progression of HBV-LC. The number of Th17 cells was markedly increased in patients with HBV-LC; an increased frequency of circulating Th17 cells was positively associated with the severity of liver fibrosis, however, nTreg frequency was negatively associated with Th17 frequency in the cohort of patients with HBV (51,52). A potential mechanism is that an imbalance in Th17/Tregs may influence fibrotic progression by promoting HSC activation and increasing liver injury (15). Therefore, the ratio of Th17/Tregs may have predictive value with regards to the early development of HBV-LC (54). Furthermore, increased intrahepatic Th17 cells were closely associated with fibrotic staging scores and clinical progression from chronic HBV infection to HBV-LC, and several Th17 cells were located in fibrotic areas from patients with HBV-LC (51). Notably, the function of Th17 cells is mediated by the production of numerous cytokines, including IL-17, IL-21 and IL-22. Further studies have focused on Th17-associated cytokines, and indicated that they have a significant role in the progression of HBV-LC (14,55).
Significantly increased expression of IL-17 has been detected in HBV-LC, which is strongly correlated with the degree of fibrosis (51,56). In addition, it has been reported that intrahepatic IL-17 is predominantly localized in the region of fibrosis (57). Transplantation of bone marrow-derived stem cells ameliorates HBV-LC, due to the downregulation of IL-17 expression (58). In HepG2 cells, periostin is induced following IL-17 treatment; therefore, this may serve as a novel biomarker in the early diagnosis of HBV-LC. In addition, activation of the CB2 receptor decreases the severity of HBV-LC by selectively reducing IL-17 production in Th17 lymphocytes, which occurs via the STAT5-dependent pathway, and inhibits IL-17-induced proinflammatory gene expression (59). Therefore, IL-17 may be critical in the pathogenesis of HBV-LC (60–63). It has been hypothesized that the mechanism underlying this process is the IL-17 signaling pathway. IL-17RA signaling in HSCs and KCs serves an important role in promoting liver fibrogenesis, by facilitating TNF-α, IL-6 and IL-1 production and increasing the expression of TGF-β, which is a fibrogenic cytokine. In addition, IL-17 directly induces production of collagen type I in HSCs through activation of the STAT3 signaling pathway (55). Activation of HSCs and the production of collagen are IL-17A-dependent, according to a previous study by Tan et al (64), whereas pharmacological inhibition of extracellular signal-regulated kinase 1/2 or p38 significantly attenuated IL-17A-induced HSC activation and collagen expression. Fabre et al (65) reported that there may be a novel profibrotic function for IL-17A, since activation of the c-Jun N-terminal kinase pathway by IL-17A was revealed to enhance the response of HSCs to TGF-β. Furthermore, IL-17 has been demonstrated to act via stabilization and upregulation of TGF-βRII, which leads to an increase in SMAD2/3 signaling (65). Treatment with IL-17 upregulated the levels of collagen, α-SMA and TGF-β in primary mouse HSCs and LX-2 cells, and activated various signaling pathways in KCs, which subsequently stimulated KCs to produce proinflammatory (TGF-β, IL-6 and TNF-α) and profibrotic cytokines, including collagen, α-SMA and TGF-β, which promote liver fibrogenesis (35,55). In addition, IL-17 exposure has been revealed to upregulate the expression of chemokines, such as IL-8 and growth related oncogene-alpha (GRO-α), in vitro (51). Therefore, IL-17 may be considered to serve a critical role in the pathogenesis of HBV-LC (Fig. 1) and suppressing the production of IL-17A may potentially benefit patients with HBV-LC.
Figure 1.
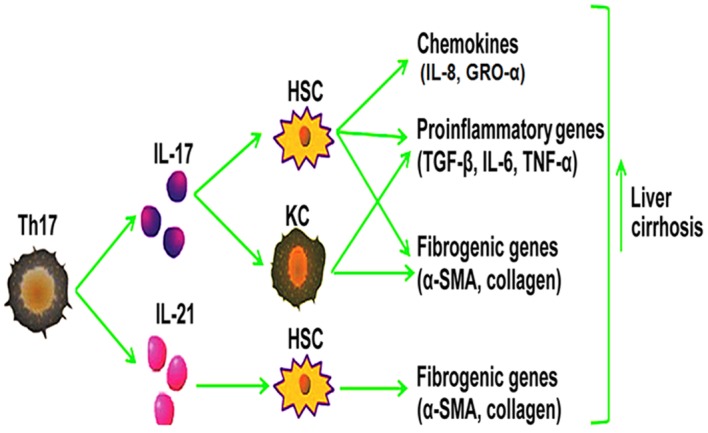
IL-17 and IL-21 serve similar roles in modulating liver cirrhosis. IL-17 promotes liver fibrosis by inducing HSC and KC activation, subsequently resulting in the upregulation of chemokines (IL-8, GRO-α) in HSCs, and fibrogenic (α-SMA, collagen) and proinflammatory genes (TGF-β, IL-6, TNF-α) in HSCs and KCs. In addition, IL-21, which is produced by Th17 cells, induces HSC activation, resulting in the production of fibrogenic genes (α-SMA, collagen), which promotes the development of liver fibrosis. IL, interleukin; GRO-α, growth related oncogene-α; HSC, hepatic stellate cell; KC, Kupffer cell; Th17, T helper 17 cells; α-SMA, α-smooth muscle actin; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
As aforementioned, increased levels of peripheral and intrahepatic Th17 cells have been demonstrated to contribute to the severity of HBV-LC progression via the induction of HSC activation; this raises the possibility that IL-21 may be involved in the process of liver fibrogenesis (66). Therefore, IL-21 may serve a role in liver fibrogenesis. Patients with HBV-LC have been reported to exhibit elevated plasma IL-21 levels and increased peripheral numbers of IL-21+CD4+ T cells, which were shown to be closely associated with fibrotic staging scores and fibrotic progression from chronic HBV infection to HBV-LC (67). Animal studies have demonstrated that IL-21 acts as a vital profibrogenic factor in vivo by contributing to the development of CD4+ Th2 responses (68). Furthermore, a previous study indicated that in vitro IL-21 administration was accompanied by increased expression of α-SMA, upregulated collagen production by LX-2 cells and LX-2 cell apoptotic inhibition (67). These results indicated that IL-21 may contribute to fibrogenesis of HBV-LC via activation of HSCs (Fig. 1).
Notably, IL-22 is able to exert a dual function in HBV infection, serving protective or proinflammatory roles depending on the hepatic injury conditions (i.e., acute vs. chronic) and the type of liver damage model analyzed. Therefore, the role of IL-22 in patients with HBV-LC remains controversial. In a previous study by Xiang et al (69), hepatic IL-22 expression was inversely correlated with inflammatory grades and fibrosis staging in HBV-infected patients. However, Kronenberger et al (70) reported that the elevation of systemic IL-22 may be used to predict reduced survival in patients with advanced liver cirrhosis. In addition, intrahepatic IL-22-producing cells were positively associated with the severity of liver cirrhosis in HBV-infected patients. Zhao et al (71) demonstrated that the expression of numerous IL-22 pathway-associated genes was significantly upregulated in HBV-infected liver tissues. This study also indicated that liver-infiltrating IL-22+ cells were increased in patients with HBV-LC compared with in patients without HBV-LC or healthy controls. Liver-infiltrating IL-22+ cells were also positively associated with liver fibrosis staging scores.
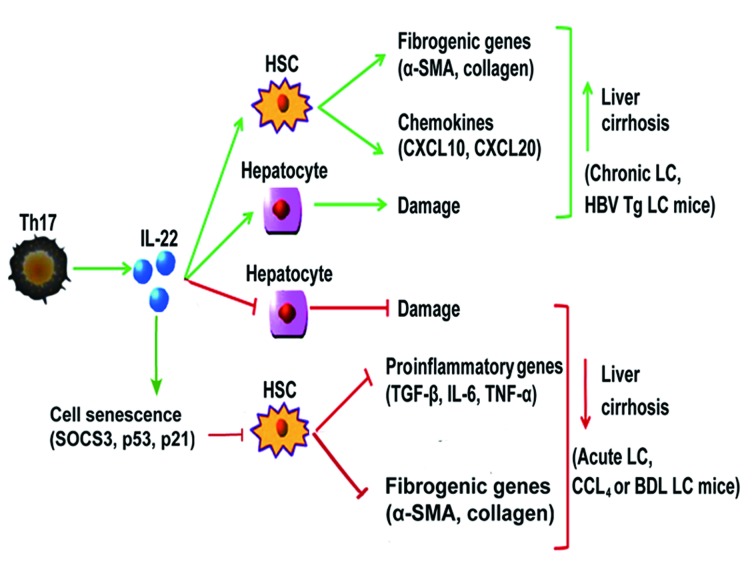
Mechanisms have been further explored in murine models. Studies that have used carbon tetrachloride (CCL4)- or bile duct ligation (BDL)-induced liver cirrhosis murine models demonstrated that IL-22 acts as an important antifibrotic cytokine. Overexpression of IL-22 in CCL4-or BDL-induced liver cirrhosis murine models was associated with accelerated resolution of liver cirrhosis during recovery, reduced liver cirrhosis, an increased number of senescence-associated β-galactosidase-positive HSCs, and decreased α-SMA expression in cultured HSCs in vitro and fibrotic livers in vivo (55,72,73). Furthermore, HSC activation was attenuated and the expression levels of inflammatory cytokines, including TGF-β, IL-6 and TNF-α, were downregulated in response to recombinant mouse IL-22 in a murine model of CCL4-induced liver fibrosis (74). These effects resulted in the amelioration of liver fibrogenesis. In addition, IL-22 treatment resulted in the upregulation of suppressor of cytokine signaling (SOCS)3 expression in HSCs, which bound p53, and subsequently increased the expression of p53 and its target genes (72); this contributed to IL-22-mediated HSC senescence in CCL4-induced liver cirrhosis. It is well known that IL-22R1 expression is restricted to cells with an epithelial origin, including hepatocytes and liver progenitor cells, during acute liver inflammation; this is due to targeting of the IL-22R complex, which is composed of IL-22R1 and IL-10R2. IL-22 promotes liver regeneration and protects against liver injury (75–79). Collectively, IL-22 may be considered an important antifibrotic cytokine in CCL4 or BDL-induced liver fibrosis models via its hepatoprotective effects and ability to directly induce HSC senescence (Fig. 2).
Figure 2.
IL-22 serves a dual role in the process of liver cirrhosis. In chronic or HBV Tg-induced liver cirrhosis, IL-22 promotes liver cirrhosis by activating HSCs, which upregulates chemokines (CXCL10, CXCL20) and fibrogenic genes (α-SMA, collagen) or promotes hepatocellular damage. Conversely, in acute, or CCL4- and BDL-induced liver cirrhosis, IL-22 induces the expression of SOCS3, p53 and p21 in HSCs, which contributes to IL-22-mediated HSC senescence, subsequently downregulating fibrogenic (α-SMA, collagen) and proinflammatory genes (TGF-β, IL-6, TNF-α), and thus protecting against hepatocellular damage. IL, interleukin; HBV, hepatitis B virus; Tg, transgenic; HSCs, hepatic stellate cells; Th17, T helper 17 cells; CXCL10, chemokine (C-X-C motif) ligand 10; CXCL20, chemokine (C-C motif) ligand 20; α-SMA, α-smooth muscle actin; CCL4, carbon tetrachloride; BDL, bile duct ligation; TGF-β, transforming growth factor-β; SOCS3, suppressor of cytokine signaling 3; LC, liver cirrhosis; TNF-α, tumor necrosis factor-α.
BDL- or CCL4-induced liver fibrosis murine models are not suitable for studying immune-mediated liver fibrotic progression in human HBV infection as they do not mimic conditions in humans, which induce severe hepatocyte damage (72). HBV transgenic (Tg) mice given repeated injections of anti-CD137 antibodies exhibited an induced T cell immune response, which subsequently resulted in chronic liver inflammation; this condition mimics human HBV-associated liver disease progression (80). A previous study by Zhao et al (71), which employed a HBV Tg mouse model that can mimic human HBV-associated liver disease, demonstrated that the suppression of IL-22 attenuates Th17 cell infiltration and liver cirrhosis. This study also indicated that IL-22 stimulates HSCs to secrete chemokine (C-X-C motif) ligand 10 and chemokine (C-C motif) ligand 20, which subsequently promotes Th17 cell chemotaxis. These results suggested that IL-22 serves a pathological role by exacerbating fibrosis and chronic liver inflammation via the recruitment of hepatic Th17 cells in HBV Tg mice. In addition, the enhanced number of Th17 cells in HBV-infected livers produced more IL-22. This resulted in the generation of a positive feedback loop, which ultimately promoted liver fibrosis during inflammation and chronic liver injury. Wu et al (81) reported similar results, in that administration of IL-22 inhibited LX-2 cell apoptosis, increased LX-2 cell proliferation, increased α-SMA expression and upregulated production of collagen by LX-2 cells. In addition, elevated IL-22 promoted liver inflammation and subsequently resulted in liver injury and exacerbation of liver fibrosis in anti-CD137-treated HBV Tg mice (80). These findings indicated that IL-22 may contribute to fibrogenesis by activating HSCs and promoting liver injury (Fig. 2).
In conclusion, the pro- and antifibrotic functions of IL-22 may depend on hepatocyte damage or protection, and on HSC senescence or activation.
4. Future perspectives
Numerous studies regarding Th17 cells in chronic HBV infection have demonstrated the critical function of Th17 cells in the progression of liver fibrosis/cirrhosis. These findings improved understanding regarding the role of Th17 cells or Th17-secreted cytokines in the process of HBV-LC. Although significant progress has been made on the functions of Th17 cells and Th17-secreted cytokines, the mechanisms underlying HBV-LC remain poorly defined in patients. To more thoroughly characterize the intrahepatic features that influence Th17 cell functions in patients with HBV-LC, it is necessary to study large HBV-infected patient populations with well-defined clinical cohorts, and to use more physiological animal models of viral infection. In conclusion, confirming whether specific Th17 cells or Th17-secreted cytokines may be used as biomarkers to aid in the diagnosis and evaluation of disease progression or prognosis will greatly benefit patients. In addition, future studies should aim to determine whether the application of treatment interventions, which specifically target Th17 cells and Th17-secreted cytokines, is useful to relieve disease symptoms, progression and prognosis.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81101240 and 81371821) and the Major Science and Technology Special Project of China (grant nos. 2012zx10002003 and 2013zx10002004). The authors would like to thank Dr Chong Huang (Department of Infectious Diseases, Huanshan Hospital, Fudan University) for his critical comments on this manuscript.
References
- 1.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI200524282C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrion-Caude A, Flamant C, Roussey M, Housset C, Flahault A, Fryer AA, Chadelat K, Strange RC, Clement A. Liver disease in pediatric patients with cystic fibrosis is associated with glutathione S-transferase P1 polymorphism. Hepatology. 2002;36:913–917. doi: 10.1053/jhep.2002.35534. [DOI] [PubMed] [Google Scholar]
- 7.Xiao F, Wei H, Song S, Li G, Song C. Polymorphisms in the promoter region of the angiotensinogen gene are associated with liver cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2006;21:1488–1491. doi: 10.1111/j.1440-1746.2006.04527.x. [DOI] [PubMed] [Google Scholar]
- 8.Martín-Vílchez S, Sanz-Cameno P, Rodríguez-Muñoz Y, Majano PL, Molina-Jiménez F, López-Cabrera M, Moreno-Otero R, Lara-Pezzi E. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology. 2008;47:1872–1883. doi: 10.1002/hep.22265. [DOI] [PubMed] [Google Scholar]
- 9.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 11.Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: Involvement of natural killer T cells. Hepatology. 2011;53:219–229. doi: 10.1002/hep.23983. [DOI] [PubMed] [Google Scholar]
- 14.Gao B, Waisman A. Th17 cells regulate liver fibrosis by targeting multiple cell types: Many birds with one stone. Gastroenterology. 2012;143:536–539. doi: 10.1053/j.gastro.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Qiu SJ, She WM, Wang FP, Gao H, Li L, Tu CT, Wang JY, Shen XZ, Jiang W. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One. 2012;7:e39307. doi: 10.1371/journal.pone.0039307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 19.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 20.Caprioli F, Sarra M, Caruso R, Stolfi C, Fina D, Sica G, MacDonald TT, Pallone F, Monteleone G. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800–1807. doi: 10.4049/jimmunol.180.3.1800. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 24.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.O'Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 29.Ge D, You Z. Expression of interleukin-17RC protein in normal human tissues. Int Arch Med. 2008;1:19. doi: 10.1186/1755-7682-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosani U, Varotto L, Gerdol M, Pallavicini A, Venier P. IL-17 signaling components in bivalves: Comparative sequence analysis and involvement in the immune responses. Dev Comp Immunol. 2015;52:255–268. doi: 10.1016/j.dci.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: Interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 32.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 34.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem. 2007;282:27229–27238. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafdil F, Wang H, Park O, Zhang W, Moritoki Y, Yin S, Fu XY, Gershwin ME, Lian ZX, Gao B. Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology. 2009;137(2125–2135):e1–e2. doi: 10.1053/j.gastro.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 37.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: Novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 38.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 40.Spolski R, Leonard WJ. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 41.Spolski R, Leonard WJ. The Yin and Yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fantini MC, Monteleone G, MacDonald TT. IL-21 comes of age as a regulator of effector T cells in the gut. Mucosal Immunol. 2008;1:110–115. doi: 10.1038/mi.2007.17. [DOI] [PubMed] [Google Scholar]
- 43.Kwok SK, Cho ML, Park MK, Oh HJ, Park JS, Her YM, Lee SY, Youn J, Ju JH, Park KS, et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012;64:740–751. doi: 10.1002/art.33390. [DOI] [PubMed] [Google Scholar]
- 44.Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 45.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W, Jr, Murphy AJ, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobleigh MA, Robek MD. Protective and pathological properties of IL-22 in liver disease: Implications for viral hepatitis. Am J Pathol. 2013;182:21–28. doi: 10.1016/j.ajpath.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 48.Ciccia F, Accardo-Palumbo A, Alessandro R, Rizzo A, Principe S, Peralta S, Raiata F, Giardina A, De Leo G, Triolo G, et al. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 2012;64:1869–1878. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- 49.El Bassuoni MA, Soliman MA, El Megeed NA, Al Gazar A. IL-17 producing cells and RORgammat mRNA transcriptional factor in cirrhotic and HCC egyptian patients. Egypt J Immunol. 2015;22:59–68. [PubMed] [Google Scholar]
- 50.Baba N, Rubio M, Kenins L, Regairaz C, Woisetschlager M, Carballido JM, Sarfati M. The aryl hydrocarbon receptor (AhR) ligand VAF347 selectively acts on monocytes and naive CD4 (+) Th cells to promote the development of IL-22-secreting Th cells. Hum Immunol. 2012;73:795–800. doi: 10.1016/j.humimm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396–403. doi: 10.1111/j.1365-2893.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang C, Cui F, Chen LM, Gong XY, Qin B. Correlation between Th17 and nTreg cell frequencies and the stages of progression in chronic hepatitis B. Mol Med Rep. 2016;13:853–859. doi: 10.3892/mmr.2015.4618. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Gong Y, Wang B, Shi K, Hou Y, Wang L, Lin Z, Han Y, Lu L, Chen D, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: Regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29:1620–1628. doi: 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Wang FP, She WM, Yang CQ, Li L, Tu CT, Wang JY, Jiang W. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat. 2014;21:129–140. doi: 10.1111/jvh.12152. [DOI] [PubMed] [Google Scholar]
- 55.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, et al. Interleukin-17 signaling in inflammatory, Kupffer cells and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143(765–776):e1–e3. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zepeda-Morales AS, Del Toro-Arreola S, García-Benavides L, Bastidas-Ramírez BE, Fafutis-Morris M, Pereira-Suárez AL, Bueno-Topete MR. Liver fibrosis in bile duct-ligated rats correlates with increased hepatic IL-17 and TGF-β2 expression. Ann Hepatol. 2016;15:418–426. doi: 10.5604/16652681.1198820. [DOI] [PubMed] [Google Scholar]
- 57.Macek Jilkova Z, Afzal S, Marche H, Decaens T, Sturm N, Jouvin-Marche E, Huard B, Marche PN. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17 (+) neutrophils. Liver Int. 2016;36:1116–1124. doi: 10.1111/liv.13060. [DOI] [PubMed] [Google Scholar]
- 58.Zheng L, Chu J, Shi Y, Zhou X, Tan L, Li Q, Cui L, Han Z, Han Y, Fan D. Bone marrow-derived stem cells ameliorate hepatic fibrosis by down-regulating interleukin-17. Cell Biosci. 2013;3:46. doi: 10.1186/2045-3701-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, Mallat A, Lotersztajn S, Lafdil F. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology. 2014;59:296–306. doi: 10.1002/hep.26598. [DOI] [PubMed] [Google Scholar]
- 60.Shi M, Wei J, Dong J, Meng W, Ma J, Wang T, Wang N, Wang Y. Function of interleukin-17 and −35 in the blood of patients with hepatitis B-related liver cirrhosis. Mol Med Rep. 2015;11:121–126. doi: 10.3892/mmr.2014.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H, Zhang D, Wang S, Wang X, Yang C. Significance of correlation between interferon-γ and soluble intercellular adhesion molecule-1 and interleukin-17 in hepatitis B virus-related cirrhosis. Clin Res Hepatol Gastroenterol. 2013;37:608–613. doi: 10.1016/j.clinre.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Du WJ, Zhen JH, Zeng ZQ, Zheng ZM, Xu Y, Qin LY, Chen SJ. Expression of interleukin-17 associated with disease progression and liver fibrosis with hepatitis B virus infection: IL-17 in HBV infection. Diagn Pathol. 2013;8:40. doi: 10.1186/1746-1596-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Chen S, Xu K. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis. Int J Mol Med. 2011;27:385–392. doi: 10.3892/ijmm.2011.594. [DOI] [PubMed] [Google Scholar]
- 64.Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835–1844. doi: 10.4049/jimmunol.1203013. [DOI] [PubMed] [Google Scholar]
- 65.Fabre T, Kared H, Friedman SL, Shoukry NH. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol. 2014;193:3925–3933. doi: 10.4049/jimmunol.1400861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X, Guo R, Ming D, Su M, Lin C, Deng Y, Lin Z, Su Z. Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor-β1/interleukin-17 to be associated with the development of hepatitis B virus-associated liver cirrhosis. J Gastroenterol Hepatol. 2014;29:1065–1072. doi: 10.1111/jgh.12459. [DOI] [PubMed] [Google Scholar]
- 67.Feng G, Zhang JY, Zeng QL, Yu X, Zhang Z, Lv S, Xu X, Wang FS. Interleukin-21 mediates hepatitis B virus-associated liver cirrhosis by activating hepatic stellate cells. Hepatol Res. 2014;44:E198–E205. doi: 10.1111/hepr.12215. [DOI] [PubMed] [Google Scholar]
- 68.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiang X, Gui H, King NJ, Cole L, Wang H, Xie Q, Bao S. IL-22 and non-ELR-CXC chemokine expression in chronic hepatitis B virus-infected liver. Immunol Cell Biol. 2012;90:611–619. doi: 10.1038/icb.2011.79. [DOI] [PubMed] [Google Scholar]
- 70.Kronenberger B, Rudloff I, Bachmann M, Brunner F, Kapper L, Filmann N, Waidmann O, Herrmann E, Pfeilschifter J, Zeuzem S, et al. Interleukin-22 predicts severity and death in advanced liver cirrhosis: A prospective cohort study. BMC Med. 2012;10:102. doi: 10.1186/1741-7015-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, Jin L, Zhou C, Fu J, Gao B, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology. 2014;59:1331–1342. doi: 10.1002/hep.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsumoto A, Kanai T, Mikami Y, Chu PS, Nakamoto N, Ebinuma H, Saito H, Sato T, Yagita H, Hibi T. IL-22-producing RORγt-dependent innate lymphoid cells play a novel protective role in murine acute hepatitis. PLoS One. 2013;8:e62853. doi: 10.1371/journal.pone.0062853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, Wang JX, Ma SJ, Yang XW, Jiang HX. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol. 2015;21:1531–1545. doi: 10.3748/wjg.v21.i5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang YM, Liu ZR, Cui ZL, Yang C, Yang L, Li Y, Shen ZY. Interleukin-22 contributes to liver regeneration in mice with concanavalin A-induced hepatitis after hepatectomy. World J Gastroenterol. 2016;22:2081–2091. doi: 10.3748/wjg.v22.i6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikoopour E, Bellemore SM, Singh B. IL-22, cell regeneration and autoimmunity. Cytokine. 2015;74:35–42. doi: 10.1016/j.cyto.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Saalim M, Resham S, Manzoor S, Ahmad H, Jaleel S, Ashraf J, Imran M, Naseem S. IL-22: A promising candidate to inhibit viral-induced liver disease progression and hepatocellular carcinoma. Tumour Biol. 2016;37:105–114. doi: 10.1007/s13277-015-4294-1. [DOI] [PubMed] [Google Scholar]
- 78.Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Zhao W, Cheng L, Guo M, Li D, Li X, Tan Y, Ma S, Li S, Yang Y, et al. CD137-mediated pathogenesis from chronic hepatitis to hepatocellular carcinoma in hepatitis B virus-transgenic mice. J Immunol. 2010;185:7654–7662. doi: 10.4049/jimmunol.1000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu LY, Liu S, Liu Y, Guo C, Li H, Li W, Jin X, Zhang K, Zhao P, Wei L, Zhao J. Up-regulation of interleukin-22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C. Clin Immunol. 2015;158:77–87. doi: 10.1016/j.clim.2015.03.003. [DOI] [PubMed] [Google Scholar]