Abstract
Prolonged action potential duration, reduced action potential firing rate, upstroke velocity and rate of diastolic depolarization have been demonstrated in atrioventricular node (AVN) cells from streptozotocin (STZ)-induced diabetic rats. To further clarify the molecular basis of these electrical disturbances, the mRNA profiles encoding a variety of proteins associated with the generation and conduction of electrical activity in the AVN, were evaluated in the STZ-induced diabetic rat heart. Expression of mRNA was measured in AVN biopsies using reverse transcription-quantitative polymerase chain reaction techniques. Notable differences in mRNA expression included upregulation of genes encoding membrane and intracellular Ca2+ transport, including solute carrier family 8 member A1, transient receptor potential channel 1, ryanodine receptor 2/3, hyperpolarization-activated cyclic-nucleotide 2 and 3, calcium channel voltage-dependent, β2 subunit and sodium channels 3a, 4a, 7a and 3b. In addition to this, potassium channels potassium voltage-gated channel subfamily A member 4, potassium channel calcium activated intermediate/small conductance subfamily N α member 2, potassium voltage-gated channel subfamily J members 3, 5, and 11, potassium channel subfamily K members 1, 2, 3 and natriuretic peptide B (BNP) were upregulated in AVN of STZ heart, compared with controls. Alterations in gene expression were associated with upregulation of various proteins including the inwardly rectifying, potassium channel Kir3.4, NCX1 and BNP. The present study demonstrated notable differences in the profile of mRNA encoding proteins associated with the generation, conduction and regulation of electrical signals in the AVN of the STZ-induced diabetic rat heart. These data will provide a basis for a substantial range of future studies to investigate whether variations in mRNA translate into alterations in electrophysiological function.
Keywords: mRNA, heart, atrioventricular node, diabetes mellitus, Streptozotocin-induced diabetic rat
Introduction
Diabetes mellitus (DM) is a serious global health problem and there is clear evidence of the negative influence of diabetes on the prevalence, severity, and prognosis of cardiovascular disease (1). Disorders of the vasculature, particularly coronary artery disease and hypertension, increase the incidence of mortality in individuals with DM (2). Diabetic patients are at greater risk of developing heart problems that are independent of vascular dysfunction which is indicative of a distinct diabetic cardiomyopathy (3,4). Impaired contractile function including a reduction in amplitude and depressed time course of contraction and relaxation of ventricular myocytes have been demonstrated in experimental models of DM including the streptozotocin (STZ)-induced diabetic rat (5–10). These changes in contractility have been partly attributed to alterations in Ca2+ transport including elevated diastolic Ca2+ and depression in amplitude and prolonged time course of the intracellular Ca2+ transient (6–8,11,12). Mechanisms underlying the alterations in Ca2+ transient include impaired sarcoplasmic reticulum (SR) Ca2+ transport and suppressed L-type Ca2+ current and Na+/Ca2+ exchange current (5–7,11–15). DM also has profound effects on the electrical conduction system of the heart which may give rise to arhythmogenic activity. Prolongation of the QT interval and QRS complex correlate with an increased incidence of sudden cardiac death in diabetic patients (16,17). Atrial fibrillation is prevalent and there is a higher incidence of atrioventricular block in diabetic patients compared to healthy controls (17–20).
Previous in vivo biotelemetry and isolated perfused heart studies have demonstrated reduced heart rate in the STZ rat (21–23). Slowing of electrical conduction has also been demonstrated in diabetic rat myocardium (24). Various experimental studies in animal models of DM have variously demonstrated changes in ion channel activity including depressed L-type calcium current, transient outward potassium current, rapid and slow delayed potassium rectifier currents all of which can result in a prolongation of action potential duration and reduced heart rate (23,25–33). DM can increase the duration of the sinoatrial node (SAN) action potential and prolong sino-atrial node conduction time and pacemaker cycle length which is associated with alterations in intercellular gap junctional coupling (23,34). Previous studies in STZ rat have demonstrated a variety of changes in mRNA, and in some cases proteins, that are important to the generation of action potentials in the SAN (35). Increased duration of the action potential in STZ-induced diabetic rat AVN has been attributed to a leftward shift in the zero current potential under voltage clamp, a reduction in peak L-type Ca2+ current density and reduced amplitude of delayed rectifier and hyperpolarization-activated currents (32). L-type calcium channels are fundamental to normal activity in the atrioventricular node (AVN) region and L-type calcium current contributes to the late stages of the pacemaker potential and generation of the action potential upstroke, and is responsible for the timing of conduction velocity through the AVN, thereby contributing to PR interval duration.
Previous studies have demonstrated increased action potential duration associated with a reduced action potential firing rate that is associated with reductions in L-type calcium current, delayed rectifier and hyperpolarization-activated currents in AVN cells from STZ-induced diabetic rat (32,33). Modification of ion channel properties either by altered trafficking and expression, or post-translational modification of channel gating properties, can therefore have a significant impact on AVN function, and result in clinical AVN abnormalities. To further clarify the molecular basis of electrical disturbances in the AVN of diabetic heart the profile of mRNA that encodes a wide variety of proteins that are associated with the generation and conduction of electrical activity in the AVN has been evaluated in the STZ-induced diabetic rat heart.
Materials and methods
Experimental protocol
Forty male Wistar rats aged 8 weeks were divided into 2 subgroups. All animals received normal rat chow and drinking water ad libitum. One subgroup of rats received STZ/citrate buffer (60 mg/kg, intraperitoneal) whilst the other subgroup received citrate buffer alone. Blood glucose was measured 5 days following STZ treatment to confirm diabetes. Experiments began 12 weeks after STZ treatment. Body weight, heart weight and blood glucose were measured immediately prior to experiments. Approval for this study was obtained from the Animal Ethics Committee, College of Medicine and Health Sciences, United Arab Emirates University.
Expression of mRNA
Expression of genes encoding a wide range of cardiac proteins was assessed using previously described techniques with small modifications (35). After animals were sacrificed the hearts were removed rapidly and placed in a dish containing: NaCl 140 mM; KCl 5.4 mM; MgCl2 1 mM; HEPES 5 mM; D-glucose 5.5 mM; CaCl2 1.8 mM and adjusted to pH 7.4 with NaOH. Hearts were dissected and 2 mm biopsy samples of AVN were carefully collected from 20 STZ and 20 control hearts as illustrated in Fig. 1 and according to previously described techniques (36–38). Immediately after removal AVN samples were immersed in RNAlater (AM7021; Life Technologies, Carlsbad, CA, USA) and stored overnight at room temperature to allow thorough penetration of the tissue. AVN samples were then frozen at −20°C pending further processing. Samples were homogenized at 6,500 rpm for 2 runs of 20 sec each with a 15 sec gap (Preceylls 24; Bertin Technologies, Raleigh, NC, USA). The SV Total RNA Isolation system (Promega, Madison, WI, USA) was used to isolate total RNA. The concentration and purity of the RNA was determined by measuring the ratio of absorbance at 260 nm and 280 nm (ND-1000; NanoDrop). A two-step RT-PCR procedure was used to generate cDNA. Total RNA (500 ng) was converted into cDNA in a 25 µl PCR reaction with 10x RT Buffer 2.0 µl, 25x dNTP Mix (100 mM) 0.8 µl, 10x RT Random Primers 2.0 µl, MultiScribe™ Reverse Transcriptase 1.0 µl, RNase inhibitor 1.0 µl, and nuclease-free H2O (High Capacity cDNA Reverse Transcription kit (4374966; Applied Biosystems, Foster, CA, USA). Reverse transcription was carried out using the following protocol: 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min on the Veriti thermal cycler (Applied Biosystems). Gene Expression Assays were performed using custom TaqMan Low Density Arrays (Format 32, 4346799; Applied Biosystems). The TaqMan assays are pre-loaded in each reaction well of the array in triplicate for each RNA sample. As in previous experiments in heart 18S RNA was used as an endogenous control (39,40). Expression of 18S was not significantly different (P>0.05) between AVN samples collected from STZ and control hearts. cDNA (RNA-equivalent) (100 ng) was loaded together with 2x TaqMan Gene Expression Master Mix (No AmpErase UNG; Applied Biosystems) for a total of 100 µl per port. Two AVN samples were combined for each real-time RT-PCR assay. Real-time RT-PCR was performed in a Fast ABI Prism 7900HT Sequence Detection system (Applied Biosystems). The PCR thermal cycling parameters were run in standard mode as follows: 50°C for 2 min, 94.5°C for 10 min, followed by 40 cycles of 97°C for 30 sec and 59.7°C for 1 min. Results were initially analyzed using ABI Prism 7900HT SDS, v2.4. Calculations and statistical analysis were performed by the SDS RQ Manager 1.1.4 software using the 2−ΔΔCt method with a relative quantification RQmin/RQmax confidence set at 95%. A list of the target genes, proteins and protein descriptions are shown in Table I.
Figure 1.
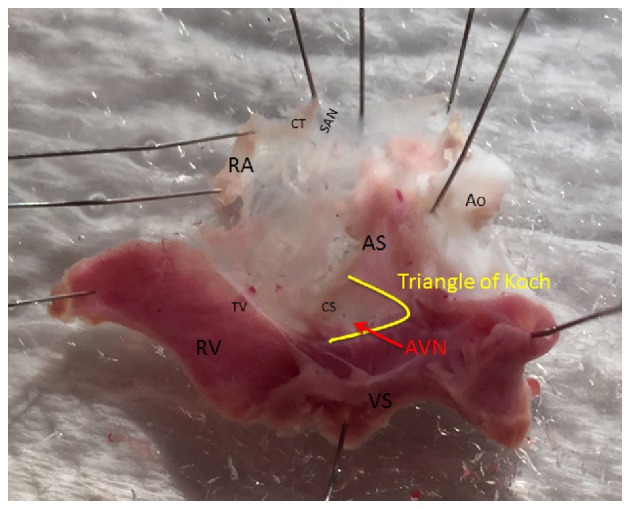
Dissection of the atrioventricular node junction in a typical control heart showing the location where tissue samples were collected. CT, crista terminalis; RA, right atrium; TV, tricuspid valve; RV, right ventricle; VS, ventricular septum; AS, atrial septum; CS, coronary sinus; SAN, sinoatrial node; AVN, atrioventricular node; Ao, Aorta.
Table I.
Target genes and proteins.
| Genes | Proteins | Protein descriptions |
|---|---|---|
| Intercellular proteins | ||
| Gja1 | Cx43 | Connexin43 |
| Gja5 | Cx40 | Connexin40 |
| Gjc1 | Cx45 | Connexin45 |
| Gjd3 | Cx31.9 | Connexin31.9 |
| Cell membrane transport | ||
| Atp1a1 | Na/K ATPase, α1 | ATPase, Na+/K+ transporting, α1 polypeptide |
| Atp1a2 | Na/K ATPase, α2 | ATPase, Na+/K+ transporting, α2 polypeptide |
| Atp1a3 | Na/K ATPase, α3 | ATPase, Na+/K+ transporting, α3 polypeptide |
| Atp1b1 | Na/K ATPase, β1 | ATPase, Na+/K+ transporting, β1 polypeptide |
| Atp2b1 | Na/K ATPase, β2 | ATPase, Ca++ transporting, plasma membrane 1 |
| Slc8a1 | NCX1 | Solute carrier family 8 (sodium/calcium exchanger), member 1 |
| Trpc1 | TRPC1 | Transient receptor potential channel 1 |
| Trpc3 | TRPC3 | Transient receptor potential channel 3 |
| Trpc4 | TRPC4 | Transient receptor potential channel 4 |
| Trpc6 | TRPC6 | Transient receptor potential channel 6 |
| Intracellular Ca2+ transport and Ca2+ regulation | ||
| Atp2a2 | SERCA2 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 |
| Calm1 | Calm1 | Calmodulin1 |
| Calm3 | Calm3 | Calmodulin3 |
| Casq2 | Casq2 | Calsequestrin 2 |
| Itpr1 | IP3R1 | Inositol 1,4,5-trisphosphate receptor, type 1 |
| Itpr2 | IP3R2 | Inositol 1,4,5-trisphosphate receptor, type 2 |
| Itpr3 | IP3R3 | Inositol 1,4,5-trisphosphate receptor, type 3 |
| Ryr2 | RYR2 | Ryanodine receptor 2 |
| Ryr3 | RYR3 | Ryanodine receptor 3 |
| Pln | PLB | Phospholamban |
| Hyperpolarization-activated cyclic nucleotide-gated channels | ||
| Hcn1 | HCN1 | Hyperpolarization-activated cyclic nucleotide-gated channels 1 |
| Hcn2 | HCN2 | Hyperpolarization-activated cyclic nucleotide-gated channels 2 |
| Hcn3 | HCN3 | Hyperpolarization-activated cyclic nucleotide-gated channels 3 |
| Hcn4 | HCN4 | Hyperpolarization-activated cyclic nucleotide-gated channels 4 |
| Calcium channels | ||
| Cacna1c | Cav1.2 | Voltage-dependent, L type, α1C subunit |
| Cacna1d | Cav1.3 | Voltage-dependent, L type, α1D subunit |
| Cacna1g | Cav3.1 | Voltage-dependent, T type, α1G subunit |
| Cacna1h | Cav3.2 | Voltage-dependent, T type, α1H subunit |
| Cacna2d1 | Cavα2δ1 | Voltage-dependent, α2/δ subunit 1 |
| Cacna2d2 | Cavα2δ2 | Voltage-dependent, α2/δ subunit 2 |
| Cacna2d3 | Cavα2δ3 | Voltage-dependent, α2/δ subunit 3 |
| Cacnb1 | Cavβ1 | Voltage-dependent, β1 subunit |
| Cacnb2 | Cavβ2 | Voltage-dependent, β2 subunit |
| Cacnb3 | Cavβ3 | Voltage-dependent, β3 subunit |
| Cacng4 | Cavγ4 | Voltage-dependent, γ subunit 4 |
| Cacng7 | Cavγ7 | Voltage-dependent, γ subunit 7 |
| Sodium channels | ||
| Scn1a | Nav1.1 | Voltage gated, type Iα subunit |
| Scn3a | Nav1.3 | Voltage gated, type IIIα subunit |
| Scn4a | Nav1.4 | Voltage gated, type IVα subunit |
| Scn5a | Nav1.5 | Voltage gated, type V, α subunit |
| Scn7a | Nav2.1 | Voltage gated, type VII, α subunit |
| Scn8a | Nav1.6 | Voltage gated, type VIII, α subunit |
| Scn1b | Navβ1 | Voltage gated, type I, β subunit |
| Scn2b | Navβ2 | Voltage gated, type II, β subunit |
| Scn3b | Navβ3 | Voltage gated, type III, β subunit |
| Potassium channels | ||
| Kcna1 | Kv1.1 | Voltage gated shaker related subfamily A, member 1 |
| Kcna2 | Kv1.2 | Voltage gated shaker related subfamily A, member 2 |
| Kcna3 | Kv1.3 | Voltage gated shaker related subfamily A, member 3 |
| Kcna4 | Kv1.4 | Voltage gated shaker related subfamily A, member 4 |
| Kcna5 | Kv1.5 | Voltage gated shaker related subfamily A, member 5 |
| Kcna6 | Kv1.6 | Voltage gated shaker related subfamily A, member 6 |
| Kcnb1 | Kv2.1 | Voltage gated shab related subfamily B, member 1 |
| Kcnd1 | Kv4.1 | Voltage gated shal related subfamily D, member 1 |
| Kcnd2 | Kv4.2 | Voltage gated shal related subfamily D, member 2 |
| Kcnd3 | Kv4.3 | Voltage gated shal related subfamily D, member 3 |
| Kcnh2 | ERG-1 | Ether-a-go-go-related protein 1 |
| Kcnip2 | KChIP2 | Kv channel interacting protein 2 |
| Kcnn1 | SK1 | Ca++ activated intermediate/small conductance subfamily n α, member 1 |
| Kcnn2 | SK2 | Ca++ activated intermediate/small conductance subfamily n α, member 2 |
| Kcnn3 | SK3 | Ca++ activated intermediate/small conductance subfamily n α, member 3 |
| Kcnq1 | Kv7.1 | Voltage gated KQT-like subfamily Q, member 1 |
| Kcnj2 | Kir2.1 | Inwardly rectifying subfamily J, member 2 |
| Kcnj3 | Kir3.1 | Inwardly rectifying subfamily J, member 3 |
| Kcnj5 | Kir3.4 | Inwardly rectifying subfamily J, member 5 |
| Kcnj8 | Kir6.1 | Inwardly rectifying subfamily J, member 8 |
| Kcnj11 | Kir6.2 | Inwardly rectifying subfamily J, member 11 |
| Kcnj12 | Kir2.2 | Inwardly rectifying subfamily J, member 12 |
| Kcnj14 | Kir2.4 | Inwardly rectifying subfamily J, member 14 |
| Kcnk1 | TWIK1 | Two pore domain subfamily K, member 1 |
| Kcnk2 | TREK1 | Two pore domain subfamily K, member 2 |
| Kcnk3 | K2P3.1 | Two pore domain subfamily K, member 3 |
| Kcnk5 | K2P5.1 | Two pore domain subfamily K, member 5 |
| Kcnk6 | TWIK2 | Two pore domain subfamily K, member 6 |
| Miscellaneous proteins | ||
| Abcc8 | SUR1 | ATP-binding cassette transporter sub-family C member 8 |
| Abcc9 | SUR2 | ATP-binding cassette, sub-family C member 9 |
| Nppa | ANP | Atrial natriuretic peptide |
| Nppb | BNP | Brain natriuretic peptide |
| Pias3 | KChAP | Protein inhibitor of activated STAT, 3 |
Expression of protein
Protein expression was measured using previously described SDS-PAGE and western blotting techniques with small modifications (35). AVN from STZ and control rats were carefully dissected, rinsed with ice-cold saline and homogenised in RIPA buffer (Tris 50 mM; NaCl 150 mM; Triton X 1%; sodium deoxylate 0.5%; SDS 0.1% adjusted to pH 7.4 and finally addition of PMSF 0.1 mM-Sigma, P7626) at 6,500 rpm for 2 runs of 20 sec each with a 15 sec gap (Preceylls 24; Bertin Technologies). Protein concentration was measured with Bio-Rad reagent. The supernatant was used for SDS-PAGE and western blotting. Protein (40 µg) was electrophoretically separated onto 8 or 10% (depending on the molecular weight of the protein to be separated) polyacrylamide gels and transferred onto nitrocellulose membranes. Expression of the specific proteins was confirmed by immunoreaction with their specific antibodies by western blot analysis. β-actin was used as a loading control. Blots were developed using the Pierce Western Blot kit. Images were obtained using the Typhoon FLA 9500, GE Healthcare Bio-Sciences AB (Uppsala, Sweden). Quantitation of protein was performed using the method described in the following website: http://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/.
For each lane the protein level was normalized to that of β-actin. The ratio of specific protein signal to that of the β-actin control was used to calculate fold-change.
Statistical analysis
Results were expressed as the mean ± SEM. of ‘n’ observations. Statistical comparisons were performed using independent sample t-test (SPSS vs. 20). P≤0.05 was considered to indicate a statistically significant difference.
Results
General characteristics: Bodyweight and heart weight were reduced and heart weight/bodyweight ratio was increased in STZ rats compared to controls. Blood glucose was elevated 5-fold in STZ rats compared to controls (Table II).
Table II.
General characteristics of streptozotocin-induced diabetic rats.
| Characteristics | Control | Streptozotocin |
|---|---|---|
| Bodyweight (g) | 401.56±9.68 | 246.44±13.28a |
| Heart weight (g) | 1.37±0.03 | 1.08±0.04a |
| Heart weight/bodyweight (mg/g) | 3.42±0.06 | 4.47±0.12a |
| Blood glucose (mg/dl) | 99.88±2.07 | 514.69±18.86a |
Data are presented as the mean ± standard error of the mean, n=16 hearts,
P<0.01.
Expression of mRNA
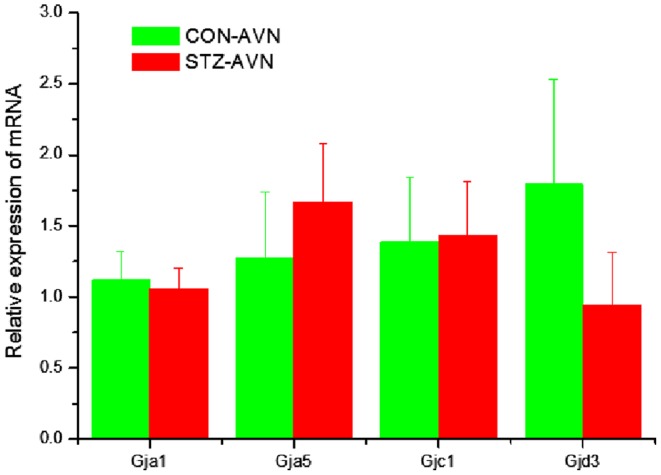
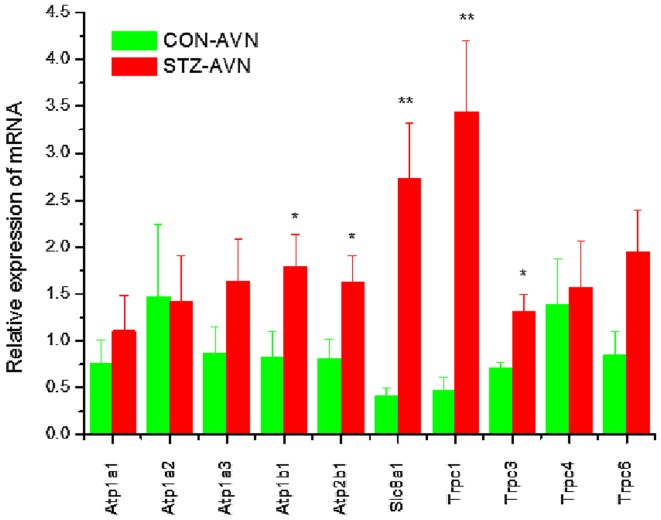
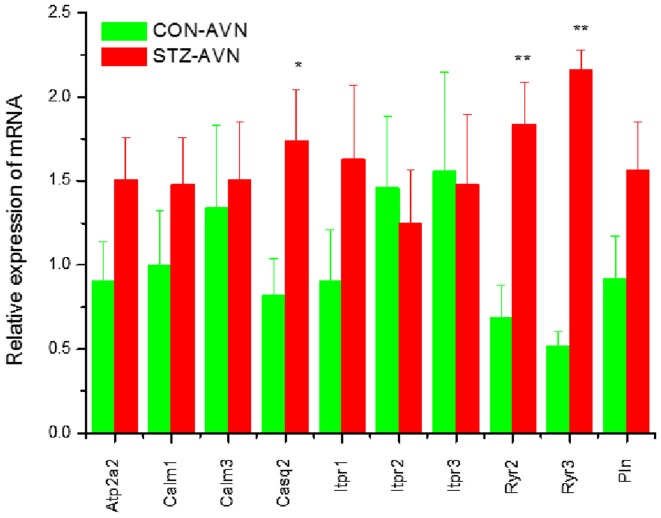
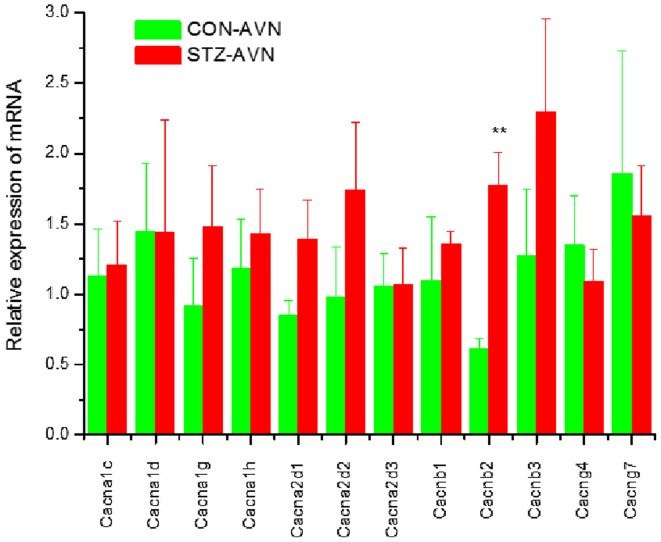
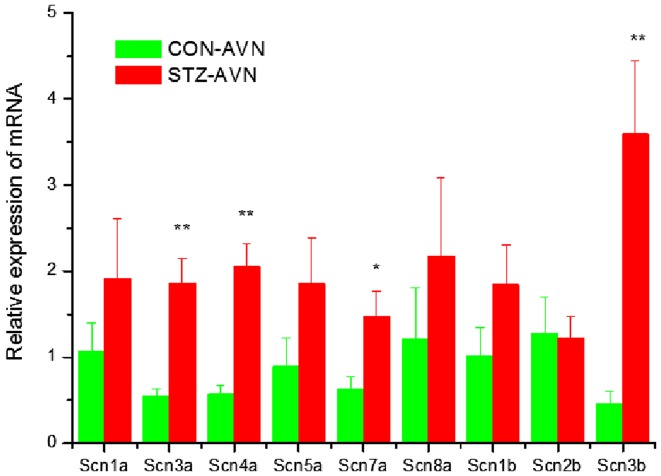
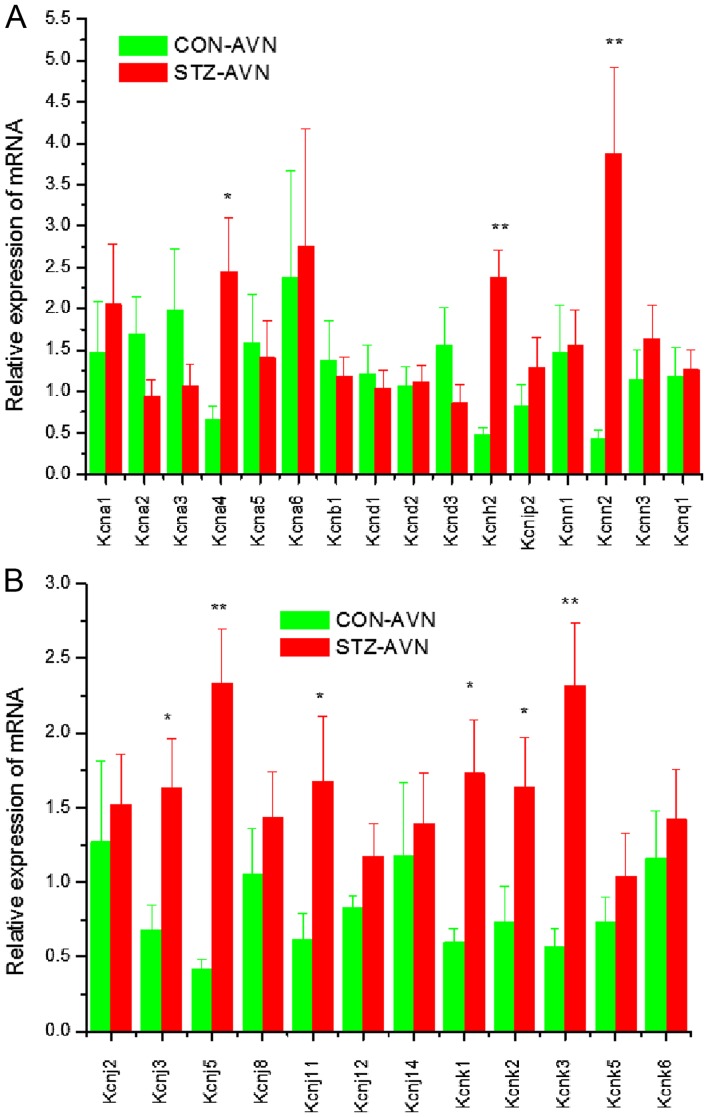
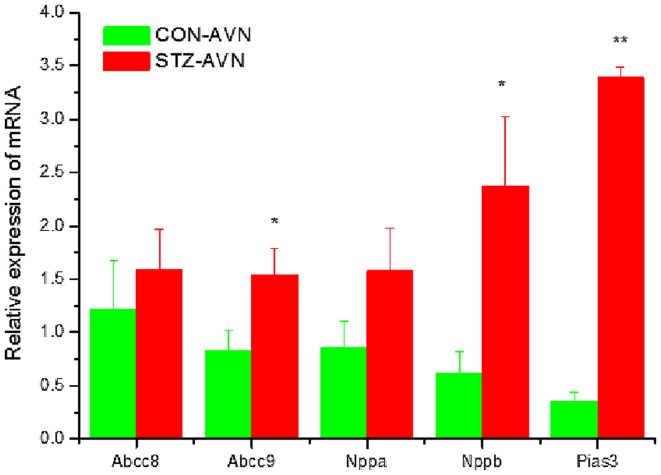
Expression of genes encoding intercellular proteins is shown in Fig. 2. There were no significant (P>0.05) differences in the expression of mRNA in AVN from STZ compared to control heart. Expression of genes encoding various cell membrane transport and intracellular Ca2+ transport and regulatory proteins are shown in Figs. 3 and 4, respectively. mRNA for Atp1b1 (2-fold), Atp2b1 (2-fold), Slc8a1 (6-fold), Trpc1 (7-fold), Trpc3 (2-fold), Casq2 (2-fold), Ryr2 (3-fold), Ryr3 (4-fold) were all significantly (P<0.05) upregulated in AVN from STZ compared to control heart. Expression of genes encoding the hyperpolarization-activated cyclic nucleotide-gated channel proteins are shown in Fig. 5. mRNA for Hcn2 (2-fold) and Hcn3 (9-fold) were significantly upregulated in AVN from STZ compared to control heart. Expression of genes encoding calcium channel proteins are shown in Fig. 6. mRNA for Cacnb2 (2-fold) was upregulated in AVN from STZ compared to control heart. Expression of genes encoding sodium channel proteins are shown in Fig. 7. mRNA for Scn3a (3-fold), Scn4a (3-fold), Scn7a (2-fold) and Scn3b (7-fold) were upregulated in AVN from STZ compared to control heart. Expression of genes encoding potassium channel proteins are shown in Fig. 8. mRNA for Kcna4 (3-fold), Kcnh2 (4-fold), Kcnn2 (9-fold), Kcnj3 (2-fold), Kcnj5 (5-fold), Kcnj11 (2-fold), Kcnk1 (2-fold), Kcnk2 (2-fold) and Kcnk3 (3-fold) were upregulated in AVN from STZ compared to control heart. Expression of genes encoding various miscellaneous proteins are shown in Fig. 9. mRNA for Abcc9 (2-fold), Nppb (3-fold) and Pias3 (8-fold) were upregulated in AVN from STZ compared to Control heart.
Figure 2.
Expression of genes encoding various intercellular proteins. Data are mean ± SEM, n=6–8 samples from STZ and control rat each containing samples from 2 hearts.
Figure 3.
Expression of genes encoding various cell membrane transport proteins. Data are mean ± SEM, n=5–8 samples from STZ and control rat each containing samples from 2 hearts. *P<0.05, **P<0.01 vs. CON-AVN.
Figure 4.
Expression of genes encoding various intracellular Ca2+ transport and Ca2+ regulation proteins. Data are mean ± SEM, n=7–8 samples from STZ and control rat each containing samples from 2 hearts. *P<0.05, **P<0.01 vs. CON-AVN.
Figure 5.
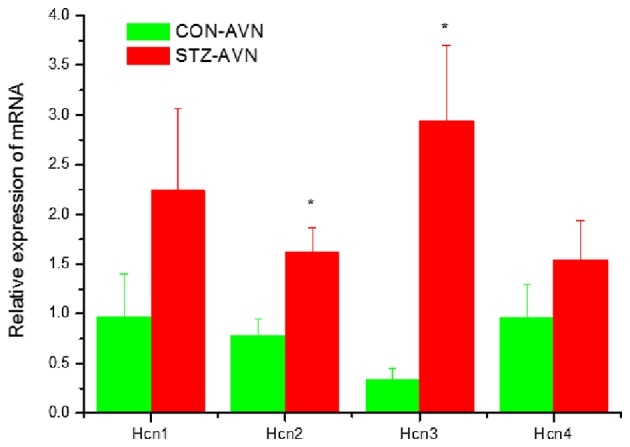
Expression of genes encoding various hyperpolarization-activated cyclic-nucleotide-gated channels. Data are mean ± SEM, n=5–8 samples from STZ and control rat each containing samples from 2 hearts. *P<0.05 vs. CON-AVN.
Figure 6.
Expression of genes encoding various calcium channel proteins. Data are mean ± SEM, n=5–9 samples from STZ and control rat each containing samples from 2 hearts. **P<0.01 vs. CON-AVN.
Figure 7.
Expression of genes encoding various sodium channel proteins. Data are mean ± SEM, n=5–9 samples from STZ and control rat each containing samples from 2 hearts. *P<0.05, **P<0.01 vs. CON-AVN.
Figure 8.
(A and B) Expression of genes encoding various potassium channel proteins. Data are mean ± SEM, n=5–8 samples from STZ and control rat each containing samples from 2 hearts. *P<0.05, **P<0.01 vs. CON-AVN.
Figure 9.
Expression of genes encoding miscellaneous cardiac proteins. Data are mean ± SEM, n=6–8 samples from STZ and control rat each containing samples from 2 hearts. *P<0.05, **P<0.01 vs. CON-AVN.
Expression of proteins
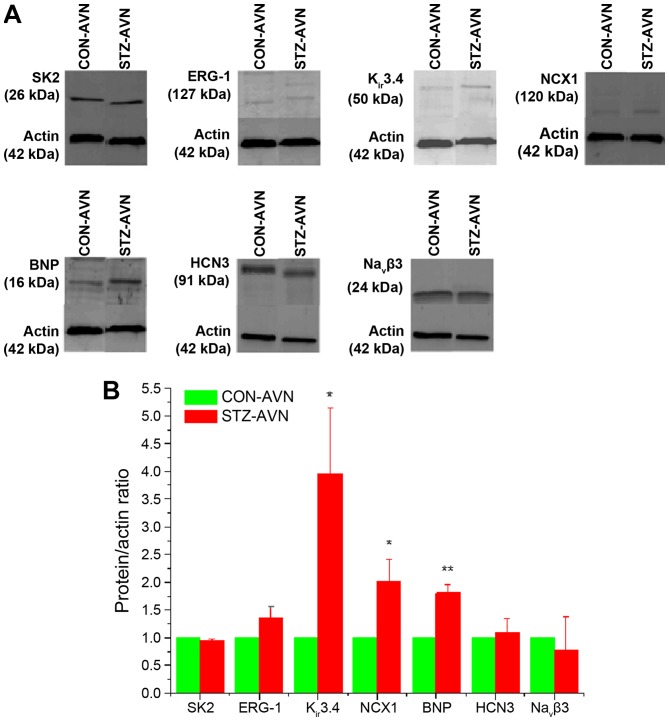
Representative Western blots comparing various proteins from STZ and control AVN are shown in Fig. 10A. The protein/actin ratio for the different proteins are shown in Fig. 10B. Expression of Kir3.4, NCX1 and BNP were significantly upregulated and SK2, ERG-1, HCN3 and Navβ3 were not significantly altered in AVN from STZ compared to control heart.
Figure 10.
(A) Typical western blot comparing expression of various proteins from STZ and control AVN. β-actin which was used as the loading control is also shown in each blot. The blots shown are representative of 6 individual samples from STZ and control rats. (B) Protein/β-actin ratios for the different proteins. Data are mean ± SEM, n=6 samples from STZ and control rat each containing 3 pooled AVNs from a total of 18 hearts AVN. *P<0.05, **P<0.01 vs. CON-AVN.
Discussion
Diabetes in the STZ-induced diabetic rat was characterized by reduced bodyweight and heart weight and increased heart weight/bodyweight ratio and a 5-fold increase in blood glucose. Major findings of this study included: i) upregulation of Slc8a1 mRNA and NCX1 protein; ii) upregulation of Trpc1 and Trpc3 mRNA; iii) upregulation of Ryr2 and Ryr3 mRNA; iv) upregulation of Hcn2 and Hcn3; v) upregulation of Cacnb2 mRNA; vi) upregulation of Scn3a/4a/7a and Scn3b; vii) upregulation of Kcna4, Kcnh2, Kcnn2, Kcnj3/11 and Kcnk2/3 mRNA and Kcnj5 mRNA and Kir3.4 protein; and viii) upregulation of Nppb mRNA and BNP protein in AVN from STZ compared to control heart.
Slc8a1 mRNA and NCX1 protein were upregulated in AVN from STZ rat heart. Upregulation of Slc8a1 has also recently been reported in the SAN from STZ-induced diabetic rat (35). The Slc8 gene family encodes the Na+/Ca2+ exchanger (NCX). Altered expression and regulation of NCX proteins contribute to abnormal Ca2+ homeostasis in heart failure, arrhythmia, hypertension and diabetes (41). Impaired Ca2+ homeostasis, due to depressed SR Ca2+ ATPase and NCX activity and NCX current have been demonstrated in ventricular myocytes from STZ-induced diabetic heart (6,42,43). The functional importance of NCX current in rabbit and mouse AVN cells has been demonstrated and depressed L-type Ca2+ current has been reported in AVN cells from STZ rat heart (32,33,44–46). Upregulation of Slc8a1 mRNA and NCX1 protein in the AVN from STZ heart may provide a compensatory pathway to facilitate Ca2+ influx and/or Ca2+ efflux from the cell which may be required for the generation and recovery of action potentials in AVN cells.
Trpc1 and Trpc3 were upregulated in AVN from STZ rat heart. Upregulation of Trpc1 has also recently been reported in the SAN from STZ-induced diabetic rat (35). The transient receptor potential channels (TRPCs) are a large family of non-selective and non-voltage-gated ion channels that convey signaling information linked to a broad range of sensory inputs including neurohormonal and mechanical load stimulation (47). TRPC1 is a mechano-sensitive, non-selective cation channel which is expressed in ventricle and atrium in a variety of mammalian species including rat (48–50). TRPC1 functions in Ca2+ influx, and its upregulation is involved in the development of cardiac hypertrophy (51). TRPC1 is also expressed in mouse SAN and in single pacemaker cells and mouse SAN may exhibit store-operated Ca2+ channel (SOCC) activity which may suggest that SOCCs are involved in regulating pacemaker firing rate (52). Upregulation of Trpc1 may be a consequence of hemodynamic disturbances in the diabetic heart which in turn stimulates mechano-sensitive TRPC channels thereby providing an alternative entry pathway for Ca2+ in the face of depressed L-type Ca2+ current in AVN cells and reduced heart rate in STZ-induced diabetic heart (32,33). Previous studies have reported elevation of mean arterial pressure in STZ-induced diabetic rats which may in turn lead to hypertrophy of the heart which would be consistent with the increase in heart weight to body weight ratio, which in turn might elicit an effect on the mechano-sensitive channels (53).
Ryr2 and Ryr3 were upregulated in AVN from STZ rat heart. The Ryr family of genes encode proteins that form the SR Ca2+ release channel. SR Ca2+ cycling is important for the genesis of spontaneous activity in the AVN (44,54–56). Whilst little is known about the effects of diabetes on SR Ca2+ signaling in the AVN, previous studies have demonstrated depressed SR Ca2+ loading, Ca2+ uptake/release and Ca2+ leak in ventricular myocytes from STZ-induced diabetic heart (6,12,15,57). These disturbances in SR Ca2+ signaling have been variously attributed to structural and/or functional defects of the RYR2 receptors or Ca2+-ATPase (SERCA) pump proteins (12,15,58–61). It was also interesting to note upregulation of Casq2, an SR Ca2+ binding protein, in AVN from STZ rat. Upregulation of Ryr2 may facilitate release of Ca2+ from the SR which in turn might compensate for depressed L-type Ca2+ current in AVN from STZ compared to control heart (32,33).
Hcn2 and Hcn3 were upregulated in AVN from STZ rat heart. Upregulation of Hcn3 was only associated with a small increase in HCN3 protein in AVN from STZ compared to control heart. The funny current which is conducted through the hyperpolarization-activated cyclic nucleotide channels plays a key role in the generation of the pacemaker current and hence, rhythmicity of the heart. Previous studies have demonstrated reduced action potential firing rates accompanied by a reduction in the amplitude of funny current, L-type Ca2+ current and delayed rectifier current in AVN cells from STZ-induced diabetic heart (32,33). In the longer term, as DM progresses, upregulation of Hcn2 and/or Hcn3, if accompanied by an increase in HCN2 and HCN3 protein, might result in an increase in the conductance of funny current and steepening of the slope of the pacemaker potential, which in turn would increase heart rate.
Cacnb2 was upregulated in AVN from STZ rat heart. Cacnb2 codes for the auxiliary β-subunit (Cavβ) which is an important modulator of Ca2+ channel activity. Expression of the β-subunit is required for normal function of cardiac L-type Ca2+ channels. Cavβ binds to the α1 pore-forming subunit of L-type Ca2+ channels and augments L-type Ca2+ current by facilitating channel opening and increasing the number of channels in the membrane (62). Several cardiovascular diseases including hypertension, heart failure and sudden cardiac death have been linked to Cacnb2 (63). Upregulation of Cacnb2 may facilitate L-type Ca2+ channel opening and provide a compensatory pathway for depressed L-type Ca2+ current in AVN cells from STZ compared to control heart (32,33). Interestingly, there were no significant changes in the expression of Cacna1c and Cacna1d suggesting that at this stage of diabetes (12 weeks after STZ treatment), whilst other changes in gene expression are taking place, changes to the α-1C and α-1D are not yet evident.
Scn3b and Scn4a were upregulated in AVN from STZ rat heart. Sodium channel SCN5a (Nav1.5) is regulated by four sodium channel auxiliary β subunits (SCN1-4b). Mutations in SCN3b have been associated with ventricular and atrial arrhythmias and altered electrophysiological properties of the sodium channel including reduced peak sodium current (64–66). Sodium channel SCN4A (Nav1.4) encodes the α-subunit of the voltage-gated sodium channel Nav1.4 and studies suggest the involvement of SCN4A variants in the pathophysiological mechanisms underlying arrhythmias in some patients with Brugada syndrome (67,68). The role of SCN3B and SCN4A in AVN remains to be clarified.
Kcna4, Kcnh2, Kcnj3/5/11, Kcnk2/3 and Kcnn2 were all upregulated in AVN from STZ rat heart. Upregulation of Kcnj5 and Kcnk3 have also recently been reported in the SAN from STZ-induced diabetic rat (35). Kir3.4 protein encoded by Kcnj5 was upregulated and ERG-1 encoded by Kcnh2 was unaltered in AVN from STZ compared to control heart. At this stage of diabetes (12 weeks after STZ treatment) alterations in gene expression might not translate into alterations in protein expression. Kcna4 encodes the Kv1.4 protein which forms the channel that carries transient outward current which makes a major contribution to the repolarizing current and termination of the cardiac action potential. Transient outward current has been widely demonstrated in different regions of the heart including ventricular myocytes and AVN cells (69–71). Kcnh2 encodes ERG-1 protein which is the α subunit of a potassium ion channel that mediates the repolarizing rapid delayed rectifier current (IKr) current in the cardiac action potential. Several studies have characterized the electrophysiological properties of IKr in AVN cells from various species including rabbit and mouse (54,71–73). IKr plays a role in both action potential repolarization and pacemaker depolarization (74,75). The Kcnj5 gene encodes the G-protein-activated inwardly rectifying potassium channel 4 and loss of the Kir3.4 gene strongly reduces cholinergic regulation of pacemaker activity in SAN cells and delays recovery of heart rate after stress, physical exercise or pharmacological β-adrenergic stimulation (76). Upregulation of Kcnj5 and Kir3.4 protein might partly underlie the slow heart rate in STZ rat heart (21–23). Kcnn2 encodes the small calcium-activated potassium channel member SK2. SK2 mRNA has been detected in a variety of organs including heart and within the heart expression of SK channels are more abundant in the atria and pacemaking tissues compared with the ventricles (77,78). Calcium-activated potassium channels are present in a variety of cells and serve to integrate changes in intracellular Ca2+ with changes in K+ conductance and membrane potential (79). Overexpression of SK2 channels result in shortening of the spontaneous action potentials of AVN cells and an increase in firing frequency whilst genetic knockout of SK2 channels result in the delay in atrial myocyte repolarization and atrial arrhythmias (80,81). Recent studies have demonstrated down-regulation of SK2 and prolonged action potentials in STZ-induced diabetic atria (82).
Nppb and BNP protein were upregulated in AVN from STZ rat heart. Upregulation of Nppa and Nppb have recently been reported in the SAN from STZ-induced diabetic rat (35). The natriuretic peptides are a family of related peptides that include atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) that are secreted from the cardiac atria and ventricles (83). ANP and BNP decrease blood pressure and cardiac hypertrophy and BNP acts locally to reduce ventricular fibrosis and they are both involved in the pathogenic mechanisms leading to major cardiovascular diseases, including heart failure, coronary heart diseases, hypertension and left ventricular hypertrophy (83–85). Previous studies have demonstrated increases in ANP and BNP in blood plasma and atrial tissues and varying effects of ANP and BNP on the amplitude and kinetics of shortening and intracellular Ca2+ in ventricular myocytes from STZ-induced diabetic rat (86,87). BNP has been shown to increase heart rate and electrical conduction velocity in isolated hearts and in the SAN and also increase spontaneous action potential frequency in isolated SAN myocytes (88). Upregulation of Nppb and BNP protein in the AVN may be associated with mechanisms that compensate for the low heart rate seen in the STZ-induced diabetic heart or alternatively be a consequence of the hypertrophy (21–23,86,87).
The SAN and AVN contribute to the generation and orderly propagation of electrical signals in the heart and it is interesting that the expression of genes that encode a variety of proteins involved in cardiac electrical transmission are similarly altered in the SAN and AVN of STZ-induced diabetic rat (35). This study has demonstrated differences in the profile of mRNA encoding a variety of proteins that are associated with the generation, conduction and regulation of electrical signals in the AVN of STZ-induced diabetic rat heart. Data from this study will provide a basis for a substantial range of future studies to investigate whether changes in mRNA translate into changes in electrophysiological function.
Acknowledgements
The present study was supported by a grant from the UAE University.
References
- 1.Julien J. Cardiac complications in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1997;11:123–130. doi: 10.1016/S1056-8727(96)00091-8. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–1019. doi: 10.1161/01.CIR.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 3.Piccini JP, Klein L, Gheorghiade M, Bonow RO. New insights into diastolic heart failure: Role of diabetes mellitus. Am J Med. 2004;116(Suppl 5A):S64–S75. doi: 10.1016/j.amjmed.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard RA, Bose D. Influence of experimental diabetes on sarcoplasmic reticulum function in rat ventricular muscle. Am J Physiol. 1991;260:H341–H354. doi: 10.1152/ajpheart.1991.260.2.H341. [DOI] [PubMed] [Google Scholar]
- 6.Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398–H1408. doi: 10.1152/ajpheart.00313.2002. [DOI] [PubMed] [Google Scholar]
- 7.Bracken N, Howarth FC, Singh J. Effects of streptozotocin-induced diabetes on contraction and calcium transport in rat ventricular cardiomyocytes. Ann N Y Acad Sci. 2006;1084:208–222. doi: 10.1196/annals.1372.018. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Sitasawad SL. Azelnidipine prevents cardiac dysfunction in streptozotocin-diabetic rats by reducing intracellular calcium accumulation, oxidative stress and apoptosis. Cardiovasc Diabetol. 2011;10:97. doi: 10.1186/1475-2840-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, Bidasee KR. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol (1985) 2009;106:1280–1292. doi: 10.1152/japplphysiol.91280.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Y, Ahmed S, Grupp IL, Matlib MA. Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol. 2001;281:H1137–H1147. doi: 10.1152/ajpheart.2001.281.3.H1137. [DOI] [PubMed] [Google Scholar]
- 11.Okatan EN, Tuncay E, Turan B. Cardioprotective effect of selenium via modulation of cardiac ryanodine receptor calcium release channels in diabetic rat cardiomyocytes through thioredoxin system. J Nutr Biochem. 2013;24:2110–2118. doi: 10.1016/j.jnutbio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Shao CH, Rozanski GJ, Patel KP, Bidasee KR. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol. 2007;42:234–246. doi: 10.1016/j.yjmcc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983;244:E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- 14.Schaffer SW, Ballard-Croft C, Boerth S, Allo SN. Mechanisms underlying depressed Na+/Ca2+ exchanger activity in the diabetic heart. Cardiovasc Res. 1997;34:129–136. doi: 10.1016/S0008-6363(97)00020-5. [DOI] [PubMed] [Google Scholar]
- 15.Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes. 2005;54:3082–3088. doi: 10.2337/diabetes.54.11.3082. [DOI] [PubMed] [Google Scholar]
- 16.Casis O, Echevarria E. Diabetic cardiomyopathy: Electromechanical cellular alterations. Curr Vasc Pharmacol. 2004;2:237–248. doi: 10.2174/1570161043385655. [DOI] [PubMed] [Google Scholar]
- 17.Movahed MR. Diabetes as a risk factor for cardiac conduction defects: A review. Diabetes Obes Metab. 2007;9:276–281. doi: 10.1111/j.1463-1326.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 18.Fairfax AJ, Leatham A. Idiopathic heart block: Association with vitiligo, thyroid disease, pernicious anaemia, and diabetes mellitus. Br Med J. 1975;4:322–324. doi: 10.1136/bmj.4.5992.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of third-degree atrioventricular block in patients with type II diabetes mellitus. Chest. 2005;128:2611–2614. doi: 10.1378/chest.128.4.2611. [DOI] [PubMed] [Google Scholar]
- 20.Kawai S, Fu L, Aziki K, Okada R, Katoh K. A degenerative lesion of the approach to the atrioventricular node producing second-degree and third-degree atrioventricular block. Pacing Clin Electrophysiol. 1992;15:2263–2269. doi: 10.1111/j.1540-8159.1992.tb04170.x. [DOI] [PubMed] [Google Scholar]
- 21.Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Effects of insulin treatment on heart rhythm, body temperature and physical activity in streptozotocin-induced diabetic rat. Clin Exp Pharmacol Physiol. 2006;33:327–331. doi: 10.1111/j.1440-1681.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- 22.Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol. 2005;90:827–835. doi: 10.1113/expphysiol.2004.029439. [DOI] [PubMed] [Google Scholar]
- 23.Howarth FC, Al Sharhan R, Al Hammadi A, Qureshi MA. Effects of streptozotocin-induced diabetes on action potentials in the sinoatrial node compared with other regions of the rat heart. Mol Cell Biochem. 2007;300:39–46. doi: 10.1007/s11010-006-9366-5. [DOI] [PubMed] [Google Scholar]
- 24.Nygren A, Olson ML, Chen KY, Emmett T, Kargacin G, Shimoni Y. Propagation of the cardiac impulse in the diabetic rat heart: Reduced conduction reserve. J Physiol. 2007;580:543–560. doi: 10.1113/jphysiol.2006.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chattou S, Diacono J, Feuvray D. Decrease in sodium-calcium exchange and calcium currents in diabetic rat ventricular myocytes. Acta Physiol Scand. 1999;166:137–144. doi: 10.1046/j.1365-201x.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang DW, Kiyosue T, Shigematsu S, Arita M. Abnormalities of K+ and Ca2+ currents in ventricular myocytes from rats with chronic diabetes. Am J Physiol. 1995;269:H1288–H1296. doi: 10.1152/ajpheart.1995.269.4.H1288. [DOI] [PubMed] [Google Scholar]
- 27.Lu Z, Jiang YP, Xu XH, Ballou LM, Cohen IS, Lin RZ. Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes. 2007;56:2780–2789. doi: 10.2337/db06-1629. [DOI] [PubMed] [Google Scholar]
- 28.Jourdon P, Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993;470:411–429. doi: 10.1113/jphysiol.1993.sp019866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimoni Y, Firek L, Severson D, Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74:620–628. doi: 10.1161/01.RES.74.4.620. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Xiao J, Wang H, Luo X, Wang J, Villeneuve LR, Zhang H, Bai Y, Yang B, Wang Z. Restoring depressed HERG K+ channel function as a mechanism for insulin treatment of abnormal QT prolongation and associated arrhythmias in diabetic rabbits. Am J Physiol. 2006;291:H1446–H1455. doi: 10.1152/ajpheart.01356.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lengyel C, Virág L, Kovács PP, Kristóf A, Pacher P, Kocsis E, Koltay ZM, Nánási PP, Tóth M, Kecskeméti V, et al. Role of slow delayed rectifier K+-current in QT prolongation in the alloxan-induced diabetic rabbit heart. Acta Physiol (Oxf) 2008;192:359–368. doi: 10.1111/j.1748-1716.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 32.Yuill KH, Tosh D, Hancox JC. Streptozotocin-induced diabetes modulates action potentials and ion channel currents from the rat atrioventricular node. Exp Physiol. 2010;95:508–517. doi: 10.1113/expphysiol.2009.050286. [DOI] [PubMed] [Google Scholar]
- 33.Yuill KH, Al Kury LT, Howarth FC. Characterization of L-type calcium channel activity in atrioventricular nodal myocytes from rats with streptozotocin-induced diabetes mellitus. Physiol Rep. 2015;3 doi: 10.14814/phy2.12632. pii: e12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howarth FC, Nowotny N, Zilahi E, El Haj MA, Lei M. Altered expression of gap junction connexin proteins may partly underlie heart rhythm disturbances in the streptozotocin-induced diabetic rat heart. Mol Cell Biochem. 2007;305:145–151. doi: 10.1007/s11010-007-9537-z. [DOI] [PubMed] [Google Scholar]
- 35.Ferdous Z, Qureshi MA, Jayaprakash P, Parekh K, John A, Oz M, Raza H, Dobrzynski H, Adrian TE, Howarth FC. Different profile of mRNA expression in sinoatrial node from streptozotocin-induced diabetic rat. PLoS One. 2016;11:e0153934. doi: 10.1371/journal.pone.0153934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo S, Dobrzynski H, Fedorov VV, Xu SZ, Yamanushi TT, Jones SA, Yamamoto M, Nikolski VP, Efimov IR, Boyett MR. Localization of Na+ channel isoforms at the atrioventricular junction and atrioventricular node in the rat. Circulation. 2006;114:1360–1371. doi: 10.1161/CIRCULATIONAHA.106.613182. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Dobrzynski H, Tellez J, Niwa R, Billeter R, Honjo H, Kodama I, Boyett MR. Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin45. Cardiovasc Res. 2006;72:271–281. doi: 10.1016/j.cardiores.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Yanni J, Boyett MR, Anderson RH, Dobrzynski H. The extent of the specialized atrioventricular ring tissues. Heart Rhythm. 2009;6:672–680. doi: 10.1016/j.hrthm.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Souza AI, Felkin LE, McCormack AM, Holder A, Barton PJ, Banner NR, Rose ML. Sequential expression of three known protective genes in cardiac biopsies after transplantation. Transplantation. 2005;79:584–590. doi: 10.1097/01.TP.0000153154.37616.94. [DOI] [PubMed] [Google Scholar]
- 40.Siu PM, Bryner RW, Murlasits Z, Alway SE. Response of XIAP, ARC, and FLIP apoptotic suppressors to 8 wk of treadmill running in rat heart and skeletal muscle. J Appl Physiol (1985) 2005;99:204–209. doi: 10.1152/japplphysiol.00084.2005. [DOI] [PubMed] [Google Scholar]
- 41.Khananshvili D. The SLC8 gene family of sodium-calcium exchangers (NCX)-structure, function, and regulation in health and disease. Mol Aspects Med. 2013;34:220–235. doi: 10.1016/j.mam.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh AQ, Hurley JR, Huang W, Taghian T, Kogan A, Cho H, Wang Y, Narmoneva DA. Diabetes alters intracellular calcium transients in cardiac endothelial cells. PLoS One. 2012;7:e36840. doi: 10.1371/journal.pone.0036840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hattori Y, Matsuda N, Kimura J, Ishitani T, Tamada A, Gando S, Kemmotsu O, Kanno M. Diminished function and expression of the cardiac Na+-Ca2+ exchanger in diabetic rats: Implication in Ca2+ overload. J Physiol 527 Pt. 2000;1:85–94. doi: 10.1111/j.1469-7793.2000.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H, Smith GL, Hancox JC, Orchard CH. Inhibition of spontaneous activity of rabbit atrioventricular node cells by KB-R7943 and inhibitors of sarcoplasmic reticulum Ca(2+) ATPase. Cell Calcium. 2011;49:56–65. doi: 10.1016/j.ceca.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Convery MK, Hancox JC. Na+-Ca2+ exchange current from rabbit isolated atrioventricular nodal and ventricular myocytes compared using action potential and ramp waveforms. Acta Physiol Scand. 2000;168:393–401. doi: 10.1046/j.1365-201x.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 46.Hancox JC, Levi AJ, Brooksby P. Intracellular calcium transients recorded with Fura-2 in spontaneously active myocytes isolated from the atrioventricular node of the rabbit heart. Proc Biol Sci. 1994;255:99–105. doi: 10.1098/rspb.1994.0014. [DOI] [PubMed] [Google Scholar]
- 47.Rowell J, Koitabashi N, Kass DA. TRP-ing up heart and vessels: Canonical transient receptor potential channels and cardiovascular disease. J Cardiovasc Transl Res. 2010;3:516–524. doi: 10.1007/s12265-010-9208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H, Wang W, Liu P, Jiang Y, Zhao Y, Wei H, Niu W. TRPC1 expression and distribution in rat hearts. Eur J Histochem. 2009;53:e26. doi: 10.4081/ejh.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe H, Murakami M, Ohba T, Ono K, Ito H. The pathological role of transient receptor potential channels in heart disease. Circ J. 2009;73:419–427. doi: 10.1253/circj.CJ-08-1153. [DOI] [PubMed] [Google Scholar]
- 50.Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG. Stretch-activated channels in the heart: Contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol. 2008;97:232–249. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Ju YK, Allen DG. Store-operated Ca2+ entry and TRPC expression; possible roles in cardiac pacemaker tissue. Heart Lung Circ. 2007;16:349–355. doi: 10.1016/j.hlc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Muthaian R, Pakirisamy RM, Parasuraman S, Raveendran R. Hypertension influences the exponential progression of inflammation and oxidative stress in streptozotocin-induced diabetic kidney. J Pharmacol Pharmacother. 2016;7:159–164. doi: 10.4103/0976-500X.195898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choisy SC, Cheng H, Orchard CH, James AF, Hancox JC. Electrophysiological properties of myocytes isolated from the mouse atrioventricular node: L-type ICa, IKr, If and Na-Ca exchange. Physiol Rep. 2015;3 doi: 10.14814/phy2.12633. pii: e12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, Shinohara T, Joung B, Maruyama M, Choi EK, On YK, Han S, Fishbein MC, Lin SF, Chen PS. Calcium dynamics and the mechanisms of atrioventricular junctional rhythm. J Am Coll Cardiol. 2010;56:805–812. doi: 10.1016/j.jacc.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridley JM, Cheng H, Harrison OJ, Jones SK, Smith GL, Hancox JC, Orchard CH. Spontaneous frequency of rabbit atrioventricular node myocytes depends on SR function. Cell Calcium. 2008;44:580–591. doi: 10.1016/j.ceca.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Yaras N, Bilginoglu A, Vassort G, Turan B. Restoration of diabetes-induced abnormal local Ca2+ release in cardiomyocytes by angiotensin II receptor blockade. Am J Physiol. 2007;292:H912–H920. doi: 10.1152/ajpheart.00824.2006. [DOI] [PubMed] [Google Scholar]
- 58.Le Douairon LS, Gratas-Delamarche A, Malardé L, Zguira S, Vincent S, Lemoine MS, Carré F, Rannou Bekono F. Combined insulin treatment and intense exercise training improved basal cardiac function and Ca(2+)-cycling proteins expression in type 1 diabetic rats. Appl Physiol Nutr Metab. 2012;37:53–62. doi: 10.1139/h11-127. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca(2+)-cycling proteins in streptozotocin-induced diabetic rats. Acta Diabetol. 2010;47(Suppl 1):S209–S218. doi: 10.1007/s00592-009-0164-x. [DOI] [PubMed] [Google Scholar]
- 60.Ligeti L, Szenczi O, Prestia CM, Szabó C, Horváth K, Marcsek ZL, van Stiphout RG, van Riel Na, den Buijs Op J, Van der Vusse GJ, Ivanics T. Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. Int J Mol Med. 2006;17:1035–1043. [PubMed] [Google Scholar]
- 61.Bidasee KR, Nallani K, Henry B, Dincer UD, Besch HR., Jr Chronic diabetes alters function and expression of ryanodine receptor calcium-release channels in rat hearts. Mol Cell Biochem. 2003;249:113–123. doi: 10.1023/A:1024738706470. [DOI] [PubMed] [Google Scholar]
- 62.Stolting G, de Oliveira RC, Guzman RE, Miranda-Laferte E, Conrad R, Jordan N, Schmidt S, Hendriks J, Gensch T, Hidalgo P. Direct interaction of CaVβ with actin up-regulates L-type calcium currents in HL-1 cardiomyocytes. J Biol Chem. 2015;290:4561–4572. doi: 10.1074/jbc.M114.573956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soldatov NM. CACNB2: An emerging pharmacological target for hypertension, heart failure, arrhythmia and mental disorders. Curr Mol Pharmacol. 2015;8:32–42. doi: 10.2174/1874467208666150507093258. [DOI] [PubMed] [Google Scholar]
- 64.Valdivia CR, Medeiros-Domingo A, Ye B, Shen WK, Algiers TJ, Ackerman MJ, Makielski JC. Loss-of-function mutation of the SCN3B-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res. 2010;86:392–400. doi: 10.1093/cvr/cvp417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P, Yang Q, Wu X, Yang Y, Shi L, Wang C, Wu G, Xia Y, Yang B, Zhang R, et al. Functional dominant-negative mutation of sodium channel subunit gene SCN3B associated with atrial fibrillation in a Chinese GeneID population. Biochem Biophys Res Commun. 2010;398:98–104. doi: 10.1016/j.bbrc.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hakim P, Gurung IS, Pedersen TH, Thresher R, Brice N, Lawrence J, Grace AA, Huang CL. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog Biophys Mol Biol. 2008;98:251–266. doi: 10.1016/j.pbiomolbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bissay V, Van Malderen SC, Keymolen K, Lissens W, Peeters U, Daneels D, Jansen AC, Pappaert G, Brugada P, De Keyser J, Van Dooren S. SCN4A variants and Brugada syndrome: Phenotypic and genotypic overlap between cardiac and skeletal muscle sodium channelopathies. Eur J Hum Genet. 2016;24:400–407. doi: 10.1038/ejhg.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Péréon Y, Lande G, Demolombe S, Nguyen The Tich S, Sternberg D, Le Marec H, David A. Paramyotonia congenita with an SCN4A mutation affecting cardiac repolarization. Neurology. 2003;60:340–342. doi: 10.1212/01.WNL.0000042093.96309.5A. [DOI] [PubMed] [Google Scholar]
- 69.Kassiri Z, Hajjar R, Backx PH. Molecular components of transient outward potassium current in cultured neonatal rat ventricular myocytes. J Mol Med (Berl) 2002;80:351–358. doi: 10.1007/s00109-002-0325-7. [DOI] [PubMed] [Google Scholar]
- 70.Mitcheson JS, Hancox JC. Characteristics of a transient outward current (sensitive to 4-aminopyridine) in Ca2+-tolerant myocytes isolated from the rabbit atrioventricular node. Pflügers Arch. 1999;438:68–78. doi: 10.1007/s004240050881. [DOI] [PubMed] [Google Scholar]
- 71.Marger L, Mesirca P, Alig J, Torrente A, Dubel S, Engeland B, Kanani S, Fontanaud P, Striessnig J, Shin HS, et al. Pacemaker activity and ionic currents in mouse atrioventricular node cells. Channels (Austin) 2011;5:241–250. doi: 10.4161/chan.5.3.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howarth FC, Levi AJ, Hancox JC. Characteristics of the delayed rectifier K current compared in myocytes isolated from the atrioventricular node and ventricle of the rabbit heart. Pflugers Arch. 1996;431:713–722. doi: 10.1007/BF02253834. [DOI] [PubMed] [Google Scholar]
- 73.Sato N, Tanaka H, Habuchi Y, Giles WR. Electrophysiological effects of ibutilide on the delayed rectifier K(+) current in rabbit sinoatrial and atrioventricular node cells. Eur J Pharmacol. 2000;404:281–288. doi: 10.1016/S0014-2999(00)00603-8. [DOI] [PubMed] [Google Scholar]
- 74.Hancox JC, Mitcheson JS. Ion channel and exchange currents in single myocytes isolated from the rabbit atrioventricular node. Can J Cardiol. 1997;13:1175–1182. [PubMed] [Google Scholar]
- 75.Mitcheson JS, Hancox JC. An investigation of the role played by the E-4031-sensitive (rapid delayed rectifier) potassium current in isolated rabbit atrioventricular nodal and ventricular myocytes. Pflugers Arch. 1999;438:843–850. doi: 10.1007/s004249900118. [DOI] [PubMed] [Google Scholar]
- 76.Mesirca P, Marger L, Toyoda F, Rizzetto R, Audoubert M, Dubel S, Torrente AG, Difrancesco ML, Muller JC, Leoni AL, et al. The G-protein-gated K+ channel, IKACh, is required for regulation of pacemaker activity and recovery of resting heart rate after sympathetic stimulation. J Gen Physiol. 2013;142:113–126. doi: 10.1085/jgp.201310996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA, Chiamvimonvat N. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010;107:851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 79.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP, Chiamvimonvat N. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008;102:465–471. doi: 10.1161/CIRCRESAHA.107.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yi F, Ling TY, Lu T, Wang XL, Li J, Claycomb WC, Shen WK, Lee HC. Down-regulation of the small conductance calcium-activated potassium channels in diabetic mouse atria. J Biol Chem. 2015;290:7016–7026. doi: 10.1074/jbc.M114.607952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volpe M, Rubattu S, Burnett J., Jr Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur Heart J. 2014;35:419–425. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Howarth FC, Al Shamsi N, Al Qaydi M, Al Mazrouei M, Qureshi A, Chandranath SI, Kazzam E, Adem A. Effects of brain natriuretic peptide on contraction and intracellular Ca2+ in ventricular myocytes from the streptozotocin-induced diabetic rat. Ann N Y Acad Sci. 2006;1084:155–165. doi: 10.1196/annals.1372.007. [DOI] [PubMed] [Google Scholar]
- 87.Howarth FC, Adem A, Adeghate EA, Al Ali NA, Al Bastaki AM, Sorour FR, Hammoudi RO, Ghaleb NA, Chandler NJ, Dobrzynski H. Distribution of atrial natriuretic peptide and its effects on contraction and intracellular calcium in ventricular myocytes from streptozotocin-induced diabetic rat. Peptides. 2005;26:691–700. doi: 10.1016/j.peptides.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Springer J, Azer J, Hua R, Robbins C, Adamczyk A, McBoyle S, Bissell MB, Rose RA. The natriuretic peptides BNP and CNP increase heart rate and electrical conduction by stimulating ionic currents in the sinoatrial node and atrial myocardium following activation of guanylyl cyclase-linked natriuretic peptide receptors. J Mol Cell Cardiol. 2012;52:1122–1134. doi: 10.1016/j.yjmcc.2012.01.018. [DOI] [PubMed] [Google Scholar]