Abstract
In the present study, the expression of Beclin 1 in osteoarthritis (OA) cartilage tissue was investigated, and also its role in proliferation, apoptosis and expression of matrix metalloproteinases (MMPs) in chondrocytes obtained from patients with OA. Beclin 1 expression in cartilage tissue from OA patients, and in the age- and sex-matched controls, was detected by immunohistochemistry, semi-quantitative polymerase chain reaction and western blotting. Chondrocytes were divided into control and Beclin 1-overexpressed groups. After transfection for 48, 72 and 96 h, cell viability, apoptosis, the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway and MMPs were examined. The mRNA and protein expression levels of Beclin 1 were significantly decreased in cartilage tissue from OA patients compared with the sex- and age-matched controls (P<0.05). In chondrocytes from OA patients, Beclin 1 overexpression significantly increased cell viability (P<0.05). Beclin 1 overexpression additionally decreased the degree of apoptosis, as demonstrated by Hoechst staining and flow cytometric analysis. B-cell lymphoma-2 (Bcl-2) was upregulated, and Bcl-2 associated X was downregulated, following Beclin 1 overexpression (P<0.05). The PI3K/Akt/mTOR signaling pathway was mitigated following Beclin 1 overexpression (P<0.05). In addition, MMP1, MMP3 and MMP13 were downregulated after Beclin 1 overexpression (P<0.05). Taken together, low expression levels of Beclin 1 may contribute towards the degeneration of chondrocytes. Beclin 1 overexpression increased cell viability, inhibited apoptosis and MMPs, likely via the PI3K/Akt/mTOR signaling pathway.
Keywords: osteoarthritis, Beclin 1, chondrocytes, proliferation, apoptosis, matrix metalloproteinases
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease that frequently presents in the elderly, and has the potential to cause disability. Age, sex, obesity, trauma, inflammation, mechanical stress and genetic factors are the primary causes of OA (1). To the best our knowledge, there are no available treatments for OA possibly due to the unclear underlying molecular mechanism of the disease. Cartilage is composed of chondrocytes and extracellular matrix (ECM). Cartilage degeneration is the primary pathological feature of OA (2). In addition, apoptosis of chondrocytes is the key factor involved in cartilage degeneration (3). The loss of chondrocytes leads to an insufficient number of cartilage cells and contributes to functional loss. Subsequently, synthesis of the ECM decreases, and this may eventually lead to the manifestation of OA (4,5). Therefore, inhibition of apoptosis of chondrocytes may aid treatment of OA.
Autophagy is a cellular stress responseto exogenous or endogenous stimuli, mediated by lysosomal removal of damaged or functionally unnecessary organelles. This inhibits cellular apoptosis and enhances the cell survival rate (6,7). Beclin 1, a homolog of the yeast Atg6, is a protein encoded by an autophagy-associated gene. A complex of phosphatidylinositol 3-kinase (PI3K) and Beclin 1 is formed that promotes the formation of autophagosomes, thereby inducing autophagy (8,9). Autophagy is thought to be cell-protective under certain pathological conditions (10,11). In recent years, studies demonstrated that, under low nutrient conditions, apoptosis of cartilage cells was inhibited due to autophagy stimulation (12). These data suggested that autophagy may be useful in protecting the loss of cartilage cells, and may provide a novel therapeutic approach for OA (13,14). However, previous studies have primarily been focused on Beclin 1 expression and its effect on chondrocyte autophagy, and the effects of Beclin 1 expression on the apoptosis of chondrocytes and the activity of matrix metalloproteinases (MMPs) has yet to be elucidated (8,12,13). In the present study, the role of Beclin 1 in chondrocyte apoptosis was investigated, as were the effects on ECM metabolism, and their underlying molecular mechanisms.
Materials and methods
Patients
Patients were enrolled at Guizhou Osteological Hospital (Guiyang, China) between May 2015 and May 2016. Cartilage tissue was obtained from 30 OA patients receiving knee joint replacement. The diagnosis of OA was based on the America OA diagnosis standards (2008) (15). Disease history, clinical examinations and x-ray scans were referred to in order to diagnose OA. The age of the patients ranged from 56–85 years with an average of 68 years, including 12 males and 18 females. In addition, cartilage tissue was collected from 10 sex- and age-matched control subjects, without OA diagnoses. Informed written consent was obtained from each participant. Experiments were approved by the Ethics Committee of Guizhou Osteological Hospital (Guiyang, China).
Immunohistochemistry
Cartilage tissues were fixed in 4% paraformaldehyde and embedded in paraffin wax. Subsequently, the specimens were sliced (20 µm), and xylene dewaxing and dehydration in graded alcohol were performed. Antigen retrieval was conducted in sodium citrate buffer (10 mM) at room temperature for 2 days and the slices were blocked in 5% horse serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room temperature for 2 h. Sections were incubated with a Beclin 1 primary antibody (1:100 dilution; cat. no. ab62557; Abcam, Shanghai, China) at 4°C overnight. After incubation with the donkey anti-rabbit IgG H&L horseradish peroxidase-conjugated secondary antibody (1:200; cat. no. ab98488; Abcam) at room temperature for 2 h, sections were incubated with 3,3′-diaminobenzidine substrate (1 mg/ml) at room temperature for 5 min. Hematoxylin (Beyotime Institute of Biotechnology, Haimen, China) was applied to stain the nuclei for 1 min at room temperature. Beclin 1 positive staining (brown staining) was localized to the cytoplasm based upon the negative control (incubated without primary antibody). The images were taken under a light microscope (BX51; Olympus Corporation, Tokyo, Japan).
Semi-quantitative polymerase chain reaction (sqPCR)
Total RNA was extracted using TRIzol® reagent according to the manufacturer's protocol (cat. no. 15596026; Thermo Fisher Scientific, Inc., Shanghai, China) and the concentration and purity of the RNA were determined using a Nanodrop 2000 spectrophotometer. RNA (2 µl) was used to synthesize cDNA using a one-step reverse transcription kit according to the manufacturer's protocol (Takara Biotechnology Co., Ltd., Dalian, China). Primers were synthesized by Shanghai Sangong Pharmaceutical Co., Ltd. (Shanghai, China). Primer sequences were as follows: Forward, 5′-TATTGAAACTCCTCGCCAGGA-3′ and reverse, 5′-GGCATAACGCATCTGGTTTT-3′ for BECN1; and forward, 5′-AGCCACATCGCTCAGACA-3′ and reverse, 5′-TCTCCTGGGAGGCATAGACC-3′ for GAPDH. Cycling conditions were as follows: An initial predenaturation step at 95°C for 5 min, followed by 37 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 13 sec and extension at 72°C for 30 sec. PCR products were detected by 2% agarose gel electrophoresis with ethidium bromide staining and the density of the bands was analyzed using ImageJ Software v1.48u (National Institutes of Health, Bethesda, MD, USA).
Preparation of chondrocytes
Cartilage tissue was cut into sections in a sterile environment and digested using 0.25% trypsin for 30 min and 0.2% collagenase for 2 h. The mixture was centrifuged at 1,896 × g for 10 min to collect the chondrocytes. Cells were resuspended in Dulbecco's Modified Eagle medium/F-12 supplemented with 10% fetal bovine serum, and cultured in an incubator at 37°C and 5% CO2. When cells reached 80% confluence, they were divided into two groups: the Beclin 1 overexpression group and the control group. The Beclin 1 overexpression group was transfected with the pcDNA™ 3.1-BECN1 (https://www.ncbi.nlm.nih.gov/gene/8678) plasmid (1 µl) using Lipofectamine™ 2000 (Shanghai Jima Industrial Co., Ltd., Shanghai, China). The control group was transfected with an empty vector (pcDNA™ 3.1; Shanghai Jima Industrial Co., Ltd.). After 24, 48, 72 and 96 h transfection at 37°C, cells were collected for subsequent experiments.
MTT assay
Chondrocytes in the logarithmic growth phase were seeded in a 96-well culture plate. Following transfection, 20 µl MTT reagent (5 mg/ml) was added to the 200 µl culture medium in each well. After a 4 h incubation at 37°C, the medium was removed, and 150 µl dimethyl sulfoxide was added into each well to dissolve the precipitation. The absorbance was measured at a wavelength of 560 nm using an automated microplate reader (IMark Group, Inc, MD, USA). A blank control (media without cells) was set to subtract the background for comparisons.
Flow cytometry
Chondrocytes in logarithmic growth phase (3×103) were seeded. After transfection for 48 h, cells were collected for Annexin V/propidium iodide staining (Beyotime Institute of Biotechnology) and detected within 1 h using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and the data were analyzed using FlowJo v10 windows software (Tree Star, Inc., Ashland, OR, USA).
Hoechst staining
Chondrocytes in the logarithmic growth phase (3×103) were seeded. After transfection for 48 h, cells were stained with Hoechst (Beyotime Institute of Biotechnology) for 5 min at room temperature, and the images were captured using a fluorescence microscope (DMI4000B; Leica Microsystems GmbH, Wetzlar, Germany).
Western blotting
Chondrocytes in the logarithmic growth phase (3×103) were seeded. After transfection for 48 h, cells were collected and lysed using radioimmunoprecipitation lysis buffer (Beyotime Institute of Biotechnology) for 5 min on ice. Protein concentration was quantified using a bicinchoninic acid assay (Beyotime Institute of Biotechnology). An equal amount of protein (20 µg) was loaded onto 10% gels prior to SDS-PAGE. After electrophoresis, proteins were transferred onto nitrocellulose membranes. Non-specific protein binding was blocked with 4% skimmed milk for 2 h at room temperature. The membranes were incubated at 4°C overnight with primary antibodies against Beclin 1 (1:1,000; cat. no. ab62557; Abcam), Bcl-2 associated X (Bax; 1:1,000; cat. no. ab32503; Abcam), B-cell lymphoma (Bcl-2; 1:1,000; cat. no. ab32124; Abcam), PI3K (1:1,000; cat. no. ab86714; Abcam), protein kinase B (Akt; 1:1,000; cat. no. ab8805; Abcam), phosphorylated (p)-Akt (1:1,000; cat. no. ab8932; Abcam), mammalian target of rapamycin (mTOR; 1:1,000; cat. no. ab2732; Abcam), p-mTOR (1:1,000; cat. no. ab109268; Abcam), MMP1 (1:1,000; cat. no. ab52631; Abcam), MMP3 (1:1,000; cat. no. ab53015; Abcam), MMP13 (1:1,000; cat. no. ab39012; Abcam) and GAPDH (1:1,000; cat. no. AG019; Beyotime Institute of Biotechnology). After washing with PBST (PBS containing 0.1% Tween-20), membranes were probed with anti-mouse or anti-rabbit horseradish peroxidase secondary antibodies (1:100; cat. nos. ab131368 and ab191866; Abcam) at room temperature for 2 h. The signal was detected using an enhanced chemiluminescence detection kit (GE Healthcare Life Sciences, Chalfont, UK) and images were captured using the ChemiDoc™ XRS instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The densities of the blots were quantified using Quantity One software v4.6.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analyses of the data were performed using Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Beclin 1 expression is reduced in cartilage tissue of OA patients
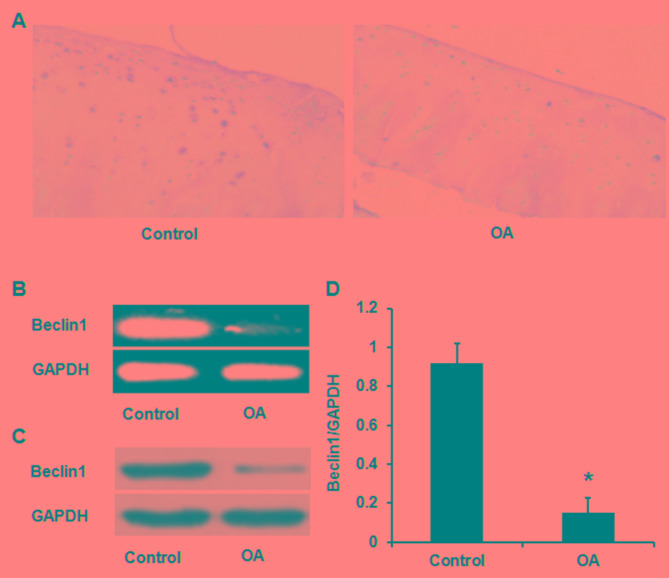
Beclin 1 expression was reduced in cartilage tissues from patients with OA when compared with the 10 sex- and age-matched control subjects, as determined by immunohistochemistry (Fig. 1A), sqPCR (Fig. 1B) and western blotting (Fig. 1C and D).
Figure 1.
Beclin 1 expression levels in cartilage tissues from patients with OA are reduced compared with the control, as demonstrated using three different methodologies, including (A) immunohistochemistry, (B) semi-quantitative polymerase chain reaction and (C) western blotting. (D) Quantification data of Beclin 1 expression. *P<0.05 vs. control. OA, osteoarthritis.
Beclin 1 overexpression increases cell viability
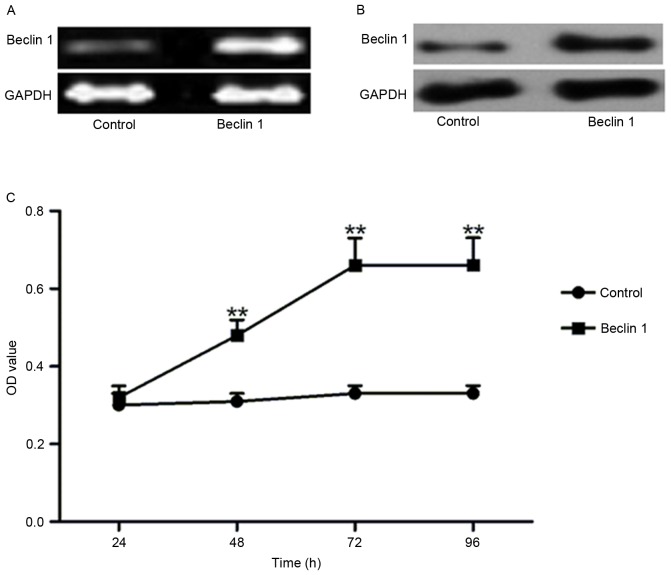
As Beclin 1 expression in the cartilage tissues of OA patients was reduced, it was investigated whether Beclin 1 overexpression altered cell viability. Beclin 1 was overexpressed at the mRNA (Fig. 2A) and protein (Fig. 2B) levels compared with the control. Compared with the control, the optical density was significantly enhanced after 48, 72 and 96 h transfection with the Beclin 1-containing plasmid (Fig. 2C), which reached a peak at 72 h.
Figure 2.
Beclin 1 overexpression increases cell viability of chondrocytes. (A) Semi-quantitative polymerase chain reaction confirmed the overexpression of Beclin 1, and (B) western blotting confirmed overexpression of Beclin 1 compared with the control. (C) Compared with the control, overexpression of Beclin 1 resulted in enhanced cell viability. **P<0.01 vs. control. OD, optical density.
Beclin 1 overexpression decreases apoptosis
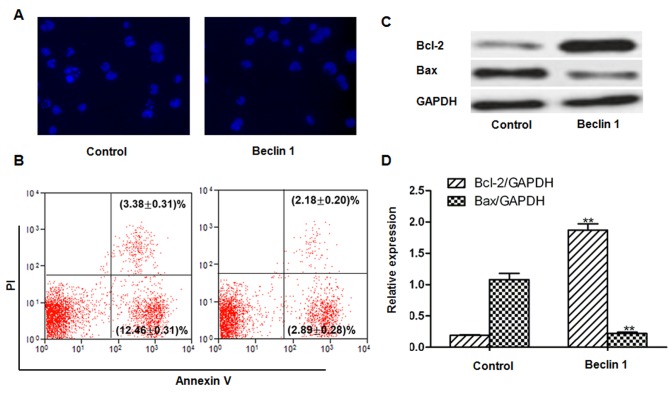
Apoptosis was reduced following transfection with the Beclin 1-containing plasmid compared with the control, as demonstrated by Hoechst staining (Fig. 3A) and Annexin V/propidium iodide double staining and flow cytometric analysis (Fig. 3B). The early apoptotic rates in the control and Beclin 1 plasmid groups were 12.46±0.31 and 2.89±0.28%. The late apoptotic rates in the control and Beclin 1 plasmid groups were 3.38±0.31 and 2.18±0.20%. Additionally, the expression levels of apoptotic-associated proteins were measured. The expression level of the anti-apoptotic protein, Bcl-2, was significantly enhanced, whereas expression of the pro-apoptotic Bax protein was significantly reduced following Beclin1 overexpression compared with the control (P<0.01; Fig. 3C and D).
Figure 3.
Beclin 1 overexpression reduces apoptosis. (A) Hoechst staining (magnification, ×200) and (B) Annexin V/propidium iodide staining and flow cytometric analysis. (C) Bcl-2 and Bax expression levels in cells transfected with Beclin-1 or the control. (D) Quantification of Bcl-2 and Bax by densitometric analysis. **P<0.01 vs. control. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2 associated X.
Beclin 1 overexpression downregulates the PI3K/Akt/mTOR signaling pathway
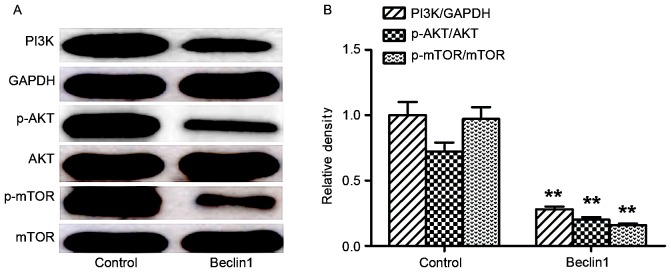
Expression levels of proteins of the PI3K/Akt/mTOR signaling pathway were compared between the two groups. Beclin 1 overexpression markedly reduced the expression levels of PI3K, p-Akt and p-mTOR compared with the control, whereas the expression levels of total Akt and mTOR were not significantly affected (Fig. 4A and B).
Figure 4.
Beclin 1 overexpression downregulates the PI3K/Akt/mTOR signaling pathway. (A) Representative blots for PI3K, p-Akt, Akt, p-mTOR, mTOR and GAPDH in cells transfected with Beclin-1 or the control. (B) Quantification of proteins by densitometric analysis. **P<0.01 vs. control. PI3K, phosphatidylinositol-3-kinase; p-Akt, phosphorylated protein kinase B; Akt, protein kinase B; p-mTOR, phosphorylated mammalian target of rapamycin; mTOR, mammalian target of rapamycin.
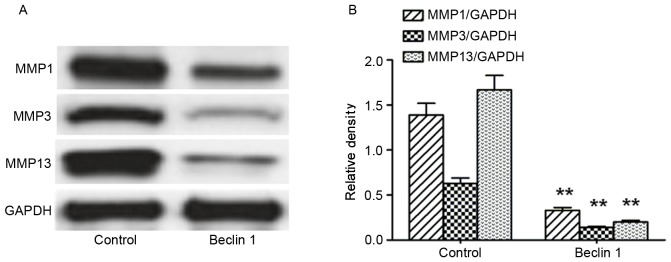
Beclin 1 overexpression decreases MMPs expression
The expression levels of MMPs were also detected after Beclin 1 overexpression. As demonstrated in Fig. 5A and B, MMP1, MMP3 and MMP13 were significantly reduced after Beclin1 overexpression compared with the control.
Figure 5.
Beclin 1 overexpression decreases expression levels of MMPs. (A) Representative blots for MMP1, MMP3 and MMP13 in cells transfected with Beclin-1 or the control. (B) Quantification of proteins by densitometric analysis. **P<0.01 vs. control. MMP, matrix metalloproteinase.
Discussion
Cartilage is a connective tissue that serves a role in elasticity and support. It is composed of chondrocytes and ECM. Chondrocytes are responsible for the generation of the ECM, whereas the ECM maintains the normal function of chondrocytes (2,4). OA and cartilage degeneration resulted in apoptosis of chondrocytes and degradation of the ECM (16,17). Due to an insufficient production of the ECM, chondrocytes lost the support from the ECM. Therefore, it was of interest to inhibit apoptosis of chondrocytes and increase cell survival for efficient treatment of OA (18).
However, the current treatment for OA with drugs or surgery has not gained satisfactory efficacy. In patients with OA, apoptosis of chondrocytes was significantly increased and cell survival significantly decreased (19). In addition, autophagy was significantly decreased, and therefore upregulation of autophagy may inhibit the apoptosis of chondrocytes, promote cell survival and prevent further degeneration of chondrocytes (19). However, the association between OA and autophagy remains to be established. Autophagy is a type of programmed cell death, which is highly conserved in eukaryotic cells. It enables the degradation and recycling of aging organelles and macromolecules in double-membraned autophagosomes. This process is important for cell proliferation and apoptosis. Autophagy is primarily regulated by genes, including those for Beclin 1 (BECN1), autophagy-related (ATG) and microtubule-associated protein 1A/1B light chain 3A (MAP1LC3A) (20). Beclin 1 may regulate other autophagy genes and determine the level of autophagy. The present study revealed that Beclin 1 expression was significantly downregulated in cartilage tissue from OA. Beclin 1 in cartilage has been positively associated with the level of autophagy (14,21). Therefore, the present study compared the expression levels of Beclin 1 in cartilage tissue of OA patients and a matched control by immunohistochemistry staining, western blotting and sqPCR. Beclin 1 expression in OA cartilage tissue was lower than that in normal cartilage, a finding that was consistent with previous publications that demonstrated that Beclin 1 is closely associated with OA development (14). One publication revealed that downregulation of Beclin 1 expression significantly affected autophagy, which additionally affected cell proliferation and apoptosis (13). Therefore, Beclin 1 overexpression may regulate apoptosis in chondrocytes.
In the present study, Beclin 1 overexpression enhanced cell viability following 48, 72 and 96 h transfection, and reached a peak at 72 h. Apoptosis was determined by using two different methodologies: Hoechst staining and Annexin V/propidium iodide staining. The results suggested that Beclin 1 overexpression inhibits apoptosis in chondrocytes. In addition, Bcl-2 and Bax expression levels were determined, proteins that serve roles in the regulation of mitochondria-dependent apoptosis. Bcl-2 was upregulated, whereas Bax was downregulated following Beclin 1 overexpression.
Beclin 1 may bind to PI3K and inhibit the level of autophagy. PI3K regulates autophagy (22,23); however, it is additionally involved in apoptosis (24). PI3K activates Akt and mTOR when insulin receptors, growth factor receptors or interferon receptors are engaged (25). Subsequently, downstream gene expression is initiated to regulate autophagy, apoptosis, differentiation and proliferation.
As demonstrated by previously published reports, the PI3K/Akt/mTOR signaling pathway was persistently activated in articular cartilage of OA rats (26,27). Caramés et al (14) revealed that rapamycin significantly upregulated Beclin1, LC3-I/II and ATG expression to inhibit apoptosis of cartilage cells and aid protection. Zhang et al (28) also demonstrated that inhibition of the mTOR signaling pathway may inhibit OA development. In the present study, Beclin 1 overexpression reduced the expression levels of PI3K, and inhibited phosphorylation of Akt and mTOR. These data suggested that Beclin1 overexpression inhibits the PI3K/Akt/mTOR signaling pathway to promote cell viability.
In addition, apoptosis of chondrocytes prohibits the accumulation of ECM molecules, and subsequently, decreases support for chondrocytes. Therefore, the present study investigated the degradation of the ECM, despite apoptosis of chondrocytes being inhibited following Beclin 1 overexpression. The degradation of the ECM is modulated by various proteases, including MMPs, which are a Zn2+-dependent protease family (29). There are ≥25 types of MMPs, including MMP1, MMP3 and MMP13. These three MMPs have an ability to degrade type II collagen. MMP1 may also degrade the protein polysaccharide and type I and type III collagen. MMP13 has a degradation ability ~10-fold higher compared with that of MMP1, and degrades the three-helical structure of type II collagen (30). MMP13 may additionally stimulate the activation of other MMPs, including MMP1 (31). In the present study, MMP1, MMP3 and MMP13 expression levels were reduced after Beclin 1 overexpression, a finding that suggested that the degradation of ECM was inhibited.
In conclusion, the present study demonstrated that Beclin 1 expression was downregulated in OA cartilage. Overexpression of Beclin 1 increased cell viability, reduced apoptosis, inhibited the PI3K/Akt/mTOR signaling pathway and reduced expression of MMPs in OA chondrocytes. Therefore, Beclin1 may be a potential target for OA therapy.
References
- 1.Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134–138. doi: 10.1016/j.rehab.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, Lavalley M, Guermazi A, Gale D, Felson DT. Structural factors associated with malalignment in knee osteoarthritis: The Boston osteoarthritis knee study. J Rheumatol. 2005;32:2192–2199. [PubMed] [Google Scholar]
- 3.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeser RF. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, Buckwalter JA. The role of chondrocyte-matrix interactions in maintaining and repairing articular cartilage. Biorheology. 2000;37:129–140. [PubMed] [Google Scholar]
- 6.Markaki M, Tavernarakis N. Metabolic control by target of rapamycin and autophagy during ageing - a mini-review. Gerontology. 2013;59:340–348. doi: 10.1159/000348599. [DOI] [PubMed] [Google Scholar]
- 7.Glick D, Barth S, Macleod KF. Autophagy: Cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakhar R, Paul S, Bhardwaj M, Kang SC. Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer Lett. 2016;372:89–100. doi: 10.1016/j.canlet.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Wargasetia TL, Shahib N, Martaadisoebrata D, Dhianawaty D, Hernowo B. Characterization of apoptosis and autophagy through Bcl-2 and Beclin-1 immunoexpression in gestational trophoblastic disease. Iran J Reprod Med. 2015;13:413–420. [PMC free article] [PubMed] [Google Scholar]
- 10.Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, Green DR, Morgan M, Cramer SD, Thorburn A. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev Cell. 2016;37:337–349. doi: 10.1016/j.devcel.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo X, Zhang J, Ji L, Ren T, An J, et al. Increased mitochondrial fission promotes autophagy and hepatocellular carcinoma cell survival through the ROS-modulated coordinated regulation of the NFKB and TP53 pathways. Autophagy. 2016;12:999–1014. doi: 10.1080/15548627.2016.1166318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Garcia O, Olmer M, Akagi R, Akasaki Y, Fisch KM, Shen T, Su AI, Lotz MK. Suppression of REDD1 in osteoarthritis cartilage, a novel mechanism for dysregulated mTOR signaling and defective autophagy. Osteoarthritis Cartilage. 2016;24:1639–1647. doi: 10.1016/j.joca.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng NT, Guo A, Meng H. The protective role of autophagy in experimental osteoarthritis, and the therapeutic effects of Torin 1 on osteoarthritis by activating autophagy. BMC Musculoskelet Disord. 2016;17:150. doi: 10.1186/s12891-016-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnero P, Aronstein WS, Cohen SB, Conaghan PG, Cline GA, Christiansen C, Beary JF, Meyer JM, Bingham CO., III Relationships between biochemical markers of bone and cartilage degradation with radiological progression in patients with knee osteoarthritis receiving risedronate: The knee osteoarthritis structural arthritis randomized clinical trial. Osteoarthritis Cartilage. 2008;16:660–666. doi: 10.1016/j.joca.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Zamli Z, Adams MA, Tarlton JF, Sharif M. Increased chondrocyte apoptosis is associated with progression of osteoarthritis in spontaneous Guinea pig models of the disease. Int J Mol Sci. 2013;14:17729–17743. doi: 10.3390/ijms140917729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15:27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62:1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Quan YY, Wang XP, Chen TS. Gold nanoparticles of diameter 13 nm induce apoptosis in rabbit articular chondrocytes. Nanoscale Res Lett. 2016;11:249. doi: 10.1186/s11671-016-1461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, Fujita N, Oka S, Kurosaka M, Kuroda R. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64:1920–1928. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, He L, Behrends C, Araki M, Araki K, Jun Wang Q, Catanzaro JM, Friedman SL, Zong WX, Fiel MI, et al. NRBF2 regulates autophagy and prevents liver injury by modulating Atg14L-linked phosphatidylinositol-3 kinase III activity. Nat Commun. 2014;5:3920. doi: 10.1038/ncomms4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Zhu G, Wang X, Wu S, Li Q. Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int. 2012;60:400–408. doi: 10.1016/j.neuint.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 26.Pal B, Endisha H, Zhang Y, Kapoor M. mTOR: A potential therapeutic target in osteoarthritis? Drugs R D. 2015;15:27–36. doi: 10.1007/s40268-015-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Crawford R, Xiao Y. Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J Cell Biochem. 2013;114:245–249. doi: 10.1002/jcb.24362. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XH, Xu XX, Xu T. Ginsenoside Ro suppresses interleukin-1β-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-κB. Chin J Nat Med. 2015;13:283–289. doi: 10.1016/S1875-5364(15)30015-7. [DOI] [PubMed] [Google Scholar]
- 29.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. pii: a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nannuru KC, Futakuchi M, Varney ML, Vincent TM, Marcusson EG, Singh RK. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010;70:3494–3504. doi: 10.1158/0008-5472.CAN-09-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]