Abstract
Senescence marker protein 30 (SMP30) has been reported to serve antiapoptotic and antioxidant roles, as well as roles in Ca2+ regulation, and may be involved in the occurrence and development of cataract. The present study aimed to investigate the expression of SMP30 in senescent human lens epithelial cells (HLECs) and explored the relationship between SMP30 and aging. SRA01/04 cells, a HLEC line, were treated with H2O2 to mimic aging, and cell morphological changes were observed by microscopy and cell activity was examined by MTT assay, senescence-associated-β-galactosidase (SA-β-Gal) staining and cell cycle analysis. The expression of SMP30 mRNA and protein was measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting. Following prolonged low-dose H2O2 exposure, cells exhibited senescence-related morphological changes, reduced growth activity, increased SA-β-Gal positive staining and cell cycle arrest in the S and G2/M phases. SMP30 mRNA expression levels were significantly downregulated following exposure to 75 and 100 µM H2O2, and the protein expression levels in the same groups were decreased by >6-fold compared with the control untreated cells. However, no significant change was observed in SMP30 expression in the 25 and 50 µM H2O2 exposure groups. These results suggest that, in the early stage of senescence induced by H2O2-mediated chronic oxidative stress, there may be no significant change in SMP30 expression, but when the oxidative stress increases and senescence is aggravated, SMP30 may be significantly downregulated in the senescent HLECs. The present study indicates that SMP30 may be an important factor involved in the aging process of HLECs and the development of cataract.
Keywords: senescence marker protein 30, oxidative stress, aging, human lens epithelial cells, cataract
Introduction
Senescence marker protein 30 (SMP30) is a novel type of calcium binding protein that does not possess a typical Ca2+ binding EF-motif, and that was first isolated as a downregulated protein in aging rat livers in an androgen-independent manner (1). It is localized in the plasma membrane, nucleus, microsomes and mitochondria of different cell types. Following cloning and analysis of the gene encoding for it, SMP30 was demonstrated to be a complex molecule composed of 442 amino acids and 34 kDa (2,3). It is widely expressed in various human tissues, including liver, kidney, breast, brain, stomach, lung, ovary, testis, skin, prostate, epididymis and it is highest expressed in liver and kidney (4,5). It has been reported as upregulated in human prostate and breast cancers, and overexpression of SMP30 has been demonstrated to alter the expression of tumor related-genes, including MYC proto-oncogene, HRas proto-oncogene, SRC proto-oncogene, tumor protein p53, and RB transcriptional corepressor 1; SMP30 inhibits the expression of oncogenes and enhances the transcription of anti-oncogenes, and thus has anti-tumor activity (6,7). In addition, SMP30 expression in cancer is significantly higher than normal tissue, and its high expression may be associated with the early stages of carcinogenesis and may be a manifestation of the cell protective mechanisms (8). These previous findings suggest a protective role of SMP30 against cancer development.
SMP30 has been reported to serve important roles in maintaining Ca2+ homeostasis and signaling via activating enzymes and the Ca2+ pump (9,10). SMP30 also prevents apoptosis induced by Ca2+ influx, as well as exhibits a protective effect against apoptosis induced by other signaling pathways (11). Previous studies have demonstrated that SMP30 can decrease reactive oxygen species levels (12), reduce the oxidative stress damage induced by peroxide and inhibit antioxidant enzyme activity (13–15). Other reports have demonstrated that SMP30 may be involved in the inhibition of DNA and RNA synthesis through regulation of protein kinases and protein phosphatases (16–19). SMP30 exhibits a suppressive effect on cellular proliferation in rat liver and kidney cells, and may be involved in proliferation-related functions in the cell nucleus (20,21). More recently, it was reported that it also involved in the cellular inflammatory response, and that its anti-inflammatory and antioxidant functions may be mediated by activation of the nuclear factor-κB pathway (22). In addition, SMP30 was reported to negatively regulate abnormal lipid accumulation and decrease glucose tolerance in the liver during the normal aging process (23–26).
As a result of its many functions, SMP30 has intrigued researchers regarding its potential as a novel anti-aging molecule. SMP30 is broadly expressed in various human tissues, however, its role in ophthalmic diseases remains unclear. Our previous study reported that expression of SMP30 in patients with cataract was higher than normal subjects, while it was significantly lower in patients >60 years old compared with patients ≤60 (27). The present study aimed to examine whether expression of SMP30 is age-related in the human lens. In order to investigate the roles of SMP30 in human lens epithelial cells, prolonged exposure of cells to low doses of hydrogen peroxide (H2O2) was used as an in vitro model to mimic senescence, and the expression of SMP30 was detected as a function of aging.
Materials and methods
Cell culture
The human lens epithelial cell line (SRA01/04) was obtained from Guangzhou JiNiu Biotechnology Co., Ltd. (Guangzhou, China). SRA01/04 cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C and in a humidified atmosphere containing 5% CO2. When the cells reached ~80% confluency, they were treated with 0.25% trypsin solution, centrifuged (106 × g, 5 min, room temperature) and plated for subculture or frozen for storage.
In vitro SRA01/04 aging model
Cells were treated with a concentration gradient of freshly-prepared H2O2 (0–150 µM) to mimic aging in vitro. Prolonged exposure to H2O2 to stimulate senescence was conducted as previously described (28). Cells cultured in the same medium without H2O2 served as the control group. The medium was changed with fresh H2O2-containing medium every 3 days for a total of 2 weeks. All experiments were performed with cells of passages 4–6 to avoid the influence of natural senescence.
Observation of cell morphology
Cell morphology observation was used as a method to evaluate cell senescence. Treated cells were observed by light microscopy (Olympus Corporation, Tokyo, Japan) at magnification, ×20.
MTT cell viability assay
A growth curve was performed to examine cell viability over time in the experimental groups. SRA01/04 cells were seeded at 1×103 cells/well in 96-well plates and incubated overnight with normal medium. The following day, cells were treated with a concentration gradient H2O2 for different periods of time (0, 1, 3, 5, 7, 9, 12 and 15 days), with 5 replicates for each experimental group and for each time point. When reaching the stated time point, cells were incubated for 4 h at 37°C with 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), then the medium was removed and 150 µl DMSO was added to dissolve the formazan products in each well. Absorbance was measured at 570 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Senescence-associated-β-galactosidase (SA-β-Gal) staining assay
A SA-β-gal staining kit (Beyotime Institute of Biotechnology, Haimen, China) was used, according to the manufacturer's instructions. SRA01/04 were seeded on 6-well plates and treated with low doses of H2O2 as aforementioned. At stated time points, and after washing with PBS, cells were incubated with 1 ml fixing solution at room temperature for 15 min, washed three times with PBS, and then incubated with 1 ml freshly prepared cell staining solution at 37°C overnight (without CO2). Stained cells were analyzed by light microscopy by observing at least 10 random fields for each sample and manually quantifying the % of SA-β-gal positive cells.
Cell cycle assay
Following the 2-week treatment period, cells were washed once in PBS and a cell suspension of 1×106 cells/ml was fixed in 70% cold ethanol at 4°C for 12 h. The cells were then washed with PBS and incubated with 100 µl RNase A for 30 min at 37°C. Finally, cells were stained with 400 µl propidium iodide in the dark for 30 min at 4°C. Cell cycle analysis was performed using a FACScan flow cytometer equipped with CFlow Plus version 1.0.264.15 (both from BD Biosciences, San Jose, CA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cultured cells using TRIzol reagent (Thermo Fisher Scientific, Inc.) and converted into cDNA using the PrimeScript RT reagent kit with gDNA Eraser (cat no. RR047A; Takara Bio, Inc., Otsu, Japan), according to the manufacturer's instructions. The concentration of total RNA was measured using a NanoDrop 2000 (Thermo Fisher Scientific, Inc.). qPCR was performed on a LightCycler 480 real-time PCR system (Roche Diagnostics, Indianapolis, IN, USA) with the SYBR Premix Ex Taq II kit (cat no. RR820A. Takara Bio, Inc.). The primers used for qPCR were: SMP30, sense 5′-CCGTGGATGCCTTTGACTATGAC-3′ and antisense 5′-GTAACAGGCCACCCAGAGCTTC-3′; GAPDH, sense 5′-GCACCGTCAAGGCTGAGAAC-3′ and antisense 5′-TGGTGAAGACGCCAGTGGA-3′. The thermocycling conditions were set up according to the manufacturer's instructions: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec, followed by a melting curve analysis of 1 cycle at 95°C for 5 sec, 60°C for 1 min and 50°C for 30 sec. The melting curve analysis confirmed that the amplification contained no primer dimers or non-specific PCR products. The experiment was repeated six independent times in triplicates and the results were calculated using 2−ΔΔCq method (29).
Protein extraction and western blot analysis
Cells were extracted using lysis buffer (Beyotime Institute of Biotechnology) at 4°C for 30 min. The concentration of total protein was measured by bicinchoninic acid assay (Beyotime Institute of Biotechnology). Protein samples (50 µg/well) were boiled, separated on 10% sodium dodecyl sulfate polyacrylamide gel, and then electrophoretically transferred onto polyvinylidene fluoride membranes. Blots were subsequently incubated for 1 h at room temperature with blocking buffer, which contained 5% (w/v) skimmed milk powder in TBS + 0.1% Tween-20 (TBST). The blots were subsequently incubated with antibodies targeting SMP30 (cat no. ab67336; dilution 1:1,000; Abcam, Cambridge, MA, USA) and β-actin (cat. no. 12262; dilution 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. Blots were then washed with TBST for 30 min. All blots were incubated with a IRDye 680RD goat anti-mouse secondary antibody (cat. no. 926-68070; dilution 1:15,000; Li-Cor Biosciences, Lincoln, NE, USA) for 2 h at room temperature. Following washing with TBST, protein signals were measured using the Odyssey Infrared Imaging System v3.0 (Li-Cor Biosciences). The experiment was repeated six independent times in triplicates.
Statistical analysis
All experiments were repeated at least three times. Statistical analysis was performed with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Results were expressed as mean ± standard deviation. Differences between groups were analyzed for significance by one-way analysis of variance followed by Fisher's least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cell morphology changes in SRA01/04 cells following prolonged low-dose exposure of H2O2
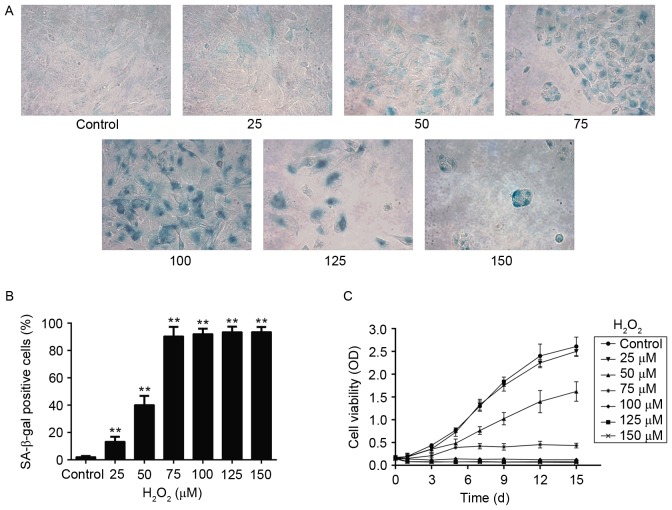
Following observation by microscopy, no significant changes in cell morphology were observed between the untreated control group and the 25 µM H2O2 group (Fig. 1). Exposure to 50 and 75 µM H2O2 resulted in somewhat enlarged cell morphology and a slightly decreased cell density in 5–7 days (Fig. 1). Continuous culture for two weeks at 75 µM H2O2 resulted in a markedly decreased cell growth and a flattened cell morphology with accumulation of granular cytoplasmic inclusions compared with the control group (Fig. 1). At the dose of 100 and 125 µM H2O2, cells appeared shrank, elongated and a larger number of floating cells were observed, while after 10 days cells gradually became detached and disrupted (Fig. 1). At 150 µM H2O2 exposure, cells were all dead in the first 3 days (Fig. 1).
Figure 1.

Prolonged H2O2 exposure results in senescence-related morphological changes. SRA01/04 were treated for two weeks with various concentrations of H2O2 (0–150 µM), and then cell morphology was examined by microscopy. Arrows denote senescent cells, which appear enlarged, flattened, and with accumulation of granular cytoplasmic inclusions. Magnification, ×20. H2O2, hydrogen peroxide.
Prolonged exposure to 50, 75 and 100 µM H2O2 induces cell senescence
SA-β-Gal staining is routinely used to detect senescent cells, and positive staining appears as cytoplasmic blue granular coloring under microscopic observation. As illustrated in Fig. 2A, positively stained cells appeared following exposure of the cells to certain doses of H2O2 for two weeks. There were nearly no positive cells in the control untreated group (2.15±0.68%) and the % of positive cells observed increased in a dose-dependent manner compared with the control group (Fig. 2B). Only a few positive cells appeared in the 25 µM group (13.37±3.50%; Fig. 2B) and 50 µM group (40.23±6.58%; Fig. 2B). The % of positive cells increased dramatically in the 75 µM group (90.48±6.80%; Fig. 2B) and 100 µM group (92.10±3.84%; Fig. 2B). In the 125 and 150 µM groups, although positive staining was observed, most cells appeared non-viable following the two-week exposure (Fig. 2A).
Figure 2.
Characteristics of senescent SRA01/04 cells induced by prolonged low-dose exposure to H2O2. (A) Representative images of SA-β-Gal staining from SRA01/04 cells treated with 0–150 µM H2O2 for two weeks. Magnification, ×20. (B) Quantification of SA-β-Gal positive cells (n=10). (C) Growth curve as measured by the MTT assay (n=6). Data were presented as mean ± standard deviation. **P<0.01 vs. control group. H2O2, hydrogen peroxide; SA-β-Gal, senescence-associated-β-galactosidase; OD, optimal density.
Prolonged exposure to 75 µM H2O2 induces growth inhibition
Cell growth was evaluated by MTT assay over the period of 15 days. Untreated control SRA01/04 cells grew slowly on days 1–3 of culture, while at day 5, cell growth turned to a logarithmic phase (Fig. 2C). In the H2O2 exposure groups, the 25 µM group exhibited no effect on cell survival compared with the control group (Fig. 2C). In the 50 µM group, the growth curve declined compared with control (Fig. 2C). The growth curve of the 75 µM group exhibited a small increase in the first 5 days of exposure, but then cell growth was arrested and maintained in a low level for the rest of the treatment (Fig. 2C). Finally, in the 100, 125 and 150 µM groups, growth declined substantially compared with control and was almost at zero for the whole duration of the experiment (Fig. 2C).
Prolonged exposure to H2O2 induces cell cycle arrest
Cell cycle analysis (Table I and Fig. 3A) was performed to examine the proportion of cells in each phase of the cell cycle. In the 25, 50, 75 and 100 µM groups, the % of cells in the G0/G1 phase were decreased significantly compared with the control group (Fig. 3B). Meanwhile, the 50, 75 and 100 µM groups exhibited significantly higher % of cells in the S phase compared with the control group (Fig. 3B), as well as a significant increase in the G2/M cell populations compared with the control group (Fig. 3B). The 125 and 150 µM groups were all cell debris (Fig. 3A). The cell proliferation index (PI), which was calculated as the sum of the % of cells in the S plus G2/M phases, was significantly increased in the 25, 50, 75 and 100 µM groups compare with the control group (Fig. 3C). The results demonstrated that prolonged exposure to 50, 75 and 100 µM H2O2 disrupted the normal cell cycle progress, arrested SRA01/04 cells in the S and G2/M phases and increased the PI value compared with control.
Table I.
Cell cycle analysis of SRA01/04 cells following H2O2 exposure in vitro.
| H2O2 (µM) | GO/G1 (% of cells) | S (% of cells) | G2/M (% of cells) | PI (% of cells) |
|---|---|---|---|---|
| 0 | 79.41±4.01 | 8.74±1.88 | 8.74±1.88 | 18.27±1.84 |
| 25 | 68.00±3.35 | 11.35±3.71 | 20.60±1.12 | 31.94±3.51 |
| 50 | 61.25±5.30 | 13.65±3.45 | 21.58±3.56 | 36.50±2.59 |
| 75 | 46.81±3.59 | 23.59±4.05 | 25.18±2.11 | 51.07±1.41 |
| 100 | 45.58±3.29 | 21.02±2.23 | 27.48±2.40 | 51.53±1.68 |
Data are presented as mean ± standard deviation from four independent repeats. PI represents the sum of the % of cells in the S and G2/M phases. H2O2, hydrogen peroxide; PI, proliferation index.
Figure 3.
Cell cycle analysis of SRA01/04 cells induced by prolonged low-dose exposure to H2O2. (A) Representative plots from flow cytometry analysis of cell cycle distribution in SRA01/04 cells treated with 0–150 µM H2O2 for two weeks. (B) Quantification of % of cells in each cell cycle phase. (C) The cell proliferation index was calculated as the sum of the % cells in the S and G2/M phases. Data were presented as mean ± standard deviation (n=4). *P<0.05 and **P<0.01 vs. control group. H2O2, hydrogen peroxide; Ctrl, control; ns, not significant.
These above experiments were employed to evaluate cell senescence in response to prolonged exposure to H2O2. Based on the results of the present study, treatment with 75 µM H2O2 for two weeks resulted in chronic oxidative stress and simulated aging of SRA01/04 cells in vitro, without causing cell death. Therefore, the dose of 75 µM H2O2 was selected to induce in vitro aging for the remainder of the experiments. The doses of 25, 50 and 100 µM were used to investigate the different grades of chronic oxidative stress.
SMP30 expression is downregulated in senescent SRA01/04 cells
In order to investigate the expression of SMP30 in senescent HLECs, SRA01/04 were exposed to H2O2 for two weeks to simulate senescence induced by chronic oxidative stress, then mRNA and protein expression levels were examined by RT-qPCR and western blotting. RT-qPCR analysis demonstrated that expression of SMP30 mRNA levels were significantly decreased following H2O2 exposure compared with the control group, to 0.7, 0.74, 0.5 and 0.4-fold of the control in the 25, 50, 75, 100 µM H2O2 groups, respectively (Fig. 4A). However, there was no difference between the 25 and 50 µM H2O2 groups (P=0.921), and no difference between the 75 and 100 µM H2O2 groups (P=0.053). As presented in Fig. 4B, at the protein level, SMP30 expression was no different in the 25 µM group (P=0.695) and the 50 µM group (P=0.126) compared with control. However, SMP30 expression was significantly decreased by >6-fold in the 75 and 100 µM groups compared with the control (Fig. 4B). No significant difference was observed in the SMP30 protein expression between the 75 and 100 µM groups (Fig. 4B).
Figure 4.
SMP30 expression is downregulated by prolonged low-dose exposure to H2O2. SRA01/04 cells were treated with 0–150 µM H2O2 for two weeks. (A) SMP30 mRNA expression levels were analyzed by reverse transcription-quantitative polymerase chain reaction. (B) SMP30 protein expression levels were analyzed by western blotting. β-actin was used as the internal control. Data were presented as mean ± standard deviation (n=6). **P<0.01 vs. control group. H2O2, hydrogen peroxide; Ctrl, control; ns, not significant.
Discussion
SMP30 has great potential as a novel anti-aging factor as it serves important roles in maintaining Ca2+ homeostasis, preventing apoptosis, and reducing oxidative stress damage (1,9). The occurrence and development of cataract is complicated owing to various factors, and the exact pathogenesis of cataract is not clearly understood, but it is thought to be closely related to calcium disorders, oxidative stress and apoptosis of HLECs (30–33). With aging or other external factors, changes can happen in these biological functions of HLECs and they can lead to the occurrence of cataract. Hammond et al (34), through analysis of patients with age-related cataract (ARC), reported that age was an important risk factor of ARC. In addition, age was confirmed to be directly related to the development of ARC, while other factors had no significant correlation (35). Potential anti-aging intervention in HLECs may therefore be especially important for the development of ARC. In recent years, SMP30 was demonstrated to serve an important role in anti-aging, as it was determined that the expression level of SMP30 gradually decreased in aging human liver cells (35). SMP30 was also demonstrated to be involved in cellular senescence and SMP30 knock-out mice were susceptible to cataract (36). Our previous report demonstrated that SMP30 is expressed in human lens capsule epithelial cells, mainly in the cytoplasm and less in the nucleus, the expression of SMP30 in patients with cataract was higher than healthy subjects, but in cataract patients of age >60 it was significantly lower than patients of age ≤60, suggesting that SMP30 expression may be closely related to the development of cataract (27). Whether SMP30 is involved the process of aging in HLECs has not been yet reported.
To explore the relationship between SMP30 and aging, a low-dose prolonged exposure to H2O2 was used to induce chronic oxidative stress and senescence in cells. H2O2 is one of the main oxides in the human lens. The concentration of H2O2 is ~20–30 µM in the normal lens and aqueous humor. However, in the cataract lens, the concentration of H2O2 is significantly increased by ~2–7-fold, and even increased to 30-fold in aqueous humor (37,38). In addition, various studies using in vitro lens cell culture and animal models have reported that H2O2 can induce cataract (39,40). Therefore, in the present study, H2O2 was selected to induce senescent HLECs and observe the expression of SMP30.
Prolonged low-dose exposure to H2O2 is the most commonly used method for inducing senescence in cells in vitro, due to its common mechanisms with pathological aging (41–44). In the present study, HLECs were exposure to H2O2 over two weeks, causing chronic oxidative stress, to induce cell senescence and to simulate aging. Then the senescent state of the cells was confirmed by morphological observations, declined growth, increased SA-β-Gal positive staining and cell cycle arrest. These results confirmed that cellular proliferation was inhibited and senescence occurred. Using this in vitro model, the expression level of SMP30 were measured at the mRNA and the protein level. Compared with the control group, SMP30 mRNA and protein were significantly decreased in the 75 and 100 µM groups, but no significant changes were observed in the 25 and 50 µM groups on the protein level. These results suggest that in the early stages of senescence induced by H2O2, there was no significant change of SMP30 protein compared with normal cells, but with the effect of oxidative stress increasing and senescence aggravated as H2O2 concentration raised, SMP30 was downregulated in senescent HLECs. SMP30 may therefore be an important factor involved in the HLEC aging process and the development of cataract.
To the best of our knowledge, the present study is the first to link SMP30 expression with aging HLECs, although the exact function and mechanism of SMP30 in the lens remains unclear and further studies will be needed to elucidate this. There are various limitations in this study. For example, measuring SMP30 expression at different time points during the senescence process would give more dynamic information on the role of SMP30 in HLEC senescence. Further studies are warranted to fully understand the anti-aging function of SMP30 and its potential in the development and prevention of cataract.
Acknowledgements
The authors would like to thank Dr Hao Liang for the instruction of experiments. The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81360146).
References
- 1.Fujita T, Uchida K, Maruyama N. Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta. 1992;1116:122–128. doi: 10.1016/0304-4165(92)90108-7. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Shirasawa T, Maruyama N. Isolation and characterization of genomic and cDNA clones encoding mouse senescence marker protein-30 (SMP30) Biochim Biophys Acta. 1996;1308:49–57. doi: 10.1016/0167-4781(96)00064-4. [DOI] [PubMed] [Google Scholar]
- 3.Fujita T, Shirasawa T, Maruyama N. Expression and structure of senescence marker protein-30 (SMP30) and its biological significance. Mech Ageing Dev. 1999;107:271–280. doi: 10.1016/S0047-6374(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 4.Feng D, Kondo Y, Ishigami A, Kuramoto M, Machida T, Maruyama N. Senescence marker protein-30 as a novel antiaging molecule. Ann N Y Acad Sci. 2004;1019:360–364. doi: 10.1196/annals.1297.062. [DOI] [PubMed] [Google Scholar]
- 5.Maia C, Santos C, Schmitt F, Socorro S. Regucalcin is under-expressed in human breast and prostate cancers: Effect of sex steroid hormones. J Cell Biochem. 2009;107:667–676. doi: 10.1002/jcb.22158. [DOI] [PubMed] [Google Scholar]
- 6.Tsurusaki Y, Yamaguchi M. Overexpression of regucalcin modulates tumor-related gene expression in cloned rat hepatoma H4-II-E cells. J Cell Biochem. 2003;90:619–626. doi: 10.1002/jcb.10652. [DOI] [PubMed] [Google Scholar]
- 7.Tsurusaki Y, Yamaguchi M. Role of regucalcin in liver nuclear function: binding of regucalcin to nuclear protein or DNA and modulation of tumor-related gene expression. Int J Mol Med. 2004;14:277–281. [PubMed] [Google Scholar]
- 8.Scott SH, Bahnson BJ. Senescence marker protein 30: Functional and structural insights to its unknown physiological function. Biomol Concepts. 2011;2:469–480. doi: 10.1515/BMC.2011.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita T, Inoue H, Kitamura T, Sato N, Shimosawa T, Maruyama N. Senescence marker protein-30 (SMP30) rescues cell death by enhancing plasma membrane Ca(2+)-pumping activity in Hep G2 cells. Biochem Biophys Res Commun. 1998;250:374–380. doi: 10.1006/bbrc.1998.9327. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama N, Ishigami A, Kondo Y. Pathophysiological significance of senescence marker protein-30. Geriatr Gerontol Int. 2010;10(Suppl 1):S88–S98. doi: 10.1111/j.1447-0594.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, Enomoto N, Sato N, Shimosawa T, Maruyama N. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-alpha- and Fas-mediated apoptosis. Am J Pathol. 2002;161:1273–1281. doi: 10.1016/S0002-9440(10)64404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handa S, Maruyama N, Ishigami A. Over-expression of senescence marker protein-30 decreases reactive oxygen species in human hepatic carcinoma Hep G2 cells. Biol Pharm Bull. 2009;32:1645–1648. doi: 10.1248/bpb.32.1645. [DOI] [PubMed] [Google Scholar]
- 13.Jung KJ, Ishigami A, Maruyama N, Takahashi R, Goto S, Yu BP, Chung HY. Modulation of gene expression of SMP-30 by LPS and calorie restriction during aging process. Exp Gerontol. 2004;39:1169–1177. doi: 10.1016/j.exger.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Son TG, Kim SJ, Kim K, Kim MS, Chung HY, Lee J. Cytoprotective roles of senescence marker protein 30 against intracellular calcium elevation and oxidative stress. Arch Pharm Res. 2008;31:872–877. doi: 10.1007/s12272-001-1240-3. [DOI] [PubMed] [Google Scholar]
- 15.Son TG, Zou Y, Jung KJ, Yu BP, Ishigami A, Maruyama N, Lee J. SMP30 deficiency causes increased oxidative stress in brain. Mech Ageing Dev. 2006;127:451–457. doi: 10.1016/j.mad.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Ueoka S. Inhibitory effect of calcium-binding protein regucalcin on ribonucleic acid synthesis in isolated rat liver nuclei. Mol Cell Biochem. 1997;173:169–175. doi: 10.1023/A:1006833111236. [DOI] [PubMed] [Google Scholar]
- 17.Katsumata T, Ya'maguchi M. Inhibitory effect of calcium-binding protein regucalcin on protein kinase activity in the nuclei of regenerating rat liver. J Cell Biochem. 1998;71:569–576. doi: 10.1002/(SICI)1097-4644(19981215)71:4<569::AID-JCB11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Omura M, Yamaguchi M. Regulation of protein phosphatase activity by regucalcin localization in rat liver nuclei. J Cell Biochem. 1999;75:437–445. doi: 10.1002/(SICI)1097-4644(19991201)75:3<437::AID-JCB9>3.3.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki S, Yamaguchi M. Suppressive role of endogenous regucalcin in the enhancement of protein kinase activity with proliferation of cloned rat hepatoma cells (H4-II-E) J Cell Biochem Suppl. 2001;(Suppl 36):S12–S18. doi: 10.1002/jcb.1080. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa T, Sawada N, Yamaguchi M. Overexpression of regucalcin suppresses cell proliferation of cloned normal rat kidney proximal tubular epithelial NRK52E cells. Int J Mol Med. 2005;16:637–643. [PubMed] [Google Scholar]
- 21.Yamaguchi M, Daimon Y. Overexpression of regucalcin suppresses cell proliferation in cloned rat hepatoma H4-II-E cells: Involvement of intracellular signaling factors and cell cycle-related genes. J Cell Biochem. 2005;95:1169–1177. doi: 10.1002/jcb.20490. [DOI] [PubMed] [Google Scholar]
- 22.Jung KJ, Lee EK, Kim SJ, Song CW, Maruyama N, Ishigami A, Kim ND, Im DS, Yu BP, Chung HY. Anti-inflammatory activity of SMP30 modulates NF-κB through protein tyrosine kinase/phosphatase balance. J Mol Med (Berl) 2015;93:343–356. doi: 10.1007/s00109-014-1219-1. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa G, Yamasaki M, Kadono M, Tanaka M, Asano M, Senmaru T, Kondo Y, Fukui M, Obayashi H, Maruyama N, et al. Senescence marker protein-30/gluconolactonase deletion worsens glucose tolerance through impairment of acute insulin secretion. Endocrinology. 2010;151:529–536. doi: 10.1210/en.2009-1163. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Ishigami A, Shima T, Mizuno M, Maruyama N, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, et al. Hepatic senescence marker protein-30 is involved in the progression of nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:426–434. doi: 10.1007/s00535-009-0154-3. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi M, Igarashi A, Uchiyama S, Sawada N. Hyperlipidemia is induced in regucalcin transgenic rats with increasing age. Int J Mol Med. 2004;14:647–651. [PubMed] [Google Scholar]
- 26.Hasegawa G. Decreased senescence marker protein-30 could be a factor that contributes to the worsening of glucose tolerance in normal aging. Islets. 2010;2:258–260. doi: 10.4161/isl.2.4.12157. [DOI] [PubMed] [Google Scholar]
- 27.Weixia L, Shaojian T, Xia L, Wenjin Z, Linzhi J, Hao L. Comparison of expression levels of senescence marker protein 30 in lens epithelial cells among different ages of cataract patients. Chin J Exp Ophthalmol. 2014:521–524. (In Chinese) [Google Scholar]
- 28.Duan J, Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol. 2005;37:1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Churchill GC, Louis CF. Ca(2+) regulation in differentiating lens cells in culture. Exp Eye Res. 2002;75:77–85. doi: 10.1006/exer.2002.1184. [DOI] [PubMed] [Google Scholar]
- 31.Zm SZ, Khoshaman K, Masoudi R, Hemmateenejad B, Yousefi R. The structural alteration and aggregation propensity of glycated lens crystallins in the presence of calcium: Importance of lens calcium homeostasis in development of diabetic cataracts. Spectrochim Acta A Mol Biomol Spectrosc. 2016;170:174–183. doi: 10.1016/j.saa.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruno KA, Lovicu FJ, Chamberlain CG, McAvoy JW. Apoptosis is a feature of TGF beta-induced cataract. Clin Exp Optom. 2002;85:76–82. doi: 10.1111/j.1444-0938.2002.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 34.Hammond CJ, Snieder H, Spector TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 2000;342:1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 35.McCarty CA, Taylor HR. The genetics of cataract. Invest Ophthalmol Vis Sci. 2001;42:1677–1678. [PubMed] [Google Scholar]
- 36.Ishikawa Y, Hashizume K, Kishimoto S, Tezuka Y, Nishigori H, Yamamoto N, Kondo Y, Maruyama N, Ishigami A, Kurosaka D. Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp Eye Res. 2012;94:85–89. doi: 10.1016/j.exer.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci. 1986;38:1463–1471. doi: 10.1016/0024-3205(86)90559-X. [DOI] [PubMed] [Google Scholar]
- 38.Spector A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 39.Yao K, Ye P, Zhang L, Tan J, Tang X, Zhang Y. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol Vis. 2008;14:217–223. [PMC free article] [PubMed] [Google Scholar]
- 40.Kleiman NJ, Wang RR, Spector A. Hydrogen peroxide-induced DNA damage in bovine lens epithelial cells. Mutat Res. 1990;240:35–45. doi: 10.1016/0165-1218(90)90006-N. [DOI] [PubMed] [Google Scholar]
- 41.Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M. Replicative senescence: A critical review. Mech Ageing Dev. 2004;125:827–848. doi: 10.1016/j.mad.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- 43.Frippiat C, Dewelle J, Remacle J, Toussaint O. Signal transduction in H2O2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med. 2002;33:1334–1346. doi: 10.1016/S0891-5849(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 44.Chen JH, Ozanne SE, Hales CN. Methods of cellular senescence induction using oxidative stress. Methods Mol Biol. 2007;371:179–189. doi: 10.1007/978-1-59745-361-5_14. [DOI] [PubMed] [Google Scholar]