Abstract
Pathological retinal angiogenesis is one of the most common causes of blindness, with limited treatment options being currently available. Epidermal growth factor (EGF)-like repeat and discoidin I-like domain-containing protein 3 (EDIL3) has been reported to serve an important role in embryonic vasculogenesis and tumor angiogenesis; however, its implication in retinal angiogenesis has yet to be elucidated. The present study aimed to investigate the putative roles of EDIL3 in retinal endothelial cells. RNA interference was used to disrupt the expression of EDIL3 in human retinal endothelial cells (HRECs) in vitro, and the resulting effects were examined. Cell proliferation was assessed using cell counting kit-8 reagent, Cell migration was investigated using a transwell chamber and a tube formation assay was used to study angiogenic capability in vitro. Flow cytometry was used to detect the cell cycle distribution and western blotting was used to study protein expression. The present results demonstrated that silencing EDIL3 expression significantly impaired the proliferative, migratory and tube forming capabilities of HRECs. Furthermore, EDIL3 knockdown was revealed to induce cell cycle arrest at the G1 phase. Western blot analysis suggested that the possible mechanisms underlying the antiproliferative effects of EDIL3 silencing may involve the inhibition of EGF receptor-mediated pathways, and the suppression of cyclin D1 and cyclin E1 expression in HRECs. In conclusion, the findings of the present study suggested that EDIL3 may be implicated in retinal angiogenesis, and may have potential as a novel therapeutic target for the treatment of pathological angiogenesis.
Keywords: epidermal growth factor-like repeat and discoidin I-like domain-containing protein 3, retinal angiogenesis, cell cycle, epidermal growth factor receptor
Introduction
Angiogenesis is defined as the development of new blood vessels from an existing network of blood vessels, and occurs physiologically during embryonic development, and under pathological conditions, including tumorigenesis, retinopathy and arthritis (1). Angiogenesis is a complex process involving endothelial activation, guided sprouting proliferation, branching, anastomosis and lumen formation (2). Pathological ocular angiogenesis, including retinal neovascularization, choroidal neovascularization and retinal vein occlusion, is one of the most common causes of blindness; however, limited therapeutic approaches are currently available for the treatment of these disorders (3). Vascular endothelial growth factor (VEGF)-neutralizing antibodies have widely been used for the treatment of aberrant ocular neovascularization (4). However, previous studies have reported that anti-VEGF treatment may be associated with retinal fibrosis, acute abdomen and osteonecrosis (5–7). Therefore, the development of novel therapeutic strategies, with fewer adverse effects, is imperative.
Epidermal growth factor (EGF)-like repeat and discoidin I-like domain-containing protein 3 (EDIL3) is an extracellular matrix protein secreted by endothelial cells, which contains 3 EGF repeat domains (E1-E3) and 2 discoidin domains (C1 and C2) (8,9). The E2 domain contains a canonical Arg-Gly-Asp motif, which can bind integrins, including αvβ3 and αvβ5, to mediate several endothelial cell functions (10–12). Under physiological conditions, EDIL3 is not expressed in adult tissues; however, it is expressed under pathological conditions in ischemic and tumor tissues (13–17). Retinal neovascularization has been demonstrated to occur during hypoxia, ischemia and uncontrolled endothelial cell growth (18). EDIL3 serves an important role in ischemic disorders; therefore, it may be hypothesized that EDIL3 could be implicated in aberrant retinal angiogenesis. However, the putative roles of EDIL3 in neovascularization have yet to be elucidated.
In the present study, the roles of EDIL3 in the regulation of retinal neovascularization were investigated, and the molecular mechanisms were explored in vitro. The expression of EDIL3 was silenced in human retinal endothelial cells (HRECs) using RNA interference. EDIL3 knockdown was revealed to significantly inhibit HREC proliferation, migration and tube formation. Furthermore, EDIL3 silencing induced cell cycle arrest at the G1 phase, and suppressed the expression of cyclin D1 and cyclin E1 in HRECs. Western blot analysis suggested that the molecular mechanisms underlying the effects of EDIL3 knockdown on HRECs, may involve the inhibition of EGF receptor (EGFR), Src and extracellular signal-regulated kinase (ERK) phosphorylation. The present results suggested that EDIL3 may have potential as a novel therapeutic target for the treatment of retinal neovascularization.
Materials and methods
Materials
Endothelial cell medium (ECM), fetal bovine serum (FBS), endothelial cell growth supplements (ECGS) and penicillin/streptomycin were purchased from ScienCell Research Laboratories, Inc. (Carlsbad, CA, USA). TRIzol® reagent, Opti-MEM™ medium, and horseradish peroxidase (HRP)-conjugated rabbit (Catalog: 31466) and mouse (Catalog: 62–6520) secondary antibodies were purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). X-tremeGENE™ small interfering (si)RNA transfection reagent was purchased from Roche Diagnostics (Shanghai) Co., Ltd. (Shanghai, China). Antibodies against EDIL3 (Catalog: 12580-1-AP), cyclin B (Catalog: 12580-1-AP, cyclin D (Catalog: 60186-1-Ig), cyclin E (Catalog: 11554-1-AP) and GAPDH (Catalog: 10494-1-AP) were purchased from Wuhan Sanying Biotechnology (Wuhan, China). Antibodies against ERK (Catalog: 4695), phosphorylated (p)-ERK (Catalog: 4370), EGFR (Catalog: 2085), p-EGFR (Catalog: 2244), Src (Catalog: 2109) and p-Src (Catalog: 12432) were purchased from CST Biological Reagents Company Limited (Shanghai, China). Propidium iodide (PI)/RNase Staining Solution (Catalog: CY2001-P) for cell cycle analysis was purchased from Tianjin Sungene Biotech Co., Ltd. (Tianjin, China). PrimeScript™ RT Reagent kit and SYBR® Premix Ex Taq™ were purchased from Takara Biotechnology Co., Ltd. (Dalian, China). Cell counting kit 8 (CCK8) was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Radioimmunoprecipitation assay (RIPA) lysis buffer, loading buffer and BeyoColor protein standard (Catalog: P0076) were purchased from Beyotime Institute of Biotechnology (Haimen, China). Immobilon Western Chemiluminescent HRP substrate was purchased from Merck KGaA (Darmstadt, Germany). Matrigel matrix, Transwell inserts and cell culture plates were purchased from Corning Incorporated (Corning, NY, USA). All other reagents were analytical grade.
Cell culture
HRECs were purchased from ScienCell Research Laboratories, Inc., and were cultured in ECM supplemented with 5% FBS, 1% ECGS and 1% penicillin/streptomycin. The cells were maintained in a 5% CO2 incubator with 100% humidity at a temperature of 37°C. HRECs between passages 3 and 8 were used in subsequent experiments.
Cell transfection and RNA interference
HRECs (~3×103) were seeded into a 24-well plate and were transfected once they reached 30–40% confluence. X-tremeGENE™ siRNA transfection reagent (2 µl) and 200 ng EDIL3 siRNA were diluted to a ratio of 1:50 in Opti-MEM and incubated for 5 min separately. Subsequently, the Opti-MEM containing X-tremeGENE™ siRNA transfection reagent was added to the Opti-MEM containing siRNA at a 1:1 ratio. Finally, X-tremeGENE-siRNA complexes were added to the cells and incubated for 48 h. The control group was incubated with the transfection reagent alone. siRNAs targeting EDIL3 (siEDIL3) and scramble siRNAs were chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The siRNA sequences were as follows: siEDIL3, forward 5′-GGUGAUAUUUGUGAUCCCATT-3′, reverse 3′-UGGGAUCACAAAUAUCACCTT-5′; and scramble, forward 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and reverse 3′-ACGUGACACGUUCGGAGAAdTdT-5′. siRNA transfection was performed using X-tremeGENE™ siRNA transfection reagent according to the manufacturer's protocol, and the concentration of siRNAs used was 50 nmol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Following siRNA transfection for 48 h, total RNA was extracted from HRECs using TRIzol® reagent. Total RNA was reverse transcribed into cDNA using the PrimeScript™ RT Reagent kit according to the manufacturer's protocol. qPCR was performed on cDNA using SYBR® Premix Ex Taq™ on a StepOnePlus™ Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling conditions were as follows: Initial denaturation at 95°C for 3 min, followed by 40 cycles at 95°C for 5 sec and at 60°C for 30 sec. The comparative Cq method was used for PCR quantification (19). Finally, the results were expressed as the mean relative value compared with control samples. The following primers were used in the present study: EDIL3, forward 5′-TACAGCAATGATGGAGAACA-3′, reverse 5′-TACCAGGACCAAGGAAGG-3′; GAPDH, forward 5′-TGGGCTACACTGAGCACCAG-3′, reverse 5′-AAGTGGTCGTTGAGGGCAAT-3′.
Cell proliferation assay
Cell proliferation was examined using a CCK8 assay according to the manufacturer's protocol. Briefly, 2,000 HRECs were seeded into a 96-well plate and incubated at 37°C overnight. Subsequently, cells were transfected with EDIL3-targeting or scramble siRNAs and cultured for an additional 48 h. The culture medium was then replaced with 110 µl fresh ECM containing 10 µl CCK8 solution and cells were incubated for 2 h. The absorbance of each well was measured at 450 nm using a Synergy™ Multi-Mode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Cell migration assay
HRECs were initially transfected with EDIL3-targeting or scramble siRNAs and incubated for 48 h. Subsequently, 2.5×104 cells were harvested and seeded in the upper chambers of Transwell inserts (pore size, 8 µm) in 0.5% FBS-containing medium. The lower chambers were filled with ECM supplemented with 1% FBS as a chemoattractant. Following incubation at 37°C for 12 h, the cells on the upper membranes were removed with a cotton swab, and cells that had migrated to the lower membranes were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 20 min at room temperature. Migrated cells were observed under an IX81 inverted microscope (Olympus Corporation, Tokyo, Japan) and the data was analyzed using Image J software (1.6.0_24; National Institutes of Health, Bethesda, MD, USA).
Tube formation assay
The tube formation assay was performed as previously described (20). Briefly, 60 µl Matrigel was added to a 96-well plate and incubated for 30 min at 37°C. HRECs were transfected with EDIL3-targeting or scramble siRNAs for 48 h, harvested and resuspended in ECM at a density of 3×104 cells/ml. A total of 100 µl cell suspension was added to the Matrigel-coated plates and cells were cultured for 3 h. Subsequently, the capillary-like structures that were formed were observed and analyzed using a DMI8 inverted microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Cell cycle assay
The cell cycle assay was performed using PI to stain cells, followed by flow cytometric analysis. Briefly, HRECs (~2×104) were seeded into a 6-well plate and transfected with EDIL3-targeting or scramble siRNA for 48 h, harvested using trypsin and fixed in 70% ethanol at 4°C overnight. Subsequently, the cells were washed with PBS, resuspended in binding buffer containing PI and RNase A and incubated for 30 min in the dark at room temperature. Stained cells were analyzed using a BD FACScan™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and data were analyzed using ModFit LT software (Version 3.1, Verity Software House, Inc., Topsham, ME, USA).
Western blot analysis
Following siRNA transfection for 48 h, HRECs were lysed using RIPA lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS and proteinase inhibitor cocktail. The samples were subsequently quantified using a bicinchoninic acid Protein Quantification kit according to the manufacturer's protocol (Beyotime Institute of Biotechnology). Extracted protein samples (~20 µg) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat milk for 2 h at room temperature and subsequently incubated with the relevant primary antibodies (1:1,000) at 4°C overnight. Then, the samples were washed three times using TBS-Tween (0.05%) and incubated with HRP-conjugated secondary antibodies (1:5,000) for 2 h at room temperature. Protein bands were visualized by enhanced chemiluminescence using Immobilon Western Chemiluminescent HRP substrate. GAPDH was used as the internal control for equal loading.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as the mean ± standard deviation of at least three independent experiments. The statistical significance of the differences between groups was assessed using one-way analysis of variance followed by Tukey's Multiple Comparison Test. P<0.05 was considered to indicate a statistically significant difference.
Results
Silencing EDIL3 expression inhibits HREC proliferation
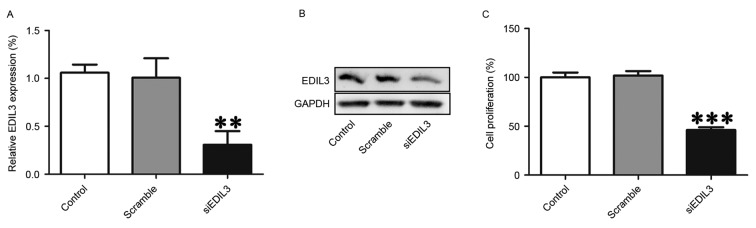
The expression of EDIL3 was silenced in HRECs using RNA interference to investigate the putative roles of EDIL3 in cell proliferation. RT-qPCR and western blot analysis confirmed that following transfection with EDIL3-targeting siRNA, the expression of EDIL3 was successfully suppressed at the mRNA and protein levels (Fig. 1A and B). In addition, a CCK8 assay demonstrated that EDIL3 knockdown significantly suppressed the proliferation of HRECs (Fig. 1C). Cell viability was suppressed by ~54% in EDIL3-silenced cells compared with the control and scramble groups (Fig. 1C). These findings suggested that EDIL3 may be implicated in retinal endothelial cell proliferation.
Figure 1.
Silencing EDIL3 expression using RNA interference inhibits HREC proliferation in vitro. HRECs were transfected with siEDIL3 or scramble siRNA. (A) EDIL3 mRNA expression levels were assessed using reverse transcription-quantitative polymerase chain reaction. (B) EDIL3 protein expression levels were detected using western blot analysis. (C) Proliferation of HRECs was examined using a Cell Counting kit 8 assay. **P<0.01, ***P<0.001 vs. control and scramble groups. EDIL3, epidermal growth factor-like repeat and discoidin I-like domain-containing protein 3; HRECs, human retinal endothelial cells; si, small interfering.
Silencing EDIL3 expression inhibits HREC migration
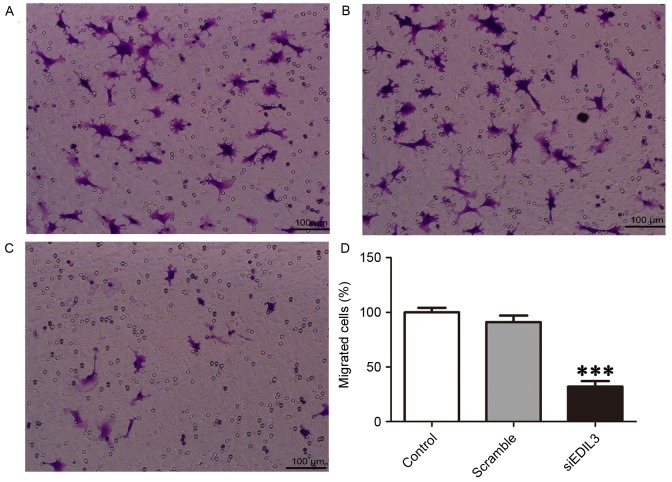
Endothelial cell migration is essential to retinal neovascularization, and involves three major mechanisms, namely chemotaxis, haptotaxis and mechanotaxis (21). To examine the effects of EDIL3 on HREC migration, a Transwell migration assay was performed (22). The present results demonstrated that following EDIL3 knockdown, the migratory capabilities of HRECs were significantly impaired (Fig. 2). HREC migration in EDIL3-silenced cells was suppressed by ~60% compared with the control and scramble groups (Fig. 2D).
Figure 2.
Silencing EDIL3 expression inhibits HREC migration in vitro. HRECs were transfected with siEDIL3 or scramble siRNA and cell migration was examined using a Transwell assay. Representative photomicrographs of migrated cells in (A) control (B) scramble and (C) siEDIL3 groups. Scale bars, 100 µm. (D) Silencing EDIL3 expression significantly reduced HREC migration. ***P<0.001 vs. control and scramble groups. EDIL3, epidermal growth factor-like repeat and discoidin I-like domain-containing protein 3; HRECs, human retinal endothelial cells; si, small interfering.
Silencing EDIL3 expression inhibits HREC tube formation
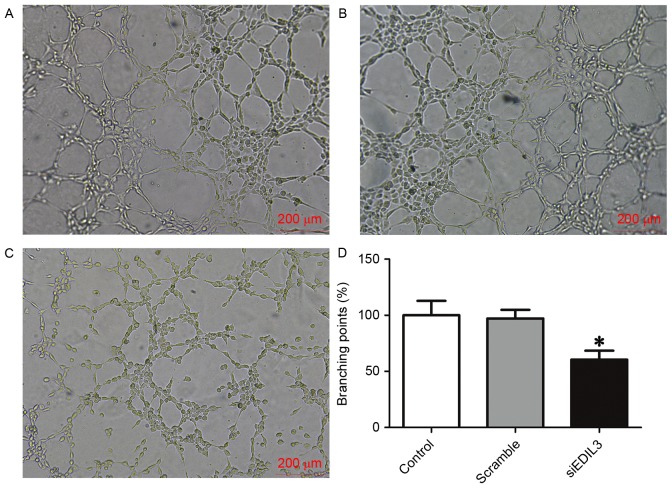
Endothelial cells are able to rapidly form capillary-like structures in vitro, when plated on a reconstituted basement membrane extracellular matrix (23). Cells plated on Matrigel-coated surfaces can rapidly attach, align and form capillary-like tubules, which can be used to study angiogenic processes and define endothelial cell populations (23,24). In the present study, Matrigel-coated plates were used to assess the angiogenic capabilities of HRECs in various treatment groups. The present findings demonstrated that following EDIL3 knockdown, the tube forming capabilities of HRECs were significantly impaired (Fig. 3). These results suggested that EDIL3 may be implicated in the process of retinal neovascularization, since the disruption of EDIL3 expression was revealed to significantly inhibit the proliferation, migration and tube formation of HRECs in vitro.
Figure 3.
Silencing EDIL3 expression inhibits HREC tube formation in vitro. HRECs were transfected with siEDIL3 or scramble siRNA and a tube formation assay was performed. Representative photomicrographs of formed capillary-like structures in (A) control (B) scramble and (C) siEDIL3 groups. Scale bars, 200 µm. (D) Silencing EDIL3 expression significantly impaired tube formation in HRECs. *P<0.05 vs. control and scramble groups. EDIL3, epidermal growth factor-like repeat and discoidin I-like domain-containing protein 3; HRECs, human retinal endothelial cells; si, small interfering.
Silencing EDIL3 expression induces cell cycle arrest at the G1 phase
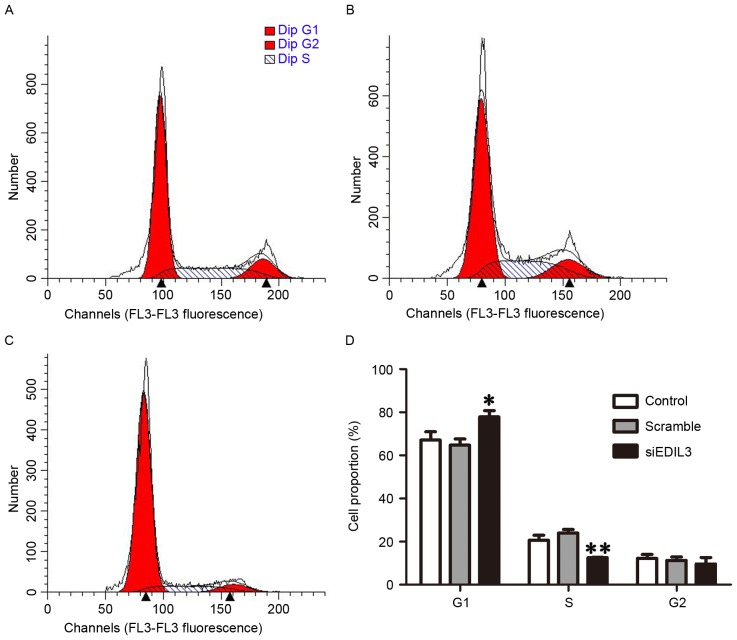
Cell cycle regulation is a complex process that serves a critical role during cellular proliferation (25). To investigate whether EDIL3 knockdown interfered with cell cycle progression, the cell cycle distribution of HRECs was analyzed using flow cytometry, 48 h following siRNA transfection. The present results demonstrated that the percentage of cells in the G1 phase was significantly increased following treatment with siEDIL3 (Fig. 4A-D). These findings suggested that EDIL3 silencing may interfere with cell cycle regulation in HRECs, possibly via modulating the expression of cell cycle-regulatory cyclins.
Figure 4.
Silencing EDIL3 expression induces cell cycle arrest at the G1 phase. HRECs were transfected with siEDIL3 or scramble siRNA and cell cycle distribution was analyzed using flow cytometry. Representative images of cell cycle distribution in (A) control (B) scramble and (C) siEDIL3 groups. (D) Silencing EDIL3 expression significantly increased the population of HRECs in the G1 phase. *P<0.05, **P<0.01 vs. control and scramble groups. EDIL3, epidermal growth factor-like repeat and discoidin I-like domain-containing protein 3; HRECs, human retinal endothelial cells; si, small interfering.
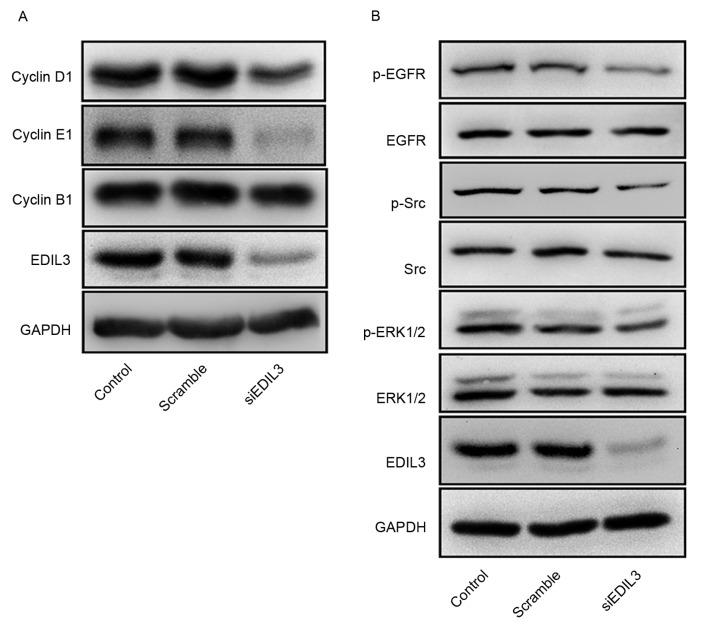
Silencing EDIL3 expression interferes with the expression of cell cycle-regulatory proteins
Cyclins are a family of proteins containing the conserved cyclin box, which mediates binding to cyclin-dependent kinases (Cdks), and are involved in cell cycle control (26). Cyclin B is necessary for the progression into the M phase of the cell cycle, cyclin D controls the G1 phase and cyclin E drives the G1/S phase transition (26). Since flow cytometry suggested that HRECs were arrested at the G1 phase following EDIL3 knockdown, the protein expression levels of cyclin B1, cyclin D1 and cyclin E1 were investigated using western blot analysis. The present results demonstrated that EDIL3 silencing appeared to suppress the expression of cyclin D1 and cyclin E1, whereas it exerted no effect on cyclin B1 (Fig. 5A). Furthermore, knockdown of EDIL3 expression appeared to suppress the phosphorylation of EGFR, Src and ERK (Fig. 5B).
Figure 5.
Silencing EDIL3 expression interferes with the expression of cell cycle-regulatory proteins and EGFR signaling. HRECs were transfected with siEDIL3 or scramble siRNA and the expression of proteins of interest was detected using western blot analysis. (A) Silencing EDIL3 expression suppressed the protein expression of cyclin D1 and cyclin E1, whereas it had no effect on cyclin B1 expression. (B) Silencing EDIL3 expression appeared to inhibit the phosphorylation of EGFR, Src and ERK in HRECs in vitro. EDIL3, epidermal growth factor-like repeat and discoidin I-like domain-containing protein 3; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; HRECs, human retinal endothelial cells; p-, phosphorylated; si, small interfering.
Discussion
Retinal neovascularization is often caused by diabetes and retinopathy of prematurity, and may result in blindness in the late stages of these diseases (18,27,28). Limited therapeutic options are available that interfere with the progression of retinal neovascularization. VEGF-neutralizing antibodies have been used for the treatment of ocular neovascularization; however, they have been associated with severe adverse effects (29,30). EDIL3 is an endothelial cell-specific protein that is usually only expressed during early embryogenesis, thus making it a promising therapeutic target for the treatment of neovascularization. The results of the present study suggested that EDIL3 silencing may inhibit retinal neovascularization in vitro, and the molecular mechanisms may involve the induction of cell cycle arrest at the G1 phase and the disruption of the EGFR signaling pathway.
EDIL3 is a regulatory extracellular matrix protein that is expressed during early embryogenesis by endothelial cells, whereas its expression is downregulated in later developmental stages (8,11). In healthy adult tissue, EDIL3 becomes quiescent or may no longer be expressed (9,22). However, EDIL3 expression can be re-initiated during ischemia and is upregulated in tumor vascular tissues (2,10,27,31). These findings suggested that EDIL3 may have potential as a therapeutic target for the treatment of abnormal angiogenesis. In the present study, disruption of EDIL3 expression using RNA interference was demonstrated to significantly suppress the proliferation, migration and tube formation of HRECs in vitro. These findings suggested that EDIL3 may participate in neovascularization processes, where it may act as a positive regulator.
To investigate the molecular mechanisms underlying the effects of EDIL3 on retinal neovascularization in vitro, the cell cycle distribution of HRECs was analyzed using flow cytometry and the expression of critical signaling proteins was assessed using western blot analysis. The cell cycle consists of four phases: G1, S, G2 and M (32). Cells progress through these phases during proliferation, and each phase is regulated through distinct proteins (26). The present results indicated that EDIL3 knockdown induced G1-phase arrest in HRECs, which may underlie the observed inhibition of cellular proliferation. In addition, the protein expression levels of cyclin D1 and cyclin E1 appeared to be downregulated, whereas cyclin B1 expression remained unaltered following EDIL3 silencing. Cyclin B1, cyclin D1 and cyclin E1 belong to the cyclin family of proteins, which can bind to Cdks (33). Cyclin B1 is responsible for driving cells through mitosis (34). The present findings revealed that the expression of cyclin B1 remained unaltered, thus suggesting that EDIL3 may not interfere with mitotic processes in HRECs. The expression of cyclin D1 is implicated in the transduction of mitogenic signals to initiate DNA synthesis during the G1 phase, and cyclin E1 is critical for driving the G1/S transition (26). The downregulation of cyclin D1 and cyclin E1 expression following EDIL3 knockdown may be implicated in the induction of cell cycle arrest at the G1 phase.
Previous studies have suggested that EDIL3 may directly activate EGFR-dependent pathways to mediate cellular proliferation and migration (12,31), and the EGFR pathway has been implicated in the regulation of cell cycle progression (35–37). Therefore, the roles of EGFR signaling in the cell cycle-arresting effects of EDIL3 silencing were investigated in HRECs. The present findings revealed that the phosphorylation of EGFR, Src and ERK were significantly suppressed in siEDIL3-transfected cells, thus suggesting that EDIL3 knockdown may inhibit EGFR signaling to induce cell cycle arrest in HRECs.
In conclusion, the results of the present study suggested that EDIL3 may be implicated in retinal neovascularization in vitro. Silencing EDIL3 expression was demonstrated to impair the proliferative, migratory and tube forming capabilities of HRECs in vitro. Furthermore, EDIL3 knockdown appeared to inhibit EGFR-mediated signaling pathways and to induce cell cycle arrest at the G1 phase. These findings suggested that EDIL3 may serve a crucial role in retinal neovascularization and may have potential as a novel therapeutic target for the treatment of aberrant neovascularization.
Acknowledgements
The present study was supported by the Natural Science Foundation of Shanghai (grant no. 15ZR1413200).
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulaiman RS, Basavarajappa HD, Corson TW. Natural product inhibitors of ocular angiogenesis. Exp Eye Res. 2014;129:161–171. doi: 10.1016/j.exer.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim LA, D'Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181:376–379. doi: 10.1016/j.ajpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Qi Y, Chen L, Shi X, Bai Y, Huang L, Yu W, Jiang Y, Zhao M, Li X. The relationship between anti-vascular endothelial growth factor and fibrosis in proliferative retinopathy: Clinical and laboratory evidence. Br J Ophthalmol. 2016;100:1443–1450. doi: 10.1136/bjophthalmol-2015-308199. [DOI] [PubMed] [Google Scholar]
- 6.Onoda Y, Shiba T, Hori Y, Maeno T, Takahashi M. Two cases of acute abdomen after an intravitreal injection of bevacizumab. Case Rep Ophthalmol. 2015;6:110–114. doi: 10.1159/000381257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabouret T, Gregory T, Dhooge M, Brezault C, Mir O, Dréanic J, Chaussade S, Coriat R. Long term exposure to antiangiogenic therapy, bevacizumab, induces osteonecrosis. Invest New Drugs. 2015;33:1144–1147. doi: 10.1007/s10637-015-0283-x. [DOI] [PubMed] [Google Scholar]
- 8.Penta K, Varner JA, Liaw L, Hidai C, Schatzman R, Quertermous T. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J Biol Chem. 1999;274:11101–11109. doi: 10.1074/jbc.274.16.11101. [DOI] [PubMed] [Google Scholar]
- 9.Aoka Y, Johnson FL, Penta K, Hirata Ki K, Hidai C, Schatzman R, Varner JA, Quertermous T. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res. 2002;64:148–161. doi: 10.1006/mvre.2002.2414. [DOI] [PubMed] [Google Scholar]
- 10.Kitano H, Kokubun S, Hidai C. The extracellular matrix protein Del1 induces apoptosis via its epidermal growth factor motif. Biochem Biophys Res Commun. 2010;393:757–761. doi: 10.1016/j.bbrc.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 11.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckham CJ, Olsen J, Yin PN, Wu CH, Ting HJ, Hagen FK, Scosyrev E, Messing EM, Lee YF. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192:583–592. doi: 10.1016/j.juro.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Rezaee M, Penta K, Quertermous T. Del1 mediates VSMC adhesion, migration and proliferation through interaction with integrin alpha(v)beta(3) Am J Physiol Heart Circ Physiol. 2002;282:H1924–H1932. doi: 10.1152/ajpheart.00921.2001. [DOI] [PubMed] [Google Scholar]
- 14.Ho HK, Jang JJ, Kaji S, Spektor G, Fong A, Yang P, Hu BS, Schatzman R, Quertermous T, Cooke JP. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: Its role in ischemia. Circulation. 2004;109:1314–1319. doi: 10.1161/01.CIR.0000118465.36018.2D. [DOI] [PubMed] [Google Scholar]
- 15.Sun JC, Liang XT, Pan K, Wang H, Zhao JJ, Li JJ, Ma HQ, Chen YB, Xia JC. High expression level of EDIL3 in HCC predicts poor prognosis of HCC patients. World J Gastroenterol. 2010;16:4611–4615. doi: 10.3748/wjg.v16.i36.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou X, Qiao H, Jiang X, Dong X, Jiang H, Sun X. Downregulation of developmentally regulated endothelial cell locus-1 inhibits the growth of colon cancer. J Biomed Sci. 2009;16:33. doi: 10.1186/1423-0127-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Y, Zhu W, Yang M, Zhu Y, Shen F, Hao Q, Young WL, Yang GY, Chen Y. Del-1 gene transfer induces cerebral angiogenesis in mice. Brain Res. 2008;1219:1–7. doi: 10.1016/j.brainres.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campochiaro PA. Ocular neovascularization. J Mol Med (Berl) 2013;91:311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) methods. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Song H, Pan D, Sun W, Gu C, Zhang Y, Zhao P, Qi Z, Zhao S. SiRNA directed against annexin II receptor inhibits angiogenesis via suppressing MMP2 and MMP9 expression. Cell Physiol Biochem. 2015;35:875–884. doi: 10.1159/000369745. [DOI] [PubMed] [Google Scholar]
- 21.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 22.Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschläger M, Dolznig H. In vitro cell migration and invasion assays. Mutat Res. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 24.DeCicco-Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L, O'Neill RC, Morin A, Wiest JS. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J Vis Exp. 2014;1:e51312. doi: 10.3791/51312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell cycle. 2002;1:103–110. doi: 10.4161/cc.1.2.108. [DOI] [PubMed] [Google Scholar]
- 26.Lim S, Kaldis P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ, Daoud Y, Baranano D, et al. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Castellanos MA, Schwartz S, Hernández-Rojas ML, Kon-Jara VA, García-Aguirre G, Guerrero-Naranjo JL, Chan RV, Quiroz-Mercado H. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013;33:329–338. doi: 10.1097/IAE.0b013e318275394a. [DOI] [PubMed] [Google Scholar]
- 29.Simó R, Sundstrom JM, Antonetti DA. Ocular Anti-VEGF therapy for diabetic retinopathy: The role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care. 2014;37:893–899. doi: 10.2337/dc13-2002. [DOI] [PubMed] [Google Scholar]
- 30.Sankar MJ, Sankar J, Mehta M, Bhat V, Srinivasan R. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2016;2:CD009734. doi: 10.1002/14651858.CD009734.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Xia H, Chen J, Shi M, Gao H, Sekar K, Seshachalam VP, Ooi LL, Hui KM. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol. 2015;63:863–873. doi: 10.1016/j.jhep.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Dalton S. Linking the cell cycle to cell fate decisions. Trends Cell Biol. 2015;25:592–600. doi: 10.1016/j.tcb.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray AW. Recycling the cell cycle: Cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/S0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 34.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 35.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: Linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lainey E, Wolfromm A, Sukkurwala AQ, Micol JB, Fenaux P, Galluzzi L, Kepp O, Kroemer G. EGFR inhibitors exacerbate differentiation and cell cycle arrest induced by retinoic acid and vitamin D3 in acute myeloid leukemia cells. Cell Cycle. 2013;12:2978–2991. doi: 10.4161/cc.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lui VW, Grandis JR. EGFR-mediated cell cycle regulation. Anticancer Res. 2002;22:1–11. [PubMed] [Google Scholar]