Abstract
Ras-related protein Rab-5A (Rab5a) has been identified to be overexpressed in several types of human cancer. However, its clinical significance and biological roles in oral cancer remain unclear. In the present study, the protein expression of Rab5a was examined in 79 cases of oral squamous cell carcinoma samples using immunohistochemistry. It was demonstrated that Rab5a protein was upregulated in 49.3% (39/79) of cancer samples. Small interfering RNA knockdown was performed on Detroit 562 cells with high endogenous expression. Rab5a transfection was performed in FaDu cells with low endogenous levels. Rab5a depletion was revealed to inhibit cell growth, invasion and colony formation while its overexpression facilitated cell growth, invasion, and colony formation. In addition, Rab5a facilitated cell cycle progression and cell migration. It was also demonstrated that Rab5a depletion downregulated and its overexpression upregulated the expression levels of various cell cycle-associated proteins, and matrix metalloproteinase-2 (MMP-2). Furthermore, Rab5a positively regulated the extracellular signal-regulated kinase (ERK) signaling pathway and promoted epithelial-mesenchymal transition (EMT). ERK inhibitor PD98059 partially inhibited the role of Rab5a on MMP-2, cyclin D1, cell proliferation and invasion. The results of the present study suggest that Rab5a is overexpressed in oral cancer tissue samples and promotes the malignant phenotype through EMT and the ERK/MMP-2 signaling pathway.
Keywords: Rab5a, oral squamous cell carcinoma, extracellular signal-regulated kinase, epithelial-mesenchymal transition
Introduction
Oral squamous cell carcinoma (OSCC) is a common oral malignancy, which belongs to head and neck sequmous cell carcinoma (HNSCC). Despite Advances in surgical treatment and chemoradiotherapy, the overall prognosis of advaned OSCC remains poor (1). Thus, it remains important to identify new markers that can be used to predict risk of OSCC progression.
Rab5a is one of the Rab GTPases proteins, which participates in multiple biological process including endocytosis and vesicle transport (2,3). Rab proteins have been shown to participate in the regulation of cancer progression (4,5). Rab5a is overexpressed in breast and ovarian cancers and promotes cancer growth and invasion (6–8). Rab5a protein is elevated in hepatocellular carcinoma samples (9). These findings indicate that Rab5a may serve as an oncoprotein in human cancers. Yet, the clinical significance and biological role of Rab5a in OSCC is not investiagted. In this study, we examined Rab5a protein expression in 79 cases of OSCC specimens. We upregulated and downregulated Rab5a in HNSCC cancer cell lines and examined its roles on cell proliferation and invasion. We also checked the molecular mechanism underlying its biological effect.
Materials and methods
Patients and specimens
The study protocol was approved by the Institutional Review Board of China Medical University. Primary tumor specimens were obtained from patients diagnosed with OSCC who underwent resection prior to chemotherapy or radiotherapy between 2014 and 2016 in School and Hospital of Stomatology, China Medical University. The histological diagnosis was evaluated according to the WHO guidelines. Clinical data was obtained from medical records.
Immunohistochemistry
The FFPE tissue sections were treated with xylene, graded alcohol and then antigen retrieval was performed in 0.01 M citrate buffer. Hydrogen peroxide was used for blockage. Tissue sections were treated with goat serum for 20 min. Sigma Prestige Rab5a antibody (1:400; Sigma, St. Louis, MO, USA) was incubated with each section at 4°C overnight. EliVision plus kit from Maixin (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) and DAB kit (Fuzhou Maixin Biotech Co., Ltd.) were used for immunohistochemical staining. Staining was performed according to manufacture's procedure. All tumor slides were examined randomly by two independent pathologists. The intensity was scored as 0 (negative), 1 (weak), 2 (strong). Percentage scores were designated as: i) 1–25%, ii) 26–50%, iii) 51–75% and iv) 76–100%. Percentage scores and intensity scores were multiplied to get a final score of 0–8. Tumor sample scored ≥4 was regarded as Rab5a high expression.
Cell culture and transfection
FaDu and Detroit 562 cell lines were obtained from American Type Culture Collection, Manassas, VA, USA. Cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) with 10% FBS (Gibco, Grand Island, NY, USA).
Rab5a plasmid was obtained from OriGene Technologies, Inc. (Rockville, MD, USA). Lipofectamine 3000 transfection reagent was used for plasmid transfection (Invitrogen). The empty vector (pCMV6) was used as negative control. For siRNA transfection, Rab5a siGENOME SMARTpool siRNA and Negative control siRNA were obtained from Thermo Fisher Scientific (Waltham, MA, USA). DharmaFECT 1 (ThermoFisher Scientific) was used for siRNA transfection.
Quantitative (q)PCR
qPCR was performed using SYBR-Green master mix (Takara Bio, Dalian, China). PCR was performed using 7500 Real-Time PCR System (Applied Biosystems). β-actin was used as the reference gene. The relative expression of target genes were calculated using the 2−ΔΔCq method. The primer sequences are as follows: Rab5a forward, 5′ CAA GAA CGA TAC CAT AGC CTA GCA C 3′; Rab5a reverse, 5′ CTT GCC TCT GAA GTT CTT TAA CCC 3′; β-actin forward, 5′ ATA GCA CAG CCT GGA TAG CAA CGT AC 3′, β-actin reverse, 5′ CAC CTT CTA CAA TGA GCT GCG TGT G 3′.
Western blot analysis
Proteins were extracted in RIPA lysis buffer and quantified using the Bradford method. 40 µg protein was separated by SDS-PAGE for all the samples. Protein samples on SDS-PAGE were transferred to PVDF membranes (Millipore Corp., Billerica, MA, USA) and incubated overnight at 4°C with primary antibody against Rab5a (11947–1-AP) (1:1,000; ProteinTech Group, Inc., Chicago, IL, USA), p-extracellular signal-regulated kinase (ERK) (no. 4370), ERK (no. 9102), MMP-2 (no. 87809), cyclin D1 (no. 2978), cyclin E (no. 4129), cyclin A (no. 4656), cyclin B (no. 4135), E-cadherin (no. 14472), p-FAK (no. 8556), p-EGFR (no. 3777), p-MEK (no. 3958), MEK (no. 4694) (1:1,000; Cell Signaling Technology, Inc., Boston, MA, USA), Snail (sc-10432), Vimentin (sc-73259) (1:500 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and GAPDH (sc-47724) (1:1,000; Santa Cruz Biotechnology, Inc.). After incubation with HRP-coupled secondary antibody (nos. 7074 and 7076) (1:2,000, Cell Signaling Technology) at 37°C for 2 h. Target proteins on PVDF membrane were visualized using ECL kit (Pierce Biotechnology, Inc., Rockford, IL, USA) and obtained using DNR Imaging System (DNR Bio-Imaging Systems, Ltd., Jerusalem, Israel). Membranes were stripped using Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) and re-blotted for GAPDH.
Colony formation and MTT assays
For colony formation assay, cells were transfected for 48 h and plated into three 6-cm cell culture dishes (1,000 cells). Cells were incubated for 2 weeks in medium. Plates were washed with PBS and stained with Giemsa. The number of colonies with more than 50 cells was counted. The colonies were manually counted using a microscope.
For MTT assay, 24 h after transfection, cells were plated in 96-well plates in at a concentration approximately 2,000 cells per well and cultured for 5 days. For quantitation of cell proliferation rate, 20 µl of 5 mg/ml MTT (thiazolyl blue) solution was added to each well and incubated for 4 h at 37°C. The medium was removed from each well and 150 µl of DMSO was added to the well. The plate was measured at 490 nm.
Gelatin zymography
Cells were cultured for 24 h in medium without serum. The supernatant was collected, which was separated on SDS-PAGE with 1 mg/ml gelatin. After electrophoresis, the gel was washed by 2.5% Triton X100 and incubated with developing buffer [50 mmol/l Tris-HCl buffer (pH 7.4), 10 mmol/l CaCl2] 12 h at 37°C. Then the gel was stained by 0.25% Coomassie Brilliant Blue R-250 and then de-stained in the solution without dye.
Transwell assay
Cell invasion assay was performed using a 24-well Transwell chamber. The inserts were coated with 20 µl Matrigel (1:5 dilution). Cells were transferred to the upper chamber in a 100 µl serum-free medium and then cultured for 18 h. Medium supplemented with 10% FBS was added to the lower chamber. Cells which did not invade through the membrane were removed with a cotton tip. Cells that passed through the membrane were fixed and stained with hematoxylin.
Wound healing assay
Cells were seeded into 6-well tissue culture plates at a density of about 90% confluence. The monolayer was gently and slowly scratched using a pipette tip under aseptically conditions. Serum free medium was used to culture cell for 2 days. The detached cells were removed and washed, then incubated in medium for the indicated times. Photos of the stained monolayer were taken under a microscope.
Statistical analysis
SPSS 12 for Windows was used for all statistical analyses. A χ2 test was used to analyze the association between Rab5a status and clinical parameters. Student's t-test was carried out to compare other data. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical significance of Rab5a in human OSCC
Rab5a protein was examined in 79 cases of OSCC tissues (Fig. 1). Negative Rab5a staining was found in normal mucosa epithelial tissues. Rab5a protein displayed high cytoplasmic staining in 39 of 79 cancer tissues. As shown in Table I. Rab5a overexpression correlated with poor differentiation (P=0.0273), advanced TNM stage (P=0.0145), higher T stage (P=0.0279) and positive nodal status (P=0.0060).
Figure 1.
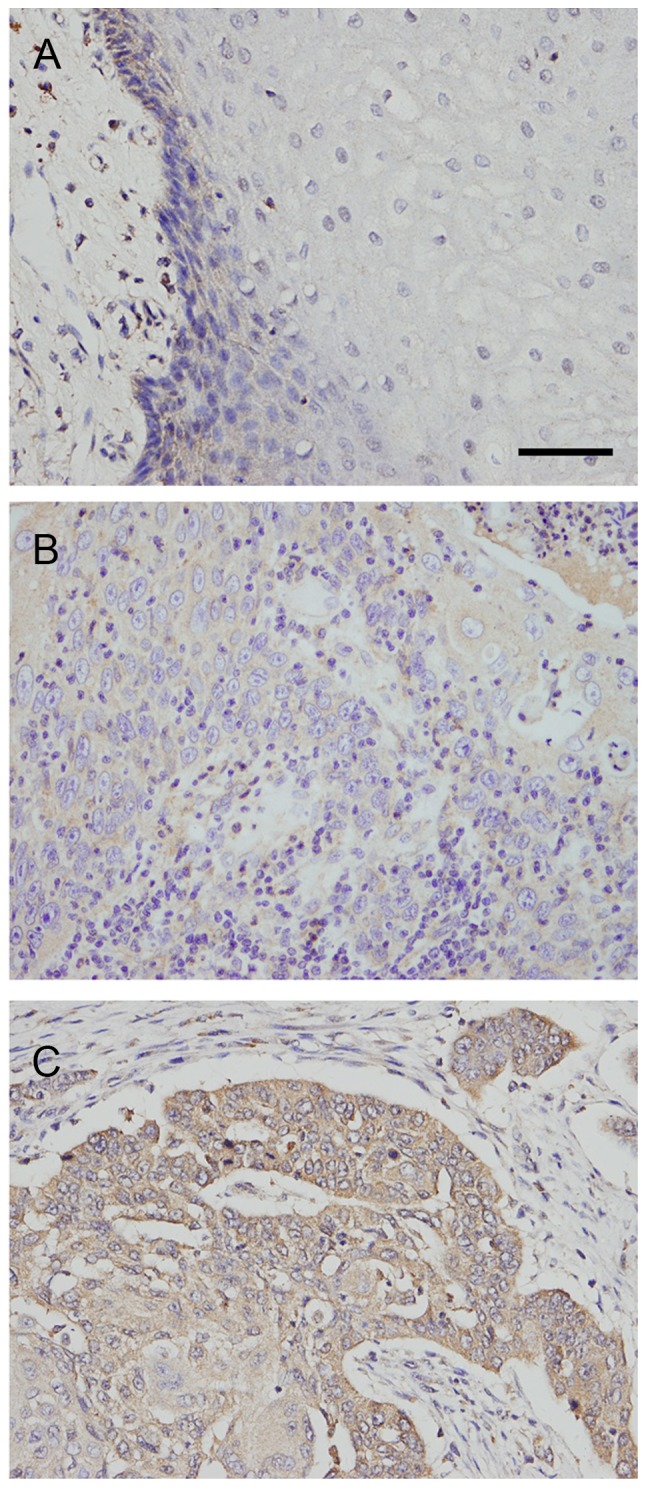
Expression of Rab5a protein in OSCC tissues. (A) Negative Rab5a expression in normal oral squamous mucosa. Scale bar: 50 µm. (B) Weak Rab5a expression in a case of stage 1, grade 1 OSCC. (C) Positive cytoplamic Rab5a expression in a case of stage 3 OSCC. (Magnification, 400X). OSCC, oral squamous cell carcinoma; Rab5a, Ras-related protein Rab-5A.
Table I.
Distribution of Rab5a status in oral squamous cell carcinoma according to clinicopathological characteristics.
| Characteristics | No. of patients | Rab5a low expression | Rab5a high expression | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 51 | 26 | 25 | 0.9336 |
| ≥60 | 28 | 14 | 14 | |
| Gender | ||||
| Male | 46 | 24 | 22 | 0.7464 |
| Female | 33 | 16 | 17 | |
| Differentiation | ||||
| Well | 61 | 35 | 26 | 0.0273 |
| Moderate-poor | 18 | 5 | 13 | |
| TNM stage | ||||
| I | 31 | 21 | 10 | 0.0145 |
| II+III | 48 | 19 | 29 | |
| Tumor status | ||||
| T1 | 32 | 21 | 11 | 0.0279 |
| T2-T4 | 47 | 19 | 28 | |
| Nodal metastasis | ||||
| Negative | 69 | 39 | 30 | 0.0060 |
| Positive | 10 | 1 | 9 |
Rab5a, Ras-related protein Rab-5A; TNM, tumor node metastasis.
Rab5a promotes OSCC cell proliferation and invasion
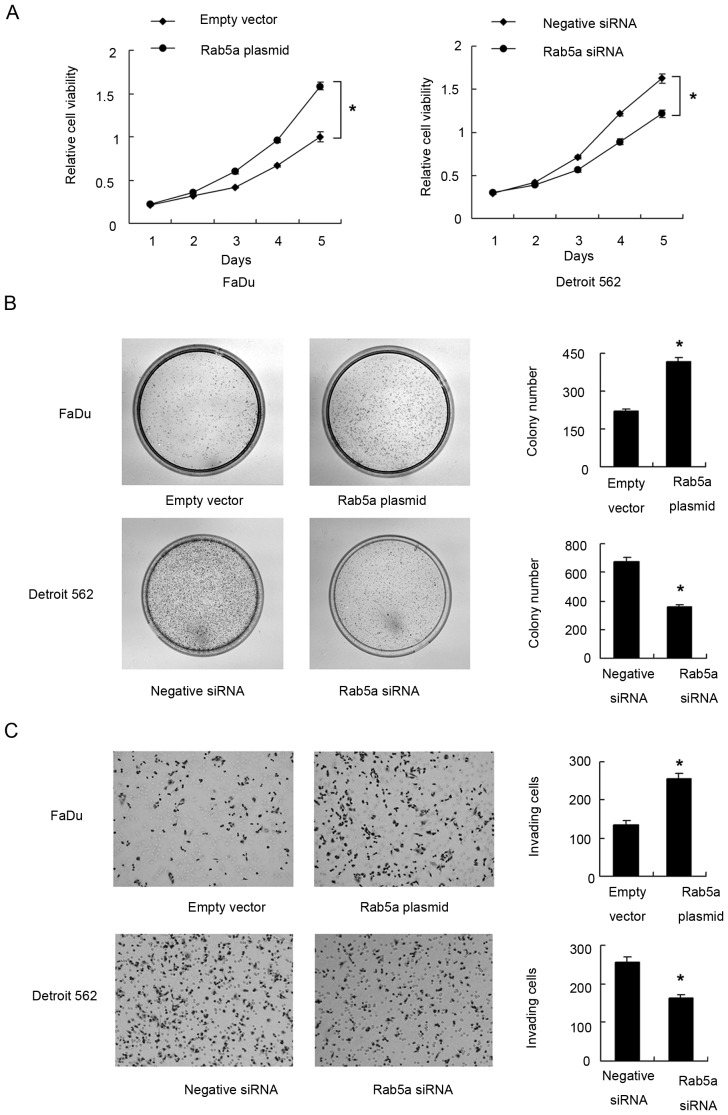
Endogenous expression of Rab5a was examined by western blot in OSCC cell lines. We found that Detroit 562 cell line had high endogenous expression while FaDu cell line had low endogenous expression (Fig. 2A). Transient transfection of Rab5a plasmid (0.5 µg/ml) and siRNA (50 nM) was performed in these cells. As shown in Fig. 2B, Rab5a plasmid significantly upregulated its expression at both protein and mRNA levels (P<0.05). siRNA knockdown efficiency was also confirmed by western blot analysis and PCR (P<0.05). MTT demonstrated that Rab5a transfection facilitated cell proliferation rate in FaDu cell line, while its siRNA blocked proliferation in Detroit 562 cell line (Fig. 3A). Colony formation assay showed the similar results. Rab5a plasmid significantly promoted colony formation ability (P<0.05) while its siRNA inhibited colony formation ability (Fig. 3B) (P<0.05). To characterize the role of Rab5a on invasion, transwell assay was performed. As shown in Fig. 3C, significant reduction of invading ability was found in cells with Rab5a siRNA depletion (P<0.05) while Rab5a plasmid upregulated invading cell number (P<0.05).
Figure 2.
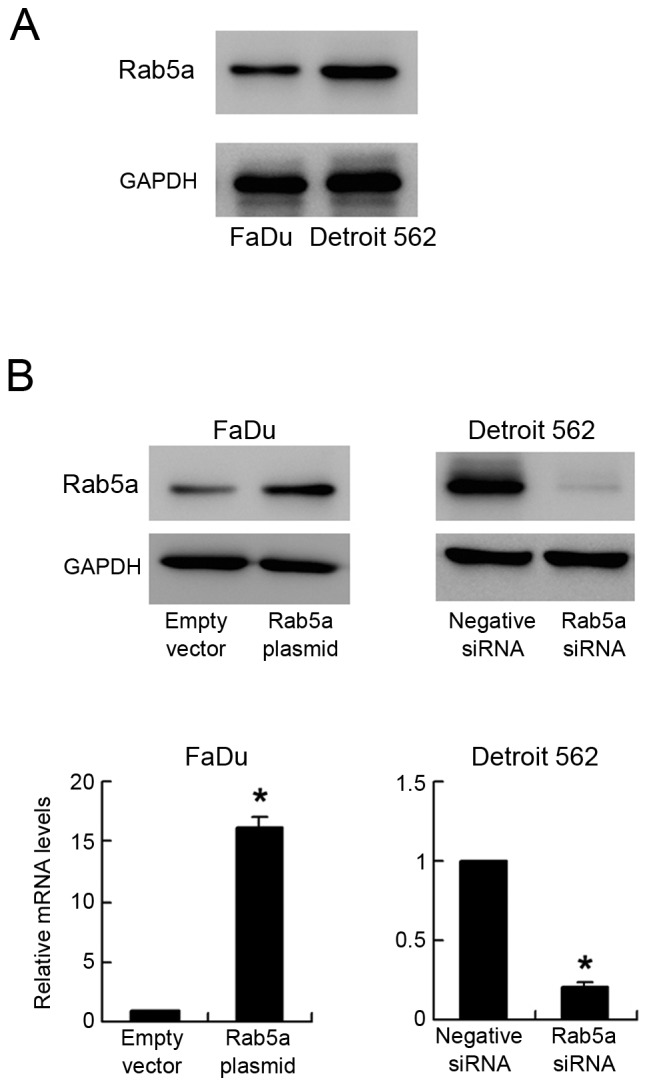
Transfection efficiency of Rab5a plasmid and siRNA. (A) Western blot showed that high Rab5a expression in Detroit 562 and low in FaDu cells. (B) Western blot and polymerase chain reaction analysis showed that Rab5a transfection in FaDu cells increased its protein and mRNA expression. Rab5a siRNA treatment in Detroit 562 cells decreased both levels. *P<0.05 (Students' t-test). Rab5a, Ras-related protein Rab-5A; si, small interferring.
Figure 3.
Rab5a promotes proliferation and invasion in OSCC cell lines. (A) MTT assay showed that Rab5a overexpression upregulated cell proliferation rate in FaDu cell line while Rab5a siRNA downregulated cell proliferation rate in Detroit 562 cell line. (B) Colony formation assay showed that Rab5a transfection increased while its depletion decreased colony formation ability. (C) Matrigel invasion assay showed that Rab5a overexpression promoted invading ability of FaDu cell line while its depletion inhibited invasion of Detroit 562 cells. *P<0.05 (Students' t-test). OSCC, oral squamous cell carcinoma; Rab5a, Ras-related protein Rab-5A; si, small interferring.
Rab5a regulates cell cycle, migration and related proteins
Cell cycle analysis by PI staining was carried out. As shown in Fig. 4A, Rab5a facilitated cell cycle transition by upregulating S phase percentage (P<0.05) while Rab5a depletion downregulated S phase percentage (P<0.05). Wound healing assay was carried out to examine cell migration. As shown in Fig. 4B, Rab5a overexpression upregulated cell migration ability (P<0.05) while its depletion downregulated migration ability (P<0.05).
Figure 4.
Rab5a regulates cell cycle and migration. (A) Rab5a facilitated cell cycle transition by upregulating S phase percentage while Rab5a depletion downregulated S phase percentage. (B) Wound healing assay showed that Rab5a overexpression upregulated cell migration speed while its depletion downregulated migration speed. *P<0.05 (Students' t-test). Rab5a, Ras-related protein Rab-5A; si, small interferring.
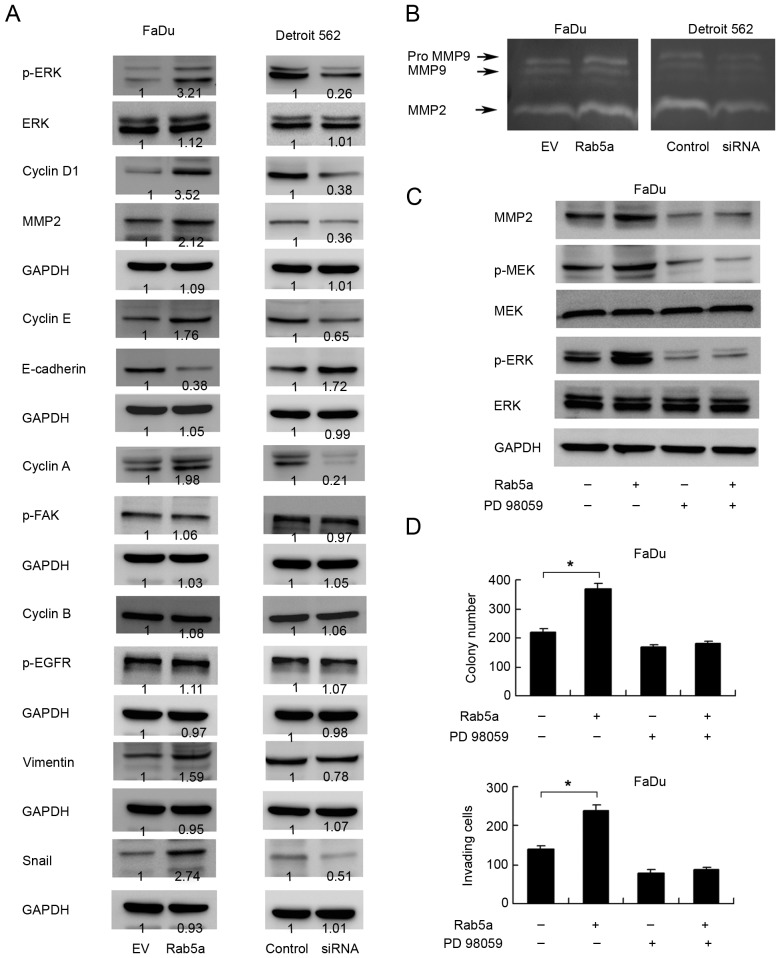
In order to investigate the potential mechanism of Rab5a induced cell proliferation and invasion, we checked the expression of cell cycle and invasion related proteins in cells transfected with Rab5a plasmid or siRNA. The results showed that Rab5a transfection upregulated cyclin D1, E, A and MMP-2 expression while Rab5a depletion downregulated these proteins (Fig. 5A). Consistent with these results, gelatin zymography showed a lytic zones at the molecular mass corresponding to MMP-2. Rab5a overexpression resulted in significantly increased secretion and enzyme activity of MMP-2 (Fig. 5B). These data suggested that Rab5a regulates cancer cell proliferation and invasion through cell cycle and MMP production.
Figure 5.
Rab5a regulates ERK signaling and epithelial-mesenchymal transition. (A) Western blot showed that Rab5a transfection upregulated cyclin D1, cyclin E, cyclin A, MMP-2, p-ERK, Snail, Vimentin expression and downregulated E-cadherin. Rab5a depletion downregulated cyclin D1, cyclin E, MMP-2, p-ERK, Snail, Vimentin expression and sligntly upregulated E-cadherin. Change of cyclin B, p-FAK and p-EGFR was not significant. Relative densitometric intensity was analyzed by ImageJ software. (B) Gelatin zymography showed that Rab5a positively regulated MMP-2 activity. (C) MEK/ERK inhibitor PD98059 blocked ERK and MEK phosphorylation. PD98059 also abolished the effect of Rab5a on MMP-2 production. (D) Rab5a significantly upregulated colony and invasion cell number. In cells with PD98059 treatment, the effect of Rab5a on colony formation and invasion was not significant. *P<0.05 (Students' t-test). ERK, extracellular signal-regulated kinase; p-, phosphorylated; MMP, matrix metalloproteinase; FAK, focal adhesion kinase; EGFR, epidermal growth factor receptor; Rab5a, Ras-related protein Rab-5A; si, small interferring; EV, empty vector.
Rab5a regulates MMP-2 through ERK signaling
To explore the mechanism of Rab5a induced MMP-2 production. We checked MAPK signaling pathway which was previous reported to regulate MMP-2. We found that Rab5a overexpression significantly upregulated ERK phosphorylation while total ERK was not changed (Fig. 5A). The change of JNK and p38 phosphorylation was not significant (data not shown), suggesting Rab5a induced ERK activation is responsible for upregulated MMP-2 production. In addition, we found that Rab5a did not change the level of p-EGFR and p-FAK which function as the upstream regulator of ERK. To further confirm the role of ERK, MEK/ERK inhibitor PD98059 (20 µM, 6 h) was used in FaDu cells with Rab5a plasmid transfection. As shown in Fig. 5C, PD98059 downregulated MEK and ERK phosphorylation. ERK inhibitor also abolished the role of Rab5a on MMP-2 upregulation. In addition, we checked the role of PD98059 on cancer cell growth and invasion. As shown in Fig. 5D, ERK inhibitor blocked the effect of Rab5a on cancer cell proliferation and invasion.
Rab5a regulates epithelial-mesenchymal transition (EMT)
In addition, we checked expression of EMT related factors and found that Rab5a overexpression downregulated E-cadherin in FaDu cell line while Rab5a depletion slightly upregulated E-cadherin expression. Rab5a also positively regulated Snail and Vimentin protein expression (Fig. 5A). Together, these data confirmed that Rab5a promotes malignant phenotype of OSCC cells through ERK/MMP-2 signaling and EMT.
Discussion
OSCC is a common oral malignancy, which is also a subset of HNSCC. In the present study, we found that Rab5a was overexpressed in 39 of 79 OSCC samples and correlated with advanced stage, tumor status and nodal metastasis, which suggests that Rab5a status indicate malignant phenotype of OSCC. Rab5a overexpression and its biological activity have been reported in several human cancers including breast, ovarian and hepatocellular carcinoma (7–9). We also found that Rab5a overexpression increased FaDu cell proliferation and invasion, while its depletion inhibited Detroit 562 cell growth and invasion. These data is in accord with previous studies indicating Rab5a as an important regulator of malignant biological behavior of OSCC cells.
To further explore the possible underlying mechanism of Rab5a on cancer cell proliferation and invasion, we examined cell cycle progression, migration and related proteins. We confirmed that Rab5a could positively regulate cell cycle progression and migration. In addition, we found that Rab5a overexpression upregulated cyclin D1, cyclin A and cyclin E in FaDu cells, while its depletion downregulated cyclin D1 cyclin A and cyclin E in Detroit 562 cells. We also found Rab5a transfection upregulated MMP-2 protein. MMP-2 was reported to play important roles in invasion and metastasis of oral cancer (10,11). This study demonstrated that MMP-2 is the target of Rab5a, which supports its role on cell invasion. MMP-2 expression is reported to be controlled at many levels such as gene transcription, enzyme activation, which can be activated by many signaling pathways. It is well established that the MAPK-ERK signaling cancer cell invasion through AP-1 transcription factors, which was shown to activate MMP-2 transcription (12–18). Thus we investigated ERK pathway activation and the results showed that Rab5a overexpression upregulated ERK phosphorylation while its depletion downregulated ERK phosphorylation. To confirm the link between Rab5a induced ERK activation and MMP-2 upregulation. We employed PD98059, an ERK signaling inhibitor, in FaDu cells and test the effect of Rab5a on MMP-2, cell proliferation and invasion. The results showed that ERK inhibition abolished the effect of Rab5a on MMP-2 upregulation. PD98059 also blocked the effect of Rab5a on proliferation and invasion, demonstrating ERK activation is responsible for cancer growth and invasion stimulated by Rab5a.
EMT plays an important role in tumor invasion and metastasis. A growing number of publications showed that oral cancer invading ability was regulated by EMT (19–21). Thus we examined EMT related markers such including E-cadherin, Snail and Vimentin. We found that Rab5a positively regulated expression of Snail and Vimentin, with E-cadherin downregulation, suggesting EMT plays a part in the regulation of Rab5a on oral cancer invasion. ERK is originally reported to control EMT in sea urchin embryo (22). ERK activation is essential for EMT process of normal murine mammary gland epithelial cell (23). ERK also functions as an EMT inducer in various human cancers (24,25). Based on these research, we postulated that Rab5a induced EMT process of oral cancer cell through an ERK dependent manner.
In conclusion, the present study demonstrates Rab5a is overexpressed in human oral cancer and contributes to cancer cell growth and invasion through ERK/MMP-2 signaling pathway and EMT.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews3007. REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–116. doi: 10.1016/j.bbcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–2519. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z, Liu XF, Wu HC, Zou SB, Wang JY, Ni PH, Chen XH, Fan QS. Rab5a overexpression promoting ovarian cancer cell proliferation may be associated with APPL1-related epidermal growth factor signaling pathway. Cancer Sci. 2010;101:1454–1462. doi: 10.1111/j.1349-7006.2010.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long G, Yang K. MicroRNA-130a inhibits cell proliferation, invasion and migration in human breast cancer by targeting the RAB5A. Int J Clin Exp Pathol. 2015;8:384–393. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang PS, Yin PH, Tseng LM, Yang CH, Hsu CY, Lee MY, Horng CF, Chi CW. Rab5A is associated with axillary lymph node metastasis in breast cancer patients. Cancer Sci. 2011;102:2172–2178. doi: 10.1111/j.1349-7006.2011.02089.x. [DOI] [PubMed] [Google Scholar]
- 9.Geng D, Zhao W, Feng Y, Liu J. Overexpression of Rab5a promotes hepatocellular carcinoma cell proliferation and invasion via FAK signaling pathway. Tumour Biol. 2016;37:3341–3347. doi: 10.1007/s13277-015-4606-5. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Xue X, Lan J, Gao Y, Xiong Z, Zhang H, Jiang W, Song W, Zhi Q. MicroRNA-29a upregulates MMP2 in oral squamous cell carcinoma to promote cancer invasion and anti-apoptosis. Biomed Pharmacother. 2014;68:13–19. doi: 10.1016/j.biopha.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Kawamata H, Nakashiro K, Uchida D, Harada K, Yoshida H, Sato M. Possible contribution of active MMP2 to lymph-node metastasis and secreted cathepsin L to bone invasion of newly established human oral-squamous-cancer cell lines. Int J Cancer. 1997;70:120–127. doi: 10.1002/(SICI)1097-0215(19970106)70:1<120::AID-IJC18>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, Yu D. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001;20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- 13.Ward Y, Wang W, Woodhouse E, Linnoila I, Liotta L, Kelly K. Signal pathways which promote invasion and metastasis: Critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol Cell Biol. 2001;21:5958–5969. doi: 10.1128/MCB.21.17.5958-5969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou HF, Guo L, Liu W, Wang SJ, Yu XG. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol. 2012;40:1714–1724. doi: 10.3892/ijo.2011.1320. [DOI] [PubMed] [Google Scholar]
- 15.Das G, Shiras A, Shanmuganandam K, Shastry P. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog. 2011;50:412–423. doi: 10.1002/mc.20723. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: An overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/A:1026028303196. [DOI] [PubMed] [Google Scholar]
- 17.Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS, Park NG, Nakajima H, Magae J, Chang YC. Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9 gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis. 2007;28:1104–1110. doi: 10.1093/carcin/bgl217. [DOI] [PubMed] [Google Scholar]
- 18.Yen JH, Kocieda VP, Jing H, Ganea D. Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem. 2011;286:38913–38923. doi: 10.1074/jbc.M111.252932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan J, Elhousiny M, Johnson NW, Gao J. Transforming growth factor-β1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclasts. Clin Exp Metastasis. 2013;30:659–670. doi: 10.1007/s10585-013-9570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CJ, Hsu CC, Chang CH, Tsai LL, Chang YC, Lu SW, Yu CH, Huang HS, Wang JJ, Tsai CH, et al. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol Rep. 2011;26:1003–1010. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 21.Vered M, Dayan D, Yahalom R, Dobriyan A, Barshack I, Bello IO, Kantola S, Salo T. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int J Cancer. 2010;127:1356–1362. doi: 10.1002/ijc.25358. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Serra M, Consales C, Livigni A, Arnone MI. Role of the ERK-mediated signaling pathway in mesenchyme formation and differentiation in the sea urchin embryo. Dev Biol. 2004;268:384–402. doi: 10.1016/j.ydbio.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Mendoza MC, Pei X, Ilter D, Mahoney SJ, Zhang Y, Ma D, Blenis J, Wang Y. Down-regulation of CMTM8 induces epithelial-to-mesenchymal transition-like changes via c-MET/extracellular signal-regulated kinase (ERK) signaling. J Biol Chem. 2012;287:11850–11858. doi: 10.1074/jbc.M111.258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lue HW, Yang X, Wang R, Qian W, Xu RZ, Lyles R, Osunkoya AO, Zhou BP, Vessella RL, Zayzafoon M, et al. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated ERK signaling. PLoS One. 2011;6:e27720. doi: 10.1371/journal.pone.0027720. [DOI] [PMC free article] [PubMed] [Google Scholar]