Abstract
Tribulus terrestris (T. terrestris) has been used as a traditional medicine for the treatment of a variety of diseases, including inflammation, edema and hypertension. The aqueous and ethanol extracts of T. terrestris contain alkaloids, flavonoids, tannins, quinines and phenolic compounds. Tribulusamide D is a compound that has been isolated from the ethanol extract of T. terrestris. The present study investigated the anti-inflammatory effect of tribulusamide D on lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Tribulusamide D inhibited the production of LPS-induced nitric oxide and prostaglandin E2, by reducing the expression of inducible nitric oxide synthase and cyclooxygenase-2 expression, respectively. The expression of these genes associated with inflammation was determined using reverse transcription-polymerase chain reaction and western blot analysis. Furthermore, tribulusamide D reduced the expression of LPS-induced inflammatory cytokines, including interleukin (IL)-6, IL-10 and tumor necrosis factor-α. They were quantified using an enzyme-linked immunosorbent assay. In addition, the present study confirmed that the inhibitory effects of tribulusamide D on the inflammatory response were mediated through inactivation of mitogen-activated protein kinase p38 and inhibition of nuclear localization of nuclear factor-B, which were also determined by western blot analysis. To the best of our knowledge, the current study is the first to demonstrate that tribulusamide D exerts anti-inflammatory activity by altering the expression of inflammatory mediators and cytokines, indicating that tribulusamide D could be developed as a potential therapeutic agent for the treatment of inflammatory disorders.
Keywords: tribulusamide D, anti-inflammatory effect, cyclooxygenase-2, pro-inflammatory cytokines, RAW 264.7 macrophages
Introduction
Tribulus terrestris L. is an annual creeping herb of the family Zygophyllaceae (1). Since ancient times, it has been used in folk medicine for the treatment of hypertension, edema, eye problems, sexual dysfunction and rheumatoid arthritis (2–8). Previously, it was reported that T. terrestris contains steroidal saponins, alkaloids and flavonoids (9) and that the aqueous and ethanol extracts of T. terrestris contain alkaloids, flavonoids, tannins, quinines and phenolic compounds (10). A novel compound isolated from the ethanol extract of T. terrestris is tribulusamide D (11). However, the molecular mechanisms underlying the anti-inflammatory effects of tribulusamide D have not previously been reported. Inflammation is an immune response to infection involving biological chemicals and physiological stimuli (12). It causes enhanced vascular permeability, fever and septic shock, thereby increasing the blood flow and migration of immune cells to the sites of infection (13). Macrophages have essential roles during inflammation, including the elimination of foreign organisms and antigen presentation (14). Upon activation of macrophages by lipopolysaccharide (LPS), various inflammatory mediators and inflammatory cytokines, including nitric oxide (NO), prostaglandin (PG) E2, interleukin (IL)-6, IL-10 and tumor necrosis factor-α (TNF-α), are secreted (15,16).
There are three isoforms of nitric oxide synthase (NOS): Neuronal NOS, endothelial NOS and inducible NOS (iNOS). Stimulation of macrophages with LPS induces increased expression of iNOS, which leads to the increased production of NO during an inflammatory response (17). Cyclooxygenase-2 (COX-2) is an enzyme in the PGH synthase family, and its expression is increased by cytokines and bacterial products, such as LPS. COX-2 converts arachidonic acid to PGs such as PGE2, which is associated with inflammatory pain and swelling (18–21). These inflammatory mediators (NO and PGE2) and cytokines (IL-6, IL-10 and TNF-α) are implicated in numerous diseases, including rheumatoid arthritis, asthma and atherosclerosis (22). Therefore, reducing NO and PGE2 release by inhibiting iNOS and COX-2, respectively, may be crucial for the development of novel anti-inflammatory drugs. In addition, Kim et al (23) reported that p38 mitogen-activated protein kinase (p38 MAPK) mediates LPS-induced nuclear factor-κB (NF-κB) activation in acute lung injury and RAW264.7 macrophages.
To the best of our knowledge, the present study is the first to demonstrate the anti-inflammatory effect of tribulusamide D, a compound isolated from the ethanolic extract of Tribulus terrestris L., on LPS-stimulated inflammatory responses in RAW 264.7 macrophages.
Materials and methods
Plant material
The fruits of Tribulus terrestris L. were purchased from the Gyeongdong Oriental Medicine Market (Seoul, Korea) in March 2012, and were identified by Professor Joa Sub Oh (College of Pharmacy, Dankook University, Cheonan, Korea). A voucher specimen (G46) was deposited at the Natural Products Research Institute, Gyeonggi Institute of Science and Technology Promotion (Suwon, Korea).
Preparation of tribulusamide D
The air-dried and crushed fruits of T. terrestris L. (10 kg) were ground and extracted with 80% ethanol (3×18 L) for 2 days at room temperature. The 80% ethanol extract was filtered and concentrated under vacuum at 40°C to yield 673.5 g of residue. This residue was then suspended in water and partitioned with hexane (3×1.5 L) to afford a hexane soluble layer (40 g). The aqueous layer was partitioned with chloroform (CHCl3) to provide a CHCl3-soluble residue (10 g). The remaining aqueous layer was partitioned with ethyl acetate to give an ethyl acetate-soluble extract (8.1 g). The ethyl acetate layer (8.1 g) was subjected to liquid chromatography on a silica gel column (230–400 mesh; 7×20 cm) using CHCl3:methanol (100:0, 99:1, 98:2, 97:3, 96:4, 94:6, 92:8, 90:10, 80:20, 70:30, 60:40, 50:50) v/v gradient mixtures as eluents. The eluent fractions G46-51-1-13 were obtained from this initial liquid chromatographic separation. The fractions were examined to an in vitro assay to assess their inhibitory effect on NO production (24).
Among them, the fraction G46-51-7 (2.71 g) exhibited promising inhibitory activity against NO production and it was selected for further investigation. G46-51-7 (2.71 g) was passed through a column containing Sephadex® LH-20 gel using CHCl3:MeOH (1:1) as eluent, to produce sub-fractions (G46-52-1-21). Of the above 21 fractions, tribulusamide D (17.3 mg) was isolated from fraction G46-52-14, which was precipitated with MeOH. 1H- and 13C-nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Ascend™ 700 MHz NMR spectrometer (Bruker Corporation, Billerica, MA, USA) using dimethylsulfoxide (DMSO) as a solvent. Electrospray ionization mass (ESI-MS) was measured on a Thermo Finnigan TSQ Quantum mass spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Tribulusamide D
Tribulusamide D is a white, amorphous powder; 1H-NMR (DMSO-d6, 700 MHz) δ: 10.4 (1H, s, OH), 9.39 (1H, s, OH), 9.16 (1H, s, OH), 8.29 (1H, t, J=5.6 Hz, NH), 7.90 (2H, d, J=8.4 Hz, H-2′ and H-6′), 7.27 (1H, d, J=16.1 Hz, H-7), 6.99 (1H, d, J=2.1 Hz, H-2), 6.88 (2H, d, J=9.1 Hz, H-3′ and H-5′), 6.87 (1H, dd, J=8.4, 2.1 Hz, H-6), 6.75 (1H, d, J=7.7 Hz, H-5), 6.52 (1H, d, J=15.4 Hz, H-8), 4.65 (2H, s, H-8′); 13C-NMR (DMSO-d6, 175 MHz) δ 193.9 (C-7′), 166.2 (C-9), 162.8 (C-4′), 147.9 (C-4), 146.0 (C-3), 140.0 (C-7), 130.9 (C-2′), 130.9 (C-6′), 127.0 (C-1′), 126.8 (C-1), 121.0 (C-6), 118.6 (C-8), 116.2 (C-5), 115.8 (C-3′), 115.8 (C-5′), 114.3 (C-2), 45.9 (C-8′); ESI-MS m/z 312 [M-H]-. The structure of tribulusamide D is presented in Fig. 1A.
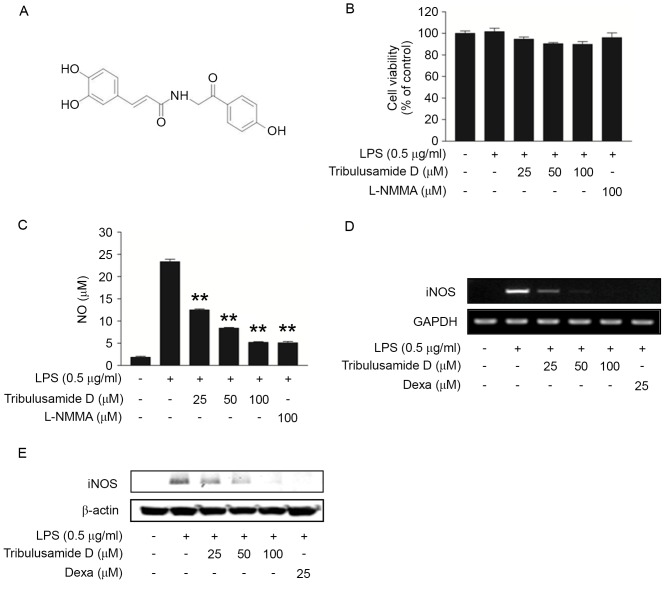
Figure 1.
Regulatory effects of tribulusamide D on NO production and iNOS expression in LPS-stimulated RAW 264.7 cells. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. (A) Chemical structure of tribulusamide D. (B) Cytotoxicity was determined by MTT assay and (C) NO assay was performed using Griess reagent. L-NMMA (100 µM) was used as a positive control. (D) Expression of iNOS mRNA was determined by reverse transcription-polymerase chain reaction analysis. (E) Expression of iNOS protein was determined by western blot analysis. Data are presented as the mean ± standard deviation of three independent experiments. **P<0.01 vs. LPS-treated cells. NO, nitric oxide; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; L-NMMA, NG-methyl-L-arginine acetate salt; Dexa, dexamethasone.
Reagents
DMSO (cat. no. D1370) and MTT (cat. no. M1415) were purchased from Duchefa Biochemie B.V (Haarlem, The Netherlands). LPS from Escherichia coli serotype 0111:B4, bovine serum albumin (cat. no. A9647), RIPA buffer (cat. no. R0278), protease inhibitor cocktail (cat. no. P8340), phosphatase inhibitor cocktail (cat. no. P5726) and NG-methyl-L-arginine acetate salt (L-NMMA) (cat. no. M7033), dexamethasone (cat. no. D4902), Celecoxib (cat. no. PZ0008) were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Cells were treated with L-NMMA (100 µM), dexamethasone (25 µM) and celecoxib (5 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h.
Image-iT™ FX Signal Enhancer, Alexa Fluor® 488-conjugated secondary antibody (goat anti-mouse, Thermo Fisher Scientific, Waltham, MA, USA, cat. no. A32723), ProLong® Gold Antifade reagent with DAPI (Thermo Fisher Scientific, Inc., cat. no. P36931). Anti-β-actin (cat. no. 5125), anti-phospho-JNK (T183/Y185) (cat. no. 4668), anti-JNK (cat. no. 9252), anti-phospho-extracellular signal-regulated kinase (ERK; T202/Y204) (cat. no. 9101), anti-ERK (cat. no. 9102), anti-phospho-p38 (T180/Y182) (cat. no. 9211) and anti-p38 (cat. no. 9212) primary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA) and anti-iNOS antibody was purchased from Abcam (Cambridge, UK) (cat. no. ab3523). Anti-COX2 (cat. no. sc-376861), anti-NF-κB p65 (cat. no. sc-7151) and anti-lamin B (cat. no. sc-6216) antibodies, and goat (cat. no. sc-2354) and rabbit (cat. no. sc-2004) IgG-horseradish peroxidase conjugated secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture conditions
RAW 264.7 mouse macrophage cells (TIB-71) were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 95% air with 5% CO2 at 37°C.
NO production assay
RAW 264.7 cells were seeded in 96-well plates (4×104 cells/well) and were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. The negative control was treated with serum-free media (SFM; Gibco; Thermo Fisher Scientific, Inc.).
The amount of nitrite, a stable metabolite of NO, was measured by using Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid; EMD Millipore, Billerica, MA, USA). Absorbance was subsequently measured at 540 nm, using a SpectraMax 190PC microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). The quantity of nitrite was determined from a standard curve for sodium nitrite (Sigma-Aldrich; Merck KGaA).
Cell cytotoxicity assay
RAW264.7 cells were plated at a density of 4×104 cells/well in 96-well plates. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. MTT (5 mg/ml in PBS) was added to each well and incubated for 90 min. The medium was removed from the wells by aspiration, 0.1 ml buffered DMSO was added to each well and the plate was shaken. The absorbance of each well was measured at a wavelength of 540 nm using a SpectraMax 190PC microplate reader. Data are presented as the mean ± standard deviation of three replicates.
ELISA
ELISA was performed to determine the concentration of cytokines in RAW 264.7 cells in vitro. RAW264.7 cells were plated at a density of 4×104 cells/well in 96-well plates and treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h.
Levels of IL-6, IL-10 and TNF-α in culture medium were quantified using platinum IL-6 (cat. no. BMS603/2), IL-10 (cat. no. BMS614/2), TNF-α (cat. no. BMS607/3) ELISA kits (eBioscience, Inc., San Diego, CA, USA), and the PGE2 (cat. no. KGE004B) concentration in culture medium was quantified using a competitive enzyme ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer's instructions.
Reverse transcription-polymerase chain reaction (RT-PCR)
RAW264.7 cells were plated at a density of 1×106 cells/well in 6-well plates and treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. The total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The integrity of the RNA was evaluated using 1% agarose gel electrophoresis and ethidium bromide staining. Briefly, 1 µg RNA was used as a template for each RT-PCR, using the SuperScript® III First-Strand Synthesis System and Taq DNA polymerase (Invitrogen; Thermo Fisher Scientific, Inc.). Invitrogen Platinum PCR SuperMix (Thermo Fisher Scientific, Inc.) was used which contains DNA polymerase. RT-PCR amplification was performed in My Gene Series Peltier Thermal Cycler (Long Gene Scientific Instruments, Hangzhou, China), using and AccuPower Pfu PCR premix (Bioneer Corporation, Daejeon, Korea). PCR conditions were as follows for 25 cycles: 30 sec denaturation at 95°C, 40 sec annealing between at 55 and 60°C, 60 sec extension at 72°C, and for final extension of 10 min. The final PCR products were electrophoresed in 1% agarose gel and stained with ethidium bromide. Images of the bands were captured using a Chemidoc XRS system (Bio-Rad Laboratories, Hercules, CA, USA) and observed using the Quantity One software version 4.6.3. The results compared with the housekeeping gene GAPDH. They were performed at least in triplicate. The sequences of the primers used for RT-PCR are shown in Table I.
Table I.
Primer sequences used for reverse transcription-polymerase chain reaction.
| Primer sequences | |||
|---|---|---|---|
| Target | Sense | Antisense | Accession number |
| GAPDH | 5′-GTATGACTCCACTCACGGCAAA-3′ | 5′-GGTCTCGCTCCTGGAGAGATG-3′ | NM_008084 |
| IL-6 | 5′-CACTTCACAAGTCGGAGGCTT-3′ | 5′-GCAAGTGCATCATCGTTGTTC-3′ | NM_031168 |
| IL-10 | 5′-CCTGGTAGAAGTGATGCCCCAGGCA-3′ | 5′-CTATGCAGTTGATGAAGATGTCAAA-3′ | NM_010548 |
| COX-2 | 5′-GGAGAGACTATCAAGATAGTGATC-3′ | 5′-ATGGTCAGTAGACTTTTACAGCTC-3′ | NM_011198 |
| TNF-α | 5′-AGCCTGTAGCCCACGTCGTA-3′ | 5′-TCTTTGAGATCCATGCCGTTG-3′ | NM_013693 |
| iNOS | 5′-ATCTGGATCAGGAACCTGAA-3′ | 5′-CCTTTTTTGCCCCATAGGAA-3′ | NM_0109273 |
IL, interleukin; COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide synthase.
Western blot analysis
RAW264.7 cells were plated at a density of 1×106 cells/well in 6-well plates and treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for different time points, as indicated. Cells were harvested and washed with PBS, and collected by centrifugation at 16,000 × g for 1 min at 4°C. To obtain the cellular lysate, cells were lysed on ice for 30 min in a RIPA buffer [50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 1% NP-40, 0.1% sodium SDS, 1 mM dithiothreitol (DTT) and 1 mM phenylmethylsulfonyl fluoride (PMSF)], which contained protease inhibitors (Sigma-Aldrich; Merck KGaA). Total protein was quantified with the Quick Start Bradford 1x Dye reagent (Bio-Rad Laboratories) using bovine serum albumin (BSA, Sigma-Aldrich; Merck KGaA) for the standard. Protein extracts representing 40 µg total protein were separated on 10% SDS-PAGE gel using the BioRad Mini Protean 3 System (Bio-Rad Laboratories) and electroblotted onto Protran nitrocellulose membranes (Whatman; GE Healthcare Life Sciences, Chalfont, UK). Membranes were blocked in 5% BSA in PBS/0.025% Tween-20 (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The primary antibodies used were specific for β-actin, p-JNK, JNK, p-ERK, ERK, p-p38, p38 (Cell Signaling Technology), iNOS (Abcam), COX-2, NF-κB p65 and Lamin B (Santa Cruz Biotechnology, Inc.). The primary antibodies were diluted (1:1,000) in 5% BSA in PBST and incubated with the membrane overnight at 4°C. The secondary antibody was applied at a 1:2,000 dilution in 5% BSA in PBST and incubated for 1 h at room temperature then processed for detection with the Supersignal West Pico Chemiluminescent substrate (Thermo Fisher Scientific, Inc.), using a Chemidoc XRS system (Bio-Rad Laboratories) and observed using the Quantity One software version 4.6.3. They were performed at least in triplicate.
Preparation of nuclear extract
RAW264.7 cells were plated at a density of 1×106 cells/well in 6-well plates and treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 30 min. Cells were washed twice with ice-cold PBS prior to trypsinization and centrifugation at 90 × g and 4°C for 5 min. Cells were then centrifuged at 20,000 × g and 4°C for 5 min and resuspended in 200 µl buffer (10 mM HEPES at pH 7.9, 10 mM KCl, 1 mM DTT, 0.5 mM PMSF and 0.1 mM EDTA). After incubation on ice for 10 min, cells were lysed by the addition of 12.5 µl of 10% NP-40. Cells were then centrifuged at 20,000 × g for 2 min at 4°C, and the supernatants were collected as cytosolic extract. Pellets were resuspended in 50 µl of extraction buffer (20 mM HEPES at pH 7.9, 0.4 M NaCl, 1 mM DTT, 1 mM PMSF, 1 mM EDTA and 1% NP-40) and incubated on ice for 10 min. Nuclear extract was collected by centrifugation at 15,000 × g for 15 min at 4°C. Western blot analysis was performed in the same manner as aforementioned.
Immunofluorescence staining
RAW264.7 cells were cultured on glass cover-slips in 6-well plates with 10% FBS at a density of 4×105 cells/well. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. The cells were washed with PBS for 5 min, fixed with 4% formaldehyde for 5 min on ice, permeabilized with 0.1% Triton X-100 for 2 min on ice, washed with PBS for 5 min, and blocked with PBS containing 5% BSA for 1 h at room temperature. Primary antibody (COX-2) was incubated for overnight (5% BSA in PBS; 1:200), washed with PBS, and followed by secondary antibody (Alexa Fluor 488-conjugated, goat anti-mouse) incubation for 1 h in darkroom (5% BSA in PBS, 1:300). After washing with PBS, the stained cells were visualized and photographed using a confocal laser scanning microscope (Zeiss AG, Oberkochen, Germany).
Statistical analysis
Statistical analysis was performed using a Student's t-test (Microsoft Excel 2007) and was based on at least three different experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of tribulusamide D on LPS-induced NO production and iNOS expression
To determine the cytotoxic effect of tribulusamide D, cells were treated with various concentrations of tribulusamide D (25, 50 or 100 µM), which was followed by LPS stimulation (0.5 µg/ml). As demonstrated in Fig. 1B, cell viability was not significantly decreased compared with control cells at concentrations of tribulusamide D up to 100 µM. Therefore, 25–100 µM of tribulusamide D was used in the present study. The anti-inflammatory effect of tribulusamide D on NO production was investigated following treatment with tribulusamide D (25–100 µM) and it was observed that tribulusamide D reduced NO production in a concentration-dependent manner. Treatment with 100 µM of tribulusamide D decreased NO production by 77.60% compared with the LPS-only group, which resembled the effect of 100 µM of L-NMMA, an inhibitor of NO synthase (Fig. 1C) (25). The present study also investigated whether the effect of tribulusamide D was associated with the expression of iNOS in LPS-stimulated macrophages. As demonstrated in Fig. 1D and E, tribulusamide D treatment reduced LPS-induced expression of iNOS at mRNA and protein levels. The results indicate that tribulusamide D may suppress LPS-induced NO production by downregulating iNOS expression.
Effects of tribulusamide D on LPS-induced expression of IL-6, IL-10 and TNF-α in LPS-stimulated cells
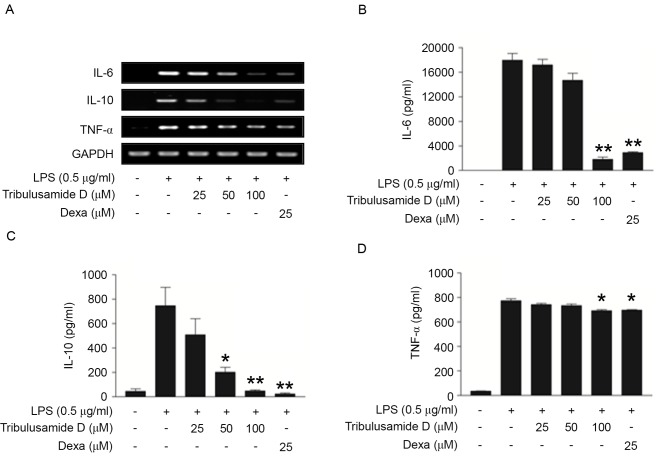
The effect of tribulusamide D on the expression of the pro-inflammatory cytokines IL-6, IL-10 and TNF-α in LPS-induced RAW 264.7 cells was investigated. As presented in Fig. 2A, LPS treatment markedly induced the mRNA expression of IL-6, IL-10 and TNF-α in RAW 264.7 cells, and tribulusamide D treatment inhibited this increase in a dose-dependent manner. It was confirmed that dexamethasone treatment (25 µM), a glucocorticoid class steroid, inhibited the mRNA expression of IL-6, IL-10 and TNF-α. In addition the effect of tribulusamide D on the protein expression of IL-6, IL-10 and TNF-α was measured using ELISA kits. Treatment with 100 µM of tribulusamide D significantly reduced protein levels of IL-6 (Fig. 2B), IL-10 (Fig. 2C) and TNF-α (Fig. 2D) compared with the LPS-only group, which was similar to the patterns observed for mRNA levels. Collectively, the results demonstrate that tribulusamide D treatment inhibits the expression and release of IL-6, IL-10 and TNF-α at the mRNA and protein level in LPS-stimulated RAW 264.7 cells.
Figure. 2.
Regulatory effect of tribulusamide D on the production of IL-6, IL-10 and TNF-α in LPS-stimulated RAW 264.7 cells. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. (A) Expression of IL-6, IL-10 and TNF-α mRNA was determined by reverse transcription-polymerase chain reaction analysis. ELISA was performed on cell culture supernatants to examine protein levels of (B) IL-6, (C) IL-10 and (D) TNF-α cytokines. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05; **P<0.01 vs. LPS-treated cells. IL, interleukin; TNF-α, tumor necrosis factor-α; LPS, lipopolysaccharide; Dexa, dexamethasone.
Effects of tribulusamide D on LPS-Induced COX-2 expression and PGE2 production. To determine the effects of tribulusamide D on LPS-induced COX-2 expression and PGE2 production, changes in COX-2 expression in LPS-stimulated cells treated with tribulusamide D were examined. As demonstrated in Fig. 3A, tribulusamide D treatment dose-dependently suppressed LPS-induced COX-2 mRNA expression. In addition, western blots revealed that treatment with 100 µM tribulusamide D decreased COX-2 protein level (Fig. 3B). Immunofluorescence analysis was also performed to further demonstrate the effect of tribulusamide D on LPS-induced COX-2 expression. As presented in Fig. 3C, anti-COX-2 staining was strong in LPS-stimulated cells. By contrast, tribulusamide D treatment (100 µM) clearly reduced LPS-induced COX-2 immunoreactivity signals. In addition, the effect of tribulusamide D on the production and release of PGE2, which is a mediator of inflammation, was investigated. The level of PGE2 in the conditioned media of LPS-induced cells was increased compared with control cells, however, treatment with 100 µM of tribulusamide D significantly decreased the level of PGE2 compared with LPS-only cells (Fig. 4). Treatment with celecoxib (5 µM), which is a COX-2 selective nonsteroidal anti-inflammatory drug, also markedly inhibited the expression of COX-2 at mRNA and protein levels, and significantly reduced PGE2 levels compared with LPS-only cells. The results indicate that tribulusamide D may suppress the production of the inflammatory mediator PGE2 by downregulating the expression of COX-2 in LPS-stimulated RAW 264.7 cells.
Figure 3.

Regulatory effect of tribulusamide D on COX-2 expression in LPS-stimulated RAW 264.7 cells. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. (A) Expression of COX-2 mRNA was determined by reverse transcription-polymerase chain reaction. (B) Expression of COX-2 protein was determined by western blotting. (C) Cellular expression of COX-2 was microscopically detected by immunofluorescence. Magnification, ×200. DNA was stained with DAPI. COX-2, cyclooxygenase-2; LPS, lipopolysaccharide.
Figure 4.

Regulatory effect of tribulusamide D on PGE2 production in LPS-stimulated RAW 264.7 cells. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 24 h. Quantitative measurement of PGE2 level was performed by using cell culture supernatants. Data are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. LPS-treated cells. PGE2, prostaglandin E2; LPS, lipopolysaccharide.
Effects of tribulusamide D on LPS-Induced nuclear localization of NF-κB and phosphorylation of MAPKs in RAW 264.7 cells
To further investigate the molecular mechanisms by which tribulusamide D regulates the LPS-induced inflammatory response, the present study investigated whether tribulusamide D regulates the translocation of NF-κB into the nucleus and the changes in activation of mitogenic signaling pathways, including ERK, JNK and p38 MAPK, which have pivotal roles in inflammatory responses (26–28). Initially, the effect of tribulusamide D on nuclear translocation of NF-κB in LPS-stimulated RAW 264.7 cells was examined. As demonstrated in Fig. 5A, LPS stimulation for 30 min induced the nuclear localization of NF-κB in the nuclear compartments, and tribulusamide D treatment reduced LPS-induced nuclear localization of NF-κB. In order to confirm the regulatory effect of tribulusamide D on the LPS-induced inflammatory response, changes in activation of ERK, JNK and p38 MAPK were analyzed. As presented in Fig. 5B, tribulusamide D treatment (100 µM) markedly inhibited LPS-induced phosphorylation/activation of p38 MAPK, however, no effects on the phosphorylation of ERK and JNK were observed. The results indicate that tribulusamide D may exert anti-inflammatory effects through inhibition of NF-κB and p38 MAPK signaling pathways.
Figure 5.

Regulatory effect of tribulusamide D on NF-κB nuclear localization and phosphorylation of p38 mitogen-activated protein kinase in LPS-stimulated RAW 264.7 cells. Cells were treated with tribulusamide D (25–100 µM) for 1 h prior to LPS (0.5 µg/ml) stimulation for 30 min. (A) Cytosolic and nuclear extracts were analyzed via western blot analysis with anti-NF-κB p65, , anti-β-actin or anti-lamin B antibodies. (B) Cell lysates were subjected to western blot analysis with anti-p-ERK, anti-ERK, anti-p-JNK, anti-JNK, anti-p-p38 and anti-p38 antibodies. Results are representative of at least three independent replicates. NF-κB, nuclear factor-κB; LPS, lipopolysaccharide; p-, phospho-; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase.
Discussion
Previous studies have explored the development of novel medicines using natural products. T. terrestris has been previously used as a traditional Chinese medicine for the treatment of high blood pressure, eye disease and edema (29,30). We previously reported that the 80% ethanol extract of T. terrestris dose-dependently inhibited NO production in LPS-stimulated RAW 264.7 cells (24). In another study, we isolated a novel compound, tribulusamide D, from the ethanol extract of T. terrestris and demonstrated that it has the potential to suppress NO production in LPS-stimulated RAW 264.7 cells (11). However, the anti-inflammatory effect and the underlying molecular mechanism of tribulusamide D were unclear.
The present study investigated the effect of tribulusamide D on LPS-induced stimulation of the inflammatory response and the expression of pro-inflammatory cytokines. NO is a pleiotropic inflammatory mediator, and it is generated by iNOS. iNOS is considered an important inducible enzyme that regulates inflammatory responses (31). The regulatory effect of tribulusamide D on NO production and iNOS expression in LPS-stimulated RAW 264.7 cells was examined, and the results demonstrated that tribulusamide D treatment significantly inhibited NO production, and reduced the mRNA and protein expression of iNOS. These results indicate that tribulusamide D may suppress LPS-induced NO production by downregulating the expression of iNOS in macrophages. Subsequently, the present study determined whether tribulusamide D has an effect on the expression of COX-2, which has been implicated as an important enzyme in the inflammatory process (32). Previous studies have indicated that COX-2 and iNOS are transcriptionally induced in response to bacterial endotoxins and pro-inflammatory cytokines in macrophages (33,34). COX-2 is induced by inflammation or cytokines and is associated with the synthesis of PGE2 (32). The current study demonstrated that the expression of COX-2 was increased following LPS treatment, and that its expression was reduced by tribulusamide D treatment at the mRNA and protein levels. The results were confirmed by immunofluorescence analysis. As demonstrated the present study, tribulusamide D treatment markedly reduced LPS-induced COX-2 immunoreactivity signals. The levels of another important inflammatory mediator, PGE2, were also measured, and it was observed that levels were decreased significantly by tribulusamide D treatment (100 µM). The results demonstrate that tribulusamide D may inhibit the production of PGE2 by suppression of COX-2 expression in LPS stimulated-RAW 264.7 cells.
It is established that IL-6, IL-10 and TNF-α have key roles in the induction and inhibition of the immune reactions of other inflammatory mediators. TNF-α is an element of the innate immune response against stimuli, and IL-6 and IL-10 are the most important pro-inflammatory cytokines. The present study demonstrated that the mRNA and protein concentration of IL-6, IL-10 and TNF-α were decreased in LPS-stimulated cells treated with 100 µM of tribulusamide D. NF-κB and MAPKs signaling pathway has a critical role in the regulation of inflammatory gene expression (35,36). The present study also demonstrated that tribulusamide D treatment inhibited LPS-induced nuclear localization of NF-κB and phosphorylation of p38 MAPK.
In conclusion, the results of the present study demonstrated that tribulusamide D isolated from T. terrestris has potential anti-inflammatory effects, which may occur through the suppression of inflammatory mediators and cytokines, by downregulating the enzymes responsible for their production, and associated signaling pathways in LPS-stimulated RAW264.7 cells. To the best of our knowledge, this is the first report demonstrating the anti-inflammatory activity of tribulusamide D in inflammatory responses. Combined, the results of the current study indicate that tribulusamide D may be a potential therapeutic agent for the treatment of inflammation-associated diseases.
Acknowledgements
The present study was conducted by the research fund of Dankook University in 2015.
Glossary
Abbreviations
- COX-2
cyclooxygenase-2
- iNOS
inducible nitric oxide synthase
- IL
interleukin
- LPS
lipopolysaccharide
- NO
nitric oxide
- PG
prostaglandin
- PMSF
phenylmethylsulfonyl fluoride
- TNF-α
tumor necrosis factor-α
- NF-κB
nuclear factor-κB
- MAPKs
mitogen-activated protein kinases
References
- 1.Yuan WH, Wang NL, Yi YH, Yao XS. Two Furostanol Saponins from the Fruits of Tribulus terrestris. Chin J Nat Med. 2008;6:172–175. doi: 10.3724/SP.J.1009.2008.00172. [DOI] [Google Scholar]
- 2.Al-Ali M, Wahbi S, Twaij H, Al-Badr A. Tribulus terrestris: Preliminary study of its diuretic and contractile effects and comparison with Zea mays. J Ethnopharmacol. 2003;85:257–260. doi: 10.1016/S0378-8741(03)00014-X. [DOI] [PubMed] [Google Scholar]
- 3.Baburao B, Rajyalakshmi G, Venkatesham A, Kiran G, Sunder A, Rao B. Anti-inflammatory and antimicrobial activities of methanolic extract of Tribulus terrestris Linn plant. I Chem Sci. 2009;7:1867–1872. [Google Scholar]
- 4.Do J, Choi S, Choi J, Hyun JS. Effects and mechanism of action of a Tribulus terrestris extract on penile erection. Korean J Urol. 2013;54:183–188. doi: 10.4111/kju.2013.54.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammoda HM, Ghazy NM, Harraz FM, Radwan MM, ElSohly MA, Abdallah II. Chemical constituents from Tribulus terrestris and screening of their antioxidant activity. Phytochemistry. 2013;92:153–159. doi: 10.1016/j.phytochem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Hussain AA, Mohammed AA, Ibrahim HH, Abbas AH. Study the biological activities of Tribulus terrestris extracts. Int J Innov Res Sci Eng Technol. 2009;57:433–435. [Google Scholar]
- 7.Mishra NK, Biswal GS, Chowdary KA, Mishra G. Anti-arthritic activity of Tribulus terrestris studied in Freund's Adjuvant induced arthritic rats. J Pharm Educ Res. 2013;4:41. [Google Scholar]
- 8.Phillips OA, Mathew KT, Oriowo MA. Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J Ethnopharmacol. 2006;104:351–355. doi: 10.1016/j.jep.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Wu TS, Shi LS, Kuo SC. Alkaloids and other constituents from Tribulus terrestris. Phytochemistry. 1999;50:1411–1415. doi: 10.1016/S0031-9422(97)01086-8. [DOI] [Google Scholar]
- 10.Gomathi S, Shanmugapriya A, Bharathi V, Gayathri G, Karpagam T. Antimicrobial activity and phytochemical studies of aqueous and ethanolic fruit extracts of Tribulus terrestris. IJPI's J Pharmacogn Herb Formul. 2012;2:47–51. [Google Scholar]
- 11.Hong SS, Jeong W, Kwon JG, Choi YH, Ahn EK, Ko HJ, Seo DW, Oh JS. Phenolic amides from the fruits of Tribulus terrestris and their inhibitory effects on the production of nitric oxide. Bull Korean Chem Soc. 2013;34:3105–3108. doi: 10.5012/bkcs.2013.34.10.3105. [DOI] [Google Scholar]
- 12.Yoon WJ, Ham YM, Kim SS, Yoo BS, Moon JY, Baik JS, Lee NH, Hyun CG. Suppression of pro-inflammatory cytokines, iNOS, and COX-2 expression by brown algae Sargassum micracanthum in RAW 264.7 macrophages. Eur Asia J BioSci. 2009;3:130. doi: 10.5053/ejobios.2009.3.0.17. [DOI] [Google Scholar]
- 13.Kim KN, Heo SJ, Yoon WJ, Kang SM, Ahn G, Yi TH, Jeon YJ. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 2010;649:369–375. doi: 10.1016/j.ejphar.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Li DY, Xue MY, Geng ZR, Py C. The suppressive effects of Bursopentine (BP5) on oxidative stress and NF-ĸB activation in lipopolysaccharide-activated murine peritoneal macrophages. Cell Physiol Biochem. 2012;29:9–20. doi: 10.1159/000337581. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 17.Yoon WJ, Ham YM, Kim KN, Park SY, Lee NH, Hyun CG, Lee WJ. Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage RAW 264.7 cells. J Med Plant Res. 2009;3:1–8. [Google Scholar]
- 18.Esposito E, Cuzzocrea S. The role of nitric oxide synthases in lung inflammation. Curr Opin Invest Drugs. 2007;8:899–909. [PubMed] [Google Scholar]
- 19.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan MG, Chilton FH, Huggins EM, Jr, McCall CE. Lipopolysaccharide priming of alveolar macrophages for enhanced synthesis of prostanoids involves induction of a novel prostaglandin H synthase. J Biol Chem. 1992;267:14547–14550. [PubMed] [Google Scholar]
- 22.Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ, Ha KS, Han JA, Yim SV, Chang K, Kwon YG, et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol Cells. 2003;16:97–105. [PubMed] [Google Scholar]
- 23.Kim HJ, Lee HS, Chong YH, Kang JL. 38 mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology. 2006;225:36–47. doi: 10.1016/j.tox.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 24.Ko HJ, Ahn EK, Oh JS. N-trans-ρ-caffeoyl tyramine isolated from Tribulus terrestris exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 cells. Int J Mol Med. 2015;36:1042–1048. doi: 10.3892/ijmm.2015.2301. [DOI] [PubMed] [Google Scholar]
- 25.Olken NM, Marletta MA. NG-Methyl-L-arginine functions as an alternate substrate and mechanism-based inhibitor of nitric oxide synthase. Biochemistry. 1993;32:9677–9685. doi: 10.1021/bi00088a020. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 28.Hommes DW, Peppelenbosch MP, van Deventer SJ. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52:144–151. doi: 10.1136/gut.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaheen G, Ahmad I, Usmanghani K, Akhter N, Ahmad M, Sultana S, Akram M. Monograph of Tribulus terrestris. J Med Plants Res. 2012;6:641. [Google Scholar]
- 30.Xu T, Xu Y, Liu Y, Xie S, Si Y, Xu D. Two new furostanol saponins from Tribulus terrestris L. Fitoterapia. 2009;80:354–357. doi: 10.1016/j.fitote.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2006;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 32.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 33.Britten N, Waldron N, Watts J, Hallberg J. Cyclooxygenase-2 inhibitor and antibacterial agent combination for intramammary treatment of mastitis. US Patent 20040033938 A1. 2004 Filed March 20, 2003; issued February 19. [Google Scholar]
- 34.Kim MS, Ahn EK, Hong SS, Oh JS. 2,8-Decadiene-1,10-Diol inhibits lipopolysaccharide-induced inflammatory responses through inactivation of Mitogen-Activated Protein Kinase and Nuclear Factor-κB signaling pathway. Inflammation. 2016;39:583–591. doi: 10.1007/s10753-015-0283-1. [DOI] [PubMed] [Google Scholar]
- 35.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 36.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression: The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]