Abstract
The aim of the present study was to investigate the effects of combined general-epidural anesthesia (CGEA) and total intravenous anesthesia (TIVA) on cellular immunity and prognosis in patients with non-small cell lung cancer (NSCLC) in a Chinese population. One-hundred and twenty NSCLC patients were randomly divided into a TIVA group (n=60) and a CGEA group (n=60) using a random number table. All patients underwent video-assisted thoracoscopic surgery for radical resection. Blood pressure (BP) and peripheral oxygen saturation (SpO2) were measured. Post-operative analgesic effects were evaluated with a visual analog scale pain score. Flow cytometry was applied to measure T lymphocyte subsets [cluster of differentiation (CD)3+, CD4+, CD8+ and CD4+/CD8+] and natural killer cell CD56+. A 3-year follow-up was conducted to observe the prognosis. The analgesic effects of CGEA were identified to be better than those of TIVA. Compared with the TIVA group, the CGEA group demonstrated a shorter time of spontaneous breathing recovery, eyes opening, and extubation, lower heart rate, blood pressure and mean arterial pressure, and higher SpO2. At 24 and 48 h after surgery, CD3+, CD4+, CD4+/CD8+ and CD56+ in the CGEA group were higher than those in the TIVA group. At 72 h after surgery, CD3+, CD4+, CD4+/CD8+ in the CGEA group were higher than those in the TIVA group. These results indicate that CGEA and TIVA effected cellular immunity, and CGEA had a reduced effect on cellular immunity and improved postoperative analgesic effects.
Keywords: non-small cell lung cancer, combined general-epidural anesthesia, total intravenous anesthesia, cellular immunity, prognosis
Introduction
Lung cancer is the leading cause of morbidity and mortality in the world (1,2). It was reported that in 2008, the incidence rate of lung cancer was 35 per 100,000 people, while the mortality was 28 per 100,000 people in China (3). Smoking history of smokers, environmental/occupational exposures and genetic susceptibility in never-smokers are considered to be risk factors of lung cancer (4,5). Non-small cell lung cancer (NSCLC) is defined as any malignant epithelial lung tumor that lacks a small-cell component (6). It is the most common type of lung cancer, accounting for 80–85% of all lung cancer cases, and comprises three histological subtypes, namely squamous cell carcinoma, adenocarcinoma and large cell carcinoma (7,8). The majority of NSCLC patients are diagnosed with metastatic disease or advanced cancer (9,10). Advanced NSCLC (stage IV) is often regarded as fatal and unresectable (11). For these patients, palliative chemotherapy is recommended as the primary treatment option, which may consist of a first-, second- and even third-line treatment (12). Patients with stage IIIA NSCLC may opt to receive induction therapy followed by surgery, although it is argued that this approach should only apply to those who experience a reaction to induction therapy. Patients with stage I and II may benefit from surgical resection (13,14).
Modern surgical resection and the associated anesthesia techniques contribute greatly to disease treatments that require surgical resection. Surgical resection of the primary tumor and local nodal metastasis has been the only potentially curative treatment for resectable NSCLC, although the benefits of complete lymph node (LN) dissection remain controversial (15). The perioperative period of major surgery is often characterized by a significant inflammatory response accompanied by immune suppression, which results from the combined effect of the various factors, including anesthetics, hypothermia, analgesics and lung ventilation (16–18). An impaired immune system in the perioperative period may imply an increased risk of postoperative infections and disease progression in cancer patients (19,20). Therefore, it is considered to be essential to minimize the factors that cause immune suppression. Anesthesia is necessary and increasingly performed for surgical resection of cancer patients (21). In addition to surgical stress, anesthetics also affect the immune system, as each type of anesthetic exerts direct suppressive effects on cellular and neurohumoral immunity by affecting the functions of immunocompetent cells, inflammatory mediator gene expression and secretion (22). Natural killer (NK) cells and T cytotoxic lymphocytes mediate multiple functions of the immune system, including defense against viral infections, response to organ transplantation and cell-mediated control of cancer formation (23–25). The aim of the current study was to establish a method by which an anesthesia treatment could be performed with minimum damage to the cellular immune function. Thus, the effects of total intravenous anesthesia (TIVA) and combined general-epidural anesthesia (CGEA) on the immune suppression of T lymphocytes and NK cells were compared. In addition, whether the two anesthesia methods affect the prognosis of patients with radically resected NSCLC was examined.
Subject and methods
Study subjects and groups
A total of 120 patients (87 males and 33 females; age, 42–65 years; average age, 56.2±6.1 years) who underwent video-assisted thoracoscopic surgery for radical resection of NSCLC between January 2009 and January 2012 at Linyi People's Hospital (Linyi, China) were included in the present study. There were 34 cases of right upper lobectomy, 16 cases of right middle lobectomy, 15 cases of right lower lobectomy, 30 cases of left upper lobectomy, 22 cases of left lower lobectomy and 3 cases of total left lung resection. The number of lymph nodes (LN) was 5 (0–16). According to the 7th version of tumor node metastasis (TNM) staging system by the International Association for the study of Lung Cancer in 2009 (26), 53 cases were stage II and 67 cases were stage IIIA. All patients with NSCLC were confirmed by bronchoscopy and punch biopsy. In addition, according to histological and cytological typing (27), there were 38 cases of squamous cell carcinoma, 61 cases of adenocarcinoma, 10 cases of large cell carcinoma and 11 cases of adenosquamous carcinoma. The inclusion criteria were as follows: i) Patients did not exhibit lung metastases; ii) patients received adjuvant chemotherapy; and iii) patients had complete medical records. The exclusion criteria included: i) Serious functional disorders of the heart, liver, kidney and vital organs; ii) combined immune and endoctrine system disorders; iii) taking non-steroidal anti-inflammatory or hormone drugs in the past 2 weeks; iv) receiving radiotherapy or chemotherapy prior to surgery; v) taking immunosuppressants in the past 2 weeks. All 120 patients were randomly divided into the TIVA group (n=60) and the CGEA group (n=60) using a random number table. The study received approval from the Ethics Committee of Linyi People's Hospital and all patients provided written informed consent.
Surgery and anesthesia regimens
Patients in the two groups underwent video-assisted thoracoscopic surgery for radical resection. A small incision was made between the 7th and the 8th ribs, 1 cm to the left. A thoracoscope was used to examine the tumor location and size, and establish whether there was metastasis. After determining the tumor location, an incision (depth, 4–5 cm) was made outside the chest with an auxiliary incision (depth, 1.5–2.0 cm) in the subscapularis. The diseased lung was resected and LN dissection was performed. The chest cavity was rinsed using a 0.9% sodium chloride injection. Without bleeding or leakage, a chest drainage tube was placed and then the incision was sutured. Patients in the two groups received preoperative intramuscular injection of 0.5 mg atropine and 0.1 g phenobarbital. Patients in the CGEA group were administered with an epidural puncture, injection of 0.375% ropivacaine for anesthesia, and block in T6-T8, simultaneously a venous injection of 0.1 mg/kg vecuronium, 5 µg/kg fentanyl and 1.0 mg/kg propofol was administered, which was followed by tracheal intubation and single lung ventilation. Intraoperative inhalation of 4% sevoflurane and intravenous injection of 2 mg/kg vecuronium maintained the state of anesthesia. Tracheal extubation was performed following recovery of respiratory function and the swallowing reflex. Patients in the TIVA group firstly underwent double thoracic tracheal intubation, which were connected to the anesthesia machine for mechanical ventilation, followed by single lung ventilation after the ventilation reached the lung. Patients in the TIVA group were treated with intravenous drugs in the same way as the patients in the CGEA group, apart from epidural drug administration. The above-mentioned drugs were purchased from aaShandong Xinhua Pharmaceutical Company Ltd. (Zibo, China).
Sample collection and visual analog scale (VAS) pain score
Venous blood samples (5 ml) from the right internal jugular were collected prior to anesthesia (T0), upon completion of surgery (T1), and 24 h (T2), 48 h (T3) and 72 h (T4) after surgery, followed by anticoagulation with EDTA and immediate inspection. Intraoperative measurements were obtained every 5 min, including blood pressure (BP), systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), heart rate (HR) and peripheral oxygen saturation (SpO2). SpO2 was maintained at >90%, and the patients with SpO2 <90% were withdrawn from the study (28,29). The postoperative follow-up included investigation of intraoperative awareness, and the analgesic effect at 24, 48 and 72 h after surgery, which was evaluated using the VAS pain score as follows: 0 Points, no pain; 1–3 points, mild pain (patients are able to tolerate); 4–6 points, moderate pain (patients are able to endure); 7–10 points, gradually intensifying intense pain (30). The time of spontaneous breathing recovery, eyes opening, and extubation were recorded.
Flow cytometry and radioimmunoassay
Detection of cluster of differentiation (CD)3+, CD4+, CD4+/CD8+, natural killer (NK) cell CD56+ and associated immune cell activation were performed. FACS Calibur flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) and its supporting lymphocyte subset detection reagent kit (BD Biosciences) were employed to measure the ratios of T lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+), NK cell CD56+ and the expression levels of T lymphocyte activation-associated membrane molecules human leukocyte antigen-antigen D related (HLA-DR), CD69+ (553237) and CD107a+(555798) (1:250; BD Biosciences) Flow cytometry detection was performed as follows: Phycoerythrin-labeled mouse anti-human CD3+ (561809), CD4+ (561841), CD8+ (566451) and CD56+ (557747) monoclonal antibodies (20 µl; BD Biosciences) were added to the flow tubes and combined with 100 µl blood specimens. The samples were incubated for 30 min at room temperature in the dark, then supplemented with 3 ml hemolysis and treated for 5 min at room temperature in the dark. Subsequently, the samples were centrifuged at room temperature for 2 min at 450 × g and the supernatant was removed. Subsequent to two centrifugal washes with phosphate-buffered saline (PBS), the samples were fixed with 300 µl paraformaldehyde and detected.
Cytokine levels were detected as follows: Radioimmunoassay was applied to measure plasma cortisol (Cor), interleukin (IL)-1 and IL-6 levels and double-antibody sandwich enzyme linked immunosorbent assay to detect interferon (IFN)-γ, IL-4, IL-17, transforming growth factor (TGF)-β, and tumor necrosis factor (TNF)-α levels. The ratio between T helper (Th)1 and Th2 was evaluated by IFN-γ/IL-4. The plasma TGF-β and IL-17 values were used to evaluate the content of Th17 cells. Cor, IL-1 and IL-6 reagents were provided by the BeiYa Institute of Biotechnology (Suzhou, China). IFN-γ, IL-4, IL-17, TGF-β and TNF-α kits were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and detection was performed in strict accordance with the reagent kit instructions.
Follow-up
All patients had a 3-year follow-up consisting of a reexamination and telephone call every 3 months. The local recurrence and distant metastasis of the tumor, and 1-, 2- and 3-year survival rates were observed. The follow-up ranged from the time of diagnosis to January 31, 2015. No patients were lost to follow-up.
Statistical analysis
Statistical data were analyzed using SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). Measurement data were expressed as the mean ± standard variation and tested for normality. Pairwise comparisons were validated using an independent t-test. Ranked data were verified using the Wilcoxon rank-sum test and comparison of enumeration data was performed using the χ2 test. The survival rate was calculated using Kaplan-Meier survival curve and significance analysis of survival rate using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparisons of baseline characteristics of NSCLC patients in the CGEA and TIVA groups
Baseline characteristics between the two groups, including age, gender, body mass index, TNM staging, histological types, operation and anesthesia time were not significantly different (P>0.05). Compared with the TIVA group, the time of spontaneous breathing recovery, eyes opening, and tracheal extubation in the CGEA group were significantly shorter (P<0.05; Table I).
Table I.
Comparison of baseline characteristics of patients between the CGEA and TIVA groups.
| Characteristic | TIVA group (n=60) | CGEA (n=60) | P-value |
|---|---|---|---|
| Age (years) | 56.2±6.4 | 56.1±5.8 | 0.929 |
| Sex (male/female) | 43/17 | 44/16 | 0.838 |
| Body mass index (kg/m2) | 23.6±2.5 | 22.9±2.2 | 0.108 |
| Tumor node metastasis staging | 0.854 | ||
| Stage II | 27 | 26 | |
| Stage IIIA | 33 | 34 | |
| Types of non-small cell lung cancer | 0.976 | ||
| Squamous cell carcinoma | 18 | 20 | |
| Adenocarcinoma | 31 | 30 | |
| Large cell carcinoma | 5 | 5 | |
| Adenosquamous carcinoma | 6 | 5 | |
| Time of surgery (min) | 169.60±9.29 | 168.41±9.96 | 0.521 |
| Time of anesthesia (min) | 172.68±46.30 | 165.30±40.22 | 0.353 |
| Time of spontaneous breathing recovery (min) | 13.64±4.77 | 8.19±3.84 | <0.001 |
| Time of eyes opening (min) | 26.67±5.17 | 21.29±6.26 | <0.001 |
| Time of extubation (min) | 32.67±7.26 | 25.80±6.38 | <0.001 |
TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia.
Hemodynamic change and SpO2 of NSCLC patients in the CGEA and TIVA groups during the perioperative period
Prior to anesthesia, the values of HR, BP, MAP and SpO2 in the two groups were not significantly different (P>0.05). At the time of intubation and the end of the surgery, the values of HR, BP and MAP in the two groups were significantly higher than those prior to anesthesia (P<0.05), but the SpO2 was significantly lower than that before anesthesia (P<0.05), with no significant difference between the two groups (P>0.05). At the time of tracheal extubation, the values of HR, BP, MAP and SpO2 in the TIVA group were significantly different when compared to before anesthesia (P<0.05), while the values of HR, BP, MAP and SpO2 in the CGEA group were not significantly different compared with those before anesthesia (P>0.05), of which the values of HR, BP and MAP were significantly lower and the SpO2 was higher than those in the TIVA group (P<0.05; Table II).
Table II.
Hemodynamic change and SpO2 of non-small cell lung cancer patients in the CGEA and TIVA groups during the perioperative period.
| Time | Group | HR (bpm) | SBP (mmHg) | DBP (mmHg) | MAP (mmHg) | SpO2(%) |
|---|---|---|---|---|---|---|
| Before anesthesia | TIVA | 80.3±9.5 | 121.5±17.2 | 75.4±11.7 | 85.6±12.1 | 96.2±2.2 |
| CGEA | 79.6±9.1 | 121.3±16.9 | 75.1±12.2 | 84.8±10.9 | 95.7±1.8 | |
| At tracheal intubation | TIVA | 83.5±8.7a | 127.1±14.2a | 78.8±8.9a | 87.9±10.5a | 94.3±1.5a |
| CGEA | 83.3±8.2a | 127.4±15.0a | 79.2±7.2a | 88.1±7.7a | 94.0±1.4a | |
| At the end of surgery | TIVA | 83.5±7.8a | 127.8±12.7a | 77.9±8.4a | 89.2±6.4a | 93.8±1.6a |
| CGEA | 83.5±7.7a | 126.5±12.4a | 78.5±5.1a | 88.6±6.3a | 93.9±1.7a | |
| Following tracheal extubation | TIVA | 82.8±7.3a | 108.2±14.3a | 79.5±7.1a | 86.8±8.7a | 93.2±1.1a |
| CGEA | 79.8±8.4b | 122.0±13.2a | 75.8±10.2b | 83.1±10.0b | 95.1±1.3b |
P<0.05 vs. before anesthesia
P<0.05 vs. the TIVA group at the same time. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SpO2, peripheral oxygen saturation; TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia.
Comparison of VAS scores of NSCLC patients in the CGEA and TIVA groups at 24, 48 and 72 h after surgery
All the patients reported no intraoperative awareness, indicating no difference between the effects of the two types of anesthesia. Patients in the CGEA group demonstrated mild or moderate pain 24 h after surgery, in which the proportion of mild pain was higher than in the TIVA group (P<0.05), while 30.00% of the patients in the TIVA group exhibited gradually intense pain. At 48 h after surgery, there were three patients with no pain in the CGEA group, while a significant difference was noted in patients with mild pain between the two groups (P<0.05). At 72 h after surgery, significant differences between the two groups were observed in patients with mild or moderate pain (P<0.05), and no obvious difference was identified between patients with no pain and those with gradually intense pain (P>0.05; Table III). The results implied that CGEA provided a better analgesic effect than TIVA.
Table III.
Comparison of VAS scores of non-small cell lung cancer patients in the CGEA and TIVA groups at 24, 48 and 72 h after surgery.
| VAS score | |||||
|---|---|---|---|---|---|
| Time, h | Group | 0 point, n (%) | 1–3 points, n (%) | 4–6 points, n (%) | 7–10 points, n (%) |
| 24 | TIVA | 0 (0) | 26 (43.33)a | 16 (26.67) | 18 (30.00)a |
| CGEA | 0 (0) | 47 (78.33) | 13 (21.67) | 0 (0) | |
| 48 | TIVA | 0 (0) | 38 (63.33)a | 17 (28.33) | 5 (8.33) |
| CGEA | 3 (5.00) | 48 (80.00) | 9 (15.00) | 0 (0) | |
| 72 | TIVA | 2 (3.33) | 47 (78.33) | 10 (16.67)a | 1 (1.67) |
| CGEA | 8 (13.33) | 50 (83.33) | 2 (3.33) | 0 (0) | |
P<0.05 vs. the CGEA group. TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia.
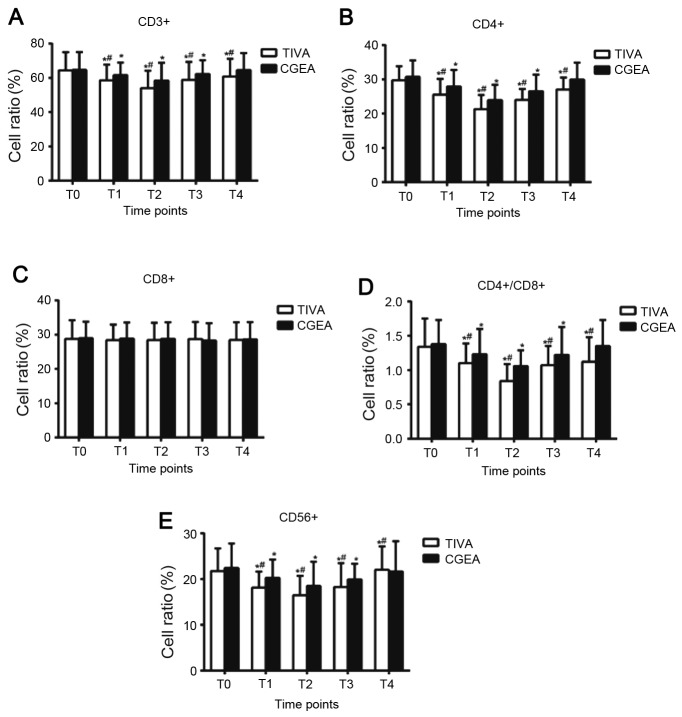
Changes of T lymphocyte subsets and NK cells of NSCLC patients in the CGEA and TIVA groups before and after surgery
Table IV and Fig. 1 demonstrate the changes of T lymphocyte subsets and NK cells before and after surgery in the two groups. At T0, T lymphocyte subsets, including CD3+, CD4+, CD8+ and CD4+/CD8+, and NK cell CD56+ in the two groups were not significantly different (P>0.05). CD3+, CD4+, CD4+/CD8+ and CD56+ were significantly lower at T1 and T2 compared with those at T0 (P<0.05), minimized at T2 (24 h following surgery) and began to rebound at T3 (48 h following surgery). At T4 (72 h following surgery), all the indexes of the CGEA group recovered to the level at T0 (P>0.05). At T1, 2, 3, and 4, CD3+, CD4+ and CD4+/CD8+ in the TIVA group remained lower than T0 (P<0.05). At T1, 2 and 3, CD3+, CD4+, CD4+/CD8+ and CD56+ in the CGEA group were higher when compared with those in the TIVA group (P<0.05). At T4, CD3+, CD4+ and CD4+/CD8+ in the CGEA group were higher than those in the TIVA group (P<0.05). CD8+ exhibited no significant change at all five time points in the two groups. The results indicate that CGEA interfered less with cellular immune function when compared with TIVA.
Table IV.
Comparisons of T lymphocyte subsets and natural killer cells of non-small cell lung cancer patients in the CGEA and TIVA groups before and after surgery.
| Time point | Group | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | CD56+ |
|---|---|---|---|---|---|---|
| T0 | TIVA | 64.35±10.64 | 29.71±4.10 | 28.77±5.40 | 1.34±0.41 | 21.77±4.91 |
| CGEA | 64.57±10.45 | 30.76±4.75 | 28.95±4.82 | 1.38±0.35 | 22.43±5.35 | |
| T1 | TIVA | 58.60±9.10a,b | 25.55±4.56a,b | 28.40±4.53 | 1.10±0.29a,b | 18.12±3.51a,b |
| CGEA | 61.60±7.32a | 27.90±4.84a | 28.85±4.71 | 1.23±0.37a | 20.25±4.02a | |
| T2 | TIVA | 54.02±10.16a,b | 21.30±4.15a,b | 28.41±5.05 | 0.84±0.25a,b | 16.15±4.26a,b |
| CGEA | 58.34±10.12a | 23.96±4.44a | 28.77±4.83 | 1.06±0.33a | 18.49±5.31a | |
| T3 | TIVA | 58.84±10.43a,b | 24.01±3.19a,b | 28.72±4.96 | 1.07±0.28a,b | 18.24±5.27a,b |
| CGEA | 62.25±8.04a | 26.55±4.81a | 28.27±5.07 | 1.22±0.41a | 19.91±3.43a | |
| T4 | TIVA | 60.75±10.32a,b | 27.04±3.51a,b | 28.45±5.13 | 1.12±0.36a,b | 22.05±5.07 |
| CGEA | 64.49±9.97 | 29.96±4.88 | 28.62±5.02 | 1.35±0.38 | 21.66±6.60 |
P<0.05 vs. T0 in the same group
P<0.05 vs. the CGEA group at the same time point. T0, before anesthesia; T1, at the end of surgery; T2, 24 h after surgery; T3, 48 h after surgery; T4, 72 h after surgery. TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia; CD, cluster of differentiation.
Figure 1.
Cell ratios of (A) CD3+, (B) CD4+, (C) CD8+, (D) CD4+/CD8+ and (E) CD56+ of NSCLC patients in the CGEA and TIVA groups before and after surgery. *P<0.05 vs. T0 in the same group; #P<0.05 vs. CGEA group at the same time. CD, cluster of differentiation; TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia; T0, before anesthesia; T1, at the end of surgery; T2, 24 h after surgery; T3, 48 h after surgery; T4, 72 h after surgery.
Changes of immune cell activation of NSCLC patients in the CGEA and TIVA groups before and after surgery
Changes in immune cell activation of the NSCLC patients in the CGEA and TIVA groups before and after surgery are presented in Table V. Prior to anesthesia, no differences were observed in the immune cell activation indexes (HLA-DR+, CD69+ and CD107a+) between the two groups (P>0.05). Compared with before anesthesia, the immune cell activation indexes (HLA-DR+, CD69+ and CD107a+) in the two groups significantly decreased at 0, 24 and 48 h after surgery (P<0.05). And the HLA-DR+ levels in the TIVA group remained low at 72 h after surgery (P<0.05). At the different time points after surgery, the immune cell activation indexes in the TIVA group were lower than those in the CGEA group (P<0.05).
Table V.
Changes of immune cell activation of non-small cell lung carcinoma patients in the CGEA and TIVA groups before and after surgery.
| Time point | Group | HLA-DR+ | CD69+ | CD107a+ |
|---|---|---|---|---|
| T0 | TIVA | 27.85±2.73 | 4.32±1.44 | 4.88±1.40 |
| CGEA | 28.17±2.66 | 4.59±1.53 | 5.03±1.52 | |
| T1 | TIVA | 19.23±2.44a,b | 3.48±1.17a,b | 3.95±1.31a,b |
| CGEA | 23.96±2.98a | 4.05±1.29a | 4.66±1.45a | |
| T2 | TIVA | 15.72±2.01a,b | 2.51±0.96a,b | 2.80±1.16a,b |
| CGEA | 23.15±2.74a | 3.66±1.24a | 4.15±1.37a | |
| T3 | TIVA | 19.80±2.36a,b | 3.37±1.22a,b | 3.81±1.35a,b |
| CGEA | 25.36±2.91a | 3.92±1.07a | 4.53±1.28a | |
| T4 | TIVA | 24.92±2.48a,b | 4.22±1.05b | 4.71±1.15b |
| CGEA | 28.06±3.13 | 4.63±1.18 | 5.11±1.05 |
P<0.05 vs. T0 in the same group
P<0.05 vs. the CGEA group at the same time. T0, before anesthesia; T1, at the end of surgery; T2, 24 h after surgery; T3, 48 h after surgery; T4, 72 h after surgery. TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia; cluster of differentiation.
Comparison of cytokine levels of NSCLC patients in the CGEA and TIVA groups before and after surgery
Changes in immune function of patients in the CGEA and TIVA groups before and after surgery are presented in Table VI. Before anesthesia, no differences in the immune function indexes (Cor, IL-1, IL-6, TNF-α, IFN-γ, IL-4, IFN-γ/IL-4, IL-17 and TGF-β) were noted between the two groups (P>0.05). In the TIVA group, compared with before anesthesia, the Cor levels were significantly different at the end of surgery (P<0.05). At 24 and 48 h after surgery, Cor, IL-1, IL-6, TNF-α, IFN-γ, IL-4 and IFN-γ/IL-4 and IL-17 were significantly different (P<0.05). At 72 h after surgery, TNF-α, IFN-γ, IL-4 and IFN-γ/IL-4 continued to be significantly different (P<0.05). However, at 48 and 72 h after surgery, TGF-β significantly decreased (P<0.05).
Table VI.
Comparison of cytokine levels of non-small cell lung carcinoma patients in the CGEA and TIVA groups before and after surgery.
| Time point | Group | Cor (ng/ml) | IL-1 (pg/ml) | IL-6 (pg/ml) | TNF-α (ng/ml) | IFN-γ (pg/ml) | IL-4 (pg/ml) | FN-γ/IIL-4 | IL-17 (ng/l) | TGF-β (ng/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | TIVA | 152.6±41.1 | 251.7±42.6 | 105.4±18.9 | 1.34±0.18 | 11.7±4.8 | 5.5±2.1 | 2.3±1.4 | 18.5±6.4 | 33.7±6.9 |
| CGEA | 164.3±37.4 | 244.1±46.0 | 102.6±24.7 | 1.39±0.20 | 12.3±4.2 | 5.8±1.7 | 2.1±0.5 | 19.4±7.9 | 34.8±7.2 | |
| T1 | TIVA | 207.8±39.2a,b | 265.9±47.3 | 112.5±29.3b | 1.38±0.23 | 12.1±3.4 | 5.9±2.3 | 2.3±1.2 | 20.7±7.5 | 35.0±9.1 |
| CGEA | 169.3±51.5 | 252.8±43.4 | 95.2±23.4 | 1.34±0.21 | 11.6±3.8 | 5.6±2.2 | 2.3±1.1 | 20.4±7.4 | 34.2±7.3 | |
| T2 | TIVA | 228.5±49.7a,b | 365.5±57.6a,b | 147.8±24.0a,b | 1.66±0.20a,b | 8.4±3.9a,b | 7.2±2.9a,b | 1.2±0.6a,b | 28.9±7.9a,b | 32.1±6.1 |
| CGEA | 175.6±54.2 | 274.6±59.1a | 109.1±19.6 | 1.40±0.25 | 11.0±5.2 | 5.7±3.0 | 2.7±2.3 | 21.9±7.2 | 32.9±6.6 | |
| T3 | TIVA | 242.5±67.8a | 279.4±53.8a | 127.0±28.4a | 1.57±0.18a | 7.1±2.7a,b | 6.6±2.4a | 1.2±0.7a,b | 24.8±8.5a | 23.6±6.5a |
| CGEA | 218.3±71.4a | 261.0±49.4 | 118.4±26.8a | 1.43±0.19 | 9.2±4.1a | 6.3±2.7a | 1.5±0.6a | 22.0±7.3 | 21.9±6.3a | |
| T4 | TIVA | 166.2±48.7 | 264.3±44.5 | 107.9±28.1 | 1.48±0.22a | 7.0±3.3a,b | 6.5±2.1a | 1.3±2.1a | 20.9±7.2 | 22.3±6.8a |
| CGEA | 169.9±51.2 | 249.7±42.8 | 97.5±30.6 | 1.44±0.22 | 8.7±2.9a | 5.9±3.0 | 1.8±1.1a | 20.3±6.4 | 21.8±6.8a |
P<0.05 vs. T0 in the same group
P<0.05 vs. CGEA group at the same time. T0, before anesthesia; T1, at the end of surgery; T2, 24 h after surgery; T3, 48 h after surgery; T4, 72 h after surgery. TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia. Cor, compound F; IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; TGF-β, transforming growth factor-β.
Compared with before anesthesia, at 24 h after surgery, IL-1 in the CGEA group was significantly different (P<0.05). At 48 h after surgery, the Cor, IL-6, TNF-α, IFN-γ, IL-4 and TGF-β indicated significant differences (P<0.05), and at 72 h after surgery, IFN-γ, IFN-γ/IL-4 and TGF-β were significantly different (P<0.05). At the end of surgery and at 24 h after surgery, IL-6 in the CGEA group was significantly lower than that in the TIVA group (P<0.05). At 24 h after surgery, IL-1, TNF-α, IL-4 and IL-17 in the CGEA group were significantly lower than those in the TIVA group (P<0.05). At 24 and 48 h after surgery, IFN-γ and IFN-γ/IL-4 in the CGEA group were significantly higher than those in the TIVA group (P<0.05). In addition, at 72 h after surgery, IFN-γ in the CGEA group was significantly higher than that in the TIVA group (P<0.05).
Comparison of the incidence of complications and disease progression of NSCLC patients in the CGEA and TIVA groups following surgery
No significant differences in the incidence rate of postoperative complications, including incisional wound infection, pulmonary infection, bronchopleural fistula, respiratory failure and arrhythmia (P>0.05) were identified in the CGEA and TIVA groups, nor in disease progression, including local tumor recurrence rate, distant metastasis rate or local recurrence and distant metastasis rate (P>0.05) (Table VII).
Table VII.
Comparison of the incidence of complications and disease progression of non-small cell carcinoma patients in the CGEA and TIVA groups after surgery.
| Variable | TIVA group (n=60) (%) | CGEA group (n=60) (%) | P-value |
|---|---|---|---|
| Complication | 0.822 | ||
| Incisional wound infection | 3 (5.00) | 3 (5.00) | |
| Pulmonary infection | 4 (6.67) | 3 (5.00) | |
| Bronchopleural fistula | 1 (1.67) | 1 (1.67) | |
| Respiratory failure | 1 (1.67) | 0 (0.00) | |
| Arrhythmia (cases) | 4 (6.67) | 5 (8.33) | |
| Total | 13 (21.67) | 12 (20.00) | |
| Disease progression | |||
| Local tumor recurrence rate | 13 (21.67) | 14 (23.33) | 0.827 |
| Distant metastasis rate | 14 (23.33) | 12 (20.00) | 0.658 |
| Rate of local recurrence and distant metastasis | 8 (13.33) | 7 (11.67) | 0.783 |
TIVA, total intravenous anesthesia; CGEA, combined general-epidural anesthesia.
Comparison of the prognosis of NSCLC patients in the CGEA and TIVA groups following surgery
Comparisons of tumor-free survival time between the CGEA and TIVA groups following a long term follow-up are presented in Table VIII. The mean tumor-free survival time in patients of the TIVA and CGEA groups was 26.78 and 27.12 months, respectively, and the median tumor-free survival time was 34 and 33 months, respectively. No significant difference was identified in tumor-free survival time between patients in the two groups. As for the overall survival rate, the 1-, 2-, 3- year survival rates following surgery in the TIVA group were 78.33, 65.00 and 43.33%, respectively, and those in the CGEA group were 80.00, 61.67 and 45.00%, respectively. Log-rank test results indicated no statistical difference in the postoperative survival curve of the two groups (P>0.05; Fig. 2).
Table VIII.
Comparison of tumor-free survival of non-small cell lung cancer patients in the CGEA and TIVA groups.
| Tumor-free survival time, months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean value | Median value | |||||||||
| 95% CI | 95% CI | |||||||||
| Group | Estimated value | Standard error | Lower limit | Upper limit | Estimated value | Standard error | Lower limit | Upper limit | χ2 | P-value |
| TIVA | 26.78 | 1.413 | 24.01 | 29.55 | 34 | 2.42 | 29.26 | 38.74 | 0.024 | 0.877 |
| CGEA | 27.12 | 1.409 | 24.36 | 29.88 | 33 | 1.94 | 29.2 | 36.8 | ||
CI, confidence interval; TIVA, total intravenous anesthesia; CGEA, combined general/-pidural anesthesia.
Figure 2.
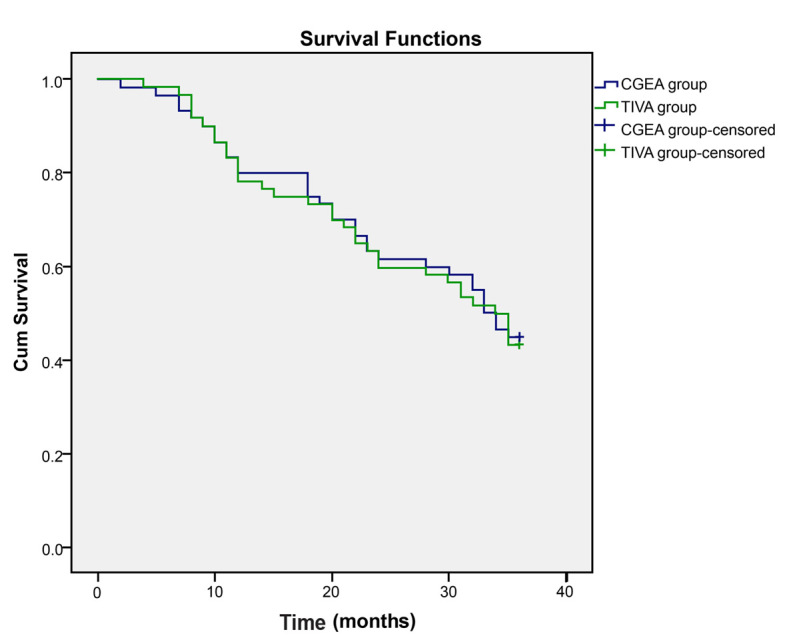
Survival curves of non-small cell lung cancer patients in the CGEA and TIVA groups. TIVA, total intravenous anesthesia; CGEA, combined general/epidural anesthesia.
Discussion
NK cells are an important cell population in the innate immune system, capable of attacking pathogen-infected and malignant body cells and spontaneously producing immune-regulatory cytokines. In addition, T lymphocytes are involved in the adaptive immune system, contributing widely to immune regulation, inflammation and protective immune responses (31). Cytokines are classified into Th1 or Th2 according to the cell of release (18). Th1 cells and T cytotoxic lymphocytes release Th1 cytokines, which have stimulatory actions on cell-mediated immunity, particularly on NK cells (32). Th2 lymphocytes secrete IL-4, which is one of the known Th2 cytokines (33). The balance between Th1/Th2 cytokines appears to be associated with infections, sepsis, and cancer formation and progression (34). Previous studies demonstrated that regional anesthesia may repair the Th1/Th2 balance, preserve the CD4/CD8 ratio and may exert a positive effect on the number and function of NK cells following surgery (35,36). Qu et al (37) demonstrated that additional epidural anesthesia did not affect inflammatory cytokine production. In the present study, T lymphocytes and NK cells were depressed perioperatively in the two groups, and the patients undergoing CGEA showed a smaller decrease of T lymphocyte subsets at 48 h after surgery compared with those undergoing TIVA, which may imply that CGEA imposes a smaller immune suppression effect than TIVA. Consistently, Kurosawa (38) proposed that TIVA and regional anesthesia may reduce the negative consequences associated with perioperative immunosuppression. Vanni et al (39) indicated that open surgery with thoracic epidural anesthesia led to a reduced impact on postoperative lymphocyte responses when compared with general anesthesia, as the study observed that the percentage of NK cells was preserved in the former. Kun et al (40) demonstrated that GEA helped to maintain the perioperative immune function of the body when compared with GA alone in patients undergoing gastric cancer surgery.
In the present study, patients undergoing CGEA took less time than those undergoing TIVA to recover spontaneous breathing, open their eyes on hearing whispered words and for tracheal extubation. Consistent with the current findings, Liu et al (41) demonstrated that the CGEA group had a shortened postoperative extubation time and recovery duration than the TIVA group. CGEA was reported to have the advantages of maintaining a normal hormone level, controlling blood pressure, maintaining hemodynamics and requiring less medication (42–44). Therefore, it may be hypothesized that all those advantages contributed to a shorter recovery period.
There are significant differences in the effects of CGEA and TIVA on cellular immune function and the time indicators of spontaneous breathing recovery, eyes opening and tracheal extubation in patients. However, our study found that patients with CGEA and TIVA reported a similar postoperative situation, including the complications occurrence, recurrence and distant metastasis rate. Almost all cancer-associated mortalities in the postoperative period are attributed to metastases or recurrence and the anesthesia method applied for surgery imposes a potential risk by affecting the pathophysiology of postoperative metastatic spread (45). Niwa et al (46) suggested an optimization of the balance between potential tumor metastases and antimetastatic immune defenses. It has been hypothesized that the anesthetic management influenced the long-term outcome following surgery and that an anesthetic treatment with low potential for immunosuppression reduces relapse (47). Pei et al (44) reported that GEA may improve the outcome for prostate cancer patients and hypothesized that it was associated with immune response and tumor cell biology. The statistical analysis in the present study did not support this hypothesis. However, consistent with the current results, Kurosawa and Kato (22) demonstrated that ~20% of the immunosuppressive effect may not normally greatly influence a patient if they are not already compromised by aging, tumor burden or other factors. In a previous study, surgical removal of tumors was linked to inadvertent dispersal of neoplastic cells into the blood and lymphatic systems (48). Meanwhile, it was reported that anesthesia had a minor influence on immune systems (22). Therefore, it is hypothesized that surgical resection itself, rather than the anesthesia treatment, may have a significant effect on the prognosis.
In conclusion, the present study demonstrated that CGEA and TIVA affected cellular immunity. However, CGEA exerted less of an effect on cellular immunity and had a better postoperative analgesic effect. Thus, CGEA may still be recommended for surgical resection for NSCLC patients. Generally, more elderly patients exhibit a worse tolerance to surgery. In addition, the risk from surgery increases as the surgery duration increases. Furthermore, heart, lung and immune function of the elderly restrict the choice of surgical approach. All of these may, to a certain extent, affect the surgery and postoperative recovery. Thus, the addition of an age-associated indicator in the current study may provide more evidence. In addition, a larger sample-size study is required to further verify this conclusion.
References
- 1.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 2.Liao C, Yu ZB, Meng G, Wang L, Liu QY, Chen LT, Feng SS, Tu HB, Li YF, Bai L. Association between Th17-related cytokines and risk of non-small cell lung cancer among patients with or without chronic obstructive pulmonary disease. Cancer. 2015;121(Suppl 17):S3122–S3129. doi: 10.1002/cncr.29369. [DOI] [PubMed] [Google Scholar]
- 3.Yang D, Zhou J, Zeng T, Yang Z, Wang X, Hu J, Song Y, Chen L, Peer D, Wang X, Bai C. Serum chemokine network correlates with chemotherapy in non-small cell lung cancer. Cancer Lett. 2015;365:57–67. doi: 10.1016/j.canlet.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. ESMO Guidelines Working Group: Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–vii64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, Thibodeau S, Adjei AA, Jett J, Deschamps C. Clinical features of 5,628 primary lung cancer patients: Experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 6.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:5311–5320. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 7.D'Addario G, Früh M, Reck M, Baumann P, Klepetko W, Felip E. ESMO Guidelines Working Group: Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 8.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L, Wallis J, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crinò L, Weder W, van Meerbeeck J, Felip E. ESMO Guidelines Working Group: Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, Govindan R, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 11.Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, Johannsdottir HK, Klughammer B, Gonzalez EE. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): A randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 12.Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, Baldotto C, Bennouna J, Shepherd FA, Le-Guennec S, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: A randomized, controlled phase III trial. J Clin Oncol. 2012;30:3640–3647. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- 13.Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e314S–e340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 14.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 15.Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ, Rusch VW, Putnam JB., Jr Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662–670. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenfeld K, Avraham R, Benish M, Goldfarb Y, Rosenne E, Shapira Y, Rudich T, Ben-Eliyahu S. Immune suppression while awaiting surgery and following it: Dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21:503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Cata JP, Bauer M, Sokari T, Ramirez MF, Mason D, Plautz G, Kurz A. Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth. 2013;25:255–262. doi: 10.1016/j.jclinane.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29:517–526. doi: 10.1016/j.cct.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Sessler DI. Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur J Cancer Prev. 2008;17:269–272. doi: 10.1097/CEJ.0b013e3282f0c005. [DOI] [PubMed] [Google Scholar]
- 21.Ash SA, Buggy DJ. Does regional anaesthesia and analgesia or opioid analgesia influence recurrence after primary cancer surgery? An update of available evidence. Best Pract Res Clin Anaesthesiol. 2013;27:441–456. doi: 10.1016/j.bpa.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 23.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: Polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motohashi S, Nakayama T. Invariant natural killer T cell-based immunotherapy for cancer. Immunotherapy. 2009;1:73–82. doi: 10.2217/1750743X.1.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 27.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw. 2015;13:515–524. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 28.Schlag C, Wörner A, Wagenpfeil S, Kochs EF, Schmid RM, von Delius S. Capnography improves detection of apnea during procedural sedation for percutaneous transhepatic cholangiodrainage. Can J Gastroenterol. 2013;27:582–586. doi: 10.1155/2013/852454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim DY, Lee DW, Jang EA, Lee SH, Jeong HJ, Jeong CW, Jeong SW, Yoo KY. Effects of inspired oxygen fraction in discriminating venous from arterial blood in percutaneous central venous catheterization under general anesthesia. Korean J Anesthesiol. 2012;62:225–229. doi: 10.4097/kjae.2012.62.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huijuan Cao XJ, Liu Jianping. Application of visual analogue scales in assessment of symptomatic outcome data. J Tradit Chin Med. 2009;50:1518–1519. [Google Scholar]
- 31.Gerner W, Käser T, Saalmüller A. Porcine T lymphocytes and NK cells-an update. Dev Comp Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: Friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 34.Han YF, Zhao J, Ma LY, Yin JH, Chang WJ, Zhang HW, Cao GW. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol. 2011;17:4258–4270. doi: 10.3748/wjg.v17.i38.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada H, Seki S, Takahashi T, Kawarabayashi N, Higuchi H, Habu Y, Sugahara S, Kazama T. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106:499–506. doi: 10.1097/00000542-200703000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Conrick-Martin I, Kell MR, Buggy DJ. Meta-analysis of the effect of central neuraxial regional anesthesia compared with general anesthesia on postoperative natural killer T lymphocyte function. J Clin Anesth. 2012;24:3–7. doi: 10.1016/j.jclinane.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Qu DM, Jin YF, Ye TH, Cui YS, Li SQ, Zhang ZY. The effects of general anesthesia combined with epidural anesthesia on the stress response in thoracic surgery. Zhonghua Yi Xue Za Zhi. 2003;83:408–411. (In Chinese) [PubMed] [Google Scholar]
- 38.Kurosawa S. Anesthesia in patients with cancer disorders. Curr Opin Anaesthesiol. 2012;25:376–384. doi: 10.1097/ACO.0b013e328352b4a8. [DOI] [PubMed] [Google Scholar]
- 39.Vanni G, Tacconi F, Sellitri F, Ambrogi V, Mineo TC, Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg. 2010;90:973–978. doi: 10.1016/j.athoracsur.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 40.Kun L, Tang L, Wang J, Yang H, Ren J. Effect of combined general/epidural anesthesia on postoperative nk cell activity and cytokine response in gastric cancer patients undergoing radical resection. Hepatogastroenterology. 2014;61:1142–1147. [PubMed] [Google Scholar]
- 41.Liu XZ, Wei CW, Wang HY, Ge YH, Chen J, Wang J, Zhang Y. Effects of general-epidural anaesthesia on haemodynamics in patients with myasthenia gravis. West Indian Med J. 2015;64:99–103. doi: 10.7727/wimj.2013.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuethrich PY, Thalmann GN, Studer UE, Burkhard FC. Epidural analgesia during open radical prostatectomy does not improve long-term cancer-related outcome: A retrospective study in patients with advanced prostate cancer. PLoS One. 2013;8:e72873. doi: 10.1371/journal.pone.0072873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneda K, Takeuchi J, Yakushiji T, Kotani J. A case of anesthesia for a patient with a huge pheochromocytoma accompanying difficulty in hemodynamics control. Masui. 2006;55:900–903. (In Japanese) [PubMed] [Google Scholar]
- 44.Pei L, Tan G, Wang L, Guo W, Xiao B, Gao X, Wang L, Li H, Xu Z, Zhang X, et al. Comparison of combined general-epidural anesthesia with general anesthesia effects on survival and cancer recurrence: A meta-analysis of retrospective and prospective studies. PLoS One. 2014;9:e114667. doi: 10.1371/journal.pone.0114667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–1250. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 46.Niwa H, Rowbotham DJ, Lambert DG, Buggy DJ. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? J Anesth. 2013;27:731–741. doi: 10.1007/s00540-013-1615-7. [DOI] [PubMed] [Google Scholar]
- 47.Tzoneva D, Cherkezov J, Georgiev S, Masljankov S, Todorov G. Long-term consequences of anesthetic technique after cancer surgery. Khirurgiia (Sofiia) 2014:69–74. (In Bulgarian, English) [PubMed] [Google Scholar]
- 48.Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth. 2012;109(Suppl 1):i17–i28. doi: 10.1093/bja/aes421. [DOI] [PubMed] [Google Scholar]