Abstract
Store-operated Ca2+ entry (SOCE) via store-operated Ca2+ channels (SOCC), encoded by transient receptor potential canonical (TRPC) channel proteins, is an important underlying mechanism regulating intracellular Ca2+ concentration ([Ca2+]i) and various intracellular functions in endothelial cells (ECs). TRPC1, the probable candidate for SOCC, is expressed in ECs. Ca2+-sensing receptor (CaSR) is functionally expressed in vascular endothelium and is important in Ca2+ mobilization and cardiovascular functions. To date, there have been no reports demonstrating an association between CaSR and TRPC1 in ECs. The present study investigated the effects of TRPC1 on CaSR-induced Ca2+ influx and nitric oxide (NO) production in human umbilical vein ECs (HUVECs). TRPC1 and CaSR proteins in HUVECs were measured by immunostaining and western blot analysis. [Ca2+]i levels were measured using the Fura-2-acetoxymethyl ester method. The indicator 3-amino, 4-aminomethyl-2, 7-difluorescein diacetate was used to measure NO production in HUVECs. The expression of TRPC1 protein in HUVECs was silenced by transfecting HUVECs with small interfering RNA (siRNA) against TRPC1. Although changes in extracellular Ca2+ failed to alter [Ca2+]i in HUVECs, the CaSR agonist spermine increased [Ca2+]i and NO production in HUVECs. NO production in HUVECs was diminished in Ca2+-free medium or following treatment with a CaSR negative allosteric modulator (Calhex231), SOCC inhibitor (MRS1845) or TRPC inhibitor (SKF96365). The spermine-induced increases in [Ca2+]i and NO production were reduced in HUVECs transfected with TRPC1 siRNA. These results suggested that TRPC1 is a primary candidate in forming SOCC that stimulates CaSR-induced SOCE and NO production in HUVECs and is a potential therapeutic target for vascular diseases.
Keywords: calcium-sensing receptor, calcium signaling, endothelial cell, ion channel, nitric oxide, transient receptor potential canonical
Introduction
Alterations in the cytoplasmic free calcium concentration ([Ca2+]i) impact various processes of the vascular endothelium, and have important roles in the regulation of vascular tone, arterial blood pressure and generation of nitric oxide (NO) (1). The alterations in [Ca2+]i are mediated by two primary mechanisms: Ca2+ release from intracellular stores and Ca2+ influx across the plasma membrane via various pathways (2). The dominant mechanism in non-excitable cells is via store-operated Ca2+ entry (SOCE), which is mediated by store-operated calcium channels (SOCCs) (3). SOCE is induced by the activation of phospholipase C by G protein-coupled receptors including Ca2+-sensing receptor (CaSR), which leads to the production of inositol 1,4,5-trisphophate (IP3). The subsequent release of Ca2+ from the endoplasmic reticulum (ER) triggers Ca2+ influx by capacitative Ca2+ entry (CCE). Members of the canonical subgroup of transient receptor potential (TRP) proteins constitute tetramers of SOCC (4).
The CaSR is part of an intricate network of calcium channels, pumps and exchangers involved in the control of [Ca2+]i, and thereby in the modulation of cardiovascular functions (5). Abnormal Ca2+ handling within blood vessels may contribute to inappropriate contraction, a primary symptom of hypertension. The understanding of how intracellular Ca2+ is regulated under physiological and pathophysiological situations forms an important aspect of the search for novel therapeutic targets for the treatment of hypertension. In recent years, major advances in the understanding of Ca2+ homeostasis have been driven in part by the identification of TRP canonical (TRPC) as critical regulators of Ca2+ influx in numerous tissue types (4). It has been reported that TRPC1 is a probable contributor to the formation of SOCC in endothelial cells (ECs) (6), and that TRPC1-mediated Ca2+ entry contributes to the thrombin-induced increase in endothelial permeability (7).
The results from our previous study demonstrated that the CaSR agonist, spermine, stimulated increases in [Ca2+]i and NO production in human aortic ECs (HAECs) via the release of intracellular Ca2+ stores in HAECs (8). However, the molecular mechanisms underlying activation of Ca2+ influx channels by CaSR, their involvement in extracellular Ca2+ influx and their role in CaSR-induced NO production in vascular ECs remain to be elucidated. The present study hypothesized that TRPC1 contributes to CaSR-induced SOCE and NO production in human umbilical vein ECs (HUVECs).
Materials and methods
Materials
Fetal bovine serum (FBS) was obtained from HyClone; GE Healthcare Life Sciences (Logan, UT, USA), and all other cell culture reagents were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Spermine (a CaSR agonist), Calhex231 (a CaSR negative allosteric modulator), MRS1845 (a SOCC inhibitor) and SKF96365 (a TRPC inhibitor) were obtained from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). Rabbit anti-TRPC1 monoclonal antibody (catalog no. ACC-010) was obtained from Alomone Laboratories, Ltd. (Jerusalem, Israel). Polyclonal mouse anti-human CaSR antibody was obtained from Abcam (Cambridge, MA, USA; catalog no. ab62653, for western blotting) and from Shanghai Seebio Science & Technology Co., Ltd. (Shanghai, China; catalog no. HL1499 for immunohistochemistry) and other antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Small interfering RNA (siRNA) was purchased from Yangzhou Ruibo Biotech Co., Ltd. (Yangzhou, China). Lipofectamine™ 2000, Fura-2-acetoxymethyl ester (AM) and the NO Fluorescence kit were obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Cell culture
HUVECs were harvested by 0.25% pancreatin digestion from normal human umbilical cords. The protocol was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The EC culture medium was supplemented with 10% FBS, 50 mg/l EC growth supplement (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), 50 mg/l heparin, 100,000 U/l penicillin and 100 mg/l streptomycin. Cells at passage 3–4 were used in the experiments as described previously (9).
Drug treatment
Cells were treated with various concentrations of extracellular Ca2+ ([Ca2+]o by sequentially adding 0.5, 2, 4 and 10 mM Ca2+ to cells in a perfusion chamber and monitoring for 5 min at each concentration. For spermine treatment, cells were divided into 6 groups: Spermine, in which cells were monitored for 1 min with Ca2+-free 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES)-buffered saline (HBS) of the following composition: 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 1.2 mM NaH2PO4, 10 mM D-glucose and 20 mM HEPES (pH 7.40), followed by 20 min of continuous perfusion with 2 mM spermine in Ca2+-free HBS; spermine + Ca2+, in which HBS containing 2 mM Ca2+ was used; Calhex231 + spermine, in which cells were monitored for 1 min with Ca2+-free HBS, followed by perfusion for 1 min with 1 mM Calhex231 in Ca2+-free HBS and continuous perfusion for 20 min with 1 mM Calhex231 and 2 mM spermine in Ca2+-free HBS solution; Calhex231 + spermine + Ca2+, in which HBS containing 2 mM Ca2+ was used; MRS1645 + spermine + Ca2+, in which 5 µM MRS1645 was used instead of Calhex231; and SKF96365 + spermine + Ca2+, in which 5 µM SKF96365 was used instead of Calhex231.
Immunostaining of CaSR and TRPC1 proteins
Cultured HUVECs on coverslips were fixed with 95% ice-cold ethanol for 10 min at room temperature and permeabilized with 0.1% Triton X-100 solution in PBS for 10 min at room temperature. The nonspecific binding sites were blocked with 10% goat serum in PBS for 30 min at room temperature. Fixed cells were treated with primary antibodies against CaSR (1:200) or TRPC1 (1:50) for 1 h at room temperature. Following washing, cells were stained with fluorescein isothiocyanate-goat anti-mouse IgG (catalog no. BA1101) or TRITC-goat anti-rabbit (catalog no. BA1090) conjugated secondary antibodies (1:30; Wuhan Boster Biological Technology, Ltd., Wuhan, China) for 30 min. Cells were washed and examined at green and red wavelengths under a Bio-Rad MRC 1000 confocal microscope (Sanyo Electric Co., Ltd., Moriguchi, Japan). For each experiment, >50 cells were recorded.
Western blot analysis
Cells were rinsed twice with ice-cold PBS and harvested in cell lysis solution (Beijing Biodev-tech Scientific & Technical Co., Ltd., Beijing, China). Protein concentration was measured using a Bicinchoninic Acid assay kit (Abcam, Shanghai, China) Equal quantities of protein (40 µg) were run on a 10% SDS-PAGE gel and subsequently transferred onto a polyvinylidene difluoride membrane by electroblotting (100 V for 1.5 h). Following incubation with 5% non-fat milk in TBS containing Tween 20 for 2 h at room temperature, membranes were incubated overnight at 4°C with primary antibodies against human CaSR (1:500) or TRPC1 (1:200). Goat anti-mouse (1:10,000; catalog no. bs-0296Gs) or goat anti-rabbit (1:8,000; catalog no. bs-0295G) IgG horseradish peroxidase-conjugated secondary antibodies (Wuhan Boster Biological Technology, Ltd.) were added to membranes for 1 h. Protein was visualized using an enhanced chemiluminescence system (Pierce; Thermo Fisher Scientific, Inc.). Intensities of the protein bands were quantified using Bio-Rad Quantity One software (version, 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
TRPC1 knockdown by siRNA
To evaluate the functional role of TRPC1 in CaSR-induced Ca2+ influx and NO production in HUVECs, siRNA was used to reduce TRPC1 expression. For human TRPC1, the sense strand siRNA, 5′-GCGACAAGGGUGACUAUUAdTdT-3′ and antisense strand siRNA, 3′-dTdTCGCUGUUCCCACUGAUAAU-5′ were used. The selective siRNA duplex and a nonspecific control duplex were obtained from Yangzhou Ruibo Biotech Co., Ltd. Transfection of siRNA into HUVECs was performed using Lipofectamine 2000 transfection reagent according to the manufacturer's protocol. Briefly, cultured cells were washed with Opti-Minimal Essential Medium without serum or antibiotics and seeded in 6-well plates to 30–40% confluence (typically 1×105 cells/35-mm plate incubated at 37°C for 48 h). The transfection reagent and siRNA were diluted separately in serum-free media, mixed and incubated for 10 min at room temperature to form the siRNA/lipid complex. This complex was then added to each well at a final concentration of 70 nM/well of siRNA. At 48 h after transfection, cells were collected to determine TRPC1 protein expression levels by western blot analysis.
[Ca2+]i measurement
[Ca2+]i levels were measured in HUVECs using Fura-2AM as a Ca2+-sensitive fluorescent indicator. HUVECs were seeded on gelatin-coated, 25-mm diameter circular glass coverslips and grown to 60–70% confluence. Following loading with 10 µM Fura-2AM for 30 min at room temperature, the coverslips were washed and the cells were maintained for 30 min prior to experimentation in indicator-free HBS. The fluorescence of Fura-2AM was recorded from a single HUVEC on coverslips in a perfusion chamber mounted onto the stage of a modified Nikon Diaphot inverted epifluorescence microscope (Nikon Corporation, Tokyo, Japan) following excitation at 340±10 and 380±10 nm, corresponding to the Ca2+-bound and Ca2+-free forms of the indicator, respectively. Bandpass interference filters (Omega Optical, Inc., Brattleboro, VT, USA) selected wavelength bands of emitted fluorescence at 510±10 nm.
Measurement of NO production
NO production was measured with the membrane-permeable indicator dye 3-amino, 4-aminomethyl-2,7-difluorescein (DAF-FM) diacetate (Beyotime Institute of Biotechnology, Shanghai, China), a fluorescent dye sensitive to NO levels (10). A monolayer of HUVECs was seeded onto coverslips and loaded with 0.5 nM DAF-FM in HBS at 37°C for 30 min in the dark, followed by incubation with DAF-FM-free HBS for an additional 20 min to allow for de-esterification of the indicator. DAF-FM fluorescence was monitored on the aforementioned fluorescence microscopy system at an excitation wavelength of 480±10 nm and an emission wavelength of 510±10 nm. To validate the use of DAF-FM fluorescence as an index of the production of cellular NO induced by agonists, HUVECs were pre-incubated with L-arginine and the specific NO synthase (NOS) inhibitor, NG-nitro-L-arginine methyl ester (L-NAME; 1 mM), as described previously (10). For experiments involving a Ca2+-free system, Ca2+-free/ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) solution was made by substituting CaCl2 with 1 mM EGTA in HBS.
Statistical analysis
Statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). Multiple comparisons were performed using a one-way analysis of variance or the Kruskal-Wallis test, followed by the Student-Newman-Keuls post hoc test or unpaired Student's t-test. Data are presented as mean ± standard error. P<0.05 was considered to indicate a statistically significant difference.
Results
Localization of CaSR and TRPC1 expression in HUVECs
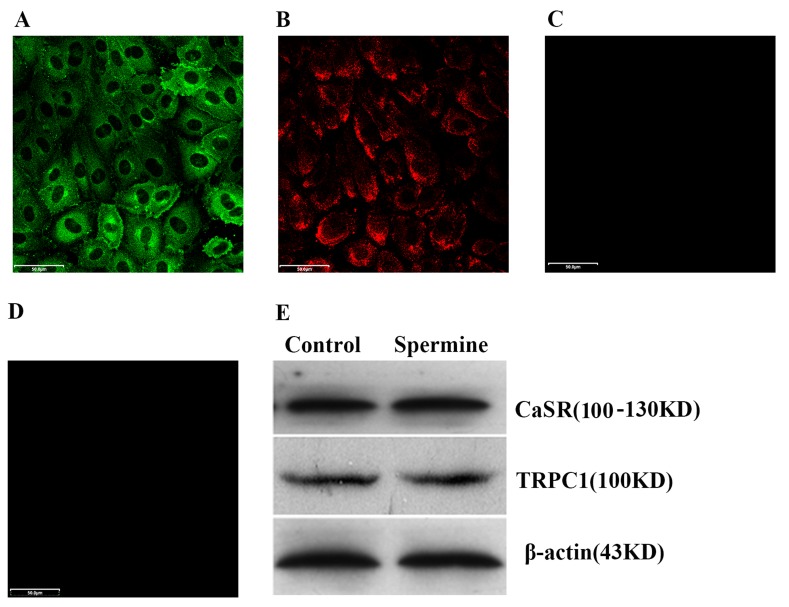
To determine whether the CaSR and TRPC1 are expressed in HUVECs, immunocytochemical analysis with confocal microscopy was performed using polyclonal or monoclonal antibodies against human CaSR and TRPC1. Green fluorescent stained CaSR was localized in the cytosol (Fig. 1A). Red fluorescent stained TRPC1 was localized in the plasma membrane (Fig. 1B). To exclude any non-specific binding, the secondary antibodies alone were used for staining. No immunostaining of the secondary antibodies was observed (Fig. 1C and D). To confirm the expression of CaSR and TRPC1 proteins in HUVECs and the stimulatory effect of spermine on CaSR and TRPC1 expression, western blot analysis was performed using cell lysates isolated from the cytoplasm. The results demonstrated that CaSR and TRPC1 were expressed and that spermine treatment (2 mM, 48 h) did not alter the protein expression levels of CaSR and TRPC1 in HUVECs (Fig. 1F).
Figure 1.
Immunostaining and western blotting to determine the location and expression, respectively, of CaSR and TRPC-1 in HUVECs. Confocal micrographs revealed that (A) CaSR protein was primarily expressed in the cytosol of HUVECs and (B) TRPC1 protein was expressed on the plasma membrane of HUVECs. Negative controls for (C) CaSR and (D) TRPC1 were performed using the secondary antibodies only. (E) Western blotting indicated that spermine treatment (2 mM, 48 h) did not alter the protein expression levels of CaSR and TRPC1 in HUVECs. Representative results from three independent experiments are presented. Magnification, ×200. Scale bar=50 µm. CaSR, Ca2+-sensing receptor; TRPC1, transient receptor potential canonical 1; HUVEC, human umbilical vein endothelial cells.
CaSR-induced SOCE activation and NO production by TRPC
To demonstrate the presence and function of CaSR in HUVECs, Fura-2AM-loaded HUVECs were stimulated by various known CaSR agonists, including extracellular Ca2+ and spermine (Fig. 2). Treatment with 0.5 to 10 mM [Ca2+]o did not cause any detectable alterations in [Ca2+]i in Fura-2AM-loaded HUVECs (n=7 each; Fig. 2A). Thus, although CaSR is expressed in HUVECs, it does not appear to respond to extracellular Ca2+ as an agonist under these experimental conditions. However, spermine, the other CaSR agonist assessed, induced an increase in [Ca2+]i in HUVECs (P=0.003; Fig. 2B and I) at a concentration (2 mM) that stimulated the CaSR in other cell types (11). Spermine evoked a rapid rise of [Ca2+]i followed by an elevated plateau phase, which potentially reflected the activation of SOCE. To determine the Ca2+ source in spermine-stimulated HUVECs, cells were treated with 2 mM spermine in Ca2+-free/EGTA HBS. Spermine stimulated an increase in [Ca2+]i in HUVECs (P=0.002; Fig. 2C) that was reduced compared with that observed in 2 mM [Ca2+]o (P=0.001; Fig. 2I). Spermine resulted in a transient increase in [Ca2+]i followed by a rapid decay to baseline, which reflected IP3-mediated Ca2+ release from the ER. These results suggested that spermine induced extracellular Ca2+ influx and intracellular Ca2+ release in HUVECs.
Figure 2.
CaSR induces store operated calcium entry activation and NO production by TRPC in HUVECs. (A) Various concentrations of extracellular Ca2+ ([Ca2+]o) as CaSR agonist did not cause any detectable alterations in [Ca2+]i. (B) Treatment with 2 mM spermine, a CaSR agonist, induced a sustained high [Ca2+]i in the presence of 2 mM [Ca2+]o.. (C) The elevated [Ca2+]i induced by spermine decreased rapidly in the absence of [Ca2+]o. The ability of spermine to increase [Ca2+]i was (D) reduced in the presence of 2 mM [Ca2+]o or (E) completely abolished in the absence of extracellular Ca2+ by 1 µM Calhex231, a CaSR negative allosteric modulator. (F) Store-operated Ca2+ channels nonselective cation channel blocker MRS1845 (5 µM) or (G) TRPC nonselective channel blocker SKF96365 (5 µM) reduced the elevated [Ca2+]i induced by spermine. (H) L-NAME, a selective inhibitor of NOS, had no effect on the spermine-mediated [Ca2+]i response. Representative traces are presented in A-H. Bar graphs indicated the effects of various treatments on (I) [Ca2+]i and (J) NO production in HUVECs. Results are presented as the mean ± standard error of 13–15 cells/test from 7 independent experiments. *P<0.05 vs. different concentrations of Ca2 or control; #P<0.05 vs. spermine alone; ∆P<0.05 vs. spermine + Ca2+. NO, nitric oxide; CaSR, Ca2+-sensing receptor; TRPC, transient receptor potential canonical; HUVECs, human umbilical vein endothelial cells; Fura-2AM, Fura-2-acetoxymethyl ester; DAF-FM, 3 amino, 4 aminomethyl-2, 7, difluorescein.
To determine whether SOCE responds to spermine stimulation, Fura-2AM-loaded HUVECs were pretreated with 1 mM Calhex231, a highly selective CaSR negative allosteric modulator (12), for 2 min and stimulated with 2 mM spermine in 2 mM [Ca2+]o medium (Fig. 2D). Calhex231 inhibited the spermine-induced increase in [Ca2+]i in HUVECs (P=0.0001; Fig. 2I). In Ca2+-free/EGTA media, Calhex231 abrogated spermine-induced changes in [Ca2+]i (P=0.00002; Fig. 2E and I). These findings suggested that SOCE may be triggered by CaSR activation in HUVECs.
To assess the behavior of channels involved in the spermine-induced Ca2+ entry, the effects of SOCC and TRPC nonselective cation channel blockers were examined. Pretreatment with 5 µM MRS1845 (P=0.002) or SKF96365 (P=0.003) significantly inhibited spermine-induced Ca2+ influx (Fig. 2F, G and I). These results suggested that the Ca2+ influx triggered by CaSR activation in HUVECs is associated with TRPC and opening of SOCC.
The production of NO was measured in spermine-treated HUVECs using the fluorescent NO indicator DAF-FM. The spontaneous increment in DAF-FM fluorescence was 281.1±13.7 (n=7) at 20 min (Fig 2J) without Ca2+ mobilizing agents, which indicated the baseline NO production rate. Spermine (2 mM) in Ca2+-free medium stimulated a significantly slower increase in DAF-FM fluorescence (P=0.004) than in 2 mM Ca2+ medium (P=0.004; Fig. 2J), suggesting that an elevation in [Ca2+]i, by store depletion or CCE, may induce NO production in HUVECs. When 1 mM L-NAME, a selective inhibitor of NOS, was added to 2 mM Ca2+ medium, the spermine-induced increase in DAF-FM fluorescence was abrogated (P=0.003), whereas no effect was observed on the spermine-mediated [Ca2+]i response (P=0.123), indicating the specificity of DAF-FM to NO (Fig. 2H-J). Similarly, the spermine-induced increase in DAF-FM fluorescence was inhibited or completely abrogated when 1 mM Calhex231 was added to 2 mM Ca2+ (P=0.003) or Ca2+-free media (P<0.0001; Fig. 2J). Pretreatment with 5 µM MRS1845 (P=0.003) or 5 µM SKF96365 (P=0.003) in 2 mM Ca2+ media resulted in inhibition of the spermine-induced increase in DAF-FM fluorescence (Fig. 2J). The results suggested that, via TRPC, CaSR-mediated activation of SOCE stimulates NO production in HUVECs.
TPRC1 serves an important role in CaSR-induced SOCE activation and NO production in HUVECs
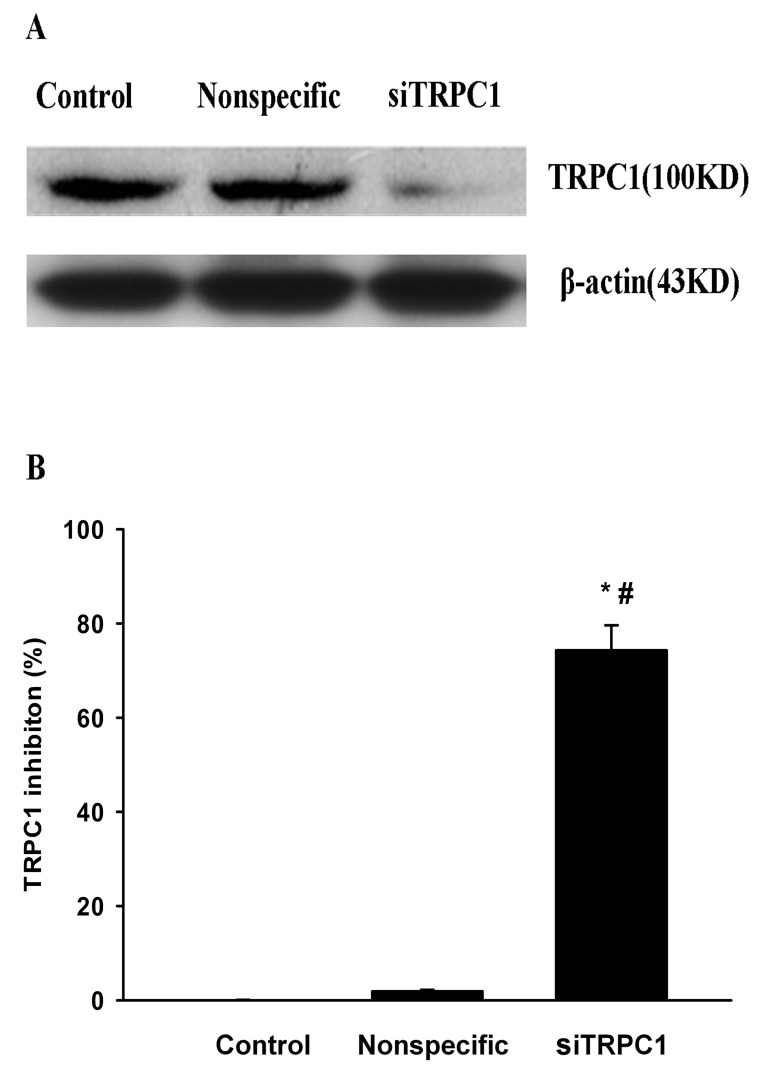
It has been previously demonstrated that TRPC1 and/or TRPC4 may function as components of SOCC (13). To determine whether TRPC1 contributes to the response to spermine, selective TRPC1 siRNA was introduced into HUVECs. Western blot analysis demonstrated that TRPC1 siRNA decreased the expression levels of TRPC1 protein by >74% (P=0.0012; Fig. 3). In parallel experiments with fura-2 loaded HUVECs or the NO fluorescent indicator DAF-FM, transfection with TRPC1 siRNA abrogated spermine-induced increases in [Ca2+]i (P=0.002) and NO production (P=0.003), whereas transfection with control nonspecific siRNA had no effect (P=0.191; P=0.07; Fig. 4). These results indicated that the SOCE and NO production triggered by CaSR activation in HUVECs are mediated by TRPC1.
Figure 3.
TRPC1 siRNA decreases the protein expression levels of TRPC1 in HUVECs. (A) Western blotting demonstrated that TRPC1 siRNA transfection decreased the expression of TRPC1 protein in HUVECs, whereas nonspecific siRNA had no significant effect. (B) Quantification of western blots revealed that TRPC1 siRNA decreased TRPC1 protein expression levels by 74%. Data are expressed as the mean ± standard error of four experiments. ∆P<0.05 vs. control; #P<0.05 vs. nonspecific siRNA. siRNA, small interfering RNA, TRPC1, transient receptor potential canonical 1; HUVECs, human umbilical vein endothelial cells.
Figure 4.
Transfection of TRPC1 siRNA reduces spermine-induced increases in [Ca2+]i and NO production in HUVECs. (A) TRPC1 siRNA, but not (B) nonspecific siRNA, reduced the spermine-stimulated increase in [Ca2+]i in the presence of 2 mM [Ca2+]o, compared with (C) the control group. Representative traces are presented in A-C. Bar graphs indicated the effects of siRNA on (D) [Ca2+]i and (E) NO production in HUVECs. ∆P<0.05 vs. control; #P<0.05 vs. nonspecific siRNA. Results are presented as the mean ± standard error of 13–15 cells/test from 7 independent experiments. NO, nitric oxide; siRNA, small interfering RNAs, TRPC1, transient receptor potential canonical 1; HUVECs, human umbilical vein endothelial cells; Fura-2AM, Fura-2-acetoxymethyl ester; DAF-FM, 3 amino, 4 aminomethyl-2,7, difluorescein.
Discussion
CaSR was been cloned from bovine parathyroid cells in 1993 (14), and it has since been demonstrated that CaSR serves a key physiological role in the cardiovascular system by modulating arterial blood pressure (15). However, the precise mechanisms underlying CaSR-mediated [Ca2+]i homeostasis in vascular ECs remain to be elucidated. In HUVECs transfected with selective CaSR siRNA, our previous study revealed that CaSR is important in spermine-evoked Ca2+ influx and NO production (16). The present study demonstrated that CaSR activation mediated Ca2+ entry through SOCC, and that TRPC1-encoded SOCC was involved in the CaSR-mediated [Ca2+]i increase in HUVECs. In addition, the present study demonstrated a role for CaSR in regulating Ca2+ entry via SOCC that advances the understanding of the molecular mechanisms underlying CaSR-mediated [Ca2+]i increases in human blood vessels. This pattern of Ca2+ entry has previously been reported in various cell types, including neonatal ventricular myocytes and the Saos-2 human osteosarcoma cell line following CaSR agonist application (17,18). In these experiments, functionally expressed CaSRs were revealed to respond to extracellular Ca2+ as an agonist (17–19) whereas in the present study, a similar protocol did not induce a significant increase in [Ca2+]i in HUVECs. This is consistent with our previously published observation in HAECs, in which spermine was the only known CaSR agonist that induced an increase in [Ca2+]i in HAECs (8), suggesting that the response of CaSR to agonists may exhibit a cell preference.
Generation of NO requires an elevation of [Ca2+]i. Although NOS is tightly controlled by multiple underlying mechanisms, including the interaction of endothelium NOS (eNOS) with effector proteins such as heat shock protein 90 and caveolin-1, the elevation of [Ca2+]i remains the primary initiator of NO production. However, the results in ECs remain controversial. Certain reports have demonstrated a synergism between NO synthesis and sustained [Ca2+]i levels (20), whereas others emphasize a primary role of Ca2+ influx (21) or Ca2+ release from thapsigargin-sensitive store sites (22). In the present study, [Ca2+]i increase and NO production were stimulated by spermine in the presence or absence of extracellular Ca2+ in HUVECs. Therefore, it appears hat eNOS does not discriminate between the sources of mobilized Ca2+ to stimulate NO production. However, the present study revealed that NO production is preferentially linked to Ca2+ entry by SOCC activation at comparable stimulation, including 2 mM spermine, indicated by a slight increase in DAF-FM fluorescence in Ca2+-free solution, and a marked increase in [Ca2+]o in HUVECs. The dependence of CaSR-induced NO production on SOCE was further demonstrated by experiments in which DAF-FM fluorescence was markedly decreased by Calhex231 and MRS1845 in the presence of extracellular Ca2+. The present study revealed that SOCE is critical for the activation of CaSR-induced NO production in HUVECs and is an essential component of CaSR-mediated intracellular Ca2+ mobilization.
Although the molecular mechanism underlying SOCCs remains to be elucidated, there is evidence that various TRPC channels may function as SOCCs (23). The TRPC family subgroup responsible for SOCCs operated by CaSR-activated Ca2+ influx remains unclear. The present study provides initial evidence that CaSR mediated ER-calcium depletion and SOCE activation occurs through TRPC1 channels. In addition, the present study is, to the best of our knowledge, the first to demonstrate an association between TRPC1-mediated Ca2+ influx and activation of CaSR-evoked SOCC resulting in NO production in HUVECs.
A few limitations should be considered when drawing conclusions from the present study. It is unclear whether co-localization of TRPC1 and CaSR occurs; further studies are required to demonstrate a direct connection between these two molecules. In addition, SOCE is activated in response to the depletion of ER-calcium stores, and the TRPC1 channel is a well-studied candidate for SOCE. Future studies should examine whether TRPC1 interacts with other TRPC channel subunits, including TRPC3 and TRPC4, and/or Orail1 and stromal interaction molecule 1/2 as important components of SOCC.
In conclusion, the results of the present study elucidated the association between TRPC1 and CaSR in ECs, an association that has clinical significance. It was demonstrated that CaSR activation increases Ca2+ influx and NO production in HUVECs. In addition, the role of TRPC1 as a potential candidate in CaSR-induced SOCE and NO production in HUVECs was demonstrated, thus furthering understanding of Ca2+-mediated channel signaling. TRPC1 may represent a potential therapeutic target for the treatment of vascular diseases.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 31160239, 30971162 and 81170048).
References
- 1.Lopez-Jaramillo P, Gonzalez MC, Palmer RM, Moncada S. The crucial role of physiological Ca2+ concentrations in the production of endothelial nitric oxide and the control of vascular tone. Br J Pharmacol. 1990;101:489–493. doi: 10.1111/j.1476-5381.1990.tb12735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen SF, Owsianik G, Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Weston AH, Geraghty A, Egner I, Edwards G. The vascular extracellular calcium-sensing receptor: An update. Acta Physiol (Oxf) 2011;203:127–137. doi: 10.1111/j.1748-1716.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- 6.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 7.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 8.Ziegelstein RC, Xiong Y, He C, Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem Biophys Res Commun. 2006;342:153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 9.McGregor PE, Agrawal DK, Edwards JD. Technique for assessment of leukocyte adherence to human umbilical vein endothelial cell monolayers. J Pharmacol Toxicol Methods. 1994;32:73–77. doi: 10.1016/1056-8719(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 10.Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl. 1999;38:3209–3212. doi: 10.1002/(SICI)1521-3773(19991102)38:21<3209::AID-ANIE3209>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Canaff L, Petit JL, Kisiel M, Watson PH, Gascon-Barré M, Hendy GN. Extracellular calcium-sensing receptor is expressed in rat hepatocytes, Coupling to intracellular calcium mobilization and stimulation of bile flow. J Biol Chem. 2001;276:4070–4079. doi: 10.1074/jbc.M009317200. [DOI] [PubMed] [Google Scholar]
- 12.Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, Rognan D, Ruat M. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca(2+)-sensing receptor. J Biol Chem. 2003;278:49487–49494. doi: 10.1074/jbc.M308010200. [DOI] [PubMed] [Google Scholar]
- 13.Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney JE., Jr Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol. 2007:593–614. doi: 10.1007/978-3-540-34891-7_35. [DOI] [PubMed] [Google Scholar]
- 14.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 15.Smajilovic S, Tfelt-Hansen J. Novel role of the calcium-sensing receptor in blood pressure modulation. Hypertension. 2008;52:994–1000. doi: 10.1161/HYPERTENSIONAHA.108.117689. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Luo XL, Zhong H, Hu QH, He F. Extracellular Ca(2+) influx and NO generation are inhibited by small interference RNA targeting extracellular Ca(2+)-sensing receptor in human umbilical vein endothelial cells. Sheng Li Xue Bao. 2012;64:289–295. (In Chinese) [PubMed] [Google Scholar]
- 17.Sun YH, Li YQ, Feng SL, Li BX, Pan ZW, Xu CQ, Li TT, Yang BF. Calcium-sensing receptor activation contributed to apoptosis stimulates TRPC6 channel in rat neonatal ventricular myocytes. Biochem Biophys Res Commun. 2010;394:955–961. doi: 10.1016/j.bbrc.2010.03.096. [DOI] [PubMed] [Google Scholar]
- 18.Jung SY, Kwak JO, Kim HW, Kim DS, Ryu SD, Ko CB, Cha SH. Calcium sensing receptor forms complex with and is up-regulated by caveolin-1 in cultured human osteosarcoma (Saos-2) cells. Exp Mol Med. 2005;37:91–100. doi: 10.1038/emm.2005.13. [DOI] [PubMed] [Google Scholar]
- 19.Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, Petrel C, Ruat M, Edwards G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with Calindol and Calhex 231. Circ Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Shin WS, Kawaguchi H, Inukai M, Kato M, Sakamoto A, Uehara Y, Miyamoto M, Shimamoto N, Korenaga R, et al. Contribution of sustained Ca2+ elevation for nitric oxide production in endothelial cells and subsequent modulation of Ca2+ transient in vascular smooth muscle cells in coculture. J Biol Chem. 1996;271:5647–5655. doi: 10.1074/jbc.271.10.5647. [DOI] [PubMed] [Google Scholar]
- 21.Louzalen L, Lantoine F, Pernollet MG, Millanvoye-Van Brussel E, Devynck MA, David-Dufilho M. SK&F 96365 inhibits intracellular Ca2+ pumps and raises cytosolic Ca2+ concentration without production of nitric oxide and von Willebrand factor. Cell Calcium. 1996;20:501–508. doi: 10.1016/S0143-4160(96)90092-5. [DOI] [PubMed] [Google Scholar]
- 22.Hutcheson IR, Griffith TM. Central role of intracellular calcium stores in acute flow- and agonist-evoked endothelial nitric oxide release. Brit J Pharmacol. 1997;122:117–125. doi: 10.1038/sj.bjp.0701340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/S0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]