Abstract
The proliferation and apoptosis of tumor cells are regulated by a variety of microRNAs (miRs). miR-21 can inhibit the apoptosis of cancer cells in vitro. Tumor necrosis factor α (TNF-α) serves an important role in the induction of proliferation of cervical cancer cells. Previous studies have demonstrated that the expression level of miR-21 is associated with TNF-α expression in alveolar macrophages. However, to the best of our knowledge, whether miR-21 regulates TNF-α in cervical cells has not been reported. The present study was designed to investigate whether miR-21 regulates TNF-α expression, proliferation and apoptosis of cervical cancer cells. miR-21, miR-21 inhibitor and control miRNA were synthesized and transfected into HeLa cervical cancer cells. Reverse transcription-quantitative polymerase chain reaction was used to measure the expression levels of miR-21 and TNF-α at the mRNA level. Western blotting was used to measure the expression levels of TNF-α at the protein level. MTT assay and Hoechest-33342 staining were used to measure the proliferation and apoptosis of HeLa cells. miR-21 was identified to upregulate the mRNA and protein expression levels of TNF-α. Furthermore, upregulation of TNF-α enhanced the proliferation capability of HeLa cells. Changes in the expression levels of miR-21 and TNF-α did not significantly affect the apoptosis of Hela cells. In conclusion, the present study demonstrated that miR-21 regulates the expression of TNF-α in HeLa cells. Additionally, the expression level of TNF-α was positively associated with the proliferation capability of Hela cells, but not apoptosis. Therefore, miR-21 regulates the proliferation of HeLa cells through regulation of TNF-α. These results provide novel potential therapeutic targets for the treatment of cervical cancer.
Keywords: apoptosis, cervical cancer, microRNA-21, tumor necrosis factor-α, proliferation
Introduction
Cervical cancer has a high incidence in females and poses a serious health risk. The number of new patients of cervical cancer worldwide of each year is nearly 50 million (1). At present, there is no effective treatment for patients with advanced cervical cancer. Therefore, discovery of targets has great significance in the targeted treatment and prognosis cervical cancer (2,3).
Tumor necrosis factor-α (TNF-α) serves an important role in a wide range of biological processes and regulates cell proliferation, differentiation and apoptosis (4). Previous studies have demonstrated that TNF-α presents in a variety of tumor tissues, and not only can inhibit tumor cell growth then induce tumor necrosis, but also improve the proliferation, migration and invasion capacity of tumor cells (2,5). It has been demonstrated that TNF-α has certain associations with cervical cancer in older women (6).
MicroRNAs (miRNAs/miRs) are involved in the regulation of cellular functions of many genes, and are widely presented in eukaryotes. miRNAs function mainly through complementary binding to the target gene which can induce the degradation of its mRNA, or suppress gene transcription to reduce the expression levels of its target genes (7,8). One protein may be regulated by a variety of miRNAs. It has been demonstrated that miRNAs serve an important regulatory role in many human diseases. Some miRNAs have been used as markers for the clinical detection of early tumors, and some have been used as therapy targets (9). Studies have revealed that altered expression of miRNAs is correlated with the proliferation, apoptosis and migration of cervical cancer cells, and certain miRNAs including miR-27b, miR-24 and miR-21, have been identified to be overexpressed in cervical cancer (10,11). To the best of our knowledge, the association between miR-21 and TNF-α in cervical cancer has not been reported.
Therefore, in the present study, TNF-α was chosen as a downstream target of miR-21. The effect of miR-21 on the expression of TNF-α and the association between TNF-α and proliferation and apoptosis of cervical cancer cells was investigated.
Materials and methods
Materials
HeLa human cervical cancer cells were purchased from Shanghai Beinuo Biological Technology Co., Ltd. (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum and trypsin digestion enzymes were purchased from Hyclone; GE Healthcare (Logan, UT, USA). PBS and antibiotics were purchased from Beyotime Institute of Biotechnology (Haimen, China). A liposome cell transfection kit was purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). An RNA extraction kit (TRIzol) and a cDNA synthesis kit were purchased from Beyotime Institute of Biotechnology. Anti-human TNF-α antibody was purchased from Shanghai Xin Le Biotechnology Co., Ltd. (Shanghai, China). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) amplification reagents were purchased from Omega Bio-Tek, Inc. (Norcross, GA, USA). Hoechest-33,342 stain was purchased from Beijing Suolaibao Biotechnology Co., Ltd. (Beijing, China). Primers were synthesized by Shanghai Shenggong Co., Ltd. (Shanghai, China).
Cell cultures and transfection
HeLa cells were conventionally cultured in DMEM containing 10% fetal bovine serum. The third generation of cells were collected, counted and seeded at a density of 10×104 cells/well and cultured for 18 h before transfection. miR-21 mimic (forward, UAGCUUAUCAGACUGAUGUUGA and reverse, AACAUCAGUCUGAUAAGCUAUU), miR-21 inhibitor (UCAACAUCAGUCUGAUAAGCUA) and negative control (forward, UUCUCCGAACGUGUCACGUTT and reverse, ACGUGACACGUUCGGAGAATT for miR-21 mimic; CAGUACUUUUGUGUAGUACAA for miR-21 inhibitor) (Dharmacon; GE Healthcare Life Sciences, Little Chalfont, UK) were diluted with 50 µl Opti-minimum essential medium (MEM; Thermo Fisher Scientific, Inc.) to a final concentration of 100 nM. Liposome (2 µl) was added to 50 µl Opti-MEM. A total of 5 min later, the two diluted liquids were mixed and incubated at room temperature for 30 min, and then added to the culture media. The culture media was replaced 4 h later. Each experiment was repeated three times.
RT-qPCR
Total RNAs of cells before transfection, and 36 or 48 h after transfection were extracted and then reverse transcribed into cDNA according to the manufacturer's protocol. cDNA was used as template to perform PCR with the following profile: An initial 5-min incubation at 95°C, 30 cycles of (45 sec at 95°C, 45 sec at 50°C and 40 sec at 72°C), and a final extension of 3 min at 72°C. Reaction mixtures contained the following ingredients at the given final concentrations: 2 ng/µl DNA template; 10 mM Tris-HCl (pH: 8.3); 1.5 mM MgCl2; 200 µM each of dNTP; 0.2 units of Taq DNA polymerase; and 0.5 µM of each primer. The primer sequences for TNF-α, miR-21 and GAPDH (internal control) are presented in Table I. mRNA expression was quantified as normalization to the internal control GAPDH using the 2-∆∆Cq method (12).
Table I.
Primer sequences.
| Gene | Sequence (5′-3′) |
|---|---|
| TNF-α-F | CCCGCATCCCAGGACCTCTCT |
| TNF-α-R | CGGGGGACTGGCGA |
| miR-21-F | CTAAGACCTGTGGAATGGC |
| miR-21-R | CTCAAAGATGTCATTGCC |
| GAPDH-F | CGGAGTCAACGGATTTGGTCGTA |
| GAPDH-R | AGCCTTCTCCATGGTGGTGAAG |
F, forward; R, reverse; TNF-α, tumor necrosis factor-α; miR, microRNA.
Western blot analysis
Cells were collected at 36 or 48 h after transfection, washed with PBS, and lysed using radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Inc.) followed by centrifugation at 5,000 × g for 20 min at 37°C for protein extraction. Proteins were quantified using a Bicinchoninic Acid kit, according to the manufacturer's protocol. Equal amounts of protein (20 µg/lane) were separated by 10% SDS-PAGE, transferred to a polyvinylidene fluoride membrane (Thermo Fisher Scientific, Inc.), and blocked with 5% nonfat milk at 37°C for 1 h. After washing with TBS with Tween-20 (TBST), membranes were incubated with primary mouse anti-human TNF-α antibody (1:1,000; RayBiotech, Inc., Norcross, GA, USA; cat. no. 119–1633) overnight at 4°C. After washing with TBST, membranes were incubated with horseradish peroxidase-conjugated anti-mouse secondary antibodies (1:2,500; Abcam, Cambridge, UK; cat. no. ab97046.) for 3 h at 37°C, and developed with enhanced chemiluminescence (Thermo Fisher Scientific, Inc.). Expression levels of TNF-α were analyzed with β-actin (1:2,500; Abcam; cat. no. ab8227) serving as an internal control.
MTT assay
The cell suspension was counted after transfected cells were seeded into 96-well plates at a density of 1×104 cells/well. MTT solution (20 µl) was added to each well at 36 or 48 h after transfection, and incubated for 4 h. Dimethyl sulfoxide (150 µl) was added to each well to stop reaction. The optical density at 560 nm was recorded using a microplate reader to calculate the proliferation rate of HeLa cells.
Hoechest-33,342 staining
Transfected cells were transferred into 6-well plates and cultured for 36 or 48 h. After removal of the cell culture media and washing with PBS for 3–5 times, 200 µl 4% paraformaldehyde was added to each well and kept at 4°C for 20 min to fix the cells. After washing with PBS for 3–5 times, 1 ml Hoechest-33,342 solution was added to each well and kept at 37°C for 20 min, rinsed again with PBS, and observed under an inverted fluorescence microscope.
Statistical analysis
SPSS version 11.3 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. All data are expressed as the mean ± standard error. Differences between groups were analyzed by two-way analysis of variance with the Bonferroni post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
RT-qPCR assay of miR-21 mRNA expression
The RT-qPCR results demonstrated that the expression levels of miR-21 were significantly upregulated at 36 and 48 h after transfection of miR-21, compared with the control group (P<0.05; Fig. 1). Transfection of an miR-21 inhibitor resulted in the downregulation of miR-21 at both time points after transfection of miR-21, compared with the control group (P<0.01; Fig. 1), suggesting that manipulation of miR-21 levels through transfection of miR-21 and miR-21 inhibitor was successful.
Figure 1.
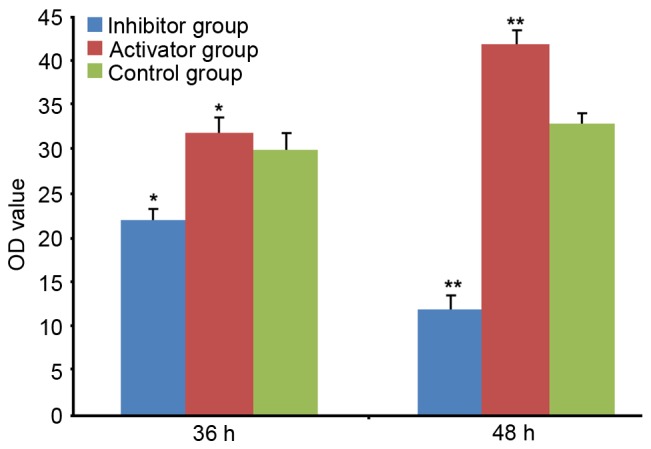
miR-21 levels at 36 and 48 h after transfection. HeLa cells were transfected with miR-21 or miR-21 inhibitor. Data are expressed as the mean ± standard error. *P<0.05 and **P<0.01 vs. other two groups at the same time-point. OD, optical density; miR-21, microRNA-21.
RT-qPCR assay of TNF-α mRNA expression
The RT-qPCR results revealed that the mRNA expression levels of TNF-α were significantly upregulated at 36 and 48 h after transfection of miR-21, compared with the control group (P<0.05; Fig. 2). Transfection of the miR-21 inhibitor resulted in the downregulation of TNF-α at 36 and 48 h after transfection of miR-21, compared with the control group (P<0.05 and P<0.01, respectively; Fig. 2), suggesting that the expression of TNF-α is positively associated with miR-21.
Figure 2.
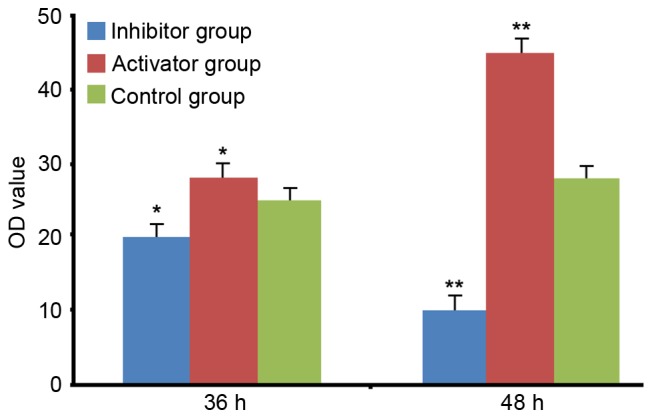
Tumor necrosis factor-α levels at 36 and 48 h after transfection. HeLa cells were transfected with miR-21 or miR-21 inhibitor. Data are expressed as the mean ± standard error. *P<0.05 and **P<0.01 vs. other two groups at the same time-point. OD, optical density; miR-21, microRNA-21.
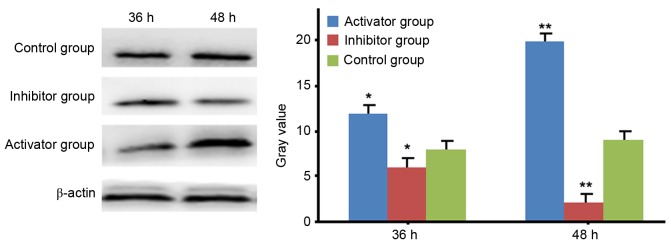
Western blot analysis of TNF-α
Western blot analysis demonstrated that the expression levels of TNF-α were significantly increased in a time-dependent manner after transfection of miR-21, compared with the control (P<0.05; Fig. 3). In contrast, the expression levels of TNF-α were significantly decreased in a time-dependent manner after transfection of miR-21 inhibitor, compared with the control (P<0.05; Fig. 3).
Figure 3.
Western blot analysis of levels of tumor necrosis factor-α after HeLa cells were transfected with miR-21 or miR-21 inhibitor. β-actin served as an internal control. Data are expressed as the mean ± standard error. *P<0.05 and **P<0.01 vs. control. miR-21, microRNA-21.
MTT assay of HeLa cell proliferation
The MTT results demonstrated that at 36 and 48 h after transfection, upregulation of TNF-α enhanced HeLa cell proliferation in a time-dependent manner. Conversely, inhibition caused a decrease in cell proliferation in a time-dependent manner (Fig. 4).
Figure 4.
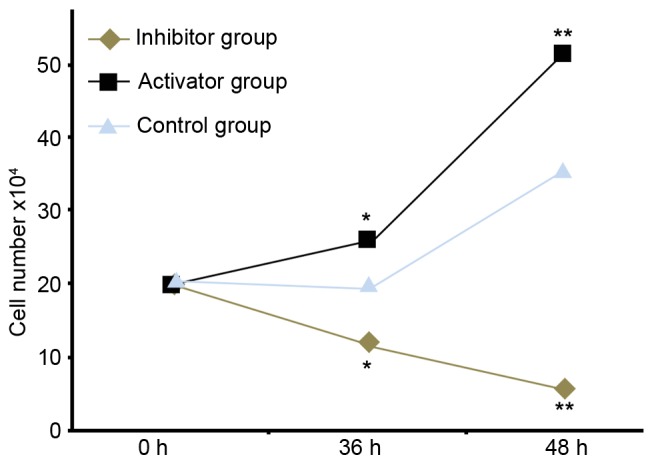
Effect of TNF-α on HeLa cell proliferation. HeLa cells were transfected with miR-21 or miR-21 inhibitor. Data are expressed as the mean ± standard error. *P<0.05 and **P<0.01 vs. control. miR-21, microRNA-21.
Hoechest-33,342 staining
Hoechest-33,342 staining results demonstrated that no significant difference was found in the apoptosis of HeLa cells among different groups.
Discussion
Cervical cancer is a complex pathological process in which proliferation and apoptosis of cervical cancer cells serves an important role. The proliferation and apoptosis of cervical cancer cells are regulated by multiple signaling pathways, including p53, phosphatase and tension homolog (PTEN) and TNF-α pathways. TNF-α not only can inhibit proliferation to induce apoptosis of cervical cancer cells by regulating the downstream nuclear factor-κB, receptor activating protein kinase 3, epidermal growth factor receptor and other proteins, but also can induce the proliferation, migration and invasion of cancer cells. Therefore, assessing the association between TNF-α and proliferation and apoptosis of cervical cancer cells is of great significance (13–16). Although studies have confirmed that miRNAs serve an important role in the proliferation and apoptosis of cancer cells, the study of miRNA regulation of TNF-α is still relatively rare. Previous studies have demonstrated that miR-29a regulates the incurrence of atherosclerosis via regulation of TNF-α, indicating the existence of a regulatory relationship between TNF-α and miRNA (17,18). It has been demonstrated that miR-21 serves an important role in the regulation of cancer cell proliferation and apoptosis. Inhibition of miR-21 promotes the migration of cervical cancer cells through RAS P21 protein activator 1. miR-21 could be used as a potential marker for detection of metastasis of cervical cancer (19). Overexpression of miR-21 in cervical cancer cells affects cell migration and proliferation via inhibition of PTEN expression (20).
The present study demonstrated that the expression levels of TNF-α were associated with expression levels of miR-21. Up- or down-regulation of TNF-α promoted or inhibited the proliferation of cervical cancer cells, respectively, but had no effect on the apoptosis of cervical cancer cells. These results indicated that miR-21/TNF-α signaling serves a regulatory role in the proliferation of cervical cancer cells.
In conclusion, there was an association between TNF-α and miR-21 in HeLa cervical cancer cells. The expression of TNF-α was positively associated with the proliferation of cervical cancer cells, but had no effect on apoptosis. Therefore, miR-21 regulates the proliferation of cervical cancer HeLa cells via downregulation of TNF-α. These results provide novel potential therapeutic targets for the treatment of cervical cancer.
Figure 5.
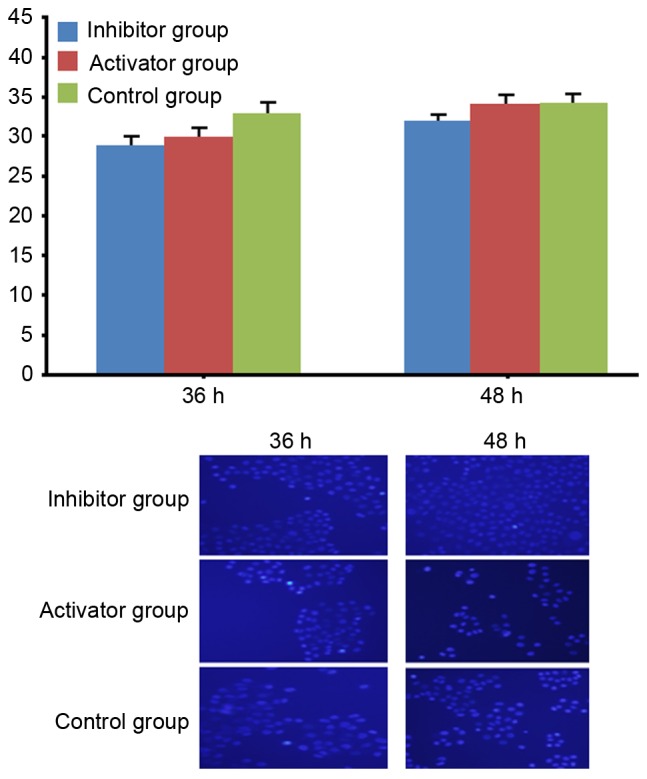
Apoptosis of HeLa cells following transfection with miR-21 or miR-21 inhibitor (magnification, ×200). Data are expressed as the mean ± standard error. P>0.05. miR-21, microRNA-21.
References
- 1.Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: A gap in cancer knowledge. Ann Oncol. 2016;27:1664–1674. doi: 10.1093/annonc/mdw183. [DOI] [PubMed] [Google Scholar]
- 2.Buda A, Elisei F, Palazzi S, De Ponti E, Arosio M, Vecchione F, Dell'Anna T, Cuzzocrea M, Bussi B, Giuliani D, et al. Quality of care for cervical and endometrial cancer patients: The impact of different techniques of sentinel lymph node mapping on patient satisfaction. Ann Surg Oncol. 2016;23:2975–2981. doi: 10.1245/s10434-016-5233-0. [DOI] [PubMed] [Google Scholar]
- 3.Meier T, Kharofa J. Magnetic resonance imaging-guided high-dose rate brachytherapy for cervical cancer. Semin Roentgenol. 2016;51:106–111. doi: 10.1053/j.ro.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: A gap in cancer knowledge. Ann Oncol. 2016;27:1664–1674. doi: 10.1093/annonc/mdw183. [DOI] [PubMed] [Google Scholar]
- 5.Seyyednejad F, Rezaee A, Haghi S, Goldust M. Survey of pre-inflammation cytokines levels in radiotherapy-induced-mucositis. Pak J Biol Sci. 2012;15:1098–1101. doi: 10.3923/pjbs.2012.1098.1101. [DOI] [PubMed] [Google Scholar]
- 6.Mbulaiteye SM, Kemp T, Gage JC, Ajenifuja KO, Kiruthu C, Wentzensen NA, Adepiti C, Wacholder S, Burk RD, Schiffman M, Pinto L. Plasma cytokine levels and human papillomavirus infection at the cervix in rural Nigerian women. Cytokine. 2013;64:146–151. doi: 10.1016/j.cyto.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Q, Wang W, Li P. Regulator role of HPV E7 protein on miR-21 expression in cervical carcinoma cells and its functional implication. Int J Clin Exp Pathol. 2015;8:15808–15813. [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Xu GX, Lu H, Yu DH, Ren Y, Wang L, Huang XH, Hou WJ, Wei ZH, Chen YP, et al. Dysregulation of miRNA-21 and their potential as biomarkers for the diagnosis of cervical cancer. Int J Clin Exp Pathol. 2015;8:7131–7139. [PMC free article] [PubMed] [Google Scholar]
- 9.Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB, Luo XL, Zhu XL, Liu C, Xu FP, Luo DL, et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol. 2016;48:471–484. doi: 10.3892/ijo.2015.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge Y, Zhang L, Nikolova M, Reva B, Fuchs E. Strand-specific in vivo screen of cancer-associated miRNAs unveils a role for miR-21(*) in SCC progression. Nat Cell Biol. 2016;18:111–121. doi: 10.1038/ncb3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bumrungthai S, Ekalaksananan T, Evans MF, Chopjitt P, Tangsiriwatthana T, Patarapadungkit N, Kleebkaow P, Luanratanakorn S, Kongyingyoes B, Worawichawong S, Pientong C. Up-regulation of miR-21 is associated with cervicitis and human papillomavirus infection in cervical tissues. PLoS One. 2015;10:e0127109. doi: 10.1371/journal.pone.0127109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Shishodia G, Shukla S, Srivastava Y, Masaldan S, Mehta S, Bhambhani S, Sharma S, Mehrotra R, Das BC, Bharti AC. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Mol Cancer. 2015;14:116. doi: 10.1186/s12943-015-0385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chopjitt P, Pientong C, Bumrungthai S, Kongyingyoes B, Ekalaksananan T. Activities of E6 protein of human papillomavirus 16 asian variant on miR-21 up-regulation and expression of human immune response genes. Asian Pac J Cancer Prev. 2015;16:3961–3968. doi: 10.7314/APJCP.2015.16.9.3961. [DOI] [PubMed] [Google Scholar]
- 15.Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, Sültmann H, Scheffner M, Hoppe-Seyler K, Hoppe-Seyler F. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015;11:e1004712. doi: 10.1371/journal.ppat.1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bantis A, Tsakaldimis G, Zissimopoulos A, Kalaitzis C, Gianakopoulos S, Pitiakoudis M, Polichronidis A, Touloupidis S. Can tumor necrosis factor a (TNF-a) and interleukin 6 (IL-6) be used as prognostic markers of infection following ureteroscopic lithrotripsy and extracorporeal shock wave lithotripsy for ureteral stones? Hell J Nucl Med. 2015;18(Suppl 1):S160. [PubMed] [Google Scholar]
- 17.Wang D, Fan Z, Liu F, Zuo J. Hsa-miR-21 and Hsa-miR-29 in tissue as potential diagnostic and prognostic biomarkers for gastric cancer. Cell Physiol Biochem. 2015;37:1454–1462. doi: 10.1159/000438514. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Xu W, Feng X, He Y, Liu X, Gao Y, Yang S, Shao Z, Yang C, Ye Z. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int J Immunopathol Pharmacol. 2015;28:351–361. doi: 10.1177/0394632015593228. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhan X, Yan D, Wang Z. Circulating microRNA-21 is involved in lymph node metastasis in cervical cancer by targeting RASA1. Int J Gynecol Cancer. 2016;26:810–816. doi: 10.1097/IGC.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Zhang W, Lv Q, Zhu D. Overexpression of miR-21 promotes the proliferation and migration of cervical cancer cells via the inhibition of PTEN. Oncol Rep. 2015;33:3108–3116. doi: 10.3892/or.2015.3931. [DOI] [PubMed] [Google Scholar]