Abstract
Reperfusion is the only approved therapy for acute ischemic stroke; however, it can cause excessive inflammation responses and aggravate brain damage. Therefore, supplementary treatment against inflammation caused by reperfusion is required. In a previous study from our group, curcumin was demonstrated to decrease infarction volume, brain edema and blood-brain barrier (BBB) disruption against cerebral ischemia/reperfusion (I/R) injury. However, the underlying mechanisms remain unclear. The present study was conducted to understand whether curcumin protects against cerebral I/R injury through anti-inflammatory and antiapoptotic properties. Ischemia for 1 h was induced in vivo in Wistar rats by middle cerebral artery occlusion (MCAO), followed by reperfusion for 24 h, and curcumin was injected intraperitoneally at 30 min prior to reperfusion. Immunohistochemistry was performed to analyze the expression levels of nuclear factor (NF)-κB, intercellular adhesion molecule (ICAM)-1, matrix metalloproteinase (MMP)-9 and caspase-3. The findings revealed that inflammation (NF-κB, ICAM-1 and MMP-9) and apoptosis (caspase-3)-related markers were significantly downregulated in the curcumin-treated MCAO group compared with the vehicle-treated MCAO group. Furthermore, brain infarction size, brain edema and neurological dysfunction were attenuated in the curcumin-treated MCAO group compared with the vehicle-treated MCAO group. Taken together, the present results provided evidence that the protective effect of curcumin against cerebral I/R injury might be mediated by anti-inflammatory and anti-apoptotic properties. Therefore, curcumin may be a promising supplementary agent against cerebral I/R injury in the future.
Keywords: cerebral ischemia/reperfusion, curcumin, intercellular adhesion molecule-1, matrix metalloproteinase-9, caspase-3, nuclear factor-κB
Introduction
Stroke is the second most common cause of mortality and leading cause of disability among adults worldwide (1). Among stroke cases, ischemic stroke accounts for the vast majority (85%) (2), and poses a significant threat to life. Ischemic stroke occurs when the blood supply to a certain brain area is suddenly interrupted by thrombosis or embolism, which leads to brain cell death and corresponding neurological dysfunction (3,4). Ischemic stroke injury can be mainly attributed to the abrupt oxygen deprivation and overexpression of intermediate factors, including excitatory glutamate release, free radicals and inflammatory products, which act synergistically to induce brain cell death (5). Understanding the detailed molecular mechanisms of stroke will be important for the development of future therapies.
Currently, restoring blood circulation to the ischemic brain area is the only approved therapy for acute ischemic stroke by the Food and Drug Administration (6). However, blood flow restoration leads to the accumulation of activated leukocytes into the initial ischemic brain area (4), which contributes to overproduction of inflammation and apoptosis-related agents to aggravate the initial brain injury. Therefore, targeting inflammation and apoptosis caused by blood restoration may be beneficial supplementary strategies against cerebral ischemia/reperfusion (I/R) injury.
It has been reported that curcumin, a pleiotropic agent extracted from the rhizome of Curcuma Longa (7), can exert pharmacological effects against stroke through its anti-inflammatory and anti-oxidative properties (8–10). In a previous study from our group, curcumin was demonstrated to significantly decrease the water content, infarction volume, and blood-brain barrier (BBB) leakage in a middle cerebral artery occlusion (MCAO) rat model (11). However, the effect of curcumin treatment on the expression of inflammation and apoptosis-related proteins during cerebral I/R injury remains unclear.
Although intercellular adhesion molecule (ICAM) −1 (12), matrix metalloproteinase (MMP)-9 (13), caspase-3 (14) and nuclear factor (NF)-κB (15) have been reported to be involved in the pathophysiology of cerebral I/R injury, regulation of their expression by curcumin has not been reported in in vivo studies. In the present study, cerebral I/R injury was induced by transient MCAO operation in Wistar rats. Curcumin was administered intraperitoneally 30 min prior to reperfusion, and it was demonstrated to significantly prevent cerebral I/R injury and to downregulate expression of inflammation and apoptosis-related proteins as analyzed by immunohistochemistry (IHC). The present findings suggested that curcumin inhibited cerebral I/R injury most likely through its anti-inflammatory and anti-apoptotic effects.
Materials and methods
Ethics statement
The study protocol was approved by the Ethics Committee, Faculty of Medicine, Chulalongkorn University (Bangkok, Thailand). The study was conducted according to the guidelines for experimental animals suggested by the National Research Council of Thailand.
Experimental animals
A total of 52 male Wistar rats were purchased from the National Laboratory Animal Center, Salaya Campus, Mahidol University (Nakornpathom, Thailand), with a body weight of 250–300 g and at 10 weeks old. All rats were housed in an animal center with a 12/12 h light-dark cycle for at least 1 week prior to experimentation. The animals were given access to normal chow and tap water ad libitum.
Experimental groups
All rats were randomly allocated into the following four groups: Sham + CORN, sham-operated group treated with vehicle; Sham + CUR, sham-operated group treated with curcumin; MCAO + CORN, stroke-induced group treated with vehicle; and MCAO + CUR, stroke-induced group treated with curcumin (Table I). To minimize the number of sacrificed rats, when performing the immunohistochemistry assay, the non-injured hemisphere of MCAO group (CUR and CORN) was used as sham control instead of separate sham rats.
Table I.
Physiological parameters, neurological deficit scores, infarction size and brain water content in each group.
| Parameter | Sham + CORN | Sham + CUR | MCAO + CORN | MCAO + CUR |
|---|---|---|---|---|
| MABP (mm Hg) | 105.6±3.957 (n=6) | 108.8±7.897 (n=6) | 91.32±4.105 (n=6) | 89.98±4.063 (n=6) |
| PaO2 (mm Hg) | 102.4±3.399 (n=6) | 100.7±3.225 (n=6) | 94.02±3.213 (n=6) | 95.93±5.678 (n=6) |
| PaCO2 (mm Hg) | 41.5±1.506 (n=6) | 41.8±2.223 (n=6) | 45.75±2.768 (n=6) | 42.87±2.276 (n=6) |
| pH | 7.38±0.03337 (n=6) | 7.377±0.0447 (n=6) | 7.413±0.05673 (n=6) | 7.367±0.08077 (n=6) |
| Neurological deficit scores | 0±0 (n=6) | 0±0 (n=6) | 3.143±0.2951a (n=21) | 2.105±0.3049b (n=19) |
| Infarction size (%) | 0±0 (n=3) | 0±0 (n=3) | 34.78±2.030a (n=5) | 20.46±2.686c (n=5) |
| Brain water content (%) | 54.12±2.699 (n=3) | 51.91±1.545 (n=3) | 68.20±1.118a (n=5) | 58.83±1.397c (n=5) |
Data are presented as means ± standard error of the mean. aP≤0.0001 vs. Sham group; bP<0.05 and cP≤0.01 vs. MCAO + CORN group. MABP, mean artery blood pressure; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; CORN, corn oil (vehicle control); CUR, curcumin; MCAO, middle cerebral artery occlusion.
Generation of the transient I/R rat model
The transient I/R model induction was performed as described in Ansari et al (16). Under isoflurane inhalation anesthesia (5% for induction, 2.5% for maintenance), the tip of a silicon-coated commercial 4–0 monofilament (cat. no. 403712PK10Re; Doccol Corporation, Sharon, MA, USA) was advanced 18–20 mm long past the carotid bifurcation into the internal carotid artery lumen through the external carotid artery stump, and to occlude the origin of the middle cerebral artery (MCA) until mild resistance was felt. The filament was kept in place for 1 h. The filament was then withdrawn to perform the reperfusion operation. Afterwards, the rat was allowed to regain consciousness. To induce the sham group, the rat was subjected to the operation identically, apart from filament insertion and middle cerebral arteries occlusion. During the procedure, the body temperature of the rat was maintained at 37°C with a heating pad. To ascertain the success of model induction, the regional cerebral blood flow (CBF) was monitored during the surgery, beginning at 20 min pre-occlusion and up to 20 min post-reperfusion, by Laser Doppler flowmetry (PeriFlux System 5000; Perimed AB, Stockholm, Sweden). The target monitor area was fixed in the distal MCA supply area (4 mm lateral and 2 mm posterior to the bregma) (16,17). Rats in the stroke-induced groups were confirmed to meet the following criteria: after occlusion, the level of CBF was <35% of the baseline level (with the baseline level set at the value obtained at 20 min pre-occlusion) and after reperfusion, the level of CBF was >60% of the baseline level.
Treatment protocol
According to previous studies, curcumin attenuates cerebral damage against neurodegenerative disease in rodent models in a dose-dependent manner (14,18). It has been reported that the optimal, well-tolerated dose for intraperitoneal injection in vivo is 300 mg/kg (14). Therefore, the dose of 300 mg/kg was used in the present study. Curcumin (cat. no. 81025; Cayman Chemical Company, Ann Arbor, MI, USA) was dissolved in 1 ml corn oil. Thirty min following MCAO, the curcumin-treated group received one intraperitoneal injection of 300 mg/kg curcumin, and the vehicle-treated group received the same volume of corn oil.
Neurological dysfunction scores
This experiment was performed prior to rat sacrifice as described in Adelson et al (19). The results were evaluated by a researcher who was blind to the experimental setup with the following scoring scale: 0, normal; 1, mild, failed to extend contralateral paw fully; 2, moderate, circling behavior to the contralateral side; 3, severe, falling to the contralateral side; 4, no spontaneous walking with depression of consciousness; and 5, death.
Physiological parameters
The rats were immobilized using a lab-made chamber, and artificially ventilated with 0.6 l/min oxygen supplementation and room air. A total of ~100 µl (2 drops) blood from the left common carotid artery was collected for blood gas measurement with a blood-gas analyzer (i-STAT analyzer, Abbott Pharmaceutical Co., Ltd., Lake Bluff, IL, USA). Then, the mean artery blood pressure was detected using a blood pressure PolyGram system (Interface QI-160G; Nihon Kohden Corporation, Tokyo, Japan).
Infarction size
The rats were perfused with 250 ml PBS (0.01 M) through the left ventricle of the heart. The brain was immediately removed without the cerebellum. By utilizing a brain matrix, coronal slices were obtained with a thickness of 2 mm each. Then, the slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) (cat. no. T8877; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution in a light-proof plastic box at 37°C for 20 min. The TTC-stained brain slices were photographed on a black background using a digital camera for further planimetric lesion size analysis with Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). The total infarction size of the whole brain was calculated by adding up the infarction size of each slice. To adjust for the influence of edema, the corrected infarction size of each slice was calculated by the equation as described in Durukan and Tatlisumak (20):
Infarction size (%) = [(contralateral hemisphere area) - (ipsilateral hemisphere non-injury area)] ×2×100/contralateral hemisphere
Brain edema
This experiment was performed as per a previous report (21). Brain edema was measured as % of brain water content. Following, the brain was separated into two hemispheres. The fresh wet weight and dry weight of each hemisphere were measured before and after 48 h of heating in an oven (70°C), respectively. The following formula was used to quantify the brain water content: water content (%) = (wet weight - dry weight)/wet weight ×100.
Immunohistochemistry analysis
Seven rats per MCAO group were utilized for this analysis. Briefly, the brain section of the MCA region (3–6 mm posterior to bregma) was fixed with 4% paraformaldehyde at room temperature for 24 h, then embedded in paraffin and sequentially sectioned into slices of 2 µm thickness. The sequence of slices was recorded from the frontal copular to occipital direction, and every 4 slices were repeatedly incubated with four different primary antibodies. A total of 2 slices were analyzed for each protein per rat. After deparaffinization and antigen retrieval by microwave heating with pH 9.0 citrate buffer (DaKo retrieval solution; cat. No. S2367; Agilent Technologies, Inc., Santa Clara, CA, USA) at high power for 5 min and at 30% power for 10 min, the slices were successively incubated with 3% H2O2 and antibody diluent (DaKo antibody diluent, cat. no. S3022; Agilent Technologies, Inc., Santa Clara, CA, USA). Followed by incubation with anti-ICAM-1 (mouse monoclonal antibody, cat. no. 554967; 1:50 dilution; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA), MMP-9 (mouse monoclonal antibody, cat. no. ab58803; 1:700 dilution; Abcam, Cambridge, UK), caspase-3 (rabbit polyclonal antibody, cat. no. 9662s; 1:700 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA), and NF-κB-p65 (mouse monoclonal antibody, cat. no. 8242s; 1:800 dilution; Cell Signaling Technology, Inc.) antibodies at 4°C overnight. This was followed by incubations in a horseradish-peroxidase conjugated goat anti-mouse secondary antibody (cat. no. sc-2005; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 30 min at room temperature, or goat anti-rabbit secondary antibody (cat. no. sc-2054; 1:100; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The immune-reactivity was detected using a 3,3′-diaminobenzidine detection kit (DaKo Liquid DAB + Substrate Chromogen System, cat. no. K3468; Agilent Technologies, Inc.). The nuclei were stained with hematoxylin at room temperature for 30 sec. A dark brown color was regarded as a positive staining signal. The sections were photographed at ×400 magnification using a light microscope (eclipse TS100; Nikon Corporation, Tokyo, Japan). The cortex was the target area and four regions of interest were analyzed (two for penumbra, two for core areas). Four images per region were captured. To assess the expression of NF-κB-p65 and caspase-3, the cell numbers with positive signal in the nucleus were manually counted. The result was represented by the number of positive cells in the total observed area. For the expression of ICAM-1 and MMP-9, Image-Pro Plus 6.0 (Media Cybernetics, Inc.) was used to analyze positive staining as described in Ruan et al (22). The optical density was calibrated and the color-specific threshold was set as follows: hue, 0–29; saturation, 0–255; intensity, 0–110 (ICAM-1), 0–100 (MMP-9). The result was represented by the integrated optical density (IOD) of the total observed area. The size of each picture was the same, as was the total observed area, which was confirmed by Image-Pro Plus 6.0 analysis. The result from the slice of the non-injured side of the brain in the MCAO-operated rats was regarded as the sham control.
Statistical analysis
Significance of differences between groups was examined by one-way analysis of variance followed by a post hoc test (Tukey's test). All data were expressed as means ± standard error of the mean. GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of curcumin on neurological dysfunction, infarction size, and brain edema
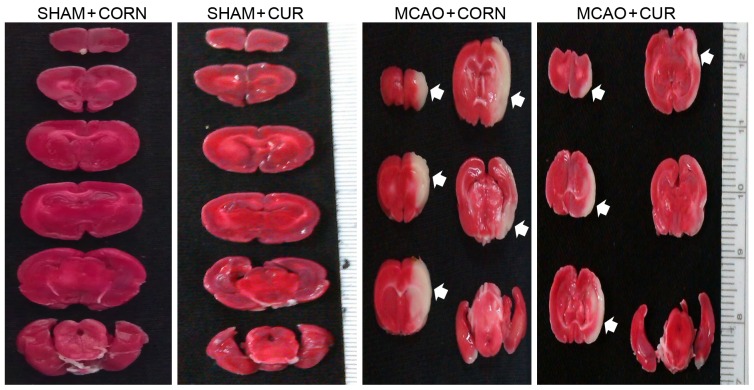
To detect the brain damage caused by cerebral I/R injury, the neurological deficit score (a biomarker of neurological dysfunction), the infarction size and brain edema were measured at 24 h post reperfusion. A total of 5 of the 21 rats succumbed in the MCAO + CORN group; 2 of the 19 rats succumbed in the MCAO + CUR group. Pre-reperfusion treatment with curcumin (MCAO + CUR group) improved the neurological deficit score to 2.105±0.3049 compared with 3.143±0.2951 in the MCAO + CORN group (P<0.05; Table I). At 24 h following reperfusion, brain slices were stained with TTC to detect brain infarction size. No apparent lesion was observed in the Sham groups (Fig. 1). The infarction size in the MCAO + CORN group was 34.78±2.030%. In the curcumin treated MCAO group the infarction size was significantly decreased to 20.46±2.686% (P<0.01; Fig. 1 and Table I). Brain edema was represented as the % of brain water content. In the MCAO + CUR group, the brain water content was significantly reduced compared with the MCAO + CORN group, at 58.83±1.397 and 68.20±1.118%, respectively (P<0.01; Table I).
Figure 1.
Effect of curcumin on infarction size as measured by TTC staining. Following TTC staining of the brain, the lesion areas appear as white colored regions. Samples from the MCAO + CUR group had smaller lesions compared with the MCAO + CORN group. No lesion areas were evident in the Sham group. TTC, 2,3,5-triphenyltetrazolium chloride; MCAO, middle cerebral artery occlusion; CUR, curcumin; CORN, corn oil (vehicle control).
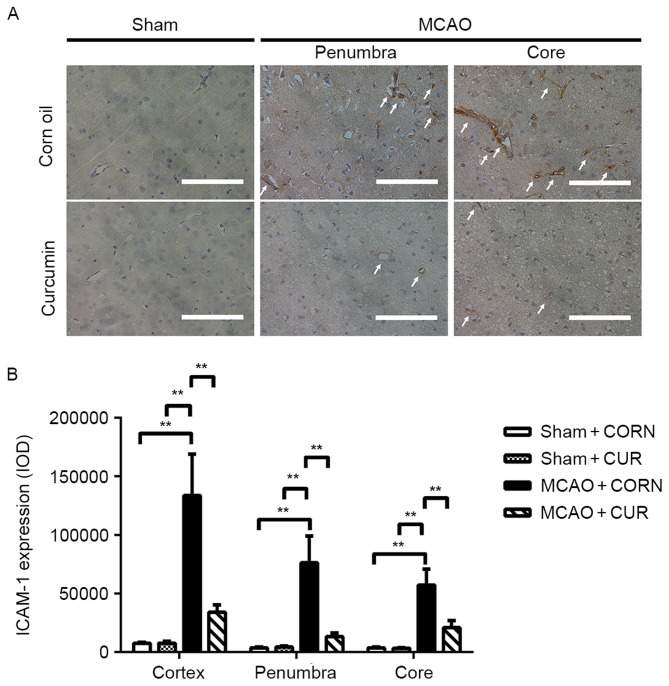
Effect of curcumin on ICAM-1 expression
The expression of ICAM-1 was detected by IHC. The results were calculated as the integrated optical density (IOD) of the total observed area. In the Sham groups, no apparent positive staining was observed (Fig. 2A). However, in the MCAO groups, obvious positive staining was observed in the penumbra, core or whole observed cortex area of the injured hemisphere (Fig. 2A). Staining was limited to the inner layer of the capillary vessels, as indicated by the white arrows in Fig. 2A. This finding was further confirmed by IHC semi-quantitative analysis. High expression of ICAM-1 was observed in the MCAO + CORN group; however, ICAM-1 expression was significantly reduced by curcumin treatment in all observed areas, including the penumbra, core and whole observed cortex areas upon 24 h cerebral I/R injury (P<0.01; Fig. 2B).
Figure 2.
Effect of curcumin on ICAM-1 expression in the whole cortex, penumbra and core area as detected by immunohistochemistry. (A) Positive staining was visualized in brown color and indicated by white arrows. Scale bar, 100 µm. (B) Quantification of ICAM-1 expression. **P≤0.01, with comparisons indicated by brackets. ICAM, intercellular adhesion molecule; MCAO, middle cerebral artery occlusion; CUR, curcumin; CORN, corn oil (vehicle control); IOD, integrated optical density.
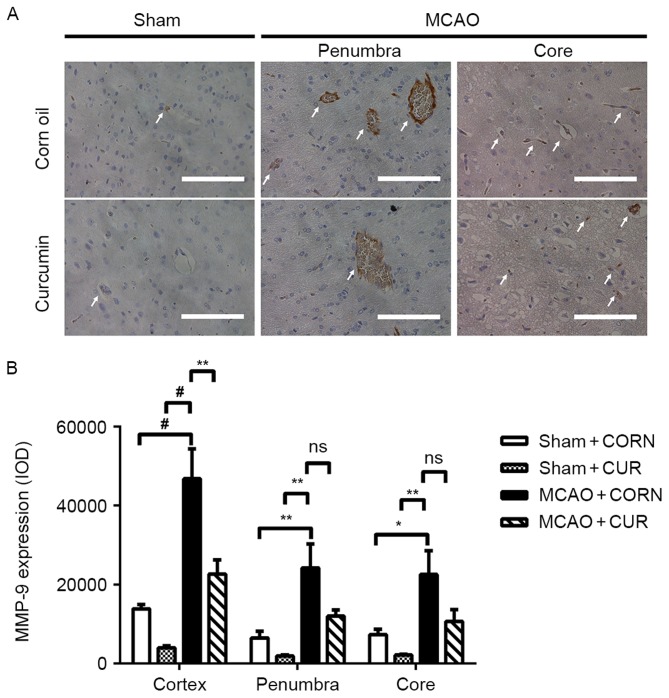
Effect of curcumin on MMP-9 expression
There was no obvious MMP-9 expression observed in the Sham groups (Fig. 3A). MMP-9 expression was observed in the vicinity of the capillary area in the MCAO groups (Fig. 3A; white arrows). The semi-quantification results confirmed that MMP-9 expression was significantly decreased in the curcumin-treated MCAO group (MCAO + CUR) in the whole observed cortex area, compared with MCAO + CORN group (P<0.01; Fig. 3B). However, there was no significant difference in MMP-9 expression between the MCAO + CORN and MCAO + CUR groups in the penumbra or core areas alone (Fig. 3B).
Figure 3.
Effect of curcumin on MMP-9 expression in the whole cortex, penumbra and core area as detected by immunohistochemistry. (A) Positive staining was visualized in brown color and indicated by white arrows. Scale bar, 100 µm. (B) Quantification of MMP-9 expression. *P<0.05, **P≤0.01 and #P≤0.0001, with comparisons indicated by brackets. MMP, matrix metalloproteinase; MCAO, middle cerebral artery occlusion; CUR, curcumin; CORN, corn oil (vehicle control); IOD, integrated optical density; ns, not significant.
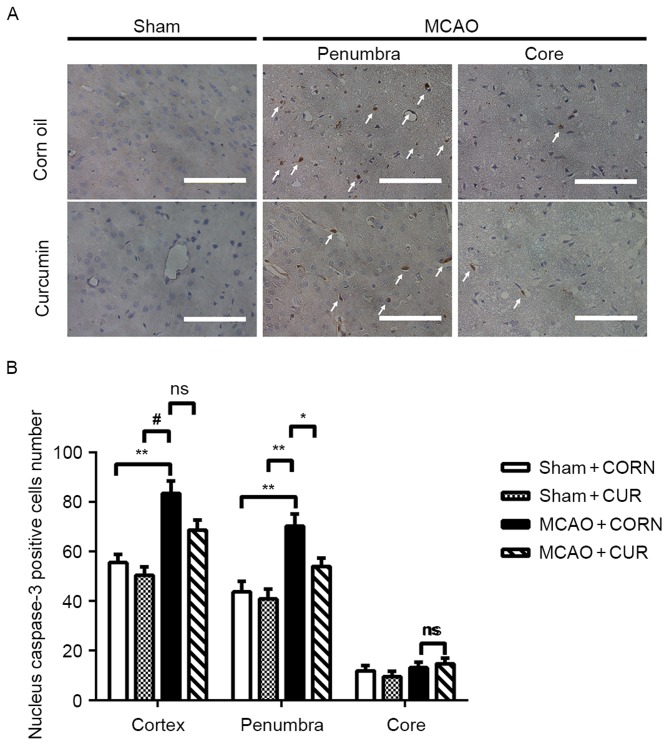
Effect of curcumin on caspase-3 expression
A decreased number of cells with positive nuclear caspase-3 staining (indicated by white arrows) was observed in the MCAO + CUR group, especially in the penumbra area, compared with the MCAO + CORN group (Fig. 4A). Quantification of the positive cells demonstrated that curcumin treatment significantly inhibited the induction of nuclear caspase-3 expression in the penumbra area compared with the MCAO + CORN group (P<0.05; Fig. 4B), whereas no significant difference was detected between the MCAO + CORN and MACO + CUR groups in the core or whole observed cortex areas (Fig. 4B).
Figure 4.
Effect of curcumin on caspase-3 expression in the whole cortex, penumbra and core area as detected by immunohistochemistry. (A) Positive caspase-3 nuclear staining was visualized in brown color and indicated by white arrows. Scale bar, 100 µm. (B) The number of cells with positive nuclear caspase-3 staining was quantified. *P<0.05, **P≤0.01 and #P≤0.0001, with comparisons indicated by brackets. MCAO, middle cerebral artery occlusion; CUR, curcumin; CORN, corn oil (vehicle control); ns, not significant.
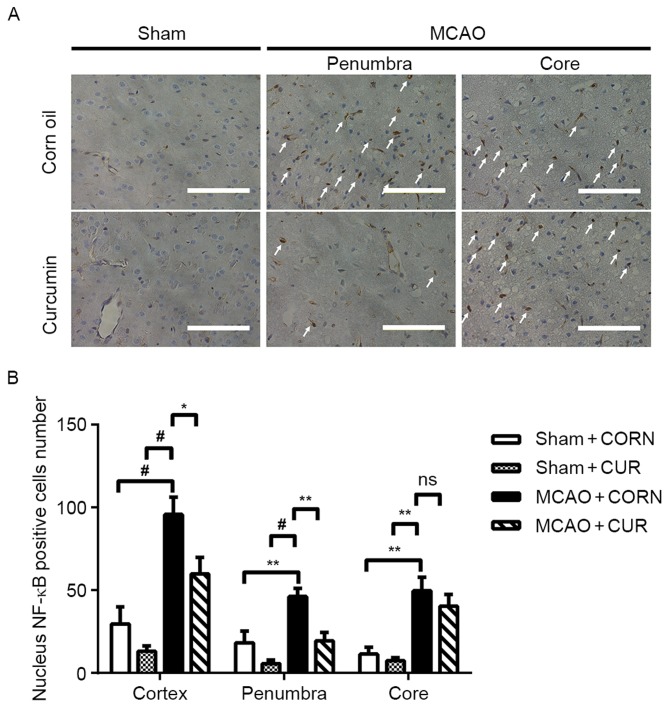
Effect of curcumin on NF-κB expression
A reduced number of cells with positive nuclear expression of NF-κB (indicated by white arrows) was observed in the MCAO + CUR group compared with the MCAO + CORN group in both the penumbra and whole observed cortex areas (Fig. 5A). Quantification confirmed that curcumin treatment significantly reduced nuclear NF-κB expression compared with the MCAO + CORN group in the penumbra and whole observed cortex areas (Fig. 5B).
Figure 5.
Effect of curcumin on nuclear NF-κB expression at the whole cortex, penumbra and core area as detected by immunohistochemistry. (A) Positive NF-κB nuclear staining was visualized in brown color and indicated by white arrows. Scale bar, 100 µm. (B) The number of cells with positive nuclear NF-κB staining was quantified. *P<0.05, **P≤0.01 and #P≤0.0001, with comparisons indicated by brackets. NF, nuclear factor; MCAO, middle cerebral artery occlusion; CUR, curcumin; CORN, corn oil (vehicle control); ns, not significant.
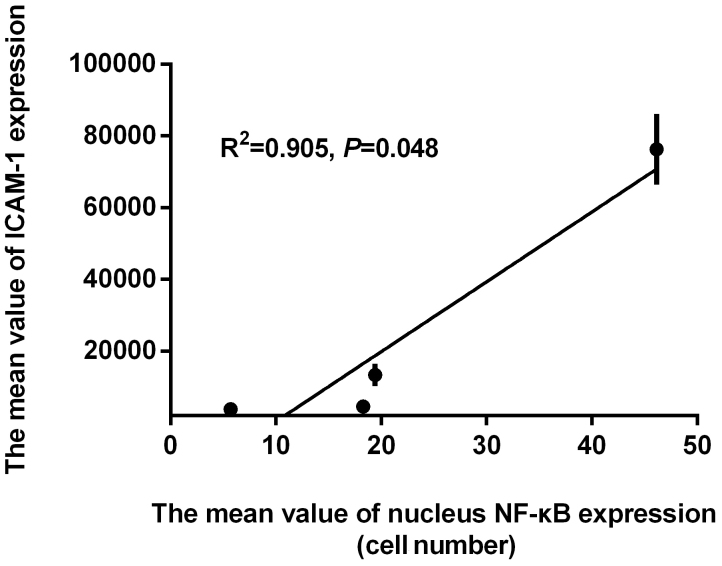
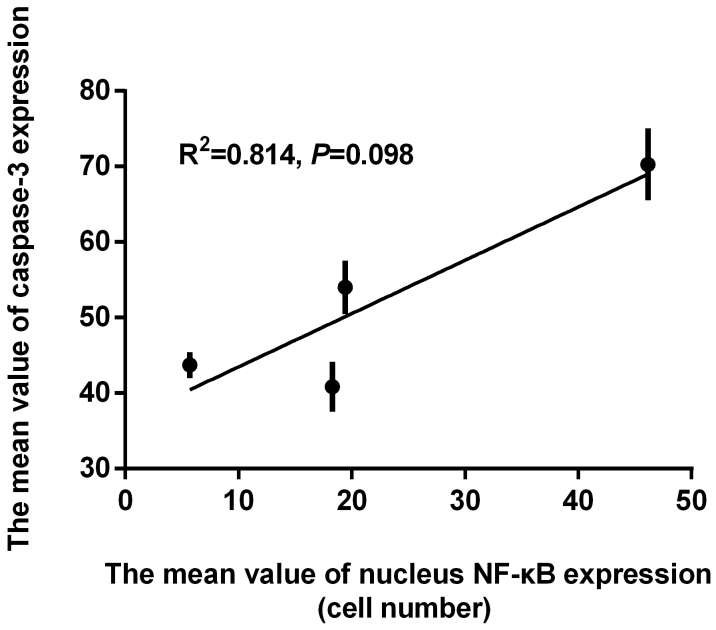
Correlation between nuclear NF-κB-positive cell numbers and the expression of ICAM-1, MMP-9, and caspase-3
In order to determine if the effect of curcumin on ICAM-1, MMP-9 and caspase-3 expression was correlated with NF-κB pathway inhibition, the aforementioned results were further examined by Pearson's correlation analysis. The Pearson's correlation coefficient of determination between the mean values of ICAM-1 and NF-κB was detected, and a significant relationship was observed (R2=0.905, P=0.048; Fig. 6). A significant relationship was also observed between the mean values of MMP-9 and NF-κB expression by Pearson's correlation analysis (R2=0.975, P=0.012; Fig. 7). Finally, the relationship between the mean values of caspase-3 and NF-κB expression was analyzed, however, the results were not significant (R2=0.814, P=0.098; Fig. 8).
Figure 6.
Pearson's correlation analysis between the mean value of ICAM-1 and NF-κB expression in the 4 treatment groups. ICAM, intercellular adhesion molecule; NF, nuclear factor.
Figure 7.
Pearson's correlation analysis between the mean value of MMP-9 and NF-κB expression in the 4 treatment groups. MMP, matrix metalloproteinase; NF, nuclear factor.
Figure 8.
Pearson's correlation analysis between the mean value of caspase-3 and NF-κB expression in the 4 treatment groups. NF, nuclear factor.
Discussion
The brain is a high oxygen demand organ. Once blood vessel occlusion occurs, oxygen and glucose deprivation can lead to permanent loss of neurological function within minutes (23). Therefore, prompt restoration of blood supply to the ischemic area is a critical strategy to effectively prevent extensive brain damage and partially recover neurological function. However, blood flow restoration can also lead to serious side effects to the initial ischemic brain area mediated by accumulation of inflammatory responses. In addition to the delivery of invasive inflammatory cells, the influx of oxygen caused by blood flow restoration can produce excessive reactive oxygen species, which can further trigger and amplify inflammatory responses. Consequently, blood flow restoration can lead to reperfusion damage to the initial ischemic brain area. Hemorrhagic transformation is the most severe complication caused by BBB disruption, which is also mainly affected by the post-reperfusion inflammatory response. It has been suggested that reperfusion can act as an independent predictor for BBB disruption (24,25). Therefore, employing anti-inflammatory agents during reperfusion may be a promising strategy to achieve optimal therapy with less side effects.
Curcumin has been consumed as food and a natural drug for thousands of years in Asian counties. During the past few years, the pharmacokinetics and safety of curcumin have been intensively investigated by acute toxicity, sub-acute toxicity and genotoxicity assays in both in vivo and in vitro studies (26). No toxic effect was observed even when curcumin was orally administered at 2,000 mg/kg to the animals (26). In addition, in phase I clinical trials, curcumin was well tolerated in humans when consumed at a daily dose of 12 g for 3 months (27), except for occasional diarrhea and nausea (27,28). It has also been demonstrated that curcumin possesses pleiotropic properties against a wide variety of disorders, including carcinoma, diabetes, and chronic inflammation diseases (29). In addition, the protective effect of curcumin on reperfusion damage has been reported in retinal (30), testicular (31), cardiac (32), pulmonary (33), and liver (34) tissue. However, to the best of our knowledge, the effect of curcumin on stroke has not been investigated. In the present study, the effect of curcumin on cerebral I/R injury was evaluated in vivo by employing an MCAO model, and the attenuation of infarction volume, neurological dysfunction, and edema were observed (Table I). The findings were consistent with the results of our previous study (11). It has been suggested that the protective effect of curcumin is mainly ascribed to its anti-inflammatory and anti-apoptotic properties, which can partly restore the morphology and/or function of damaged tissue. For example, curcumin exhibits anti-inflammation properties in stabilizing the tight junction structure and mitigating the BBB opening, as well as alleviating edema upon hypoxia intervention (35). In addition, when curcumin is prophylactically administered before stroke, it significantly reverses the augmentation of the infarct volume and neurological dysfunction; this function has been primarily correlated with its anti-apoptotic effect (36). However, the underlying molecular mechanism of curcumin, especially post-ischemia administration of curcumin, on protecting brain reperfusion injury remains poorly understood. Therefore, in the present study, we investigated whether curcumin (30 min post-ischemia administration) could affect cerebral I/R injury through anti-inflammatory and/or anti-apoptotic properties at the molecular level.
ICAM-1 belongs to the immunoglobulin family of cell surface proteins. It is constitutively expressed on many cells, including endothelial cells, fibroblasts, macrophages and activated lymphocytes, with low expression levels under quiescent conditions (37). Upon reperfusion injury, ICAM-1 functions by regulating the interaction between leukocytes and vascular endothelial cells (38) and then facilitating leukocyte infiltration to the damaged brain area. The final result is microvasculature disturbance (‘no-flow’ phenomenon) (39). The infiltrated leukocytes can then enhance the expression of MMP-9. MMP-9 is a potent proteinase that directly digests the extracellular matrix and destroys tight junctions, which are the primary components of the BBB (40), as well as triggers further inflammatory responses (41). Consequently, under the synergistic effects of the above mediators, brain cell death is aggravated (42). It has been confirmed that transient ischemia with reperfusion tends to result in milder damage. Apoptosis-related cell death is more prominent, especially within the ischemic penumbra area (43), which is associated with caspase-3 activation following cerebral I/R (44). At the transcriptional level, ICAM-1, MMP-9 and caspase-3 expression are regulated by NF-κB activation (15,45), which is present in almost all cell types of the nervous system and is well-known for regulating the inflammation and cell apoptosis process (15,46,47). By using in vivo models of reperfusion injury, the molecular mechanisms of anti-inflammatory and anti-apoptotic effects of curcumin have been widely demonstrated, including the decrease in interleukin (IL)-1β, IL-2, IL-6, cyclooxygenase-2, caspase-9, tumor necrosis factor (TNF-α, P-selectin, E-selectin, MMP-2, MMP-3, activator protein (AP)-1, p38 and/or c-Jun N-terminal kinase/ mitogen-activated protein kinase pathways (48–51). However, the regulatory effects of curcumin on NF-κB (52,53), ICAM-1 (54,55), MMP-9 (56,57) and caspase-3 (58,59) were mainly investigated in in vitro studies. To the best of our knowledge, the effects of curcumin on the expression of the above factors have not been previously investigated in in vivo models of cerebral I/R injury.
Previous studies have reported that curcumin could suppress early ICAM-1 expression and, accordingly repress neutrophil adhesion in the MCAO rat model (4 h ischemia plus 4 h reperfusion) (54). However, the regulation effect of curcumin on ICAM-1 protein expression 24 h post-cerebral I/R injury has not been investigated. The present results demonstrated that curcumin intervention (30 min pre-reperfusion) significantly repressed the ICAM-1 expression induced by 24 h reperfusion damage in vivo. Our results extended the findings by Funk et al (54) that curcumin represses ICAM-1 expression post-cerebral I/R, which suggests that the decreased inflammation responses might be attributed to the reduced leukocyte invasion mediated by ICAM-1 inhibition. It is interesting to note that the inhibition effect of curcumin on ICAM-1 in the penumbra and core area were both significant; therefore, curcumin may inhibit ICAM-1 expression in areas with mild as well as severe injury, which might be ascribed to the potent regulatory effect of curcumin on inflammation-related pathways that control ICAM-1 expression, for example NF-κB. Indeed, curcumin was demonstrated to downregulate expression of nuclear NF-κB in the present study. AP-1 is another important transcription factor involved in regulating ICAM-1 expression (60). Curcumin inhibition of ICAM-1 expression in relation to a reduced AP-1 pathway has also been reported (61,62).
Furthermore, curcumin suppressed MMP-9 expression. High expression of MMP-9 is an indicator for hemorrhagic transformation via BBB disruption, especially post-reperfusion (25). The present results indicated that curcumin treatment dramatically prevented the increase of MMP-9 upon cerebral I/R injury at the protein level, which was associated with attenuated edema. The result was consistent with a previous study reporting that curcumin-mediated decrease in MMP-9 expression was paralleled with attenuation of edema and BBB opening (63). However, there is a small discrepancy in the neuro-protective effect of curcumin on the infarction size between the present study and Kelly-Cobbs et al (63). Kelly-Cobbs et al (63) reported no significant change in infarction size between the curcumin-treated and untreated stroke groups. This may be due to different injection doses of curcumin (300 mg/kg vs. 250 mg/kg) and the damage severity of the stroke model (1 h ischemia followed by 24 h reperfusion vs. 3 h ischemia followed by 21 h reperfusion) in the two studies. In addition, although the results from either the penumbra or core areas alone were not significant, the combined results were significant (cortex area results). It can be hypothesized that the non-significant result might be related to the small sample size from the penumbra or core area alone.
Apart from the anti-inflammatory effect of curcumin, an anti-apoptotic effect was observed in the present study. Curcumin intervention significantly decreased the expression of caspase-3 compared with the control group in the penumbra area. Caspase-3 is the enzyme responsible for cell apoptosis. Therefore, inhibition of caspase-3 expression can prevent cell damage induced by apoptosis. In addition, inhibition of caspase-3 expression was concomitant with attenuation of the infarction size in vivo. Of note, the significantly decreased caspase-3 expression was only observed in the penumbra area. Because transient ischemia with reperfusion tends to induce milder damage than permanent ischemia injury to the brain, apoptosis is more prominent than necrosis, especially in the penumbra area. Thus, the inhibition effect of curcumin on apoptosis may be more apparent in the penumbra area.
To further understand the protective effect of curcumin on inflammation and apoptosis, in the present study, the expression of NF-κB, a transcription regulator involved in inflammation and apoptosis, was also analyzed. It has been confirmed that once NF-κB is activated, it translocates to the nucleus and binds to promoter or enhancer regions to facilitate expression of inflammation and apoptosis-related genes (47,64). Previous studies have documented that curcumin is a potent inhibitor of NF-κB in chronic inflammation diseases, including carcinoma (65), diabetes (66) and dementia (67). The mechanism is conducted by suppression of inhibitor of κB (IκB) phosphorylation, of IκB kinase activity, and NF-κB nuclear translocation (68). In brain diseases, the inhibitory action has been examined in cerebral traumatic (69) and alcohol-induced brain impairment models (70). However, the inhibition effect of curcumin on NF-κB in acute cerebral I/R injury remains unknown.
In the present study, elevated NF-κB activation was detected in the MCAO + CORN group, along with increased cerebral I/R injury upon 24 h of reperfusion injury, which suggested that NF-κB activation may be detrimental to cerebral I/R injury. Furthermore, the attenuation of brain infarction size, neurological dysfunction and brain edema were paralleled with decreased NF-κB expression following pre-reperfusion administration of curcumin. These results are in agreement with findings from Tu et al (71) that reported that two doses of curcumin administration attenuated infarction volume, neurological dysfunction and brain edema in a permanent cerebral ischemia rat model, and that this effect was associated with the downregulation of NF-κB activation. In addition, Pearson's correlation analysis demonstrated that expression of ICAM-1 and MMP-9 were significantly correlated with NF-κB expression, which suggested that the inhibition effect of curcumin on ICAM-1 and MMP-9 may be related to inhibition of NF-κB signaling.
The dual effects (pro-apoptotic and anti-apoptotic) of NF-κB activation on cell survival still remain controversial in stroke (72), which can vary depending on the study models employed, injury duration and severity of damage (73). Therefore, the effect of curcumin on NF-κB expression needs to be elucidated. In the present study, even though the results were not significant, the trend of caspase-3 expression changes was similar to those of NF-κB expression. This finding might suggest that the NF-κB activation-induced pro-apoptotic effects overwhelmed anti-apoptosis responses upon 1 h of ischemia and 24 h reperfusion in the MCAO model. In addition, the anti-apoptotic effect of curcumin against the cerebral I/R injury might be closely correlated with NF-κB inhibition. Notably, the inhibition effect of curcumin on NF-κB was mainly achieved in the penumbra area. This finding might explain why curcumin had a stronger effect in the penumbra area to downregulate inflammation and apoptosis-related proteins.
Taken together, the present results suggest that curcumin has neuro-protective effects, most likely via decreasing the expression of NF-κB, and subsequently attenuating the expression of downstream mediators, ICAM-1, MMP-9 and caspase-3. The present study, compared with in vitro studies, may provide better pre-clinical evidence regarding the protective effect of curcumin against cerebral I/R injury and more reliable evidence for clinical experiments in the future, since the rat model used better resembles the pathophysiological status of human stroke.
In conclusion, the augmentation of cerebral injury displayed by enlarged infarction size, edema, and neurological dysfunction, which was correlated with upregulation of ICAM-1, MMP-9, caspase-3 and NF-κB in a cerebral I/R rat model, indicated that ICAM-1, MMP-9 and caspase-3 synergistically aggravated brain damage by mediating inflammatory reaction and cell death; the underlying mechanism was associated to NF-κB activation. Furthermore, pre-reperfusion administration of curcumin protected the brain against I/R injury, which may be due to the inhibition of ICAM-1, MMP-9, caspases-3 and NF-κB expression. Therefore, curcumin may be a useful and promising neuro-protective agent against acute ischemic stroke in the future.
Acknowledgements
The authors would like to acknowledge the help of Assistant Professor Supang Maneesri Le Grand in providing technical and material assistance. This study was supported by the 90th Anniversary of Chulalongkorn University Fund and Ratchadaphiseksomphot Endowment Fund, Faculty of Medicine, Chulalongkorn University.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi M, Kaczmarek L. MMP-9 Inhibition: A therapeutic strategy in ischemic stroke. Mol Neurobiol. 2014;49:563–573. doi: 10.1007/s12035-013-8538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saenger AK, Christenson RH. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin Chem. 2010;56:21–33. doi: 10.1373/clinchem.2009.133801. [DOI] [PubMed] [Google Scholar]
- 4.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 6.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 7.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: A short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46:273–279. doi: 10.1016/S1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 10.Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid Redox Signal. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Suwanwela NC, Patumraj S. Curcumin by down regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Hu XM, Zhang Y, Zeng FD. Effects of sodium beta-aescin on expression of adhesion molecules and migration of neutrophils after middle cerebral artery occlusion in rats. Acta Pharmacol Sin. 2004;25:869–875. [PubMed] [Google Scholar]
- 13.Saja K, Babu MS, Karunagaran D, Sudhakaran PR. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. Int Immunopharmacol. 2007;7:1659–1667. doi: 10.1016/j.intimp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Zhao Y, Zheng WP, Lu YY, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari S, Azari H, McConnell DJ, Afzal A, Mocco J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. J Vis Exp. 2011:2879. doi: 10.3791/2879. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 18.Dohare P, Garg P, Jain V, Nath C, Ray M. Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav Brain Res. 2008;193:289–297. doi: 10.1016/j.bbr.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M, Xiong X, Shatz CJ, Giffard RG. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73:1100–1107. doi: 10.1016/j.neuron.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durukan A, Tatlisumak T. Ischemic stroke in mice and rats. Methods Mol Biol. 2009;573:95–114. doi: 10.1007/978-1-60761-247-6_6. [DOI] [PubMed] [Google Scholar]
- 21.Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Ruan R, Guo AH, Hao YJ, Zheng JS, Wang D. De novo assembly and characterization of narrow-ridged finless porpoise renal transcriptome and identification of candidate genes involved in osmoregulation. Int J Mol Sci. 2015;16:2220–2238. doi: 10.3390/ijms16012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soria FN, Pérez-Samartin A, Martin A, Gona KB, Llop J, Szczupak B, Chara JC, Matute C, Domercq M. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J Clin Invest. 2014;124:3645–3655. doi: 10.1172/JCI71886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35(11 Suppl 1):S2659–S2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 25.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Dávalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. doi: 10.1161/01.STR.0000046764.57344.31. [DOI] [PubMed] [Google Scholar]
- 26.Dandekar P, Dhumal R, Jain R, Tiwari D, Vanage G, Patravale V. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: Acute, sub-acute and genotoxicity studies. Food Chem Toxicol. 2010;48:2073–2089. doi: 10.1016/j.fct.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as ‘Curecumin’: From kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Strimpakos AS, Sharma RA. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 29.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HJ, Xing YQ, Jin W, Li D, Wu K, Lu Y. Effects of curcumin on interleukin-23 and interleukin-17 expression in rat retina after retinal ischemia-reperfusion injury. Int J Clin Exp Pathol. 2015;8:9223–9231. [PMC free article] [PubMed] [Google Scholar]
- 31.Takhtfooladi MA, Asghari A, Takhtfooladi HA, Shabani S. The protective role of curcumin on testicular tissue after hindlimb ischemia reperfusion in rats. Int Urol Nephrol. 2015;47:1605–1610. doi: 10.1007/s11255-015-1101-2. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Wu M, Tang L, Pan Y, Liu Z, Zeng C, Wang J, Wei T, Liang G. Novel curcumin analogue 14p protects against myocardial ischemia reperfusion injury through Nrf2-activating anti-oxidative activity. Toxicol Appl Pharmacol. 2015;282:175–183. doi: 10.1016/j.taap.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhou JH, Zhao S, Chen HE, Chen D, Hao ML, Ying L, Lin LN, Wang WT. Effect of curcumin on caspase-12 and apoptosis in pulmonary ischemia/reperfusion injury mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:1118–1124. (In Chinese) [PubMed] [Google Scholar]
- 34.Lin CM, Lee JF, Chiang LL, Chen CF, Wang D, Su CL. The protective effect of curcumin on ischemia-reperfusion-induced liver injury. Transplant Proc. 2012;44:974–977. doi: 10.1016/j.transproceed.2012.01.081. [DOI] [PubMed] [Google Scholar]
- 35.Sarada SK, Titto M, Himadri P, Saumya S, Vijayalakshmi V. Curcumin prophylaxis mitigates the incidence of hypobaric hypoxia-induced altered ion channels expression and impaired tight junction proteins integrity in rat brain. J Neuroinflammation. 2015;12:113. doi: 10.1186/s12974-015-0326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathore P, Dohare P, Varma S, Ray A, Sharma U, Jagannathan NR, Ray M. Curcuma oil: Reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem Res. 2008;33:1672–1682. doi: 10.1007/s11064-007-9515-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang MJ, Zhang M, Yin YW, Li BH, Liu Y, Liao SQ, Gao CY, Li JC, Zhang LL. Association between intercellular adhesion molecule-1 gene K469E polymorphism and the risk of stroke in a Chinese population: A meta-analysis. Int J Neurosci. 2015;125:175–185. doi: 10.3109/00207454.2014.919916. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 39.del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci. 2010;1207:143–148. doi: 10.1111/j.1749-6632.2010.05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 42.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 43.Love S. Apoptosis and brain ischaemia. Prog Neuropsycho-pharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 44.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 47.Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 48.Yeh CH, Chen TP, Wu YC, Lin YM, Lin Jing P. Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res. 2005;125:109–116. doi: 10.1016/j.jss.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Ovbiagele B. Potential role of curcumin in stroke prevention. Expert Rev Neurother. 2008;8:1175–1176. doi: 10.1586/14737175.8.8.1175. [DOI] [PubMed] [Google Scholar]
- 50.Yeh CH, Lin YM, Wu YC, Lin PJ. Inhibition of NF-kappa B activation can attenuate ischemia/reperfusion-induced contractility impairment via decreasing cardiomyocytic proinflammatory gene up-regulation and matrix metalloproteinase expression. J Cardiovasc Pharmacol. 2005;45:301–309. doi: 10.1097/01.fjc.0000155385.41479.b3. [DOI] [PubMed] [Google Scholar]
- 51.Chen TH, Yang YC, Wang JC, Wang JJ. Curcumin treatment protects against renal ischemia and reperfusion injury-induced cardiac dysfunction and myocardial injury. Transplant Proc. 2013;45:3546–3549. doi: 10.1016/j.transproceed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-kappaB activation. Biochem Pharmacol. 1998;55:775–783. doi: 10.1016/S0006-2952(97)00557-1. [DOI] [PubMed] [Google Scholar]
- 53.Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr Metab (Lond) 2008;5:17. doi: 10.1186/1743-7075-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Funk JL, Frye JB, Davis-Gorman G, Spera AL, Bernas MJ, Witte MH, Weinand ME, Timmermann BN, McDonagh PF, Ritter L. Curcuminoids limit neutrophil-mediated reperfusion injury in experimental stroke by targeting the endothelium. Microcirculation. 2013;20:544–554. doi: 10.1111/micc.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KH, Lee EN, Park JK, Lee JR, Kim JH, Choi HJ, Kim BS, Lee HW, Lee KS, Yoon S. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26:1037–1047. doi: 10.1002/ptr.3694. [DOI] [PubMed] [Google Scholar]
- 56.Park SY, Kim YH, Kim Y, Lee SJ. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J Cell Biochem. 2012;113:3653–3662. doi: 10.1002/jcb.24238. [DOI] [PubMed] [Google Scholar]
- 57.Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Liu ZJ, Liu HQ, Xiao C, Fan HZ, Huang Q, Liu YH, Wang Y. Curcumin protects neurons against oxygen-glucose deprivation/reoxygenation-induced injury through activation of peroxisome proliferator-activated receptor-gamma function. J Neurosci Res. 2014;92:1549–1559. doi: 10.1002/jnr.23438. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs: Potential role for STAT-3 and NF-kappaB signaling. J Invest Dermatol. 2010;130:2110–2119. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 60.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–1386. doi: 10.1016/S0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 61.Xia L, Xie H, Yu Y, Zhou H, Wang T, Yan J. The Effects of NF-κB and c-Jun/AP-1 on the expression of prothrombotic and proinflammatory molecules induced by anti-β2GPI in mouse. PLoS One. 2016;11:e0147958. doi: 10.1371/journal.pone.0147958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yen FL, Tsai MH, Yang CM, Liang CJ, Lin CC, Chiang YC, Lee HC, Ko HH, Lee CW. Curcumin nanoparticles ameliorate ICAM-1 expression in TNF-α-treated lung epithelial cells through p47 (phox) and MAPKs/AP-1 pathways. PloS One. 2013;8:e63845. doi: 10.1371/journal.pone.0063845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly-Cobbs AI, Prakash R, Li W, Pillai B, Hafez S, Coucha M, Johnson MH, Ogbi SN, Fagan SC, Ergul A. Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304:H806–H815. doi: 10.1152/ajpheart.00720.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenihan CR, Taylor CT. The impact of hypoxia on cell death pathways. Biochem Soc Trans. 2013;41:657–663. doi: 10.1042/BST20120345. [DOI] [PubMed] [Google Scholar]
- 65.Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T, Sa G. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: Protection by curcumin. Cancer Res. 2007;67:362–370. doi: 10.1158/0008-5472.CAN-06-2583. [DOI] [PubMed] [Google Scholar]
- 66.Jiménez-Flores LM, López-Briones S, Macías-Cervantes MH, Ramírez-Emiliano J, Pérez-Vázquez V. A PPARγ, NF-κB and AMPK-dependent mechanism may be involved in the beneficial effects of curcumin in the diabetic db/db mice liver. Molecules. 2014;19:8289–8302. doi: 10.3390/molecules19068289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaur U, Banerjee P, Bir A, Sinha M, Biswas A, Chakrabarti S. Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer's disease: The NF-κB connection. Curr Top Med Chem. 2015;15:446–457. doi: 10.2174/1568026615666150114160543. [DOI] [PubMed] [Google Scholar]
- 68.Calabrese V, Bates TE, Mancuso C, Cornelius C, Ventimiglia B, Cambria MT, Di Renzo L, De Lorenzo A, Dinkova-Kostova AT. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 69.Yang Z, Zhao T, Zou Y, Zhang JH, Feng H. Curcumin inhibits microglia inflammation and confers neuroprotection in intracerebral hemorrhage. Immunol Lett. 2014;160:89–95. doi: 10.1016/j.imlet.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Tiwari V, Chopra K. Protective effect of curcumin against chronic alcohol-induced cognitive deficits and neuroinflammation in the adult rat brain. Neurosci. 2013;244:147–158. doi: 10.1016/j.neuroscience.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 71.Tu XK, Yang WZ, Chen JP, Chen Y, Ouyang LQ, Xu YC, Shi SS. Curcumin inhibits TLR2/4-NF-κB signaling pathway and attenuates brain damage in permanent focal cerebral ischemia in rats. Inflammation. 2014;37:1544–1551. doi: 10.1007/s10753-014-9881-6. [DOI] [PubMed] [Google Scholar]
- 72.Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, Schneider A, Schwaninger M. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]